Abstract
S-palmitoylation describes the reversible attachment of fatty acids (predominantly palmitate) onto cysteine residues via a labile thioester bond. This posttranslational modification impacts protein functionality by regulating membrane interactions, intracellular sorting, stability, and membrane micropatterning. Several recent findings have provided a tantalizing insight into the regulation and spatiotemporal dynamics of protein palmitoylation. In mammalian cells, the Golgi has emerged as a possible super-reaction center for the palmitoylation of peripheral membrane proteins, whereas palmitoylation reactions on post-Golgi compartments contribute to the regulation of specific substrates. In addition to palmitoylating and depalmitoylating enzymes, intracellular palmitoylation dynamics may also be controlled through interplay with distinct posttranslational modifications, such as phosphorylation and nitrosylation.
Introduction
Cellular proteins undergo a vast array of dynamic and static modifications to their amino acid backbone, which can dramatically extend and regulate functional output. Some of these modifications, phosphorylation in particular, have been the subject of intensive research, and their regulation and intracellular functions are extensively characterized. In contrast, our understanding of S-palmitoylation (hereafter referred to as palmitoylation), the attachment of palmitate (C16:0) or other long chain fatty acids to cysteine residues, has lagged behind some of its more popular cousins. Indeed, enzymes that catalyze palmitoylation reactions have only recently been identified after landmark studies in yeast, settling a long-running debate as to whether this process was predominantly enzyme mediated or spontaneous (Lobo et al., 2002; Roth et al., 2002; Fukata et al., 2004; Keller et al., 2004).
Palmitoylation is one of a group of lipid modifications (collectively termed lipidation) that appears on eukaryotic proteins and includes the common N-myristoyl and isoprenyl modifications. N-myristoylation describes the addition of myristic acid (C14:0) to a glycine residue with an exposed NH2 group after cleavage of the immediately adjacent initiating methionine (Zha et al., 2000; Resh, 2006a). This process is predominantly cotranslational, mediated by soluble enzymes, and has a strict consensus sequence (MGXXXS/T). N-myristoylation can also occur posttranslationally, notably after caspase-mediated protein cleavage during programmed cell death (Zha et al., 2000). Prenylation is a posttranslational process also catalyzed by soluble enzymes, involving the attachment of farnesyl or geranylgeranyl isoprenoids to a C-terminal cysteine present within a defined consensus sequence (Wright and Philips, 2006).
Unlike the catalysts of N-myristoylation and prenylation reactions, the enzymes that mediate palmitoylation are polytopic membrane proteins (Fukata et al., 2004; Mitchell et al., 2006), implying that cellular palmitoylation reactions occur at the cytosol–membrane interface. There are ∼23 putative S-palmitoyl transferases in mammals, characterized by the presence of a DHHC (aspartate-histidine-histidine-cysteine) motif within an ∼50 amino acid cysteine-rich domain. The large number of these DHHC proteins coupled with their localization to distinct membrane compartments (Ohno et al., 2006) implies that the cellular palmitoylation machinery is a highly regulated and coordinated system.
There is no strict consensus sequence for palmitoylation, however, palmitoylated cysteines do share some common characteristics: (a) they are often adjacent to myristoylation and prenylation sites, (b) the surrounding amino acids tend to be basic or hydrophobic, and (c) they are frequently located in the cytoplasmic regions flanking transmembrane domains (or within transmembrane domains). An elegant combined proteomic and genetic analysis in yeast revealed that some DHHC proteins appear to exhibit a preference for a particular class of substrate, e.g., transmembrane proteins or myristoylated/prenylated proteins (Roth et al., 2006). Similarly, some palmitoylated yeast proteins displayed a marked dependence on a specific DHHC protein (of which seven are expressed in yeast). However, this study also revealed that DHHC proteins can have overlapping substrate specificities, which is consistent with previous studies in mammalian systems showing that specific substrates can be palmitoylated by more than one DHHC protein (Fukata et al., 2004; Fang et al., 2006; Fernández-Hernando et al., 2006; Greaves et al., 2008, 2010; Tsutsumi et al., 2009; Shmueli et al., 2010).
Regulatory effects of palmitoylation
Palmitoylation often couples with either N-myristoylation or prenylation to regulate membrane interactions of soluble proteins. N-myristoylation or prenylation of proteins in the cell cytosol provides a degree of hydrophobicity, although single lipid modifications are only sufficient for transient membrane interaction (Shahinian and Silvius, 1995). In contrast, two closely positioned lipid modifications promote stable membrane attachment (Shahinian and Silvius, 1995). Thus, at the cellular level, single myristoyl or prenyl chains facilitate transient membrane association, which is sufficient to allow access to membrane-bound DHHC proteins; subsequent palmitoylation by DHHC proteins will then promote stable membrane binding by inhibiting membrane dissociation (kinetic trapping; Fig. 1 A). In this way, palmitoylation is essential for stable membrane association of many proteins, including farnesylated Ras and myristoylated Gα subunits (Hancock et al., 1990; Linder et al., 1993; Parenti et al., 1993). Several soluble lipidated proteins are modified exclusively by S-palmitoylation, and these proteins may use an intrinsic weak membrane affinity for transient membrane interaction before palmitoylation (Greaves et al., 2008, 2009a).
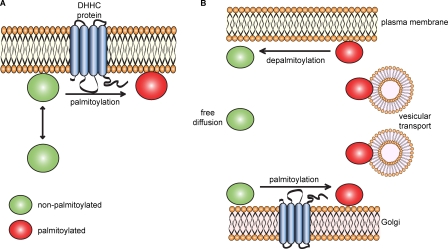
Figure 1.
Regulation of membrane binding and trafficking of peripheral proteins by palmitoylation. (A) Proteins modified with single lipid groups (prenylation or N-myristoylation; green circles) have a weak membrane affinity that allows transient membrane interaction. Palmitoylation by membrane-bound DHHC proteins promotes stable membrane association by kinetic trapping (Shahinian and Silvius, 1995). Note that some peripheral proteins are exclusively palmitoylated, and these proteins were suggested to interact with membranes before palmitoylation by way of an intrinsic weak membrane affinity (Greaves et al., 2008, 2009a). (B) Palmitoylation of Ras-farnesyl by Golgi-localized DHHC proteins leads to a dramatic increase in membrane affinity by kinetic trapping. This increased membrane residency facilitates entry of palmitoylated Ras (red circles) into transport vesicles that deliver it to the plasma membrane. It is possible that palmitoylation also serves to move Ras into cholesterol-rich domains from which Golgi exit vesicles are formed (Patterson et al., 2008). Depalmitoylation of Ras can occur anywhere in the cell, perhaps modulated by Apt1, resulting in membrane release and cytosolic diffusion before repalmitoylation at the Golgi. For simplicity, the figure only depicts depalmitoylation occurring at the plasma membrane. This palmitoylation/depalmitoylation regulation of protein sorting is not specific for Ras proteins and may be a common mechanism underlying the sorting of many peripheral palmitoylated proteins (Kanaani et al., 2008; Tsutsumi et al., 2009; Rocks et al., 2010).
Therefore, it is clear that a major function of palmitoylation is to mediate stable membrane attachment of soluble proteins. However, the effects of this posttranslational modification are more complex and diverse than that of a simple membrane anchor, and indeed, many transmembrane proteins are also palmitoylated. Analyses of different proteins and systems have revealed that palmitoylation has many distinct effects on modified proteins, regulating protein trafficking, protein stability, membrane microlocalization, and protein–protein interactions (Resh, 2006a,b; Greaves and Chamberlain, 2007; Linder and Deschenes, 2007; Greaves et al., 2009b; Noritake et al., 2009). Although these effects of palmitoylation appear diverse, they are likely determined by two particular properties of palmitate: hydrophobicity/membrane affinity and preference for ordered cholesterol-rich membrane domains or rafts (Melkonian et al., 1999; Brown, 2006).
A central function of palmitoylation is the regulation of protein sorting (Greaves et al., 2009b), and it might be predicted that this simple lipid modification would have a very limited repertoire of effects on this process. However, this is not the case, and palmitoylation has been shown to act as a highly versatile sorting signal, which regulates protein trafficking to many distinct intracellular compartments. Several studies have highlighted regulatory effects of palmitoylation on either retention or anterograde trafficking of proteins at the ER–Golgi or protein cycling within the endosomal/lysosomal system (Linder and Deschenes, 2007; Greaves et al., 2009b). Recent studies have extended our knowledge of the functional effects of palmitoylation on protein sorting by highlighting novel roles for this modification in targeting to cilia and flagella (Emmer et al., 2009; Cevik et al., 2010; Follit et al., 2010) and to the cleavage furrow of dividing cells (Hannoush and Arenas-Ramirez, 2009).
What is the underlying mechanistic basis for the effects of palmitoylation on protein sorting? For some soluble proteins, palmitoylation may be a passive sorting signal, acting only as an essential membrane anchor that allows other domains of the protein to regulate subsequent trafficking. However, palmitoylation also has active effects on protein sorting, achieved by partitioning of proteins into cholesterol-rich membrane subdomains or rafts (Levental et al., 2010), changing protein orientation at the membrane and thus affecting protein–protein interactions (Hayashi et al., 2005; Lin et al., 2009), regulating the ubiquitination status of proteins and thereby modulating ubiquitination-dependent protein sorting (Valdez-Taubas and Pelham, 2005; Linder and Deschenes, 2007; Abrami et al., 2008), or affecting the transmembrane orientation of palmitoylated proteins in the membrane bilayer (Abrami et al., 2008).
Reversible palmitoylation dynamically regulates protein localization
In contrast to other static lipid modifications, the versatility of palmitoylation as a membrane interaction and protein sorting module is greatly enhanced by its reversibility. It has long been appreciated that the palmitoylation of many (but not all) proteins is dynamic (Magee et al., 1987) and can be modulated in response to cell stimulation (Degtyarev et al., 1993; Mumby et al., 1994; Wedegaertner and Bourne, 1994). Although palmitoylation dynamics of transmembrane proteins can impact sorting to distinct membrane compartments, depalmitoylation of soluble proteins can also mediate membrane release and cytosolic diffusion. Thus, the rapid palmitoylation and depalmitoylation dynamics of many proteins add an extra level of complexity to the effects of this posttranslational modification on protein sorting.
The effects of palmitoylation–depalmitoylation dynamics have been extensively analyzed for palmitoylated Ras isoforms (Goodwin et al., 2005; Rocks et al., 2005; Roy et al., 2005). Farnesylated H- and N-Ras exhibit a weak membrane affinity that mediates interaction with Golgi membranes. Palmitoylation of Ras at this compartment promotes stable membrane association and trafficking from the Golgi to the plasma membrane. Subsequent depalmitoylation releases Ras from the plasma membrane into the cytosol, and the process of palmitoylation at the Golgi and trafficking to the plasma membrane is repeated (Fig. 1 B). This palmitoylation cycle achieves a constant flux of Ras proteins from Golgi to plasma membrane and was suggested to prevent spillover of Ras onto other cellular membranes by constantly resetting the intracellular localization (Goodwin et al., 2005; Rocks et al., 2005).
The Ras cycling pathway is an example of how constitutive palmitoylation dynamics coordinate protein sorting. For some proteins, palmitoylation turnover and corresponding changes in intracellular localization can also be regulated by cell activity. Protein palmitoylation dynamics that are subject to regulation have been particularly well characterized for postsynaptic proteins; palmitoylation of the molecular scaffold PSD95 and AMPA and NMDA glutamate receptors are all regulated by synaptic activity (El-Husseini et al., 2002; Hayashi et al., 2005, 2009). PSD95 is a peripheral palmitoylated protein that coordinates protein clustering at postsynaptic sites. This protein was suggested to display a decreased palmitoylation in response to glutamate receptor activation, which corresponded with a reduced synaptic clustering of both PSD95 and AMPA receptors. Palmitoylation of GluR1/GluR2 subunits of AMPA receptors and NR2A/2B subunits of NMDA receptors is also modulated by synaptic activity. The palmitoylation status of GluR1 affects association with the 4.1N protein, regulating activity-dependent receptor internalization and plasma membrane insertion dynamics (Hayashi et al., 2005; Lin et al., 2009). Dynamic palmitoylation of NR2 subunits also modulates cell surface expression of the NMDA receptor (Hayashi et al., 2009). Thus, neurons use dynamic palmitoylation as a response mode to couple changes in synaptic activity to changes in protein localization.
Spatiotemporal palmitoylation dynamics
Classically, palmitoylation dynamics have been studied using radiolabeled palmitate and pulse-chase protocols; for example, this approach indicated a half-life for N-Ras palmitoylation of ∼20 min (Magee et al., 1987) and showed that palmitoylation of Gα subunits of heterotrimeric G proteins can be modulated by agonist stimulation (Degtyarev et al., 1993; Mumby et al., 1994; Wedegaertner and Bourne, 1994).
Recently, the intracellular dynamics of palmitoylation and palmitoylation-dependent protein trafficking have been investigated using fluorescence imaging. The power of biochemical radiolabeling techniques comes from the ability to selectively follow the fate of a labeled pool of protein by pulse chase. In contrast, conventional confocal microscopy analysis of fluorescent proteins is not well suited to allow older proteins to be distinguished from newly synthesized proteins, static protein localizations to be differentiated from dynamic trafficking, and resolution of palmitoylated and unpalmitoylated proteins. However, these issues can be addressed by synchronizing the protein under investigation or by selective photoactivation/photobleaching of a specific pool of the protein or by using visible membrane accumulation of soluble proteins as an indicator of palmitoylation. These techniques were successfully used in the landmark publications that detailed the palmitoylation-dependent cycling pathway of Ras proteins (Goodwin et al., 2005; Rocks et al., 2005).
Recent studies used microinjection of semisynthetic N-Ras as a means to study real-time spatiotemporal dynamics of palmitoylation and membrane targeting (Rocks et al., 2005, 2010); microinjection of fluorescent protein allows the analysis of a synchronized (i.e., chemically identical and with the same initial localization) pool of protein. This elegant approach involves coupling chemically synthesized farnesylated peptides encompassing the C-terminal membrane targeting domain of N-Ras to a Cy3-labeled protein consisting of the remainder of N-Ras (amino acids 1–181) via a maleimidocaproyl linker. By using these semisynthetic farnesylated proteins with intact or mutated palmitoylation sites, high-resolution temporal dynamics together with spatial information on cellular palmitoylation and depalmitoylation reactions were open to investigation.
Immediately after microinjection, farnesylated N-Ras displayed a dispersed localization, which is consistent with its presence in the cytosol and transient association with intracellular membranes (Rocks et al., 2010). However, a rapid enrichment of the Cy3-labeled construct became apparent at the Golgi region (t/2 ∼14 s) with plasma membrane staining visible at later time points. The simplest interpretation of these observations is that palmitoylation of the farnesylated N-Ras occurs at the Golgi, promoting early accumulation at this compartment, and is followed by anterograde transport to the plasma membrane. This notion agrees well with previous analyses of Ras protein cycling (Goodwin et al., 2005; Rocks et al., 2005). Nevertheless, it should be noted that palmitoylation of the microinjected semisynthetic Ras protein was not directly assayed in this study. Although technically challenging, it will therefore be of major interest in follow-up studies to perform correlative measurements of palmitoylation and intracellular localization of microinjected Ras. In addition, a recently published study reported that palmitoylated Ras proteins traffic from Golgi to recycling endosomes en route to the plasma membrane (Misaki et al., 2010). Rocks et al. (2010) did not report association of microinjected Ras proteins with recycling endosomes, and it will be important to clarify this apparent discrepancy between these studies.
The progressive pattern of localization of microinjected Ras (i.e., dispersed → Golgi → plasma membrane) was also observed for semisynthetic constructs in which the N terminus of N-Ras (1–181) was ligated to either myristoylated peptides derived from Gαi1 or Fyn or to the N-terminal 20 amino acids from GAP43, all of which contain palmitoylation sites. This result suggests that palmitoylation of N-Ras at the Golgi is not dependent on farnesylation per se but may instead be a common feature of peripheral palmitoylated proteins with an underlying membrane affinity. Note that GAP43 lacks any other lipid modifications and had the slowest rate of Golgi accumulation (t/2 = 29 s); this protein may bind to membranes before palmitoylation via an intrinsic weak membrane affinity (Greaves et al., 2008, 2009a). It will be particularly interesting in follow-up studies to examine whether microinjected full-length Gαi1, Fyn, and GAP43 display identical intracellular localization dynamics to the peptide sequences that were fused to N-Ras. This issue is relevant because previous work has shown that regions distant from palmitoylation sites can have a marked influence on the specificity of interaction with DHHC proteins; for example, the yeast vacuolar protein Vac8 was palmitoylated specifically by Pfa3 in vitro, but the isolated palmitoylation domain from this protein was palmitoylated by all five yeast DHHC proteins that were examined (Nadolski and Linder, 2009).
Many other palmitoylated peripheral proteins do not display obvious steady-state localization at the Golgi but instead associate with the plasma membrane and endosomal membranes (Adamson et al., 1992; Kasahara et al., 2007; Sandilands et al., 2007). The absence of such proteins from the Golgi might indicate that they are not modified by Golgi-localized DHHC proteins or could instead reflect a different rate of palmitate turnover and subsequent Golgi recycling (Fig. 2). Interestingly, proteins in this category, including Fyn, TC10, R-Ras, RhoB, and Rap2C all displayed colocalization with a Golgi marker when vesicular traffic through the secretory pathway was blocked by low temperature (Rocks et al., 2010). This observation implies that after their synthesis, these proteins traffic via the Golgi and is consistent with the notion that the Golgi may be a specialized reaction center for the palmitoylation of all newly synthesized peripheral proteins and for a subset that undergo rapid depalmitoylation dynamics.
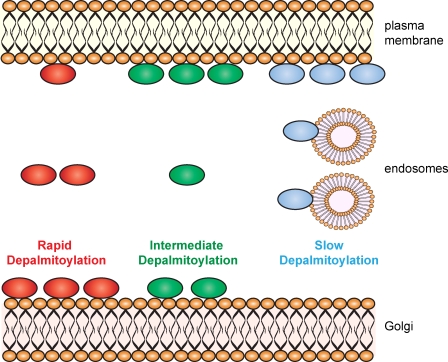
Figure 2.
Regulation of protein localization by palmitoylation dynamics. The illustration depicts three palmitoylated proteins that have different rates of depalmitoylation. In this context, the term depalmitoylation refers to the complete absence of palmitoyl groups on the protein. Rapid depalmitoylation is associated with an enriched steady-state localization on Golgi membranes. This is achieved by depalmitoylation promoting membrane release and subsequent palmitoylation by Golgi-specific DHHC proteins leading to an accumulation at this compartment. Rapid depalmitoylation prevents excessive accumulation on endosomes via vesicular trafficking from the plasma membrane. In contrast, proteins that have a slower rate of depalmitoylation are maintained on membranes for longer and reach endosomal membranes via the plasma membrane. Note that a slower depalmitoylation rate may be achieved by a relative resistance to thioesterases, and/or the presence of many palmitoylated cysteines, and/or palmitoylation by DHHC proteins beyond the Golgi. All of these situations would limit the amount of the protein in a completely depalmitoylated state. This slower rate of depalmitoylation and membrane release limits the steady-state distribution on Golgi membranes.
The Golgi as a hub for palmitoylation of peripheral proteins
The suggestion that palmitoylation of newly synthesized peripheral proteins is a Golgi-specific event is largely consistent with previously published data. Although DHHC palmitoyl transferases associate with a range of organelles, including the ER, Golgi, and plasma membrane (Ohno et al., 2006; Greaves et al., 2010), independent analyses of DHHC substrate pairs have returned a high percentage of Golgi DHHC proteins as positive hits (Fukata et al., 2004, 2006; Huang et al., 2004; Fernández-Hernando et al., 2006; Greaves et al., 2008, 2010; Tsutsumi et al., 2009). Indeed, DHHC9 displays specificity toward H- and N-Ras (Swarthout et al., 2005) and is Golgi localized (Swarthout et al., 2005). However, Rocks et al. (2010) argued against any rigid requirements for DHHC substrate specificity and reported that DHHC9 knockdown (at the mRNA level at least) did not affect the intracellular localization of H-Ras or recovery kinetics after photobleaching of Golgi-localized H-Ras. To examine more closely whether the palmitoylation domain of N-Ras contained a recognition motif for interaction with specific DHHC proteins, the amino acids around the palmitoylation site were changed to their stereoisomeric (i.e., D-amino acids) counterparts. The insertion of these D-amino acids, which was predicted to disrupt any specific protein-binding site, had no major effect on Golgi accumulation of the microinjected proteins (Rocks et al., 2010). Further analysis suggested that there was also not an essential DHHC recognition domain in the remainder of the Ras protein. These observations suggest that the DHHC proteins that modify Ras do not require a specific signature of the palmitoylated domain or upstream region and implies that palmitoylation of peripheral proteins may not be restrained by tight enzyme substrate specificities; the major requirement for palmitoylation presumably being a suitable cysteine in close membrane proximity. However, this idea is not readily consistent with other studies that have highlighted features of both DHHC proteins and substrate proteins that contribute to specificity of interaction (Greaves et al., 2009a, 2010; Huang et al., 2009; Nadolski and Linder, 2009) or that identified a requirement for a specific DHHC protein for palmitoylation of a specific substrate (Roth et al., 2006; Ohyama et al., 2007; Stowers and Isacoff, 2007; Emmer et al., 2009; Huang et al., 2009; Noritake et al., 2009; Tian et al., 2010). Indeed, depletion of a single DHHC protein, Erf2, had a marked impact on the palmitoylation of yeast Ras (Bartels et al., 1999; Roth et al., 2006). Further work is clearly required to delineate the reasons for these apparent inconsistencies.
If the Golgi really is a super-reaction center for palmitoylation of peripheral proteins in mammalian cells, it will be of great interest to determine how this is achieved. Are Golgi-localized enzymes highly efficient? Are cofactors required for palmitoylation enriched at the Golgi? Do peripheral proteins interact in a slightly different way with Golgi membranes, making them more susceptible to palmitoylation? Most peripheral palmitoylated proteins associate with post-Golgi membranes (particularly the plasma membrane) after palmitoylation. Thus, specific palmitoylation at the Golgi might be an important prerequisite to ensure plasma membrane trafficking. A particular feature of palmitate that might facilitate Golgi to plasma membrane trafficking is its affinity for cholesterol-rich lipid raft domains (Melkonian et al., 1999; Levental et al., 2010). It was recently proposed that raft domains might act as platforms for vesicle budding from the Golgi (Patterson et al., 2008), and thus, palmitoylation-dependent association with these domains would be predicted to promote protein traffic to the plasma membrane. In support of the idea that palmitoylation at the Golgi is important for correct trafficking of peripheral proteins, we recently identified a connection between the palmitoylation of cysteine-string protein (CSP) at the Golgi and its subsequent sorting (Greaves et al., 2008). A mutant form of CSP with an enhanced membrane affinity associates with ER membranes and is only palmitoylated when ER and Golgi membranes are mixed by applying brefeldin A (Greaves et al., 2008). Interestingly, however, after washout of brefeldin A, the Golgi recovers but the CSP mutant remains trapped at the ER (Greaves et al., 2008), perhaps reflecting a disconnect between forward transport of peripheral palmitoylated proteins and the lipid environment of ER membranes (cholesterol poor).
Although the Golgi has been highlighted as a possible hub for palmitoylation of peripheral mammalian proteins, the yeast Ras palmitoyl transferase (Erf2) is localized to the ER (Bartels et al., 1999). Thus, there currently appears to be marked differences in the palmitoylation pathways of mammalian and yeast Ras proteins, which might reflect reported differences in the intracellular trafficking itineraries of these proteins (Dong et al., 2003; Wang and Deschenes, 2006).
Regulation of spatial patterns of palmitoylation by initial membrane interaction
A key aspect of membrane targeting and cycling of dually lipidated proteins such as H/N-Ras, and the compartment where palmitoylation occurs, is the membrane interaction dynamics of the monofarnesylated protein. It is often implied that peripheral proteins bind to all or any intracellular membrane before palmitoylation; but is this the case? Does farnesylated Ras have an equal affinity for all intracellular membranes and, therefore, an unbiased membrane sampling before palmitoylation? In vitro experiments suggest that this may not be the case, as membrane interactions of farnesylated peptides are affected by membrane lipid composition (Gohlke et al., 2010). Binding of farnesylated N-Ras to liquid-ordered membranes containing saturated phospholipids and cholesterol was reduced compared with liquid-disordered membranes made from an unsaturated phospholipid. These findings are particularly relevant given the high concentration of cholesterol in the plasma and endosomal membranes compared with membranes such as the ER (Mondal et al., 2009). Semisynthetic proteins containing a single farnesyl or myristoyl group were suggested to interact with the plasma membrane based on total internal fluorescence microscopy analysis (Rocks et al., 2010); however, there was no indication of whether this interaction occurred with the same efficiency as interaction with ER–Golgi membranes. Visual inspection of the localization of farnesylated Ras proteins and peptides expressed in cells via plasmid transfection reveals clear ER and Golgi staining, whereas interaction with other membrane compartments (including the plasma membrane) is less obvious (Choy et al., 1999; Rocks et al., 2005, 2010). Therefore, peripheral proteins may display a preference for interaction with specific membrane compartments rather than binding randomly to any intracellular membrane. This would clearly provide palmitoylation-dependent protein cycling with more specificity and directionality. The precise membrane interaction dynamics of peripheral proteins before palmitoylation therefore merits further high-resolution analysis.
Palmitoylation reactions beyond the Golgi
Although the Golgi appears to function as a hub for palmitoylation of newly synthesized and cycling peripheral proteins, certain proteins undergo dynamic palmitoylation remodeling without accessing the Golgi. This suggests that active DHHC proteins are localized in post-Golgi compartments. Indeed, DHHC2 and DHHC5 associate with the plasma membrane in neuroendocrine cells (Greaves et al., 2010), and these proteins are present on post-Golgi membranes in neuronal dendrites and at the postsynaptic density (Noritake et al., 2009; Li et al., 2010). SNAP25, a multiply palmitoylated peripheral protein, is modified by Golgi-localized DHHC proteins (Fukata et al., 2004, 2006; Huang et al., 2004; Greaves et al., 2009a, 2010), which promote stable membrane attachment of SNAP25 (Greaves et al., 2009a). Recent work also reported that SNAP25 can be palmitoylated by DHHC2 but that this enzyme is unable to promote stable membrane attachment (Greaves et al., 2010). This observation is consistent with the idea that palmitoylation of newly synthesized SNAP25 (which promotes membrane association) is restricted to the Golgi and that modification by post-Golgi DHHCs is only relevant once SNAP25 has been trafficked from Golgi to plasma membrane. This may reflect a weaker association between SNAP25 and DHHC2, such that productive interaction can only occur when SNAP25 is stably membrane associated. However, it is also consistent with the idea that unpalmitoylated SNAP25 may have a higher affinity for Golgi membranes than the plasma membrane, again reinforcing the importance of membrane affinities and preferences of peripheral proteins before palmitoylation (see previous paragraph).
In hippocampal neurons, DHHC2 is associated with mobile dendritic vesicles of unknown origin, and total internal reflection microscopy suggested that inhibition of synaptic activity promotes an increase in DHHC2 levels either at or just beneath the plasma membrane (Noritake et al., 2009). Interestingly, this movement of DHHC2 correlates with enhanced palmitoylation and synaptic clustering of PSD95 (El-Husseini et al., 2002; Noritake et al., 2009), and depletion of DHHC2 was reported to inhibit the increase in synaptic clustering of PSD95 after synaptic blockade. This work illustrates that dynamic palmitoylation can be achieved without peripheral proteins (such as PSD95) visiting the Golgi. It is interesting to note that depletion of DHHC3, which is localized to the somatic Golgi, also inhibited synaptic accumulation of PSD95. However, in contrast with DHHC2 depletion, knockdown of DHHC3 had no effect on the activity-dependent increase in synaptic clustering of PSD95 (Noritake et al., 2009). This suggests that Golgi-localized DHHC3 is involved in the initial palmitoylation of newly synthesized PSD95, before dendritic targeting.
CSP, an important neuroprotective DnaJ chaperone, is palmitoylated by Golgi-localized DHHC enzymes (DHHC3, DHHC7, DHHC15, and DHHC17; Greaves et al., 2008). In this regard, CSP is similar to most other peripheral palmitoylated proteins. Consistent with the analyses of CSP palmitoylation in mammalian cells, disruption of DHHC17 in Drosophila melanogaster resulted in a loss of palmitoylation and mislocalization of CSP (Ohyama et al., 2007; Stowers and Isacoff, 2007). Surprisingly, however, DHHC17 does not exhibit a Golgi localization in Drosophila neurons but, instead, has a presynaptic distribution on synaptic vesicles or at the presynaptic plasma membrane (Ohyama et al., 2007; Stowers and Isacoff, 2007). Although palmitoylation cycles have not been reported for CSP, it is possible that DHHC17 is important for regulating local palmitoylation dynamics of CSP in Drosophila presynaptic terminals.
Finally, there is also strong evidence to show that peripheral membrane proteins can undergo palmitoylation beyond the confines of the Golgi in yeast cells. As discussed earlier, Ras is modified by ER-localized ERF2 (Bartels et al., 1999), and palmitoylation and membrane association of the yeast vacuolar fusion protein Vac8 are also markedly reduced after depletion of the DHHC protein Pfa3, which is localized to the vacuole membrane (Hou et al., 2005; Smotrys et al., 2005).
Mechanisms and spatiotemporal dynamics of depalmitoylation
In contrast to the wealth of information available on palmitoylating enzymes, our understanding of the proteins that regulate protein depalmitoylation is poor. Two main candidate thioesterases have been identified. Protein palmitoyl thioesterase 1 (Ppt1) depalmitoylates H-Ras and different Gα subunits in vitro (Camp and Hofmann, 1993). Although there are reports that a cytosolic pool of Ppt1 may be present in cells (Kim et al., 2008), this protein is thought to be predominantly localized to the lysosomal lumen (Hellsten et al., 1996), where it is believed to function in depalmitoylation reactions occurring during protein degradation. Acyl protein thioesterase 1 (Apt1) reportedly displays thioesterase activity toward Giα1, H-Ras, eNOS, and certain viral proteins (Duncan and Gilman, 1998; Yeh et al., 1999; Veit and Schmidt, 2001) but is inactive against other proteins such as caveolin (Yeh et al., 1999; Veit and Schmidt, 2001). Importantly, Apt1 has a cytosolic localization, suggesting that it can regulate cellular palmitoylation dynamics. In support of this idea, overexpression of Apt1 into HEK293 cells was reported to increase the rate of removal of radiolabeled palmitate from Gsα in pulse-chase experiments (Duncan and Gilman, 1998).
Despite Apt1 being identified many years ago, the physiological importance of this protein as a thioesterase is not clear. However, a recent study reported an important function for Apt1 in controlling dendritic spine volume, possibly by regulating palmitoylation and membrane localization of Gα13 (Siegel et al., 2009). The recent description of a novel Apt1 inhibitor (palmostatin B) should provide an important tool to more finely dissect the function of this protein in cellular palmitoylation dynamics (Dekker et al., 2010). Initial analysis with palmostatin B suggests that it promotes a moderate increase in Ras palmitoylation and disrupts the intracellular localization of this protein.
Although our understanding of the mechanisms of depalmitoylation is limited, the spatiotemporal dynamics of this process were investigated by microinjection of semisynthetic N-Ras containing both farnesyl and palmitoyl chains (Rocks et al., 2010). Previous analysis of an N-Ras protein in which the palmitoyl group was attached by a noncleavable thioether linkage revealed a dispersed intracellular localization without Golgi enrichment (Rocks et al., 2005). This localization was suggested to reflect a requirement for active palmitoylation/depalmitoylation cycling to achieve the correct localization of Ras. The palmitoylated protein with a cleavable thioester bond was therefore not expected to display any initial membrane-targeting specificity. Despite this, the construct rapidly accumulated at the Golgi (t/2 = 27 s). This Golgi accumulation was suggested to follow on from binding of the farnesylated/palmitoylated protein to any membrane, depalmitoylation, cytosolic diffusion, and subsequent repalmitoylation at the Golgi. There were two main interpretations made from this behavior of farnesylated and palmitoylated N-Ras: (1) depalmitoylation must be very rapid to account for the speed of Golgi accumulation and (2) depalmitoylation must occur throughout the cell because if it was confined to a specific location, association of the farnesylated and palmitoylated protein with some membranes would be irreversible.
If depalmitoylation is not restricted to a specific membrane compartment, this suggests (a) a common depalmitoylase with access to many membranes (consistent with the cytosolic localization of Apt1), (b) a group of depalmitoylating enzymes with wide membrane compartment coverage, or (c) nonenzymatic depalmitoylation.
The same spatiotemporal pattern of localization was observed for a farnesylated/palmitoylated protein containing D-amino acids at the palmitoylation site (Rocks et al., 2010), suggesting that depalmitoylation does not require a specific recognition sequence around this region. How would a cytosolic thioesterase like Apt1 recognize membrane-embedded thioester linkages without the presence of a defined consensus sequence? It is clear that much more research on Apt1 is required and that the identity of novel inhibitors will greatly facilitate this. One interesting angle is to determine whether the turnover of palmitate on cellular proteins correlates with the ability of Apt1 to promote depalmitoylation in vitro. For example, caveolin is not a substrate of Apt1 in vitro and does not undergo rapid dynamic palmitoylation in cells (Parat and Fox, 2001), and the reverse is obviously true for proteins such as eNOS and Ras.
Interplay between palmitoylation and other posttranslational modifications
DHHC proteins are clearly master regulators of intracellular palmitoylation reactions, and thioesterases (such as Apt1) may be equally important. However, are these enzymes the only means of regulating palmitoylation? In fact, there is evidence that palmitoylation/depalmitoylation dynamics can also be modulated by distinct posttranslational modifications present on the target protein.
Interplay between palmitoylation and phosphorylation was recognized many years ago for the β2-adrenergic G protein–coupled receptor (Moffet et al., 1993). Mutation of the palmitoylated cysteine in the C-terminal tail of this receptor led to an increased level of basal phosphorylation and a loss of coupling to Gs (O’Dowd et al., 1989; Moffet et al., 1993). The effects of mutating the palmitoylation site were not caused by a loss of palmitoylation per se but rather by the increased phosphorylation of the palmitoylation-deficient mutant (Moffett et al., 1996). Palmitoylation–phosphorylation interplay has also been reported to regulate trafficking of AMPA and NMDA receptor subunits (Hayashi et al., 2009; Lin et al., 2009). For GluR1 subunits of AMPA receptors, depalmitoylation was suggested to enable the more-efficient phosphorylation of neighboring serine residues. This phosphorylation in turn increased the interaction of GluR1 with 4.1N protein, which modulated plasma membrane internalization and insertion dynamics of AMPA receptors (Lin et al., 2009). How does palmitoylation regulate phosphorylation? One potential mechanism is membrane insertion of palmitoylated cysteines restricting access of protein kinases to the adjacent phosphorylation sites (Fig. 3 A).
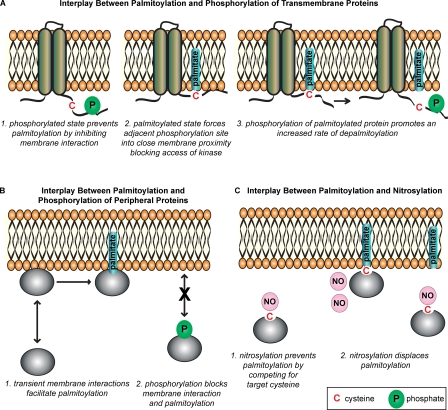
Figure 3.
Interplay between phosphorylation/nitrosylation and palmitoylation. (A) Reciprocal regulation of transmembrane proteins. (1) Negatively charged phosphate group prevents palmitoylation of an adjacent cysteine by blocking membrane interaction. (2) Palmitoylation-mediated membrane association prevents access of protein kinases to an adjacent phosphorylation site. (3) Phosphorylation could alter the depalmitoylation rate of a neighboring cysteine, e.g., by increasing access to a thioesterase enzyme. (B) Phosphorylation of a soluble protein prevents palmitoylation by inhibiting transient membrane interaction. (C, 1) Possible regulatory effects of nitrosylation on palmitoylation. Nitrosylation may prevent palmitoylation by direct competition for cysteine residues. (2) It is also possible that nitrosylation could directly displace palmitate. Note that the examples shown do not illustrate the full range of effects that phosphorylation might have on palmitoylation and vice versa.
If palmitoylation can regulate phosphorylation, can phosphorylation regulate palmitoylation? The STREX variant of BK potassium channels contains a PKA phosphorylation site within the cytoplasmic C-terminal tail that mediates channel inhibition (Tian et al., 2001). A recent study reported that the STREX variant also contains palmitoylated cysteines adjacent to the PKA site that regulate plasma membrane binding of the cytosolic C terminus (Tian et al., 2008). Introduction of a phosphomimetic mutation at the PKA phosphorylation site or activation of cellular PKA perturbed palmitoylation-dependent membrane association of the C-terminal tail of STREX. Importantly, the full-length channel lacking the palmitoylated cysteines in the STREX domain was no longer subject to PKA-mediated inhibition, implying that phospho-regulation of the STREX channel is achieved via changes in palmitoylation and membrane association of the cytoplasmic C terminus.
Phosphorylation also appears to regulate palmitoylation of the cyclic nucleotide phosphodiesterase (PDE) isoform PDE10A2 (Charych et al., 2010). The N terminus of PDE10A2 contains a palmitoylated cysteine residue (Cys11) that is essential for membrane anchoring and efficient dendritic transport in striatal neurons. Palmitoylation was perturbed when phosphomimetic mutations were introduced into a downstream PKA phosphorylation site (Thr-16). Does phosphorylation at Thr-16 actively promote depalmitoylation of Cys-11, or does it block palmitoylation of this cysteine? In fact, PKA activation markedly enhanced PDE10A2 phosphorylation but had no acute effect on membrane expression levels. This observation suggests that phosphorylation does not promote depalmitoylation and membrane release of PDE10A2 but, instead, likely inhibits membrane binding by blocking palmitoylation. This phospho-regulation of palmitoylation might be relevant to many palmitoylated peripheral proteins and could represent a mechanism to promote a shift toward the depalmitoylated state of a protein in the absence of active (thioesterase driven) depalmitoylation. Negatively charged phosphate groups are likely to inhibit palmitoylation of neighboring cysteines by interfering with membrane interactions before palmitoylation (Fig. 3).
Another posttranslational modification that may impact palmitoylation dynamics is nitrosylation. Nitric oxide (NO) is produced from l-arginine by NO synthase enzymes (NOS) and can directly modify cysteines by S-nitrosylation (Stamler et al., 1992); this modification might therefore regulate palmitoylation dynamics by direct competition. The NO donor SIN-1 inhibited the basal level and the isoproterenol-stimulated increase in palmitate incorporation into β2 adrenergic receptor (Adam et al., 1999). Palmitate incorporation into H-Ras, caveolin, SNAP25, and certain viral proteins has also been reported to be modified by NO donors (Hess et al., 1993; Baker et al., 2000; Akerström et al., 2009). Indeed, the NO donor S-nitrosocysteine accelerated removal of radiolabeled palmitate from H-Ras in pulse-chase experiments (Baker et al., 2000), raising the intriguing possibility that NO may directly displace palmitate from modified proteins (Fig. 3 C).
Overall, there is sufficient published data to suggest that NO may be an important regulator of palmitoylation dynamics. The development of more sensitive techniques to directly study nitrosylation is required to more rigorously delineate interplay between this modification and palmitoylation. It will be particularly important in future studies to determine how palmitoylation dynamics are affected by endogenously produced NO. The intracellular diffusion range of NO from its site of production is limited, and thus, interplay between palmitoylation and nitrosylation will be more physiologically relevant for palmitoylated proteins that interact either directly or indirectly with NOS enzymes.
Perspective
Landmark studies in yeast highlighted the DHHC protein family as the catalysts of intracellular membrane fusion reactions and provided an essential spark to the palmitoylation field. This, together with the continuing development of new methodologies and reagents, has served as a platform for rapid expansion in our understanding of the mechanisms, spatiotemporal dynamics, and outcomes of protein palmitoylation. Techniques such as acyl-biotin exchange and click chemistry have offered highly sensitive alternatives to radiolabeling for the study of cellular palmitoylation (Drisdel and Green, 2004; Martin and Cravatt, 2009; Yap et al., 2010). These approaches also permit the palmitoylation status of proteins to be studied in situ without cell labeling (acyl-biotin exchange) or the analysis of spatial patterns of palmitoylated proteins by fluorescence imaging (Hannoush and Arenas-Ramirez, 2009). Furthermore, both techniques have facilitated analysis of the cellular palmitoylome in both yeast and mammalian cells (Roth et al., 2006; Kang et al., 2008; Martin and Cravatt, 2009). The recent development of Apt1 inhibitors is an important step toward further delineating the function of this protein and developing an enhanced understanding of cellular depalmitoylation dynamics (Dekker et al., 2010). It is expected that further technological developments will maintain the rapid pace of palmitoylation research. In particular, the development of more specific and selective inhibitors against the DHHC protein family (Resh, 2006b) will be a key to delineating the individual functions of these proteins and their contribution to the spatiotemporal dynamics of cellular palmitoylation reactions.
Acknowledgments
Work in our laboratory is supported by the UK Medical Research Council (grant G0601597).
References
- Abrami L., Kunz B.A., Iacovache I., van der Goot F.G. 2008. Palmitoylation and ubiquitination regulate exit of the Wnt signaling protein LRP6 from the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 105:5384–5389 10.1073/pnas.0710389105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam L., Bouvier M., Jones T.L.Z. 1999. Nitric oxide modulates β(2)-adrenergic receptor palmitoylation and signaling. J. Biol. Chem. 274:26337–26343 10.1074/jbc.274.37.26337 [DOI] [PubMed] [Google Scholar]
- Adamson P., Paterson H.F., Hall A. 1992. Intracellular localization of the P21rho proteins. J. Cell Biol. 119:617–627 10.1083/jcb.119.3.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerström S., Gunalan V., Keng C.T., Tan Y.J., Mirazimi A. 2009. Dual effect of nitric oxide on SARS-CoV replication: viral RNA production and palmitoylation of the S protein are affected. Virology. 395:1–9 10.1016/j.virol.2009.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T.L., Booden M.A., Buss J.E. 2000. S-Nitrosocysteine increases palmitate turnover on Ha-Ras in NIH 3T3 cells. J. Biol. Chem. 275:22037–22047 10.1074/jbc.M001813200 [DOI] [PubMed] [Google Scholar]
- Bartels D.J., Mitchell D.A., Dong X., Deschenes R.J. 1999. Erf2, a novel gene product that affects the localization and palmitoylation of Ras2 in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:6775–6787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.A. 2006. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology (Bethesda). 21:430–439 [DOI] [PubMed] [Google Scholar]
- Camp L.A., Hofmann S.L. 1993. Purification and properties of a palmitoyl-protein thioesterase that cleaves palmitate from H-Ras. J. Biol. Chem. 268:22566–22574 [PubMed] [Google Scholar]
- Cevik S., Hori Y., Kaplan O.I., Kida K., Toivenon T., Foley-Fisher C., Cottell D., Katada T., Kontani K., Blacque O.E. 2010. Joubert syndrome Arl13b functions at ciliary membranes and stabilizes protein transport in Caenorhabditis elegans. J. Cell Biol. 188:953–969 10.1083/jcb.200908133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charych E.I., Jiang L.-X., Lo F., Sullivan K., Brandon N.J. 2010. Interplay of palmitoylation and phosphorylation in the trafficking and localization of phosphodiesterase 10A: implications for the treatment of schizophrenia. J. Neurosci. 30:9027–9037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy E., Chiu V.K., Silletti J., Feoktistov M., Morimoto T., Michaelson D., Ivanov I.E., Philips M.R. 1999. Endomembrane trafficking of ras: the CAAX motif targets proteins to the ER and Golgi. Cell. 98:69–80 10.1016/S0092-8674(00)80607-8 [DOI] [PubMed] [Google Scholar]
- Degtyarev M.Y., Spiegel A.M., Jones T.L. 1993. Increased palmitoylation of the Gs protein alpha subunit after activation by the beta-adrenergic receptor or cholera toxin. J. Biol. Chem. 268:23769–23772 [PubMed] [Google Scholar]
- Dekker F.J., Rocks O., Vartak N., Menninger S., Hedberg C., Balamurugan R., Wetzel S., Renner S., Gerauer M., Schölermann B., et al. 2010. Small-molecule inhibition of APT1 affects Ras localization and signaling. Nat. Chem. Biol. 6:449–456 10.1038/nchembio.362 [DOI] [PubMed] [Google Scholar]
- Dong X., Mitchell D.A., Lobo S., Zhao L., Bartels D.J., Deschenes R.J. 2003. Palmitoylation and plasma membrane localization of Ras2p by a nonclassical trafficking pathway in Saccharomyces cerevisiae. Mol. Cell. Biol. 23:6574–6584 10.1128/MCB.23.18.6574-6584.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drisdel R.C., Green W.N. 2004. Labeling and quantifying sites of protein palmitoylation. Biotechniques. 36:276–285 [DOI] [PubMed] [Google Scholar]
- Duncan J.A., Gilman A.G. 1998. A cytoplasmic acyl-protein thioesterase that removes palmitate from G protein alpha subunits and p21(RAS). J. Biol. Chem. 273:15830–15837 10.1074/jbc.273.25.15830 [DOI] [PubMed] [Google Scholar]
- El-Husseini Ael.-D., Schnell E., Dakoji S., Sweeney N., Zhou Q., Prange O., Gauthier-Campbell C., Aguilera-Moreno A., Nicoll R.A., Bredt D.S. 2002. Synaptic strength regulated by palmitate cycling on PSD-95. Cell. 108:849–863 10.1016/S0092-8674(02)00683-9 [DOI] [PubMed] [Google Scholar]
- Emmer B.T., Souther C., Toriello K.M., Olson C.L., Epting C.L., Engman D.M. 2009. Identification of a palmitoyl acyltransferase required for protein sorting to the flagellar membrane. J. Cell Sci. 122:867–874 10.1242/jcs.041764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang C., Deng L., Keller C.A., Fukata M., Fukata Y., Chen G., Lüscher B. 2006. GODZ-mediated palmitoylation of GABA(A) receptors is required for normal assembly and function of GABAergic inhibitory synapses. J. Neurosci. 26:12758–12768 10.1523/JNEUROSCI.4214-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Hernando C., Fukata M., Bernatchez P.N., Fukata Y., Lin M.I., Bredt D.S., Sessa W.C. 2006. Identification of Golgi-localized acyl transferases that palmitoylate and regulate endothelial nitric oxide synthase. J. Cell Biol. 174:369–377 10.1083/jcb.200601051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follit J.A., Li L., Vucica Y., Pazour G.J. 2010. The cytoplasmic tail of fibrocystin contains a ciliary targeting sequence. J. Cell Biol. 188:21–28 10.1083/jcb.200910096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M., Fukata Y., Adesnik H., Nicoll R.A., Bredt D.S. 2004. Identification of PSD-95 palmitoylating enzymes. Neuron. 44:987–996 10.1016/j.neuron.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Fukata Y., Iwanaga T., Fukata M. 2006. Systematic screening for palmitoyl transferase activity of the DHHC protein family in mammalian cells. Methods. 40:177–182 10.1016/j.ymeth.2006.05.015 [DOI] [PubMed] [Google Scholar]
- Gohlke A., Triola G., Waldmann H., Winter R. 2010. Influence of the lipid anchor motif of N-ras on the interaction with lipid membranes: a surface plasmon resonance study. Biophys. J. 98:2226–2235 10.1016/j.bpj.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J.S., Drake K.R., Rogers C., Wright L., Lippincott-Schwartz J., Philips M.R., Kenworthy A.K. 2005. Depalmitoylated Ras traffics to and from the Golgi complex via a nonvesicular pathway. J. Cell Biol. 170:261–272 10.1083/jcb.200502063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves J., Chamberlain L.H. 2007. Palmitoylation-dependent protein sorting. J. Cell Biol. 176:249–254 10.1083/jcb.200610151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves J., Salaun C., Fukata Y., Fukata M., Chamberlain L.H. 2008. Palmitoylation and membrane interactions of the neuroprotective chaperone cysteine-string protein. J. Biol. Chem. 283:25014–25026 10.1074/jbc.M802140200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves J., Prescott G.R., Fukata Y., Fukata M., Salaun C., Chamberlain L.H. 2009a. The hydrophobic cysteine-rich domain of SNAP25 couples with downstream residues to mediate membrane interactions and recognition by DHHC palmitoyl transferases. Mol. Biol. Cell. 20:1845–1854 10.1091/mbc.E08-09-0944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves J., Prescott G.R., Gorleku O.A., Chamberlain L.H. 2009b. The fat controller: roles of palmitoylation in intracellular protein trafficking and targeting to membrane microdomains (Review). Mol. Membr. Biol. 26:67–79 10.1080/09687680802620351 [DOI] [PubMed] [Google Scholar]
- Greaves J., Gorleku O.A., Salaun C., Chamberlain L.H. 2010. Palmitoylation of the SNAP25 protein family: specificity and regulation by DHHC palmitoyl transferases. J. Biol. Chem. 285:24629–24638 10.1074/jbc.M110.119289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J.F., Paterson H., Marshall C.J. 1990. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 63:133–139 10.1016/0092-8674(90)90294-O [DOI] [PubMed] [Google Scholar]
- Hannoush R.N., Arenas-Ramirez N. 2009. Imaging the lipidome: omega-alkynyl fatty acids for detection and cellular visualization of lipid-modified proteins. ACS Chem. Biol. 4:581–587 10.1021/cb900085z [DOI] [PubMed] [Google Scholar]
- Hayashi T., Rumbaugh G., Huganir R.L. 2005. Differential regulation of AMPA receptor subunit trafficking by palmitoylation of two distinct sites. Neuron. 47:709–723 10.1016/j.neuron.2005.06.035 [DOI] [PubMed] [Google Scholar]
- Hayashi T., Thomas G.M., Huganir R.L. 2009. Dual palmitoylation of NR2 subunits regulates NMDA receptor trafficking. Neuron. 64:213–226 10.1016/j.neuron.2009.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten E., Vesa J., Olkkonen V.M., Jalanko A., Peltonen L. 1996. Human palmitoyl protein thioesterase: evidence for lysosomal targeting of the enzyme and disturbed cellular routing in infantile neuronal ceroid lipofuscinosis. EMBO J. 15:5240–5245 [PMC free article] [PubMed] [Google Scholar]
- Hess D.T., Patterson S.I., Smith D.S., Skene J.H. 1993. Neuronal growth cone collapse and inhibition of protein fatty acylation by nitric oxide. Nature. 366:562–565 10.1038/366562a0 [DOI] [PubMed] [Google Scholar]
- Hou H., Subramanian K., LaGrassa T.J., Markgraf D., Dietrich L.E., Urban J., Decker N., Ungermann C. 2005. The DHHC protein Pfa3 affects vacuole-associated palmitoylation of the fusion factor Vac8. Proc. Natl. Acad. Sci. USA. 102:17366–17371 10.1073/pnas.0508885102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K., Yanai A., Kang R., Arstikaitis P., Singaraja R.R., Metzler M., Mullard A., Haigh B., Gauthier-Campbell C., Gutekunst C.-A., et al. 2004. Huntingtin-interacting protein HIP14 is a palmitoyl transferase involved in palmitoylation and trafficking of multiple neuronal proteins. Neuron. 44:977–986 10.1016/j.neuron.2004.11.027 [DOI] [PubMed] [Google Scholar]
- Huang K., Sanders S., Singaraja R., Orban P., Cijsouw T., Arstikaitis P., Yanai A., Hayden M.R., El-Husseini A. 2009. Neuronal palmitoyl acyl transferases exhibit distinct substrate specificity. FASEB J. 23:2605–2615 10.1096/fj.08-127399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaani J., Patterson G., Schaufele F., Lippincott-Schwartz J., Baekkeskov S. 2008. A palmitoylation cycle dynamically regulates partitioning of the GABA-synthesizing enzyme GAD65 between ER-Golgi and post-Golgi membranes. J. Cell Sci. 121:437–449 10.1242/jcs.011916 [DOI] [PubMed] [Google Scholar]
- Kang R., Wan J., Arstikaitis P., Takahashi H., Huang K., Bailey A.O., Thompson J.X., Roth A.F., Drisdel R.C., Mastro R., et al. 2008. Neural palmitoyl-proteomics reveals dynamic synaptic palmitoylation. Nature. 456:904–909 10.1038/nature07605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara K., Nakayama Y., Kihara A., Matsuda D., Ikeda K., Kuga T., Fukumoto Y., Igarashi Y., Yamaguchi N. 2007. Rapid trafficking of c-Src, a non-palmitoylated Src-family kinase, between the plasma membrane and late endosomes/lysosomes. Exp. Cell Res. 313:2651–2666 10.1016/j.yexcr.2007.05.001 [DOI] [PubMed] [Google Scholar]
- Keller C.A., Yuan X., Panzanelli P., Martin M.L., Alldred M., Sassoè-Pognetto M., Lüscher B. 2004. The gamma2 subunit of GABA(A) receptors is a substrate for palmitoylation by GODZ. J. Neurosci. 24:5881–5891 10.1523/JNEUROSCI.1037-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.-J., Zhang Z., Sarkar C., Tsai P.-C., Lee Y.-C., Dye L., Mukherjee A.B. 2008. Palmitoyl protein thioesterase-1 deficiency impairs synaptic vesicle recycling at nerve terminals, contributing to neuropathology in humans and mice. J. Clin. Invest. 118:3075–3086 10.1172/JCI33482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental I., Grzybek M., Simons K. 2010. Greasing their way: lipid modifications determine protein association with membrane rafts. Biochemistry. 49:6305–6316 10.1021/bi100882y [DOI] [PubMed] [Google Scholar]
- Li Y., Hu J., Höfer K., Wong A.M.S., Cooper J.D., Birnbaum S.G., Hammer R.E., Hofmann S.L. 2010. DHHC5 interacts with PDZ domain 3 of post-synaptic density-95 (PSD-95) protein and plays a role in learning and memory. J. Biol. Chem. 285:13022–13031 10.1074/jbc.M109.079426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D.-T., Makino Y., Sharma K., Hayashi T., Neve R., Takamiya K., Huganir R.L. 2009. Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat. Neurosci. 12:879–887 10.1038/nn.2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder M.E., Deschenes R.J. 2007. Palmitoylation: policing protein stability and traffic. Nat. Rev. Mol. Cell Biol. 8:74–84 10.1038/nrm2084 [DOI] [PubMed] [Google Scholar]
- Linder M.E., Middleton P., Hepler J.R., Taussig R., Gilman A.G., Mumby S.M. 1993. Lipid modifications of G proteins: alpha subunits are palmitoylated. Proc. Natl. Acad. Sci. USA. 90:3675–3679 10.1073/pnas.90.8.3675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo S., Greentree W.K., Linder M.E., Deschenes R.J. 2002. Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J. Biol. Chem. 277:41268–41273 10.1074/jbc.M206573200 [DOI] [PubMed] [Google Scholar]
- Magee A.I., Gutierrez L., McKay I.A., Marshall C.J., Hall A. 1987. Dynamic fatty acylation of p21N-ras. EMBO J. 6:3353–3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B.R., Cravatt B.F. 2009. Large-scale profiling of protein palmitoylation in mammalian cells. Nat. Methods. 6:135–138 10.1038/nmeth.1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkonian K.A., Ostermeyer A.G., Chen J.Z., Roth M.G., Brown D.A. 1999. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J. Biol. Chem. 274:3910–3917 10.1074/jbc.274.6.3910 [DOI] [PubMed] [Google Scholar]
- Misaki R., Morimatsu M., Uemura T., Waguri S., Miyoshi E., Taniguchi N., Matsuda M., Taguchi T. 2010. Palmitoylated Ras proteins traffic through recycling endosomes to the plasma membrane during exocytosis. J. Cell Biol. 191:23–29 10.1083/jcb.200911143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D.A., Vasudevan A., Linder M.E., Deschenes R.J. 2006. Protein palmitoylation by a family of DHHC protein S-acyltransferases. J. Lipid Res. 47:1118–1127 10.1194/jlr.R600007-JLR200 [DOI] [PubMed] [Google Scholar]
- Moffett S., Mouillac B., Bonin H., Bouvier M. 1993. Altered phosphorylation and desensitization patterns of a human beta 2-adrenergic receptor lacking the palmitoylated Cys341. EMBO J. 12:349–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett S., Adam L., Bonin H., Loisel T.P., Bouvier M., Mouillac B. 1996. Palmitoylated cysteine 341 modulates phosphorylation of the β2-adrenergic receptor by the cAMP-dependent protein kinase. J. Biol. Chem. 271:21490–21497 10.1074/jbc.271.27.16384 [DOI] [PubMed] [Google Scholar]
- Mondal M., Mesmin B., Mukherjee S., Maxfield F.R. 2009. Sterols are mainly in the cytoplasmic leaflet of the plasma membrane and the endocytic recycling compartment in CHO cells. Mol. Biol. Cell. 20:581–588 10.1091/mbc.E08-07-0785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby S.M., Kleuss C., Gilman A.G. 1994. Receptor regulation of G-protein palmitoylation. Proc. Natl. Acad. Sci. USA. 91:2800–2804 10.1073/pnas.91.7.2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadolski M.J., Linder M.E. 2009. Molecular recognition of the palmitoylation substrate Vac8 by its palmitoyltransferase Pfa3. J. Biol. Chem. 284:17720–17730 10.1074/jbc.M109.005447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noritake J., Fukata Y., Iwanaga T., Hosomi N., Tsutsumi R., Matsuda N., Tani H., Iwanari H., Mochizuki Y., Kodama T., et al. 2009. Mobile DHHC palmitoylating enzyme mediates activity-sensitive synaptic targeting of PSD-95. J. Cell Biol. 186:147–160 10.1083/jcb.200903101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dowd B.F., Hnatowich M., Caron M.G., Lefkowitz R.J., Bouvier M. 1989. Palmitoylation of the human beta 2-adrenergic receptor. Mutation of Cys341 in the carboxyl tail leads to an uncoupled nonpalmitoylated form of the receptor. J. Biol. Chem. 264:7564–7569 [PubMed] [Google Scholar]
- Ohno Y., Kihara A., Sano T., Igarashi Y. 2006. Intracellular localization and tissue-specific distribution of human and yeast DHHC cysteine-rich domain-containing proteins. Biochim. Biophys. Acta. 1761:474–483 [DOI] [PubMed] [Google Scholar]
- Ohyama T., Verstreken P., Ly C.V., Rosenmund T., Rajan A., Tien A.-C., Haueter C., Schulze K.L., Bellen H.J. 2007. Huntingtin-interacting protein 14, a palmitoyl transferase required for exocytosis and targeting of CSP to synaptic vesicles. J. Cell Biol. 179:1481–1496 10.1083/jcb.200710061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parat M.-O., Fox P.L. 2001. Palmitoylation of caveolin-1 in endothelial cells is post-translational but irreversible. J. Biol. Chem. 276:15776–15782 10.1074/jbc.M006722200 [DOI] [PubMed] [Google Scholar]
- Parenti M., Viganó M.A., Newman C.M., Milligan G., Magee A.I. 1993. A novel N-terminal motif for palmitoylation of G-protein alpha subunits. Biochem. J. 291:349–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson G.H., Hirschberg K., Polishchuk R.S., Gerlich D., Phair R.D., Lippincott-Schwartz J. 2008. Transport through the Golgi apparatus by rapid partitioning within a two-phase membrane system. Cell. 133:1055–1067 10.1016/j.cell.2008.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh M.D. 2006a. Trafficking and signaling by fatty-acylated and prenylated proteins. Nat. Chem. Biol. 2:584–590 10.1038/nchembio834 [DOI] [PubMed] [Google Scholar]
- Resh M.D. 2006b. Use of analogs and inhibitors to study the functional significance of protein palmitoylation. Methods. 40:191–197 10.1016/j.ymeth.2006.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocks O., Peyker A., Kahms M., Verveer P.J., Koerner C., Lumbierres M., Kuhlmann J., Waldmann H., Wittinghofer A., Bastiaens P.I.H. 2005. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science. 307:1746–1752 10.1126/science.1105654 [DOI] [PubMed] [Google Scholar]
- Rocks O., Gerauer M., Vartak N., Koch S., Huang Z.-P., Pechlivanis M., Kuhlmann J., Brunsveld L., Chandra A., Ellinger B., et al. 2010. The palmitoylation machinery is a spatially organizing system for peripheral membrane proteins. Cell. 141:458–471 10.1016/j.cell.2010.04.007 [DOI] [PubMed] [Google Scholar]
- Roth A.F., Feng Y., Chen L., Davis N.G. 2002. The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J. Cell Biol. 159:23–28 10.1083/jcb.200206120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A.F., Wan J., Bailey A.O., Sun B., Kuchar J.A., Green W.N., Phinney B.S., Yates J.R., III, Davis N.G. 2006. Global analysis of protein palmitoylation in yeast. Cell. 125:1003–1013 10.1016/j.cell.2006.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Plowman S., Rotblat B., Prior I.A., Muncke C., Grainger S., Parton R.G., Henis Y.I., Kloog Y., Hancock J.F. 2005. Individual palmitoyl residues serve distinct roles in H-ras trafficking, microlocalization, and signaling. Mol. Cell. Biol. 25:6722–6733 10.1128/MCB.25.15.6722-6733.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandilands E., Brunton V.G., Frame M.C. 2007. The membrane targeting and spatial activation of Src, Yes and Fyn is influenced by palmitoylation and distinct RhoB/RhoD endosome requirements. J. Cell Sci. 120:2555–2564 10.1242/jcs.003657 [DOI] [PubMed] [Google Scholar]
- Shahinian S., Silvius J.R. 1995. Doubly-lipid-modified protein sequence motifs exhibit long-lived anchorage to lipid bilayer membranes. Biochemistry. 34:3813–3822 10.1021/bi00011a039 [DOI] [PubMed] [Google Scholar]
- Shmueli A., Segal M., Sapir T., Tsutsumi R., Noritake J., Bar A., Sapoznik S., Fukata Y., Orr I., Fukata M., Reiner O. 2010. Ndel1 palmitoylation: a new mean to regulate cytoplasmic dynein activity. EMBO J. 29:107–119 10.1038/emboj.2009.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel G., Obernosterer G., Fiore R., Oehmen M., Bicker S., Christensen M., Khudayberdiev S., Leuschner P.F., Busch C.J.L., Kane C., et al. 2009. A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nat. Cell Biol. 11:705–716 10.1038/ncb1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotrys J.E., Schoenfish M.J., Stutz M.A., Linder M.E. 2005. The vacuolar DHHC-CRD protein Pfa3p is a protein acyltransferase for Vac8p. J. Cell Biol. 170:1091–1099 10.1083/jcb.200507048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J.S., Simon D.I., Osborne J.A., Mullins M.E., Jaraki O., Michel T., Singel D.J., Loscalzo J. 1992. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc. Natl. Acad. Sci. USA. 89:444–448 10.1073/pnas.89.1.444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers R.S., Isacoff E.Y. 2007. Drosophila huntingtin-interacting protein 14 is a presynaptic protein required for photoreceptor synaptic transmission and expression of the palmitoylated proteins synaptosome-associated protein 25 and cysteine string protein. J. Neurosci. 27:12874–12883 10.1523/JNEUROSCI.2464-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarthout J.T., Lobo S., Farh L., Croke M.R., Greentree W.K., Deschenes R.J., Linder M.E. 2005. DHHC9 and GCP16 constitute a human protein fatty acyltransferase with specificity for H- and N-Ras. J. Biol. Chem. 280:31141–31148 10.1074/jbc.M504113200 [DOI] [PubMed] [Google Scholar]
- Tian L., Duncan R.R., Hammond M.S.L., Coghill L.S., Wen H., Rusinova R., Clark A.G., Levitan I.B., Shipston M.J. 2001. Alternative splicing switches potassium channel sensitivity to protein phosphorylation. J. Biol. Chem. 276:7717–7720 10.1074/jbc.C000741200 [DOI] [PubMed] [Google Scholar]
- Tian L., Jeffries O., McClafferty H., Molyvdas A., Rowe I.C.M., Saleem F., Chen L., Greaves J., Chamberlain L.H., Knaus H.-G., et al. 2008. Palmitoylation gates phosphorylation-dependent regulation of BK potassium channels. Proc. Natl. Acad. Sci. USA. 105:21006–21011 10.1073/pnas.0806700106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., McClafferty H., Jeffries O., Shipston M.J. 2010. Multiple palmitoyltransferases are required for palmitoylation-dependent regulation of large conductance calcium- and voltage-activated potassium channels. J. Biol. Chem. 285:23954–23962 10.1074/jbc.M110.137802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi R., Fukata Y., Noritake J., Iwanaga T., Perez F., Fukata M. 2009. Identification of G protein alpha subunit-palmitoylating enzyme. Mol. Cell. Biol. 29:435–447 10.1128/MCB.01144-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez-Taubas J., Pelham H. 2005. Swf1-dependent palmitoylation of the SNARE Tlg1 prevents its ubiquitination and degradation. EMBO J. 24:2524–2532 10.1038/sj.emboj.7600724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit M., Schmidt M.F.G. 2001. Enzymatic depalmitoylation of viral glycoproteins with acyl-protein thioesterase 1 in vitro. Virology. 288:89–95 10.1006/viro.2001.1063 [DOI] [PubMed] [Google Scholar]
- Wang G., Deschenes R.J. 2006. Plasma membrane localization of Ras requires class C Vps proteins and functional mitochondria in Saccharomyces cerevisiae. Mol. Cell. Biol. 26:3243–3255 10.1128/MCB.26.8.3243-3255.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedegaertner P.B., Bourne H.R. 1994. Activation and depalmitoylation of Gs α. Cell. 77:1063–1070 10.1016/0092-8674(94)90445-6 [DOI] [PubMed] [Google Scholar]
- Wright L.P., Philips M.R. 2006. Thematic review series: lipid posttranslational modifications. CAAX modification and membrane targeting of Ras. J. Lipid Res. 47:883–891 10.1194/jlr.R600004-JLR200 [DOI] [PubMed] [Google Scholar]
- Yap M.C., Kostiuk M.A., Martin D.D.O., Perinpanayagam M.A., Hak P.G., Siddam A., Majjigapu J.R., Rajaiah G., Keller B.O., Prescher J.A., et al. 2010. Rapid and selective detection of fatty acylated proteins using omega-alkynyl-fatty acids and click chemistry. J. Lipid Res. 51:1566–1580 10.1194/jlr.D002790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh D.C., Duncan J.A., Yamashita S., Michel T. 1999. Depalmitoylation of endothelial nitric-oxide synthase by acyl-protein thioesterase 1 is potentiated by Ca(2+)-calmodulin. J. Biol. Chem. 274:33148–33154 10.1074/jbc.274.46.33148 [DOI] [PubMed] [Google Scholar]
- Zha J., Weiler S., Oh K.J., Wei M.C., Korsmeyer S.J. 2000. Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science. 290:1761–1765 10.1126/science.290.5497.1761 [DOI] [PubMed] [Google Scholar]