Abstract
We investigated the effects of ‘add on' treatment of α-blocker (AB) on blood pressure (BP) and the safety of ABs in men with symptomatic BPH with or without hypertension. We retrospectively reviewed 2,924 BPH outpatients who took ABs at our institution between 2005 and 2009. BPH symptom severity, prostate volume and BP were determined for 953 patients with their baseline data. BP level and International Prostate Symptom Score were measured within 2 months after AB treatment. Patients were assigned to four groups: group 1 had 272 normotensive patients on concomitant hypertensive medication; group 2 had 293 normotensive patients not on the medication; group 3 had 216 hypertensive patients on concomitant medication; and group 4 had 172 hypertensive patients not on the medication. The addition of AB lowered the mean systolic BP by 16.6 mm Hg for group 3 and by 8.6 mm Hg for group 4, and diastolic BP by 18.0 mm Hg for group 3 (P<0.05). However, normotensive groups on entry, irrespective of antihypertensive medication, showed no significant BP changes from baseline after AB medication. In the hypertensive groups on entry, the doxazosin gastrointestinal therapeutic system (GITS) resulted in significant reductions in systolic BP from 142.2 to 134.9 mm Hg and in diastolic BP from 97.6 to 84.6 mm Hg. When analyzed by AB regimen, the incidence of BP-related adverse events was comparable. AB therapy for BPH can have an appropriate and beneficial effect on BP, especially in baseline hypertensive patients. Doxazosin GITS treatment resulted in optimal management of BP within the normal range, especially in pharmacologically or physiologically hypertensive patients.
Keywords: BPH, blood pressure, alpha blocker, adverse events
Introduction
BPH is often encountered in aging men, and it is the most common urological disorder.1 The prevalence of BPH and hypertension increases with age, hence both are common diseases in elderly males.2 An estimated 25% of men aged >60 years have concomitant BPH and hypertension.2 Although BPH and hypertension seem to involve separate disease processes, it has been postulated that age-related increases in sympathetic tone may have a role in their pathophysiologies.2, 3
Treatments for BPH include surgical or medical therapy. The number of patients treated for BPH is rapidly increasing in Korea, and noninvasive medical therapy is being increasingly chosen as the primary treatment option.4 Of the medications for BPH, selective α1-adrenoceptor antagonists have been considered as an effective, noninvasive treatment option for men with BPH. However, the administration of α-blockers (ABs) to patients with BPH raises the concern that patients who are taking other antihypertensive drugs and those with a normal blood pressure (BP) level could experience excessive reductions in BP that would cause hypotensive symptoms. One agent that is shown to provide rapid relief is doxazosin, a selective α1-adrenoceptor antagonist that is also used to treat hypertension. Doxazosin has been shown to be effective and well tolerated in the treatment of symptomatic BPH in hypertensive patients.5 However, a previous placebo-controlled study of doxazosin in normotensive BPH patients showed a decrease in BP compared with placebo.6 Although other ABs, such as tamsulosin and alfuzosin, are effective for treating patients with BPH and as part of combined therapy in patients with hypertension,7, 8 there are few reports comparing their effects on BP in BPH patients depending on antihypertensive medication. Therefore, we aimed to retrospectively evaluate the effects of ABs on BP in BPH patients with or without concomitant hypertension. We also evaluated the efficacy and safety of ABs in these patients.
Methods
Study design
We retrospectively reviewed 2924 BPH patients who had been initially diagnosed with BPH and prescribed with α1-adrenoceptor antagonists at our institution between January 2005 and October 2009. The symptoms of BPH were recorded through a routine initial evaluation of BPH using a transrectal ultrasound of the prostate, uroflowmetry, International Prostate Symptom Score (IPSS), urine analysis and PSA determinations. At the initial visit, BP level and concomitant hypertension-related medication were also recorded. BP and IPSS were measured within 2 months after AB treatment. Hypertension was defined as a diastolic BP of 90 mm Hg or above in a sitting position. Adverse events (AEs) were defined as symptoms that require discontinuation or change of the current AB medication.
Patients
Patients were excluded from this study if they had ever taken medications such as AB or 5-α-reductase inhibitors. Patients were also excluded if they had neurogenic bladder dysfunction, confirmed prostate cancer, acute or chronic urinary retention status, acute or chronic prostatitis within the last 3 months, serum PSA levels over 10 ng ml−1, a history of recurrent urinary tract infection or bladder stones and previous TURP or other surgical intervention related to BPH. We also excluded patients who were taking other antihypertensive drugs at the baseline point and until follow-up BP measurements. Of the 2924 patients enrolled, BPH symptom severity (assessed by IPSS and urinary flow rate), prostate volume, baseline BP (before AB medication) and follow-up BP (after AB medication) measurements were determined for 953 patients using baseline data. Patients were assigned to four groups: group 1 had 272 normotensive patients on concomitant hypertensive medication; group 2 had 293 normotensive patients not on the medication; group 3 had 216 hypertensive patients on concomitant medication; and group 4 had 172 hypertensive patients not on the medication.
Statistical analysis
All analyses were conducted with SAS statistical software, version 9.1 (SAS Institute, Cary, NC, USA). The means of the groups were compared using Student's t-test and/or analysis of variance test. After performing a covariate analysis of variance adjusting for age and prostate volume, the significance of differences in BP among groups, based on baseline BP and AB regimen, was examined using two-way analysis of covariance with subsequent linear contrasts (Table 1). The χ2-test was used to determine the statistical significance of differences in AEs among groups according to AB regimen. Differences were considered statistically significant when the probability of error was less than 5% (P<0.05).
Table 1. The baseline characteristics of patients according to blood pressure.
| Normotension | Hypertension | |||
|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | |
| No | 272 | 293 | 216 | 172 |
| Age | 62.8±5.7 | 63.3±4.4 | 64.2±5.8 | 65.9±6.1 |
| Systolic BP (mm Hg) | 124.8±2.1 | 125.3±2.6 | 140.4±1.9 | 139.8±2.4 |
| Diastolic BP (mm Hg) | 74.5±0.9 | 76.2±1.0 | 93.1±1.2 | 94.4±1.3 |
| IPSS (total) | 14.7±0.5 | 15.6±0.5 | 14.8±0.3 | 14.2±0.4 |
| QOL | 3.5±0.2 | 3.6±0.5 | 3.7±0.4 | 3.0±0.5 |
| Prostate volume (cc) | 38.8±6.1 | 32.2±4.5 | 41.3±7.1 | 37.3±5.8 |
| PSA (ng ml−1) | 2.1±0.2 | 1.6±0.3 | 1.7±0.5 | 2.0±0.6 |
| Qmax (ml s−1) | 11.0±1.7 | 10.7±0.5 | 11.6±0.8 | 10.7±1.3 |
| Residual urine volume (cc) | 58.6±10.2 | 43.6±7.1 | 62.9±8.4 | 55.4±6.6 |
Abbreviations: BP, blood pressure; IPSS, international prostate symptom score; Qmax, urinary flow rate; QOL, quality of life.
The values for age, blood pressure, prostate volume, PSA, IPSS, QOL, Qmax, and residual urine volume are means±s.d.
Results
Of 953 patients enrolled, 385 patients (40.4%) took tamsulosin 0.2 mg; 203 (21.3%) took alfuzosin 10 mg; 197 (20.7%) were on the doxazosin gastrointestinal therapeutic system (GITS) 4 mg; and 168 (17.7%) took terazosin 2 mg once daily. The overall mean age was 63.2±5.8 years and the mean prostate volume was 38.1±5.5 cc. The mean duration of follow-up was 58.8±7.3 days, and there was no significant difference in the duration of follow-up by AB regimen.
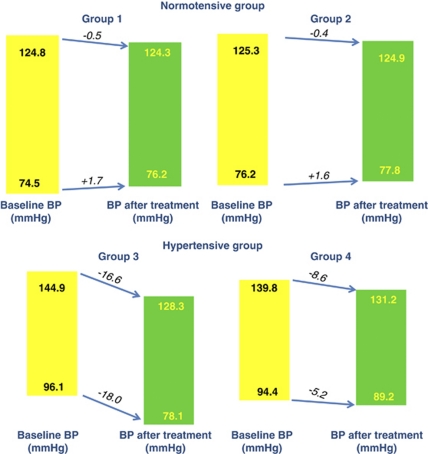
The addition of ABs lowered the mean systolic BP by 16.6 mm Hg for group 3 and by 8.6 mm Hg for group 4, and diastolic BP by 18.0 mm Hg for group 3 (P<0.05) (Figure 1). However, normotensive groups on entry, irrespective of antihypertensive medication, showed no significant BP changes from baseline values after AB medication. After adjusting for age, significant changes in mean systolic BP from baseline values were found in group 2 (Δ−0.4 mm Hg) versus group 3 (Δ−16.6 mm Hg); changes in diastolic BP were found in group 1 (Δ+1.6 mm Hg) versus group 3(Δ−18.0 mm Hg), group 1(Δ+1.6 mm Hg) versus group 4 (Δ−8.2 mm Hg), group 2 (Δ+1.7 mm Hg) versus group 3 (Δ−18.0 mm Hg) and in group 2 (Δ+1.7 mm Hg) versus group 4 (Δ−8.2 mm Hg) (Figure 1).
Figure 1.
Comparison of the mean changes in blood pressure (BP) from baseline values according to group.
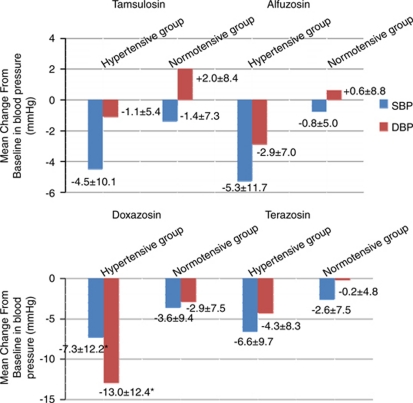
Baseline and follow-up BP levels according to AB regimen are shown in Table 2. Follow-up systolic BP was only significantly lower in the doxazosin GITS treatment group. There were significant improvements in IPSS total score from baseline data for all drugs. However, there were no significant differences among drugs. Figure 2 shows the mean change from baseline data in BP for hypertensive and normotensive patients according to AB regimen. Of the 197 patients in the doxazosin GITS group, 91 patients had baseline high BP (53 with concurrent antihypertensive medication versus 38 without antihypertensive medication). In baseline hypertensive BP patients, treatment with doxazosin GITS resulted in significant reductions in systolic BP from 142.2 mm Hg to 134.9 mm Hg and in diastolic BP from 97.6 mm Hg to 84.6 mm Hg (Δ–7.3 and Δ–13.0 mm Hg, respectively).
Table 2. The baseline and clinical characteristics of patients according to α-blocker regimen.
| Tamsulosin 0.2 mg | Alfuzosin 10 mg | Doxazosin GITS 4 mg | Terazosin 2 mg | |
|---|---|---|---|---|
| No | 385 | 203 | 197 | 168 |
| Age | 62.5±7.0 | 61.8±8.2 | 66.1±6.0 | 64.3±7.6 |
| IPSS (total) | ||||
| Baseline | 16.0±2.1* | 15.5±2.6* | 16.5±1.9* | 15.8±2.4* |
| Follow up | 11.6±2.0* | 10.6±1.8* | 12.0±1.3* | 10.6±1.5* |
| QOL | ||||
| Baseline | 3.7±0.2* | 3.9±0.5* | 4.0±0.4* | 3.9±0.5 |
| Follow up | 3.0±0.3* | 3.1±0.7* | 3.3±0.6* | 3.4±0.3 |
| Prostate volume (cc) | 35.8±6.1 | 32.2±4.5 | 41.3±7.1 | 38.3±5.8 |
| PSA (ng ml−1) | 2.5±0.2 | 1.9±0.3 | 2.4±0.5 | 1.7±0.6 |
| Qmax (ml s−1) | 11.0±1.7 | 11.7±0.5 | 10.6±0.8 | 10.0±1.3 |
| Residual urine volume (cc) | 60.6±10.2 | 51.6±7.1 | 67.9±8.4 | 62.4±6.6 |
| Baseline BP (mm Hg) | ||||
| Systolic | 129.4±6.8 | 130.1±9.3 | 131.3±8.3* | 129.8±7.9 |
| Diastolic | 83.6±9.2 | 84.5±5.9 | 84.9±6.7 | 85.8±6.2 |
| BP following ‘add on' treatment | ||||
| Systolic | 127.6±7.2 | 126.8±10.6 | 125.1±10.9* | 126.2±9.1 |
| Diastolic | 83.3±9.4 | 84.2±8.0 | 83.5±6.9 | 84.1±7.8 |
| Adverse events following ‘add on' treatment | ||||
| Dizziness, N(%) | 11 (2.2) | 7 (3.4) | 10 (5.1) | 8 (4.8) |
| Postural hypotension, N(%) | 7 (1.9) | 5 (2.5) | 11 (5.6) | 7 (4.2) |
| Etc., N(%) | 3 (0.8) | 2 (1.0) | 2 (1.0) | 5 (3.0) |
Abbreviations: BP, blood pressure; GITS, gastrointestinal therapeutic system; IPSS, international prostate symptom score; Qmax, urinary flow rate; QOL, quality of life.
The values for age, blood pressure, prostate volume, PSA, IPSS, QOL, Qmax, and residual urine volume are means ±s.d.
*P<0.05 by ANCOVA test.
Figure 2.
The mean s.d. change in blood pressure (BP) from baseline values for hypertensive and normotensive patients according to α blocker regimen. DBP, diastolic BP; SBP, systolic BP.
The incidence of BP-related side effects, such as dizziness or postural hypotension, was significantly higher in group 3 (P=0.021). When analyzed by AB regimen, the incidence of BP-related side effects was comparable, and the differences among groups were not significant (Table 2).
Discussion
Because BPH and hypertension are chronic conditions, long-term medication must be safe and continuously effective during treatment. The efficacy of ABs for BPH treatment has been well documented, and our results compare favorably with those of previous studies.4, 9, 10However, despite similar efficacy, ABs have different AEs, and many AEs are secondary to reduction in BP (for example, dizziness and orthostatic hypotension).9 In a recent meta-analysis,11 alfuzosin, terazosin and doxazosin showed a significantly higher risk of developing vascular events compared with placebo, and tamsulosin showed an increase with no significance. Our previously reported treatment with doxazosin GITS for 1 year resulted in a significantly higher reduction in BP in hypertensive patients than in normotensive patients.12 Furthermore, BP in the concomitant antihypertensive medication group was reduced significantly compared with that in the no-medication group. However, in the present study, treatment with doxazosin GITS resulted in significant reductions in systolic and diastolic BP, especially in baseline hypertensive BP patients, irrespective of concomitant antihypertensive medication. These contradictory findings might result from the relatively small number of patients in the concomitant antihypertensive medication group (n=18) in the previous study. Our results suggest that doxazosin GITS treatment has the ‘additional benefit' of lowering BP in pharmacologically or physiologically hypertensive patients. However, we were unable to find out why the AB ‘add on' treatment lowered BP, especially in patients with baseline high BP. Our results warrant future prospective studies to elucidate the underlying mechanism.
In this study, the overall incidence of AEs was comparable among the four groups, even though systolic BP was significantly lower in the doxazosin GITS group after doxazosin GITS ‘add on' treatment (Table 2). Because ABs cause vasodilation, vascular AEs take the form of dizziness, presyncope or syncope. These symptoms can be life threatening, particularly in an older patient population. Terazosin and doxazosin, originally developed as antihypertensive drugs, are nonsubtype selective ABs, and both are associated with a larger number of vasodilatory side effects than either tamsulosin or alfuzosin.13, 14, 15, 16In this study, the occurrence of medication-related AEs was lower than the 16.1% previously reported by Kirby et al.17, possibly because the present study had a short-term follow-up period.
There are several limitations. First, it is a retrospective study with a short-term follow-up period. A prospective study with a long-term follow-up period is needed to confirm our findings. Another limitation was that the safety aspect of our study was limited to vascular AEs because of their potentially life-threatening effects. We only identified with certainty those of sufficient severity to require discontinuation of ABs. Finally, we measured BP only with the patient in the seated position. We acknowledge this methodological flaw of not measuring BP in a supine position, in addition to the sitting position, to rule out any orthostatic hypotension that might be present. However, despite these limitations, our results provide adequate preliminary data to support a prospective, randomized, controlled trial of the effect of AB ‘add on' treatment on BP in patients with baselinehigh or normal BP.
Conclusions
AB therapy for BPH can have an appropriate and beneficial effect on BP, especially in baseline hypertensive patients. Furthermore, doxazosin GITS treatment resulted in optimal management of BP within the normal range, especially in pharmacologically or physiologically hypertensive patients.
The authors declare no conflict of interest.
References
- Tammela T. Benign prostatic hyperplasia. Practical treatment guidelines. Drugs Aging. 1997;10:349–366. doi: 10.2165/00002512-199710050-00004. [DOI] [PubMed] [Google Scholar]
- Boyle P, Napalkov P. The epidemiology of benign prostatic hyperplasia and observations on concomitant hypertension. Scand J Urol Nephrol Suppl. 1995;168:7–12. [PubMed] [Google Scholar]
- Parsons JK. Modifiable risk factors for benign prostatic hyperplasia and lower urinary tract symptoms: new approaches to old problems. J Urol. 2007;178:395–401. doi: 10.1016/j.juro.2007.03.103. [DOI] [PubMed] [Google Scholar]
- Chung BH, Hong SJ, Lee MS. Doxazosin for benign prostatic hyperplasia: an open-label, baseline-controlled study in Korean general practice. Int J Urol. 2005;12:159–165. doi: 10.1111/j.1442-2042.2005.00998.x. [DOI] [PubMed] [Google Scholar]
- Fawzy A, Hendry A, Cook E, Gonzalez F. Long-term (4 year) efficacy and tolerability of doxazosin for the treatment of concurrent benign prostatic hyperplasia and hypertension. Int J Urol. 1999;6:346–354. doi: 10.1046/j.1442-2042.1999.00071.x. [DOI] [PubMed] [Google Scholar]
- Fawzy A, Braun K, Lewis GP, Gaffney M, Ice K, Dias N. Doxazosin in the treatment of benign prostatic hyperplasia in normotensive patients: a multicenter study. J Urol. 1995;154:105–109. [PubMed] [Google Scholar]
- Lepor H. Phase III multicenter placebo-controlled study of tamsulosin in benign prostatic hyperplasia Tamsulosin Investigator Group. Urology. 1998;51:892–900. doi: 10.1016/s0090-4295(98)00126-5. [DOI] [PubMed] [Google Scholar]
- Chapple CR. A Comparison of Varying alpha-Blockers and Other Pharmacotherapy Options for Lower Urinary Tract Symptoms. Rev Urol. 2005;7 (Suppl 4:S22–S30. [PMC free article] [PubMed] [Google Scholar]
- Djavan B, Marberger M. A meta-analysis on the efficacy and tolerability of alpha1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. Eur Urol. 1999;36:1–13. doi: 10.1159/000019919. [DOI] [PubMed] [Google Scholar]
- Nix JW, Carson CC. Medical management of benign prostatic hypertrophy. Can J Urol. 2007;14 (Suppl 1:53–57. [PubMed] [Google Scholar]
- Nickel JC, Sander S, Moon TD. A meta-analysis of the vascular-related safety profile and efficacy of alpha-adrenergic blockers for symptoms related to benign prostatic hyperplasia. Int J Clin Pract. 2008;62:1547–1559. doi: 10.1111/j.1742-1241.2008.01880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung BH, Hong SJ. Long-term follow-up study to evaluate the efficacy and safety of the doxazosin gastrointestinal therapeutic system in patients with benign prostatic hyperplasia with or without concomitant hypertension. BJU Int. 2006;97:90–95. doi: 10.1111/j.1464-410X.2006.05858.x. [DOI] [PubMed] [Google Scholar]
- Lepor H. Long-term efficacy and safety of terazosin in patients with benign prostatic hyperplasia. Terazosin Research Group. Urology. 1995;45:406–413. doi: 10.1016/s0090-4295(99)80008-9. [DOI] [PubMed] [Google Scholar]
- Roehrborn CG, Oesterling JE, Auerbach S, Kaplan SA, Lloyd LK, Milam DE, et al. The hytrin community assessment trial study: a one-year study of terazosin versus placebo in the treatment of men with symptomatic benign prostatic hyperplasia. HYCAT Investigator Group. Urology. 1996;47:159–168. doi: 10.1016/s0090-4295(99)80409-9. [DOI] [PubMed] [Google Scholar]
- Kirby RS, Roehrborn C, Boyle P, Bartsch G, Jardin A, Cary MM, et al. Efficacy and tolerability of doxazosin and finasteride, alone or in combination, in treatment of symptomatic benign prostatic hyperplasia: the prospective european doxazosin and combination therapy (PREDICT) trial. Urology. 2003;61:119–126. doi: 10.1016/s0090-4295(02)02114-3. [DOI] [PubMed] [Google Scholar]
- McConnell JD, Roehrborn CG, Bautista OM, Andriole GL, Jr, Dixon CM, Kusek JW, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- Kirby RS, Andersen M, Gratzke P, Dahlstrand C, Hoye K. A combined analysis of double-blind trials of the efficacy and tolerability of doxazosin-gastrointestinal therapeutic system, doxazosin standard and placebo in patients with benign prostatic hyperplasia. BJU Int. 2001;87:192–200. doi: 10.1046/j.1464-410x.2001.02032.x. [DOI] [PubMed] [Google Scholar]