Abstract
The PINK1–Parkin pathway plays a critical role in mitochondrial quality control by selectively targeting damaged mitochondria for autophagy. In this issue, Tanaka et al. (2010. J. Cell Biol. doi: 10.1083/jcb.201007013) demonstrate that the AAA-type ATPase p97 acts downstream of PINK1 and Parkin to segregate fusion-incompetent mitochondria for turnover. p97 acts by targeting the mitochondrial fusion-promoting factor mitofusin for degradation through an endoplasmic reticulum–associated degradation (ERAD)-like mechanism.
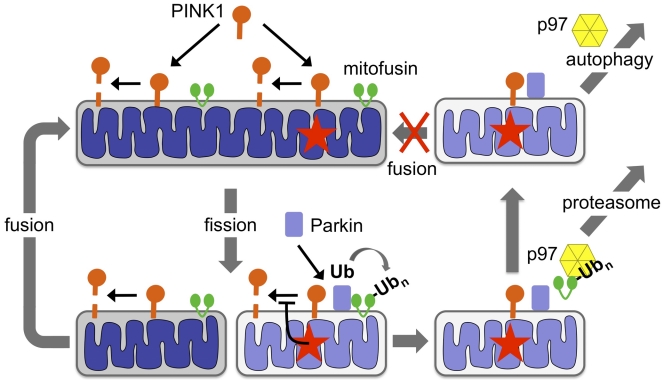
Ultrastructural and biochemical studies beginning in the 1950s demonstrated that mitochondria are degraded in the lysosome through a process called autophagy. Subsequent studies led to the identification of factors that promote autophagy, and demonstrated that mitochondria are degraded in response to starvation or other cellular signals (Yang and Klionsky, 2010). However, until recently, the evidence in support of a mechanism by which damaged mitochondria can be selectively detected and degraded was largely nonexistent. Over the past two years, studies of the PINK1 and parkin genes, loss-of-function mutations of which give rise to heritable forms of Parkinson’s disease, have demonstrated that these genes encode components of a mitochondrial quality control apparatus that promotes the selective turnover of damaged mitochondria (Whitworth and Pallanck, 2009; Jones, 2010). These studies have led to a model in which PINK1, a mitochondrially localized serine/threonine kinase, accumulates selectively in depolarized mitochondria, where it recruits Parkin, a cytosolic E3 ubiquitin ligase. Parkin promotes the ubiquitination of the mitochondrial fusion-promoting factor mitofusin and the destruction of the depolarized mitochondria through an autophagic mechanism (Fig. 1). Now, Tanaka et al. (see p. 1367 of this issue) extend our understanding of the PINK1–Parkin mitochondrial quality control pathway by defining the role of mitofusin ubiquitination in this process. Furthermore, the authors demonstrate that the hexameric AAA-type ATPase p97 is required downstream of PINK1 and Parkin to promote the proteasomal turnover of ubiquitinated mitofusin and the autophagic removal of damaged mitochondria.
Figure 1.
The PINK1–Parkin mitochondrial quality control pathway. PINK1 is targeted to the mitochondrial outer membrane and is cleaved in a mitochondrial membrane potential–dependent fashion. Mitochondrial damage (red stars) followed by fission segregates a damaged/depolarized product. Inactivation of PINK1 cleavage in the damaged/depolarized fission product leads to an accumulation of PINK1 and the recruitment of Parkin. Parkin then ubiquitinates (Ub) mitofusin, which in turn leads to the assembly of p97 on ubiquitinated mitofusin and the subsequent extraction and proteasomal degradation of mitofusin. The damaged/depolarized mitochondrion, which now lacks mitofusin, is unable to fuse with undamaged mitochondria and is instead targeted for autophagy in a p97-dependent process.
Although the finding that Parkin promotes the ubiquitination of mitofusin was also recently reported in flies (Poole et al., 2010; Ziviani et al., 2010), the interpretation of this finding was unclear. Ubiquitination of mitofusin might serve as a flag to target damaged mitochondria for autophagic turnover. Alternatively, or in addition, ubiquitin-mediated inactivation of mitofusin on damaged mitochondria after a fission event might serve to produce fusion-incompetent mitochondria that are prevented from reentering the undamaged mitochondrial network. By treating cells briefly with the mitochondrial depolarizing agent CCCP in the presence and absence of Parkin, Tanaka et al. (2010) were able to show that after the removal of CCCP from the cell culture media, the fragmented mitochondrial population from Parkin-deficient cells reentered the mitochondrial network with significantly faster kinetics than the mitochondrial population from Parkin-expressing cells. These investigators further showed that mitochondria in mouse embryonic fibroblasts that lack both of the known vertebrate mitofusins are still degraded in a Parkin-dependent fashion upon CCCP treatment. Together, these findings indicate that Parkin-mediated mitofusin ubiquitination serves to prevent damaged mitochondria from reentering the undamaged mitochondrial network. Moreover, mitofusin ubiquitination does not represent a signal-triggering mitochondrial turnover. This study also showed that Parkin-mediated mitochondrial turnover was significantly attenuated in cells lacking the mitochondrial fission-promoting factor DRP1, which suggests that another possible role of mitofusin inactivation is to promote the formation of fragmented mitochondria that can be more efficiently degraded through autophagy. Of potential relevance to these findings is recent work demonstrating that mitochondrial fission often yields asymmetric products differing in membrane potential, with those fission products of lower membrane potential typically exhibiting a decreased probability of fusion and an increased probability of autophagy relative to their siblings (Twig et al., 2008).
Tanaka et al. (2010) have also advanced our understanding of the mechanism of PINK1–Parkin-mediated mitofusin degradation by demonstrating that ubiquitinated mitofusin is degraded in a proteasome-dependent fashion through the aid of the AAA-ATPase p97. Although previous work indicates that p97 participates in a wide variety of cellular processes, including SNARE-mediated fusion of homotypic membranes (Kondo et al., 1997), DNA replication (Deichsel et al., 2009), and autophagosome maturation (Tresse et al., 2010), the role of p97 in the proteasomal turnover of ubiquitinated mitofusin is probably most relevant to its function in endoplasmic reticulum–associated degradation (ERAD). In this process, p97 is believed to assemble with polyubiquitinated ER proteins, in concert with p97-associated substrate-recruiting cofactors, to provide the driving force required to extrude the polyubiquitinated proteins from the ER membrane so that they can be degraded by the proteasome in the cytosol (Raasi and Wolf, 2007). Thus, the work of Tanaka et al. (2010) suggests that an ERAD-like mechanism also exists to degrade ubiquitinated mitochondrial proteins. This conclusion is further supported by closely related studies in yeast and vertibrate cell culture systems showing that p97 translocates to mitochondria under stress to promote the proteasomal degradation of ubiquitinated mitochondrial targets, including mitofusin (Heo et al., 2010; Xu et al., 2010). Although only a very small number of mitochondrial proteins are currently known to be degraded in a proteasome-dependent fashion, all of these mitochondrial proteasomal substrates were revealed relatively recently (Margineantu et al., 2007; Azzu and Brand, 2010; Livnat-Levanon and Glickman, 2010), so a more focused effort to identify additional such substrates might dramatically expand the list of mitochondrial p97/proteasomal targets.
Like many important advances, the work of Tanaka et al. (2010) also raises new questions. For example, the finding that mitofusins are dispensable for Parkin-mediated mitochondrial turnover still leaves open the possibility that the presence of mitofusins on damaged mitochondria inhibits mitochondrial turnover. Moreover, the finding that Parkin promotes the turnover of depolarized mitochondria in cells that lack mitofusins suggests that there are other substrates of Parkin that are important for the turnover event. The voltage-dependent anion channel 1 (VDAC1) has previously been reported to be one of these substrates (Geisler et al., 2010). However, a recent study using VDAC1 knockout cells indicated that VDAC1 is dispensable for Parkin-mediated mitochondrial turnover (Narendra et al., 2010), which suggests that the Parkin substrates required to induce mitochondrial turnover remain to be identified. Another question arising from the work of Tanaka et al. (2010) concerns the role of p97 in mitochondrial turnover. Although the authors convincingly show that p97 is required for both mitofusin and mitochondrial turnover, it is by no means clear that these roles of p97 are related: the requirement of p97 in mitochondrial turnover could pertain to the proteasomal turnover of another, unknown ubiquitinated mitochondrial protein, or to another activity of p97 altogether, such as its recently documented role in autophagosome maturation (Tresse et al., 2010). The finding that p97-mediated mitofusin turnover is evolutionarily conserved in yeast (Heo et al., 2010), which lacks obvious PINK1 and Parkin orthologues, also raises the question of whether the factors that promote mitofusin ubiquitination in this organism constitute a PINK–Parkin-like mitochondrial quality control pathway. In addition to these new questions, there are several additional questions involving the PINK1–Parkin pathway that await explanation, including the mechanism by which PINK1 recruits Parkin to mitochondria and directs it to its substrates. Given the many powerful model systems in play to address these questions and the rapid pace of progress on the PINK1–Parkin pathway over the past two years, it seems likely that answers to these and other questions will be forthcoming shortly.
References
- Azzu V., Brand M.D. 2010. Degradation of an intramitochondrial protein by the cytosolic proteasome. J. Cell Sci. 123:578–585 10.1242/jcs.060004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichsel A., Mouysset J., Hoppe T. 2009. The ubiquitin-selective chaperone CDC-48/p97, a new player in DNA replication. Cell Cycle. 8:185–190 [DOI] [PubMed] [Google Scholar]
- Geisler S., Holmström K.M., Skujat D., Fiesel F.C., Rothfuss O.C., Kahle P.J., Springer W. 2010. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 12:119–131 10.1038/ncb2012 [DOI] [PubMed] [Google Scholar]
- Heo J.M., Livnat-Levanon N., Taylor E.B., Jones K.T., Dephoure N., Ring J., Xie J., Brodsky J.L., Madeo F., Gygi S.P., et al. 2010. A stress-responsive system for mitochondrial protein degradation. Mol. Cell. 40:465–480 10.1016/j.molcel.2010.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. 2010. PINK1 targets dysfunctional mitochondria for autophagy in Parkinson disease. Nat. Rev. Neurol. 6:181 10.1038/nrneurol.2010.19 [DOI] [PubMed] [Google Scholar]
- Kondo H., Rabouille C., Newman R., Levine T.P., Pappin D., Freemont P., Warren G. 1997. p47 is a cofactor for p97-mediated membrane fusion. Nature. 388:75–78 10.1038/40411 [DOI] [PubMed] [Google Scholar]
- Livnat-Levanon N., Glickman M.H. 2010. Ubiquitin-proteasome system and mitochondria - reciprocity. Biochim. Biophys. Acta. In press [DOI] [PubMed] [Google Scholar]
- Margineantu D.H., Emerson C.B., Diaz D., Hockenbery D.M. 2007. Hsp90 inhibition decreases mitochondrial protein turnover. PLoS One. 2:e1066 10.1371/journal.pone.0001066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D.P., Kane L.A., Hauser D.N., Fearnley I.M., Youle R.J. 2010. p62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both. Autophagy. 6:1090–1106 10.4161/auto.6.8.13426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A.C., Thomas R.E., Yu S., Vincow E.S., Pallanck L. 2010. The mitochondrial fusion-promoting factor mitofusin is a substrate of the PINK1/parkin pathway. PLoS One. 5:e10054 10.1371/journal.pone.0010054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raasi S., Wolf D.H. 2007. Ubiquitin receptors and ERAD: a network of pathways to the proteasome. Semin. Cell Dev. Biol. 18:780–791 10.1016/j.semcdb.2007.09.008 [DOI] [PubMed] [Google Scholar]
- Tanaka A., Cleland M.M., Xu S., Narendra D.P., Suen D.-F., Karbowski M., Youle R.J. 2010. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J. Cell Biol. 191:1367–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresse E., Salomons F.A., Vesa J., Bott L.C., Kimonis V., Yao T.P., Dantuma N.P., Taylor J.P. 2010. VCP/p97 is essential for maturation of ubiquitin-containing autophagosomes and this function is impaired by mutations that cause IBMPFD. Autophagy. 6:217–227 10.4161/auto.6.2.11014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig G., Elorza A., Molina A.J., Mohamed H., Wikstrom J.D., Walzer G., Stiles L., Haigh S.E., Katz S., Las G., et al. 2008. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 27:433–446 10.1038/sj.emboj.7601963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth A.J., Pallanck L.J. 2009. The PINK1/Parkin pathway: a mitochondrial quality control system? J. Bioenerg. Biomembr. 41:499–503 10.1007/s10863-009-9253-3 [DOI] [PubMed] [Google Scholar]
- Yang Z., Klionsky D.J. 2010. Eaten alive: a history of macroautophagy. Nat. Cell Biol. 12:814–822 10.1038/ncb0910-814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Peng G., Wang Y., Fang S., Karbowski M. 2010. The AAA-ATPase p97 is essential for outer mitochondrial membrane turnover. Mol. Biol. Cell. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziviani E., Tao R.N., Whitworth A.J. 2010. Drosophila parkin requires PINK1 for mitochondrial translocation and ubiquitinates mitofusin. Proc. Natl. Acad. Sci. USA. 107:5018–5023 10.1073/pnas.0913485107 [DOI] [PMC free article] [PubMed] [Google Scholar]