Summary
Background
Although survival of children with acute lymphoblastic leukaemia has improved greatly in the past two decades, the outcome of those who relapse has remained static. We investigated the outcome of children with acute lymphoblastic leukaemia who relapsed on present therapeutic regimens.
Methods
This open-label randomised trial was undertaken in 22 centres in the UK and Ireland and nine in Australia and New Zealand. Patients aged 1–18 years with first relapse of acute lymphoblastic leukaemia were stratified into high-risk, intermediate-risk, and standard-risk groups on the basis of duration of first complete remission, site of relapse, and immunophenotype. All patients were allocated to receive either idarubicin or mitoxantrone in induction by stratified concealed randomisation. Neither patients nor those giving interventions were masked. After three blocks of therapy, all high-risk group patients and those from the intermediate group with postinduction high minimal residual disease (≥10−4 cells) received an allogenic stem-cell transplant. Standard-risk and intermediate-risk patients with postinduction low minimal residual disease (<10−4 cells) continued chemotherapy. The primary outcome was progression-free survival and the method of analysis was intention-to-treat. Randomisation was stopped in December, 2007 because of differences in progression-free and overall survival between the two groups. This trial is registered, reference number ISCRTN45724312.
Findings
Of 239 registered patients, 216 were randomly assigned to either idarubicin (109 analysed) or mitoxantrone (103 analysed). Estimated 3-year progression-free survival was 35·9% (95% CI 25·9–45·9) in the idarubicin group versus 64·6% (54·2–73·2) in the mitoxantrone group (p=0·0004), and 3-year overall survival was 45·2% (34·5–55·3) versus 69·0% (58·5–77·3; p=0·004). Differences in progression-free survival between groups were mainly related to a decrease in disease events (progression, second relapse, disease-related deaths; HR 0·56, 0·34–0·92, p=0·007) rather than an increase in adverse treatment effects (treatment death, second malignancy; HR 0·52, 0·24–1·11, p=0·11).
Interpretation
As compared with idarubicin, mitoxantrone conferred a significant benefit in progression-free and overall survival in children with relapsed acute lymphobastic leukaemia, a potentially useful clinical finding that warrants further investigation.
Funding
Cancer Research UK, Leukaemia and Lymphoma Research, Cancer Council NSW, and Sporting Chance Cancer Foundation.
Introduction
In the past three decades, survival in children with acute lymphoblastic leukaemia has improved from 50% to more than 80%.1 For patients who relapse, changes in subsequent management have had little effect on outcome,2 and acute lymphoblastic leukaemia remains a leading cause of death in childhood. The overall survival of relapsed acute lymphoblastic leukaemia has remained between 46% and 56% in the UK from 1991 to 2003.3,4 Similar outcomes have been reported by the Berlin Frankfurt Münster group in Germany5 and Children's Oncology Group in the USA.6 During this time, the pattern of relapse has changed, with a proportionate increase in patients who have disease recurrence within the CNS.7 To meet these challenges, a total redesign of the management of relapsed acute lymphoblastic leukaemia was undertaken in the UK, and the ALLR3 trial opened in 2003.
Intergroup study results have shown that the outcome after a relapse improves with the length of first remission.4–6 Some late relapses are thought to arise from a common precursor that retains the chemosensitivity of the original clone, which could explain the high cure rates achieved with chemotherapy alone in late relapses. In the UK most relapses occur late,7 and ALLR3 was thus designed to have a conventional four-drug induction with continuous asparagine depletion throughout the first 3 months. This approach effectively achieves remission in patients with relapsed disease.4–6,8 For more resistant disease, a second block of non-cross resistant drugs (at the time of protocol design, cyclophosphamide and etoposide) was introduced. To target the extramedullary compartment, intrathecal methotrexate and high-dose CNS penetrating schedules of cytarabine9 and methotrexate10 in a Capizzi11 design were introduced. A randomisation of idarubicin or mitoxantrone was introduced in induction. The choice of idarubicin was based on its previous use in relapsed acute lymphoblastic leukaemia11,12 and on the evidence that the metabolite idarubicinol enters the CNS.13 Mitoxantrone was chosen as the test drug on the basis of its chemosensitivity profile in relapsed acute lymphoblastic leukaemia14 and a reported B-cell specific effect.15 Whereas idarubicin is a topoisomerase IIα poison and targets cycling cells, the additional inhibition of topoisomerase-IIβ activity by mitoxantrone was thought to have an advantage against more quiescent cells.16 The detection of minimal residual disease by real-time PCR (RQ-PCR) analysis of antigen receptors17 at the end of induction was used to select patients for allogenic stem-cell transplant (allo-SCT) and to establish the kinetics of the randomised therapeutic response. In this Article, we report the outcome of this randomisation.
Methods
Patients
Patients aged 1–18 years with a first relapse of acute lymphoblastic leukaemia who had not received an allo-SCT in first complete remission were eligible. Those with mature B-acute lymphoblastic leukaemia were excluded. The study opened in January, 2003, in 22 participating centres of the Children's Cancer and Leukaemia Group (CCLG) in the UK and Ireland. In November, 2006, nine centres of the Australian and New Zealand Children's Haematology/Oncology Group (ANZCHOG) joined the trial. This analysis is based on a snapshot of the data taken in June, 2009. Ethics approval in the UK was obtained from MREC for Wales and from institutional ethics committees outside the UK. Written informed consent was obtained from patients or from parents or legal guardians.
Randomisation and masking
Patients were randomly assigned to receive either mitoxantrone or idarubicin on days 1 and 2 of induction. The trial statistician generated randomisation lists by stratified block randomisation with varying block sizes, stratified by risk group and study group (CCLG or ANZCHOG). ALLR3 was run on a custom built, web-enabled database with decision support systems allowing data entry by participating centres or groups; centres could therefore enrol patients directly, with treatment being assigned by the computer according to centrally held lists, which enabled allocation concealment.
Procedures
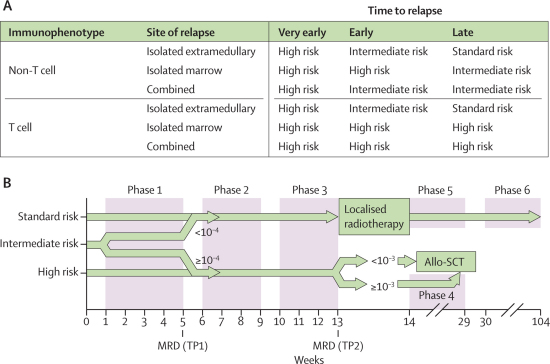
A marrow relapse was defined as more than 25% blasts in the bone marrow. CNS relapse was defined as more than 5 white blood cells per μL with morphological evidence of blasts in the cerebrospinal fluid (CSF). Testicular disease was diagnosed clinically and confirmed with ultrasonography or by biopsy. The sites of relapse were defined as isolated bone marrow, isolated extramedullary, or combined (>5% blasts in the bone marrow along with at least one extramedullary site). Time to relapse was classified as very early within 18 months of first diagnosis, early after 18 months of first diagnosis but within 6 months after end of therapy, and late if more than 6 months after end of therapy.4 The time to relapse, site of relapse, and immunophenotype were used to risk stratify patients into standard-risk, intermediate-risk, and high-risk groups (figure 1).
Figure 1.
Risk stratification (A) and trial design (B)
(A) Stratification according to immunophenotype, site of relapse, and time to relapse. Risk groups: very early refers to less than 18 months from first diagnosis; early refers to 18 months or more after first diagnosis and less than 6 months from stopping therapy; and late refers to 6 months or more after stopping therapy. (B) MRD sampling TPs are marked. At TP1, standard-risk and intermediate-risk patients with MRD lower than 10−4 cells were ineligible, and high-risk and intermediate-risk patients with MRD of 10−4 cells or more were eligible for allo-SCT. Localised radiotherapy was given to those with extramedullary disease and not proceeding to allo-SCT. When MRD assessment was not possible in intermediate-risk patients, allo-SCT was allowed provided relapse occurred within 24 months of stopping therapy. Details of the phases are provided in table 1. MRD=minimal residual disease. TP=timepoint. Allo-SCT=allogenic stem-cell transplant.
Patients were defined as having achieved a second complete remission if they had less than 5% blasts in the marrow or no blasts in the CSF at the end of phase 1 (first timepoint, figure 1). Minimal residual disease was measured from marrow samples obtained at diagnosis, at the end of induction (first timepoint), and after phase 3 (second timepoint). At first timepoint, low minimal residual disease was defined as fewer than 10−4 cells with two sensitive markers (quantitative range 10−4) and high minimal residual disease by at least one marker of 10−4 cells or more. All others were classified as indeterminate. Minimal residual disease was not estimated in isolated extramedullary disease. All patients received three consecutive blocks of chemotherapy (figure 1 and table 1) and patients in both groups were allocated to allo-SCT according to risk groups and minimal residual disease (figure 1).
Table 1.
Therapeutic protocol
| Dose | Days | |
|---|---|---|
| Phase 1—induction (weeks 1–4) | ||
| Intrathecal methotrexate* | .. | 1, 8 |
| Dexamethasone oral | 20 mg/m2 | 1–5; 15–19 |
| Mitoxantrone IV or idarubicin IV | 10 mg/m2 | 1, 2 |
| Vincristine IV | 1·5 mg/m2 | 3, 10, 17, 24 |
| PEG-asparaginase IM† | 1000 u/m2 | 3, 18 |
| Phase 2—consolidation (weeks 5–8) | ||
| Dexamethasone oral | 6 mg/m2 | 1–5 |
| Vincristine IV | 1·5 mg/m2 | 3 |
| Intrathecal methotrexate* | .. | 8 |
| Methotrexate IV‡ | 1000 mg/m2 | 8 |
| PEG-asparaginase IM† | 1000 u/m2 | 9 |
| Cyclophosphamide IV | 440 mg/m2 | 15–19 |
| Etoposide IV | 100 mg/m2 | 15–19 |
| Phase 3—intensification (weeks 9–12) | ||
| Intrathecal methotrexate* | .. | 1, 22 |
| Dexamethasone oral | 6 mg/m2 | 1–5 |
| Vincristine IV | 1·5 mg/m2 | 3 |
| Cytarabine IV, every 12 h | 3000 mg/m2 | 1, 2, 8, 9 |
| Erwinase IM | 20000 u/m2 | 2, 4, 9, 11, 23 |
| Methotrexate IV‡ | 1000 mg/m2 | 22 |
| Phase 4—before SCT§ | ||
| Fludarabine IV | 25 mg/m2 | 1–5 |
| Cytarabine IV | 2000 mg/m2 | 1–5 |
| Liposomal daunorubicin citrate IV | 100 mg/m2 | 1 |
| Phase 5—before continuation (weeks 14–29)¶ | ||
| Intrathecal methotrexate‖ | .. | 1, 43, 57, 99 |
| Dexamethasone oral | 6 mg/m2 | 1–5, 57–61 |
| 6-mercaptopurine oral | 75 mg/m2 | 1–42, 57–98 |
| Vincristine IV | 1·5 mg/m2 | 3, 59 |
| Methotrexate oral | 20 mg/m2 | 10,17, 31, 38, 67, 74, 88, 95 |
| Methotrexate every 6 h | 25 mg/m2 | 22, 78 |
| Etoposide IV | 150 mg/m2 | 42, 49, 99, 106 |
| Cyclophosphamide IV | 300 mg/m2 | 42, 49, 99, 106 |
| Cytarabine IV | 50 mg /m2 | 43–46, 50–53, 100–103, 107–110 |
| Phase 6—continuation treatment (weeks 30—104)¶ | ||
| Dexamethasone oral, every 4 weeks | 6 mg/m2 | 1–5 |
| Vincristine IV, every 4 weeks | 1·5 mg/m2 | 1 |
| Intrathecal methotrexate,* every 12 weeks | .. | .. |
| 6-mercaptopurine oral, daily | 75 mg/m2 | .. |
| Methotrexate oral, weekly | 20 mg/m2 | .. |
IV=intravenous. PEG=polyethylene glycol. IM=intramuscular. SCT=stem-cell transplant. q6h=every 6 h. MRD=minimal residual disease. TP=timepoint. Dexamethasone was given as two divided doses with a maximum daily dose of 40 mg. Patients were randomly assigned to receive either idarubicin or mitoxantrone.
Doses of intrathecal methotrexate were age-related: 8 mg for children younger than 2 years, 10 mg for those aged 2 years, and 12 mg for children older than 2 years.
Six doses of erwinase, 20 000 u IM on alternate days, replaced each dose of PEG-asparaginase for those with a known allergy to E coli asparaginase.
Methotrexate was infused over 36 h and calcium folinate rescue started 48 h from start of infusion.
Only patients with an MRD of 10−3 cells or higher at TP2 were eligible for phase 4.
Phases 5 and 6 were given to patients who were not transplanted. Patients with testicular or CNS disease received 24 Gy as fractionated treatment before start of phase 5.
Oral methotrexate replaced intrathecal methotrexate for patients who had received cranial irradiation. Oral methotrexate was not given on the weeks of intrathecal methotrexate.
Cytogenetic analysis was done locally and reviewed centrally by the Leukaemia Research Cytogenetics Group. When relapse cytogenetics was not done (eg, isolated extramedullary relapse), the original diagnostic cytogenetics was used. Outcome after first relapse was used to define the poor-risk cytogenetic group used in these analyses (see webappendix p 1).
For the randomised comparison, progression-free survival was the primary outcome. Progression-free survival was defined as time from randomisation to the first of induction failure (5% blasts or more in bone marrow at first timepoint, persistence of CSF blasts, non-regression of testicular enlargement), relapse, death from any cause, or a second malignancy. Secondary outcomes were overall survival, defined as the time from randomisation to death from any cause, and proportion of patients in the intermediate-risk group with low minimal residual disease at first timepoint. Adverse events were graded according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) v 3.0.18 Safety was summarised for all patients by the number of toxic effects during the trial scored at CTCAE18 grade 3 or higher, and the proportion of patients having a serious adverse event.19
Statistical analysis
The first planned interim analysis (May, 2007) drew attention to differences in overall survival and progression-free survival between the two groups, and, after 6 months of further observation, an independent data-monitoring committee recommended closure of the randomisation. Final analysis of randomised objectives did not take place until 2009 to allow the data to mature. No adjustment to results in this analysis was made to account for previous observations of the data.
We included all randomised patients from all study groups in the main analysis, analysed by intention-to-treat apart from three ineligible patients excluded and one patient censored at the time of major protocol violation. Analysis was repeated in UK patients only as a secondary objective. For progression-free and overall survival the primary analysis was done by Kaplan-Meier plot and (unstratified) log-rank test. Multiple Cox regression was done to assess treatment effect after adjustment for prespecified prognostic covariates: study group, risk group (treated as ordinal), ETV6-RUNX1, age group (1–<6, 6–10, and ≥10 years), and sex. We used multiple imputation to impute missing ETV6-RUNX1 data. The Cox model for progression-free survival was used to separately assess interactions of the randomly assigned drug with the following variables: immunophenotype, site, time from diagnosis to relapse (continuous), and minimal residual disease at the first timepoint (<1×10−4 or ≥1×10−4 cells). For minimal residual disease, only intermediate-risk patients with bone marrow or combined relapse were included. Logistic regression, with and without the covariates, was used to explore the relation between the drugs and minimal residual disease at the first timepoint in intermediate-risk patients, for whom a measurement of minimal residual disease was available.
The number of toxic effects at grade 3 or higher per patient was modelled with Poisson regression.20 We compared the number of patients who had at least one serious adverse event between treatments with a χ2 test, and relative risk is presented. An exploratory competing risks analysis used the Fine and Gray model21 and Gray's test,22 splitting events of progression-free survival into disease-related events (progression, relapse, disease-related deaths) and treatment-related events (treatment-related deaths, second malignancies). Webappendix pp 2–3 provides further details.
This study is registered, reference number ISCRTN45724312.
Role of the funding source
The sponsors and funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of this report. The corresponding author had full access to all the data and had final responsibility for the decision to submit for publication.
Results
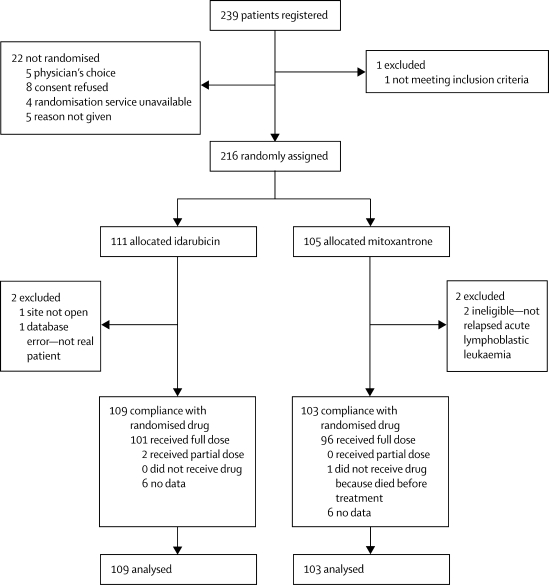
Of the 239 consecutive patients registered in the study, 216 were randomly assigned to either idarubicin (n=111 patients) or mitoxantrone (n=105 patients), of whom 109 and 103 patients, respectively, were analysed (figure 2). The two randomised groups were similar in sex, age at relapse, and immunophenotype (table 2). Although risk groups were balanced, we noted differences between the treatment groups with regard to site, time to relapse, and cytogenetic subtypes. The mitoxantrone group had more late recurrences, isolated marrow relapses, and high hyperdiploid patients than did the idarubicin group (table 2).
Figure 2.
Trial profile
All patients were scheduled to receive two doses of anthracycline. Data for having received the drug were not available for 12 patients, six in each group.
Table 2.
Characteristics of randomised patients
| Idarubicin | Mitoxantrone | ||
|---|---|---|---|
| n | 109 | 103 | |
| Sex | |||
| Boys | 70 (64%) | 61 (59%) | |
| Girls | 39 (36%) | 42 (41%) | |
| Age at first relapse (years) | |||
| Median (IQR) | 9·1 (6·4–13·1) | 9·6 (7·0–12·9) | |
| 1–<6 | 22 (20·2%) | 10 (9·7%) | |
| 6–9 | 42 (38·5%) | 45 (43·7%) | |
| ≥10 | 45 (41·3%) | 48 (46·6%) | |
| Immunophenotype (B:T) | |||
| B | 95 (87·2%) | 93 (90·3%) | |
| T | 14 (12·8%) | 10 (9·7%) | |
| Time to relapse (months) | |||
| Median (IQR) | 37·4 (24·0–55·1) | 42·8 (29·6–57·5) | |
| Very early | 14 (12·8%) | 12 (11·7%) | |
| Early | 40 (36·7%) | 25 (24·3%) | |
| Late | 55 (50·5%) | 66 (64·1%) | |
| Site of relapse | |||
| Isolated extramedullary | 32 (29·4%) | 20 (19·4%) | |
| Isolated marrow | 56 (51·4%) | 67 (65·1%) | |
| Combined | 21 (19·3%) | 16 (15·5%) | |
| Risk group | |||
| Standard | 5 (4·6%) | 3 (2·9%) | |
| Intermediate | 80 (73·4%) | 76 (73·8%) | |
| High | 24 (22·0%) | 24 (23·3%) | |
| Age at first diagnosis | |||
| Median (IQR) | 5·0 (2·9–8·7) | 5·3 (3·4–9·1) | |
| <1 years | 5 (4·6%) | 1 (1·0%) | |
| 1–9 years | 85 (78·0%) | 83 (80·6%) | |
| ≥10 years | 19 (17·4%) | 19 (18·4%) | |
| White-cell count at first diagnosis | |||
| <50 × 109 cells per L | 61 (56·0%) | 69 (67·0%) | |
| ≥50 × 109 cells per L | 22 (20·2%) | 20 (19·4%) | |
| Unknown | 26 (23·9%) | 14 (13·6%) | |
| Cytogenetics | |||
| High hyperdiploid | 22 (20·2%) | 32 (31·1%) | |
| ETV6-RUNX1 | 17 (15·6%) | 14 (13·6%) | |
| Normal | 8 (7·3%) | 7 (6·8%) | |
| Poor | 12 (11·0%) | 9 (8·7%) | |
| Other | 41 (37·6%) | 30 (29·1%) | |
| Unknown | 9 (8·3%) | 11 (10·7%) | |
| High risk | 24 | 24 | |
| Isolated extramedullary | 1 (4·2%) | 4 (16·7%) | |
| Isolated marrow | 20 (83·3%) | 17 (70·8%) | |
| Combined | 3 (12·5%) | 3 (12·5%) | |
| T cell | 7 (29·2%) | 8 (33·3%) | |
| Intermediate and standard risks | 85 | 79 | |
| Isolated extramedullary | 31 (36·5%) | 16 (20·3%) | |
| Isolated marrow | 36 (42·4%) | 50 (63·3%) | |
| Combined | 18 (21·2%) | 13 (16·5%) | |
Data are number (%) unless otherwise indicated. IQR=interquartile range, numbers in brackets show lower and upper quartiles. MLL=patients with myeloid-lymphoid or mixed lineage leukaemia rearrangements. Ph+=philadelphia-chromosome positive. TCF3=includes patients with TCF3-PBX1 and TCF3-HLF fusions.
The median follow-up was 41 months in both groups (95% CI 34–48 for idarubicin and 35–46 for mitoxantrone, reverse Kaplan-Meier method). Table 3 shows treatment outcomes to June, 2009. 108 (51%) of 212 evaluable patients are in second complete remission: 45 of 109 (41%) in the idarubicin group and 63 of 103 (61%) in the mitoxantrone group. Of the 56 subsequent relapses, a third complete remission was achieved in six of 38 patients in the idarubicin group and three of 18 patients in the mitoxantrone group. 49 patients were transplanted in each group. 16 (33%) patients relapsed after allo-SCT in the idarubicin group and two (4%) in the mitoxantrone group.
Table 3.
Trial outcome as at June, 2009
|
Idarubicin |
Mitoxantrone |
||||
|---|---|---|---|---|---|
| SCT | No SCT | SCT | No SCT | ||
| End of phase 1 (TP1) | |||||
| High risk | 21 | .. | 14 | .. | |
| Intermediate and standard risk | |||||
| High MRD | 21 | .. | 24 | .. | |
| Low MRD | .. | 16 | 17 | ||
| Extramedullary | 24 | 5 | 12 | 2 | |
| Indeterminate | 9 | 3 | 9 | 9 | |
| Total | 75 | 24 | 59 | 28 | |
| End of phase 2 | |||||
| Withdrawn | 1 | .. | .. | .. | |
| Relapse | 2 | .. | .. | .. | |
| Treatment-related mortality | 1 | 1 | 1 | .. | |
| End of phase 3 (TP2) | |||||
| Relapse | 7 | .. | 1 | .. | |
| Treatment-related mortality | 5 | .. | .. | .. | |
| Total | 59 | 23 | 57 | 28 | |
| Phase 4 | |||||
| Relapse | 1 | NA | 1 | NA | |
| Treatment received: not transplanted | |||||
| Relapse | 9 | 3 | 8 | 6 | |
| Second remission | 1 | 18 | 4 | 17 | |
| Treatment-related mortality | .. | 1 | .. | .. | |
| Treatment received: transplanted | |||||
| Relapse | 16 | .. | 2 | .. | |
| Treatment-related mortality | 6 | .. | 2 | 2 | |
| Accident | 1 | .. | 1 | .. | |
| Second remission | 25 | 1 | 39 | 3 | |
In phase 1, of the patients treated with mitoxantone, three withdrew, eight failed induction and there were five treatment-related mortalities. Of the patients who were treated with idarubicin, five failed induction and there were five treatment-related mortalities. Patients were allocated at the end of phase 1 to either receive an allo-SCT (SCT) or continue in chemotherapy with or without radiotherapy (No SCT). Allo-SCT=allogenic stem-cell transplant. MRD=minimal residual disease.
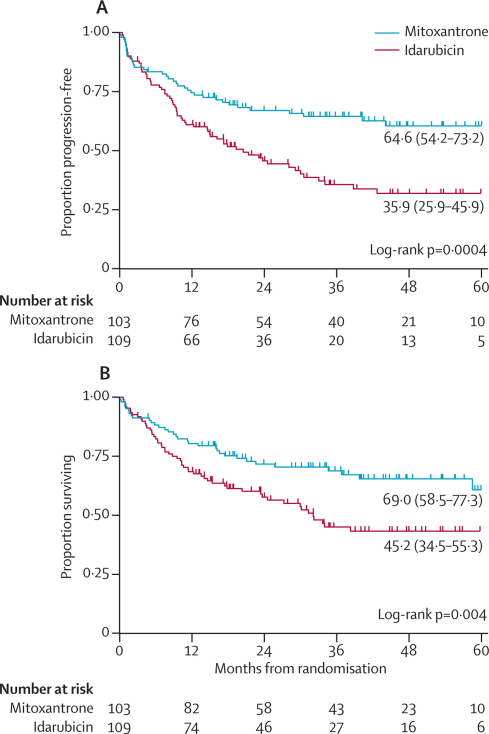
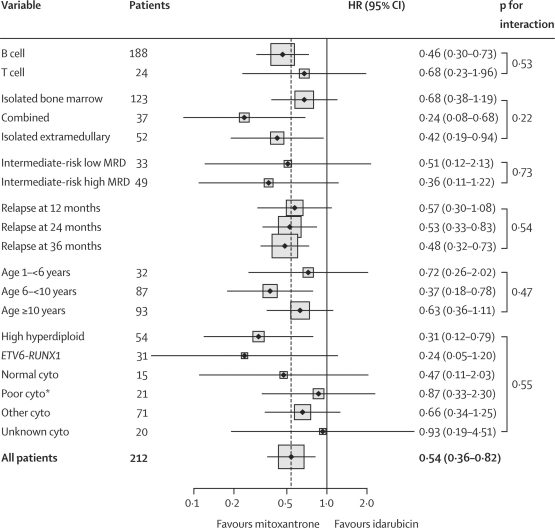
The estimated 3-year progression-free survival of the whole group was 50·3% (95% CI 42·9–57·3) and overall survival 57·1% (49·5–63·9). Progression-free survival and overall survival were significantly better for mitoxantrone than for idarubicin (figure 3). Mitoxantrone almost halved the hazard of an event at any given timepoint for both progression-free and overall survival. The adjusted hazard ratio (HR) for progression-free survival was 0·54 (95% CI 0·36–0·82, p=0·003) and for overall survival was 0·56 (0·36–0·87, p=0·01) (webappendix p 4). The results remained unchanged when analysis was restricted to UK patients only (webappendix p 4). We next tested the effect of potential imbalances due to prognostic factors in the two randomised groups (table 2). Neither covariate adjustment nor interactions significantly altered the treatment effect (figure 4 and webappendix p 4). Sensitivity analysis confirmed the robustness of this finding (webappendix p 5). The difference between groups remained significant and of similar magnitude to the primary analysis.
Figure 3.
Kaplan Meier estimates of progression-free (A) and overall (B) survival by randomised treatment
3-year estimated survival percentages (95% CI) are presented.
Figure 4.
Randomised drug effect on progression-free survival by patient characteristics, from Cox models with interactions
p values for interaction test the hypothesis that the randomised treatment effect varies between the different subgroups. Hazard ratios indicate that all subgroups show a treatment effect favouring mitoxantrone. HR=hazard ratio. MRD=minimal residual disease. Cyto=cytogenetic subgroups. *Poor refers to poor outcome after relapse (webappendix p 2).
For high-risk patients no clinical decision was made on the basis of minimal residual disease at timepoint 1. Hence, few data were available for these patients. In the high-risk group, of the seven patients with high minimal residual disease at first timepoint, five had more than 10−3 cells at second time point, which suggests that postinduction chemotherapy did not reduce disease burden for these patients. No patients with a low minimal residual disease at the first timepoint had high minimal residual disease at the second timepoint in either high-risk or intermediate-risk groups. We noted no apparent difference between the two drugs in minimal residual disease at first timepoint in the intermediate-risk group (table 3 and webappendix p 6). Thus, the decreased relapse rate in the mitoxantrone group seemed to be unrelated to the kinetics of disease clearance (adjusted odds ratio for low minimal residual disease 1·06, 95% CI 0·42–2·67, p=0·90).
During the whole trial, the number of toxic effects at grade 3 or higher was significantly lower for patients given mitoxantrone than for those given idarubicin (incidence rate ratio mitoxantrone:idarubicin 0·86, 95% CI 0·75–0·98, p=0·02; webappendix p 7). Toxic effects during phases 1 and 2 were significantly higher for patients given idarubicin and, compared with mitoxantrone, were mainly of an hepatic and gastrointestinal nature (webappendix pp 6–7). We recorded no significant randomised drug effect during phases 3 and 4. Toxic effects in phases 5 and 6 were significantly worse for patients given mitoxantrone who, compared with those who received idarubicin, showed a delay in haemopoietic recovery, although the number of toxic effects was low (webappendix pp 6–7). No significant randomised drug effect was recorded after allo-SCT. Separation of progression-free survival into disease-related and treatment-related events showed that mitoxantrone reduced the risk of disease-related events (HR 0·56, 95% CI 0·34–0·92; Gray's test p=0·007), whereas the effect on toxicity was not significant (HR 0·52, 0·24–1·11; Gray's test p=0·11; table 4 and webappendix p 7).
Table 4.
Endpoint of progression-free survival grouped by competing risks
| Idarubicin | Mitoxantrone | ||
|---|---|---|---|
| Treatment-related | 19 (17·4%) | 10 (9·7%) | |
| Second malignancy | 1 (0·9%) | 0 (0·0%) | |
| Treatment-related death | 18 (16·5%) | 10 (9·7%) | |
| Disease-related | 46 (42·2%) | 27 (26·2%) | |
| Refractory | 5 (4·6%) | 8 (7·8%) | |
| Second relapse | 38 (34·9%) | 17 (16·5%) | |
| Disease-related death | 3 (2·8%) | 2 (1·9%) | |
| Other death | 1 (0·9%) | 1 (1·0%) | |
| No events | |||
| Censored | 43 (39·4%) | 65 (63·1%) | |
Data are number (%) unless otherwise indicated. The number of disease-related deaths is low because most disease-related deaths occurred after a second relapse or after refractory disease, and hence do not constitute progression-free endpoints.
Discussion
The results from this trial clearly show that, compared with idarubicin, mitoxantrone significantly improves the outcome of children with relapsed acute lymphoblastic leukaemia. The trial ran across all 22 centres in the UK and Ireland and nine of ten centres in Australia and New Zealand. Although frontline protocols for both groups differ, the result of the randomisation was similar between the two study groups. Table 5 summarises the outcomes in studies in children with relapsed acute lymphoblastic leukaemia. Randomised trials in this disorder are infrequent, and this trial reports a valuable result. Unique to this study were the randomisation of anthracyclines in induction and the choice of mitoxantrone as test drug. Although mitoxantrone was less toxic than idarubicin, the effect was mainly due to better disease control. For the UK, the 3-year overall survival of 69% achieved in the mitoxantrone group is a substantial improvement compared with the overall survival of 56% achieved in the preceding ALLR2 trial.4
Table 5.
Reported outcomes of trials in relapsed childhood acute lymphoblastic leukaemia
| Randomisation | Result of randomisation | Number of patients (randomised) | Treatment protocol | Anthracycline | Outcome (95% CI) or SE | |
|---|---|---|---|---|---|---|
| All patients | ||||||
| BFM (1991)23 | Methotrexate 1 g/m2vs 12 g/m2 | NS | 130* | ALL-REZ BFM 85 | Daunorubicin | 6-year EFS 31% (4) |
| CCLG (2000)3 | None | .. | 256 | ALL R1 | Epirubicin | 5-year EFS 46% (40–52) |
| CCLG (2005)4 | None, retrospective | .. | 150 | ALL R2 | Epirubicin | 5-year EFS 56% (47–64) |
| BFM (2005)24 | Timing of cytarabine and methotrexate in early relapse | NS | 183 (56) | ALL-REZ BFM 87 | Daunorubicin | 15-year OS 37% (3) 15-year EFS 30% (3) |
| BFM (2010)5–10 | Methotrexate 1 g/m2vs 5 g/m2 | NS | 525 (269) | ALL-REZ BFM 90 | Daunorubicin | 10-year OS 36% (2) 10-year EFS 32% (2) |
| CCLG (2010) | Idarubicin vs mitoxantrone | In favour of mitoxantrone | 212 (212) | ALL R3 | Mitoxantrone | 3-year OS 69% (59–77) 3-year PFS 65% (54–73) |
| Selected cohort | ||||||
| † AEIOP (2002)25 | None, retrospective, and prospective | .. | 99 | Various | Idarubicin | 4-year OS 25% (5) 4-year EFS 21% (5) |
| ‡CCG (2007)6 | None | .. | 347 | Various | Not specified | 3-year OS 56% (3) 3-year EFS 45% (3) |
| §CCG/TACL (2010)26 | None, retrospective | .. | 225 | Various | Not specified | 5-year DFS 45% (3) |
Confidence Intervals (CI) are presented as percentages (%) and standard error as ±. NS=no significant difference. BFM=Berlin Frankfurt Münster. CCLG=Children's Cancer and Leukaemia Group, UK. AEIOP=Associazione Italiana Ematologia Oncologia Pediatrica. CCG=Children's Cancer Group, USA. TACL=Therapeutic Advances in Childhood Leukemia Consortium, USA. EFS=event-free survival. OS=overall survival. DFS=disease-free survival. PFS=progression-free survival.
Number randomised not stated.
All isolated extramedullary disease and late pre-B relapses and those with less than 25% blasts in the marrow were excluded.
Only relapses in NCI standard risk patients were analysed.
Isolated extramedullary relapses were excluded.
The decision to stop the randomisation was based on the significant difference in deaths between the two groups. Although we are aware of at least two other trials of childhood acute lymphoblastic leukaemia27,28 that reported early, they both recruited most of their target population. To our knowledge the closure of randomisation halfway through the trial, with this magnitude of difference attributable to one drug, is unheralded in clinical trials in childhood acute lymphoblastic leukaemia. Continuation of follow-up vindicates the decision by the data-monitoring committee to close the randomisation (webappendix p 2). Early closure resulted in lower than calculated recruitment to the two groups of the study and the resulting disproportionate numbers within the subgroups. Nevertheless, within the limitations posed by the closure, we can be certain that mitoxantrone provided a significant survival advantage in our cohort of relapsed patients.
If the difference between the two drugs was mainly that of chemosensitivity, we would have expected to detect a difference in minimal residual disease at the first timepoint, which was not the case. To enable the quick assessment of the number of new drugs now in the pipeline, study designs are incorporating the use of minimal residual disease as a surrogate marker of outcome. If we had opted to use such a study design, mitoxantrone would have been discarded. Our experience is thus a caveat for trial designs that propose to use surrogate markers of therapeutic response, such as minimal residual disease, as a primary endpoint to assess treatment response in phase 2 and 3 trials.29,30 Both idarubicin and mitoxantrone are tissue bound after infusion and can be detected months later. Thus in the context of the results of this trial, mitoxantrone seemed to have a delayed cytotoxic effect. Mitoxantrone, unlike idarubicin, is an anthracenadione. Other than the differential action on topoisomerase II isoforms and quiescent cells, it also has the ability to create DNA adducts,31 stimulate binding of nuclear factor κB,32 and potentiate immune-based cell kill by tagging leukaemic cells with calcireticulin.33
Although all these mechanisms could have contributed to the delayed cytotoxic effect, there is another intriguing possibility. First, three of five patients given mitoxantrone who had high minimal residual disease at the first timepoint and who were not transplanted for various reasons had recurrence. Thus, in the group with high minimal residual disease, sustained remission probably needs both mitoxantrone and allo-SCT. Second, although idarubicin was more toxic than mitoxantrone, this toxic effect was mainly seen during the first 8 weeks of treatment.
For the few patients who were not transplanted and continued on chemotherapy, haematological toxic effects increased during the later phases in the mitoxantrone group. Thus, mitoxantrone might in some way affect the haemopoietic stem-cell niche, making it a less favourable environment for the leukaemic cell and more conducive to the allograft. Further analyses of the effects of mitoxantrone on malignant and normal cells are needed. Such studies have the potential to identify novel mechanisms for the eradication of this disease.
A randomised trial of mitoxantrone was previously done in childhood acute myeloid leukaemia.34 Results showed an improved disease-free survival and lower relapse rates in the mitoxantrone group compared with the daunorubicin group; however, these reduced rates did not translate into overall survival.34 Mitoxantrone has only been infrequently used in therapeutic trials in childhood acute lymphoblastic leukaemia. A perception that optimisation has been reached with available drugs has shifted focus towards newer drugs and targeted therapy. These drugs will be prohibitively expensive for many patients. Mitoxantrone is a cheap and readily available drug and clearly needs further clinical assessment in childhood acute lymphoblastic leukaemia. Most frontline protocols are now moving towards risk-stratifying treatment based on minimal residual disease after induction. Patients with positive minimal residual disease usually receive anthracycline-containing delayed intensification blocks. Logically, assessment of the potential benefit of mitoxantrone in childhood acute lymphoblastic leukaemia would be in a randomised use of mitoxantrone in the delayed intensification phase of frontline treatment for high-risk patients. Our results suggest that, while we wait for targeted therapies to become a reality, conventional cytotoxics still have a role in treatment of acute lymphoblastic leukaemia.
Acknowledgments
Acknowledgments
This study was funded in part by a programme grant from Cancer Research UK (VS); grants from the Leukaemia and Lymphoma Research funded in part the analysis of minimal residual disease (NGo) and the Leukaemia Research Cytogenetics Group (AVM). The Cancer Council NSW and Sporting Chance Cancer Foundation funded analysis of minimal residual disease at CCIA (RS). MRD standardisation and quality control were supported by EuroMRD. The trial was initially sponsored by the Barts and the London NHS Trust (2003–6) and then by the University of Leicester NHS Trust (2006–09). In the UK, assessments of minimal residual disease were done at the UK MRD Network Laboratories (Barts, Bristol, Glasgow, Sheffield). We thank the participating children and their families and the physicians who enrolled their patients into the study. Marita Marshall, Helen Crowne, John Fox, and members of the Cancer Research UK IS team helped to design and run the database. We thank the members of the Data Monitoring Committee—namely, Mike Stevens, Moira Stewart, and Rob Edwards, as well as Tim Eden, Shekhar Krishnan, Louise Cheesman, and Anita Lim for their contributions. This paper is dedicated to the memory of Tony Oakhill, who pioneered the management of relapsed acute lymphoblastic leukaemia in the UK.
Contributors
VS conceived and designed the study with the help of SL. VS designed the database with the help of Cancer Research UK Information Systems. VS, PD, TR, MM, and PA were trial coordinators. CP, AM, NGr, and CL were responsible for trial data management. CP, VS, RW, and SL analysed data. JH, NGo, and RS were responsible for minimal residual disease and AVM for cytogenetics. CP, AM, RW, and VS wrote the paper and all others reviewed the final draft. All authors had full access to study data and all shared in the decision to submit for publication.
Conflicts of interest
VS has participated in speaker bureaus and advisory boards for EUSA Pharma, Genzyme, Medac, Kyowa-Hakko, and Novartis. PA has received travel support from Genzyme and Gilead. NGo has participated in advisory boards for ENZON. All other authors declare that they have no conflicts of interest.
Web Extra Material
References
- 1.Schrappe M, Nachman J, Hunger S. Educational symposium on long-term results of large prospective clinical trials for childhood acute lymphoblastic leukemia (1985–2000) Leukemia. 2010;24:253–254. doi: 10.1038/leu.2009.276. [DOI] [PubMed] [Google Scholar]
- 2.Harned TM, Gaynon P. Relapsed acute lymphoblastic leukemia: current status and future opportunities. Curr Oncol Rep. 2008;10:453–458. doi: 10.1007/s11912-008-0070-3. [DOI] [PubMed] [Google Scholar]
- 3.Lawson SE, Harrison G, Richards S. The UK experience in treating relapsed childhood acute lymphoblastic leukaemia: a report on the medical research council UKALLR1 study. Br J Haematol. 2000;108:531–543. doi: 10.1046/j.1365-2141.2000.01891.x. [DOI] [PubMed] [Google Scholar]
- 4.Roy A, Cargill A, Love S. Outcome after first relapse in childhood acute lymphoblastic leukaemia—lessons from the United Kingdom R2 trial. Br J Haematol. 2005;130:67–75. doi: 10.1111/j.1365-2141.2005.05572.x. [DOI] [PubMed] [Google Scholar]
- 5.Tallen G, Ratei R, Mann G. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J Clin Oncol. 2010;28:2339–2347. doi: 10.1200/JCO.2009.25.1983. [DOI] [PubMed] [Google Scholar]
- 6.Malempati S, Gaynon PS, Sather H, La MK, Stork LC. Outcome after relapse among children with standard-risk acute lymphoblastic leukemia: Children's Oncology Group study CCG-1952. J Clin Oncol. 2007;25:5800–5807. doi: 10.1200/JCO.2007.10.7508. [DOI] [PubMed] [Google Scholar]
- 7.Krishnan S, Wade R, Moorman AV. Temporal changes in the incidence and pattern of central nervous system relapses in children with acute lymphoblastic leukaemia treated on four consecutive Medical Research Council trials, 1985–2001. Leukemia. 2010;24:450–459. doi: 10.1038/leu.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raetz EA, Borowitz MJ, Devidas M. Reinduction platform for children with first marrow relapse of acute lymphoblastic leukemia: a Children's Oncology Group Study. J Clin Oncol. 2008;26:3971–3978. doi: 10.1200/JCO.2008.16.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stam RW, den Boer ML, Meijerink JP. Differential mRNA expression of Ara-C-metabolizing enzymes explains Ara-C sensitivity in MLL gene-rearranged infant acute lymphoblastic leukemia. Blood. 2003;101:1270–1276. doi: 10.1182/blood-2002-05-1600. [DOI] [PubMed] [Google Scholar]
- 10.von Stackelberg A, Hartmann R, Buhrer C. High-dose compared with intermediate-dose methotrexate in children with a first relapse of acute lymphoblastic leukemia. Blood. 2008;111:2573–2580. doi: 10.1182/blood-2007-07-102525. [DOI] [PubMed] [Google Scholar]
- 11.Wells RJ, Feusner J, Devney R. Sequential high-dose cytosine arabinoside-asparaginase treatment in advanced childhood leukemia. J Clin Oncol. 1985;3:998–1004. doi: 10.1200/JCO.1985.3.7.998. [DOI] [PubMed] [Google Scholar]
- 12.Testi AM, Del Giudice I, Arcese W. A single high dose of idarubicin combined with high-dose ARA-C for treatment of first relapse in childhood ‘high-risk’ acute lymphoblastic leukaemia: a study of the AIEOP group. Br J Haematol. 2002;118:741–747. doi: 10.1046/j.1365-2141.2002.03706.x. [DOI] [PubMed] [Google Scholar]
- 13.Reid JM, Pendergrass TW, Krailo MD, Hammond GD, Ames MM. Plasma pharmacokinetics and cerebrospinal fluid concentrations of idarubicin and idarubicinol in pediatric leukemia patients: a Childrens Cancer Study Group report. Cancer Res. 1990;50:6525–6528. [PubMed] [Google Scholar]
- 14.Hongo T, Fujii Y. In vitro chemosensitivity of lymphoblasts at relapse in childhood leukemia using the MTT assay. Int J Hematol. 1991;54:219–230. [PubMed] [Google Scholar]
- 15.Chan A, Weilbach FX, Toyka KV, Gold R. Mitoxantrone induces cell death in peripheral blood leucocytes of multiple sclerosis patients. Clin Exp Immunol. 2005;139:152–158. doi: 10.1111/j.1365-2249.2005.02653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Errington F, Willmore E, Leontiou C, Tilby MJ, Austin CA. Differences in the longevity of topo IIalpha and topo IIbeta drug-stabilized cleavable complexes and the relationship to drug sensitivity. Cancer Chemother Pharmacol. 2004;53:155–162. doi: 10.1007/s00280-003-0701-1. [DOI] [PubMed] [Google Scholar]
- 17.van der Velden VH, Hochhaus A, Cazzaniga G, Szczepanski T, Gabert J, van Dongen JJ. Detection of minimal residual disease in hematologic malignancies by real-time quantitative PCR: principles, approaches, and laboratory aspects. Leukemia. 2003;17:1013–1034. doi: 10.1038/sj.leu.2402922. [DOI] [PubMed] [Google Scholar]
- 18.Trotti A, Colevas AD, Setser A. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 19.Clinical trial authorisations: Safety reporting—SUSARS and ASRs. 2010. http://www.mhra.gov.uk/Howweregulate/Medicines/Licensingofmedicines/Clinicaltrials/Safetyreporting-SUSARSandASRs/index.htm (accessed June 1, 2010).
- 20.McCullagh P, Nelder JA. Generalised Linear Models. Chapman and Hall; London: 1983. [Google Scholar]
- 21.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 22.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1140–1154. [Google Scholar]
- 23.Henze G, Fengler R, Hartmann R. Six-year experience with a comprehensive approach to the treatment of recurrent childhood acute lymphoblastic leukemia (ALL-REZ BFM 85). A relapse study of the BFM group. Blood. 1991;78:1166–1172. [PubMed] [Google Scholar]
- 24.Einsiedel HG, von Stackelberg A, Hartmann R. Long-term outcome in children with relapsed ALL by risk-stratified salvage therapy: results of trial acute lymphoblastic leukemia-relapse study of the Berlin-Frankfurt-Munster Group 87. J Clin Oncol. 2005;23:7942–7950. doi: 10.1200/JCO.2005.01.1031. [DOI] [PubMed] [Google Scholar]
- 25.Testi AM, Moleti ML, Giona F. A single high dose of idarubicin combined with high-dose ARA-C (MSKCC ALL-3 protocol) in adult and pediatric patients with acute lymphoblastic leukemia. Experience at the University “La Sapienza” of Rome. Haematologica. 1997;82:664–667. [PubMed] [Google Scholar]
- 26.Ko RH, Ji L, Barnette P. Outcome of patients treated for relapsed or refractory acute lymphoblastic leukemia: a Therapeutic Advances in Childhood Leukemia Consortium study. J Clin Oncol. 2010;28:648–654. doi: 10.1200/JCO.2009.22.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nachman JB, Sather HN, Sensel MG. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338:1663–1671. doi: 10.1056/NEJM199806043382304. [DOI] [PubMed] [Google Scholar]
- 28.Seibel NL, Steinherz PG, Sather HN. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood. 2008;111:2548–2555. doi: 10.1182/blood-2007-02-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleming TR. Surrogate endpoints and FDA's accelerated approval process. Health Aff (Millwood) 2005;24:67–78. doi: 10.1377/hlthaff.24.1.67. [DOI] [PubMed] [Google Scholar]
- 30.Susman E. Accelerated approval seen as triumph and roadblock for cancer drugs. J Natl Cancer Inst. 2004;96:1495–1496. doi: 10.1093/jnci/96.20.1495. [DOI] [PubMed] [Google Scholar]
- 31.Parker BS, Buley T, Evison BJ. A molecular understanding of mitoxantrone-DNA adduct formation: effect of cytosine methylation and flanking sequences. J Biol Chem. 2004;279:18814–18823. doi: 10.1074/jbc.M400931200. [DOI] [PubMed] [Google Scholar]
- 32.Karl S, Pritschow Y, Volcic M. Identification of a novel pro-apopotic function of NF-kappaB in the DNA damage response. J Cell Mol Med. 2009;13:4239–4256. doi: 10.1111/j.1582-4934.2009.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao C, Han Y, Ren Y, Wang Y. Mitoxantrone-mediated apoptotic B16-F1 cells induce specific anti-tumor immune response. Cell Mol Immunol. 2009;6:469–475. doi: 10.1038/cmi.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibson BE, Wheatley K, Hann IM. Treatment strategy and long-term results in paediatric patients treated in consecutive UK AML trials. Leukemia. 2005;19:2130–2138. doi: 10.1038/sj.leu.2403924. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.