Abstract
OBJECTIVE:
Infant formulas differ considerably in composition and sensory profiles. In this randomized study, we examined whether healthy infants fed an extensively protein hydrolysate formula (PHF) would differ in feeding behavior and growth from those fed cow-milk formula (CMF).
PATIENTS AND METHODS:
Infants were randomly assigned to be fed CMF or PHF between 0.5 and 7.5 months of age. Each month for 7 months, infants were weighed and measured and then videotaped while being fed their assigned formula. Anthropometric z scores were calculated by using World Health Organization growth standards. Multilevel linear growth and piecewise mixed-effects models compared trajectories for growth measures and formula acceptance.
RESULTS:
When compared with infants fed CMF, infants fed PHF had significantly lower weight-for-length z scores across ages 2.5 to 7.5 months. There were no differences in length-for-age z scores, which indicate that group differences resulted from gains in weight, not length. Infants fed PHF also had significantly slower weight gain velocity compared with infants fed CMF. During the monthly assessments, PHF-fed infants consumed less formula to satiation than did CMF-fed infants across the study period. Maternal ratings of infants' acceptance of the formula did not differ at any age.
CONCLUSIONS:
z-score trajectories indicate that CMF-fed infants' weight gain was accelerated, whereas PHF-fed infants' weight gain was normative. Whether such differences in growth are because of differences in the protein content or amino acid profile of the formulas and, in turn, metabolism is unknown. Research on the long-term consequences of these early growth differences is needed.
Keywords: growth, infancy, sensitive periods, development, protein, formula
WHAT'S KNOWN ON THIS SUBJECT:
The different classes of formulas, and different brands within each class, vary in composition and flavor profiles, both of which may influence feeding and growth patterns. Because recent evidence suggests that, relative to intact proteins, hydrolyzed proteins are absorbed and metabolized in a way that promotes greater satiation, we conducted a randomized study on healthy, formula-fed infants to determine whether growth patterns and feeding behaviors differ on the basis of formula type.
WHAT THIS STUDY ADDS:
Not all formulas are alike. On the basis of World Health Organization standards, z-score trajectories indicate that cow-milk formula-fed infants' weight gain was accelerated, whereas protein-hydrolysate formula-fed infants' weight gain was normative.
Sensitive periods early in life have long-term consequences on health and food habits. Rapid rates of growth during the first year increase the risk for later obesity,1,2 metabolic syndrome,3 and mortality from cardiovascular disease,4 which leads some to speculate that interventions designed to reduce the incidence and severity of disease should begin during infancy.3,5
Using breastfed infants as the gold standard, past research has suggested that infants who are fed formula weigh more and have greater risk for later obesity.6–9 Formula choices include cow-milk–based formulas (CMFs), soy-based formulas, and protein hydrolysate–based formulas (PHFs); PHFs are the feeding regimen of choice for formula-fed infants who cannot tolerate intact proteins. In hydrolysates, milk proteins are broken down by enzymes and then ultrafiltrated to remove large residual peptides.10 These classes of formulas, as well as different brands within each class, vary in composition and flavor profiles,11 both of which may influence feeding and growth patterns. Thus, it may be inappropriate to consider formula-fed infants as a homogeneous group.
Recent evidence from animal models and adult human populations suggests that, relative to intact proteins, hydrolyzed proteins are absorbed and metabolized in a way that promotes greater satiation, possibly because of the gut nutrient-sensing system12 and/or more rapid nutrient absorption.13–16 On the basis of this research, we hypothesized that the feeding and growth patterns of infants fed PHF would differ from those fed an isocaloric CMF. Evidence consistent with this hypothesis comes from 2 clinical trials of infants with a family history of atopic disease who were randomly assigned at birth to be fed PHF or CMF for the first 617 or 1218 months of life. Infants randomly assigned to be fed either partially or extensively hydrolyzed PHF gained less weight compared with infants randomly assigned to be fed CMF. However, this reduced weight gain could be attributed to not only differences in the formulas but also to some aspect of these infants' family history.19 Moreover, in 1 of the studies,18 exclusive breastfeeding that was recommended for the first months of life may have confounded the results. Because neither of these studies objectively measured the infants' behaviors during formula feeding, differences in growth caused by differences in satiation could not be determined.
To rigorously test the hypothesis that the type of formula fed affects formula intake and growth patterns, we conducted a randomized study in which healthy infants, with no reported family history of allergies or atopic diseases and whose mothers decided to formula feed, were randomly assigned to receive either an extensively hydrolyzed PHF or CMF from 0.5 through 7.5 months of life. Monthly assessments provided information on infant formula intake and growth patterns. This randomization and frequent assessment permitted precise control of exposure history and in-depth longitudinal follow-up.
SUBJECTS AND METHODS
Study Design
Sixty-four mother-infant pairs participated in randomized experiments to study sensitive periods in human flavor-learning (data reported elsewhere is currently under review). The most recent experiment was registered at clinicaltrials.gov (identifier NCT00994747). Through ads in local newspapers and Web sites, we recruited recently parturient women who, before recruitment, had chosen to formula feed their healthy, term newborns a CMF. The majority of recruited mothers (n = 61) were exclusively formula feeding; 3 mothers were predominantly formula feeding but breastfed fewer than 3 times daily during the first 2 months of life.
Participation began when the infants were aged 0.5 months. At this time, the mother-infant pairs were randomly assigned to groups that differed according to the type of formula provided to the mother for her infant during the entire 7-month study on the basis of minimization to ensure balance among groups in terms of race/ethnicity and infant gender. One group (n = 35) was assigned to a CMF (Enfamil [Mead Johnson Nutrition, Evansville, IN]); the other group (n = 29) was assigned to an extensively hydrolyzed PHF (Nutramigen [Mead Johnson Nutrition]). A total of 8 mother-infant dyads withdrew, which yielded final group sizes of 32 for the CMF group and 24 for the PHF group. Each formula provided 67.7 kcal/100 mL; the PHF contained 35% more protein equivalent than the CMF (1.9 vs 1.4 g/100 mL, respectively)20,21 and greater amounts of small peptides and free amino acids22 (A.K.V., A. San Gabnel, DVM MS, M. Hirota, BS, and J.A.M., unpublished data, 2010). The PHF and CMF both contained 3.6 g/100 kcal fat and differed slightly in their carbohydrate content (7.0 and 7.4 g/kcal, respectively).20,21 Mothers were blind to the type of formula to which they were assigned, and study personnel were blind to the hypotheses and group assignment of the subjects when analyzing the videotapes and entering data. The University of Pennsylvania Office of Regulatory Affairs approved all procedures, and informed consent was obtained from each mother before study entry.
Monthly Procedures
On the first day of the study, when the infants were ∼0.5 months of age, and at the beginning of each monthly cycle, the mother-infant pairs came to our outpatient laboratory for evaluation. At study entry, mothers reported their infants' birth weights and lengths.24 At study entry and each monthly visit thereafter, infants and mothers were weighed and measured for length or height, respectively (Seca model 232 [Seca, Hamburg, Germany]; Health-O-Meter [Sunbeam Products, Inc, Boca Raton, FL]). Infants were weighed and measured in lightweight clothing or diapers. Mothers reported when their infants were last fed formula, solid foods, or beverages and provided information on parity, income, and other demographic measures.
During each monthly visit, infants were videotaped while their mothers fed them a meal of the assigned formula by using established methodologies.25,26 A video camera was placed 10 to 12 feet away and focused on the infant's face to document the feeding bout. Feeding occurred at approximately the same time of day each month and under naturalistic conditions in which the infant determined the pace and duration of the feed.25,26 Feeding ended when the infant refused the bottle on 3 consecutive occasions, at which point the total amount of milliliters consumed was recorded. Two other indicators of formula acceptance were measured: (1) amount of time the infant spent attached to the nipple of the bottle, which was determined from the videotapes; and (2) mothers' perception of their infant's enjoyment of the formula, which they rated on a 9-point scale (from 1, extreme dislike, to 9, extreme like) at the end of each test meal.
During the 2 days before each monthly visit, mothers recorded in a diary how often and when they fed their infants formula or any other foods or beverages. The next month's supply of formula (with labels removed) was distributed at the end of each visit. Mothers were given instructions on how to prepare the formula but were never given instructions by study personnel on how or how much to feed their infants or on when or how to introduce solid foods.
Statistical Analyses
Data were analyzed by using SAS 9.2 (SAS Institute, Inc, Cary, NC). Descriptive information was generated for all variables of interest. Each outcome variable was assessed for normality. We normalized anthropometric data (infant weight and length) to z scores 2 ways. First, we used World Health Organization (WHO) Anthro 3.0.1 software (available at www.who.int/childgrowth/en) to calculate age- and gender-specific z scores on the basis of the WHO growth standards, which use the breastfed child as the norm. Second, we used Epi Info 3.5.1 software (available at www.cdc.gov/epiinfo) to calculate age- and gender-specific z scores on the basis of the Centers for Disease Control and Prevention growth references,27 which are based on growth data from predominantly formula-fed infants. Following the methods of Burnham et al,28 weight-gain and linear-growth velocity were evaluated by using continuous difference scores for the change in weight or length between monthly assessments (calculated as Δweight [kg]/Δage [days] or Δlength [cm]/Δage [days] between subsequent monthly visits).
Independent t tests and χ2 tests were used to analyze differences among infants in the 2 formula groups at study entry. With repeated-measures analyses of variance we examined the effects of group on infant feeding and anthropometric measures. In addition, we used multilevel linear-growth models to compare the 2 group trajectories for each of these measures across the study period. A multilevel linear-growth model approach has advantages over repeated-measures analysis of variance for analyzing repeated measures over time: it accounts for the correlated data structure that arises from repeated measurements; enables use of the exact age of measurement; and allows retention of cases with 1 or more missing data points.30
We examined 3 z scores as indicators of infant growth: weight-for-age; length-for-age; and weight-for-length. The use of z scores allows statistical comparison between the sample and current growth references and standardizes weight and length data for what is considered normative for each infant's gender and age at measurement. In addition, the weight-for-length z score combines weight and length data to indicate how appropriate the infant's weight is for a given length. Analyses of the trajectories of these 3 growth indicators provide an age- and gender-standardized metric of the appropriateness of infants' weight and length gains from birth to 7.5 months and the effect of formula type on these gains.
Initial analyses of the growth trajectories for both the WHO and Centers for Disease Control and Prevention weight-for-age, length-for-age, and weight-for-length z scores indicated that separate slopes should be specified for birth to 0.5 months and 0.5 to 7.5 months for weight-for-age and weight-for-length z scores and for birth to 1.5 months and 1.5 to 7.5 months for length-for-age z scores. Thus, we tested whether piecewise mixed-effects models fit better than linear or quadratic mixed-effects models.29,30 Akaike information criteria and Bayesian information criteria values for the weight-for-age, length-for-age, and weight-for-length z score models all indicated that the piecewise models fit better than the linear or quadratic models. For other variables related to growth velocity and formula acceptance, comparison of Akaike information criteria and Bayesian information criteria values indicated that linear or quadratic models fit better than piecewise models. The amount of time elapsed since the infant was last fed was included as a covariate in analyses of formula acceptance.
RESULTS
Subject Characteristics
Table 1 lists the characteristics of the 2 groups of mother-infant dyads. Infants who were randomly assigned to be fed CMF or PHF did not significantly differ in weight or length at birth or in age, weight, or length at study entry, and mothers did not differ in age, BMI, or parity at study entry or in race/ethnicity or income levels.
TABLE 1.
Subject Characteristics According to Formula Group
| CMF Group (N = 32) | PHF Group (N = 24) | χ2 or t | P | |
|---|---|---|---|---|
| Infant characteristics | ||||
| Gender, male, % (n) | 50.0 (16) | 58.3 (14) | 0.38 | .54 |
| Age at study entry, mean ± SD, mo | 0.5 ± 0.3 | 0.4 ± 0.3 | −0.63 | .53 |
| Birth weight, mean ± SD, kg | 3.4 ± 0.5 | 3.5 ± 0.6 | 0.88 | .38 |
| Birth length, mean ± SD, cm | 51.1 ± 2.8 | 51.1 ± 2.7 | 0.06 | .95 |
| Weight at study entry, mean ± SD, kg | 4.2 ± 0.6 | 4.2 ± 0.6 | −0.10 | .92 |
| Length at study entry, mean ± SD, cm | 53.0 ± 2.5 | 53.0 ± 2.4 | −0.01 | .99 |
| Maternal/familial characteristics, mean ± SD | ||||
| Age at study entry, y | 29.8 ± 6.2 | 30.3 ± 8.5 | 0.22 | .82 |
| BMI at study entry | 35.8 ± 13.4 | 30.1 ± 5.9 | −1.24 | .23 |
| Family income level, % (n)a | ||||
| Less than $10 000 | 15.6 (5) | 12.5 (3) | 0.47 | .79 |
| $10 000–49 999 | 21.9 (7) | 29.2 (7) | — | — |
| More than $50 000 | 46.9 (15) | 31.7 (10) | — | — |
| Racial/ethnic category, % (n) | ||||
| Black | 21.9 (7) | 29.2 (7) | 4.76 | .19 |
| White | 59.4 (19) | 62.5 (15) | — | — |
| Hispanic | 15.6 (5) | 0.0 (0) | — | — |
| Other/mixed | 3.1 (1) | 8.3 (2) | — | — |
| Parity, primiparous, % (n) | 28.1 (9) | 33.3 (8) | 1.63 | .44 |
Data not available for 9 families.
No group effect or group-by-time interaction effect was seen for the number of times per day infants were fed formula across the study period (Table 2). Across both groups, mothers reported that their infants consumed formula 7.6 ± 1.9 times per day at study entry and 4.9 ± 1.6 times per day at 7.5 months. Infants in both groups also were introduced to solid foods similarly: cereal first, at ∼3.5 months; fruits at 4.5 months; vegetables at 5 months; and meats at 6 months of age. By 5.5 months, all but 7 infants had started consuming solid foods. There were no effects of timing of solid-food introduction or frequency of solid-food consumption on group differences in infants' weight-for-age, length-for-age, or weight-for-length z scores.
TABLE 2.
Number of Daily Formula Feedings and Age of Solid-Food Introduction as Determined by Maternal Reports and Intake of Assigned Formula as Determined by Laboratory-Based Monthly Assessments
| CMF Group (N = 32) | PHF Group (N = 24) | F | P | |
|---|---|---|---|---|
| No. of daily formula feedings, mean ± SE | ||||
| 0.5 mo | 7.7 ± 0.3 | 7.4 ± 0.5 | — | — |
| 1.5 mo | 7.3 ± 0.4 | 6.4 ± 0.3 | — | — |
| 2.5 mo | 6.9 ± 0.4 | 5.8 ± 0.4 | — | — |
| 3.5 mo | 6.2 ± 0.3 | 5.7 ± 0.4 | — | — |
| 4.5 mo | 5.9 ± 0.3 | 5.6 ± 0.3 | — | — |
| 5.5 mo | 5.2 ± 0.4 | 5.8 ± 0.3 | — | — |
| 6.5 mo | 5.0 ± 0.4 | 5.1 ± 0.4 | — | — |
| 7.5 mo | 4.8 ± 0.3 | 5.0 ± 0.4 | — | — |
| Average | 6.2 ± 0.1 | 5.9 ± 0.1 | 2.6a | .12 |
| Intake of assigned formula at laboratory-based assessment, least-squares mean ± SE, mLb | ||||
| 1.5 mo | 130.1 ± 8.8 | 94.7 ± 10.1 | — | — |
| 2.5 mo | 157.2 ± 12.1 | 124.3 ± 14.0 | — | — |
| 3.5 mo | 160.8 ± 12.7 | 152.1 ± 13.5 | — | — |
| 4.5 mo | 190.8 ± 14.6 | 128.4 ± 17.0 | — | — |
| 5.5 mo | 180.2 ± 15.2 | 152.8 ± 16.9 | — | — |
| 6.5 mo | 160.7 ± 17.1 | 154.1 ± 18.7 | — | — |
| 7.5 mo | 190.1 ± 22.4 | 188.8 ± 24.0 | — | — |
| Average | 164.9 ± 5.6 | 143.4 ± 6.7 | 3.96a | .05 |
| Age at solid-food introduction, mean ± SE, mo | ||||
| Cereal | 3.6 ± 0.3 | 3.5 ± 0.3 | 0.03 | .86 |
| Fruit | 4.9 ± 0.3 | 4.5 ± 0.3 | 0.87 | .36 |
| Vegetables | 5.3 ± 0.3 | 5.0 ± 0.3 | 0.43 | .51 |
| Meat | 6.3 ± 0.2 | 6.0 ± 0.3 | 0.47 | .50 |
Main effect of group; group-×-time interaction was not significant.
Analysis adjusted for time since last formula or solid-food feeding.
Infant Growth Patterns
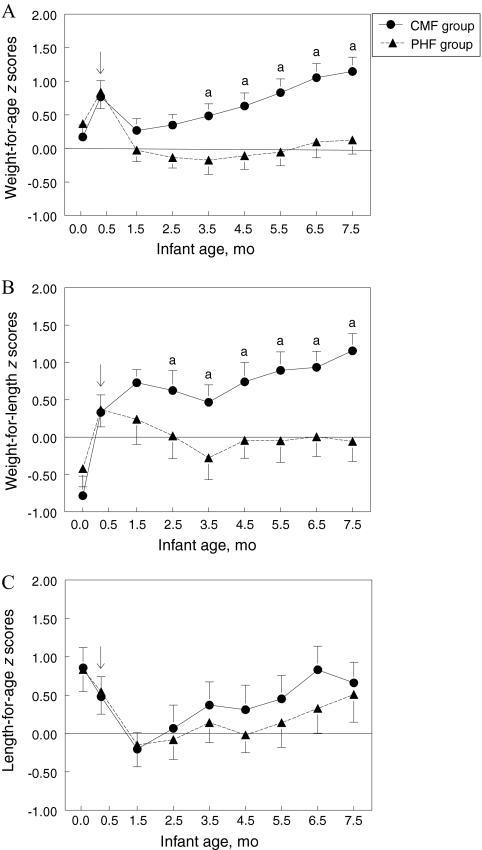
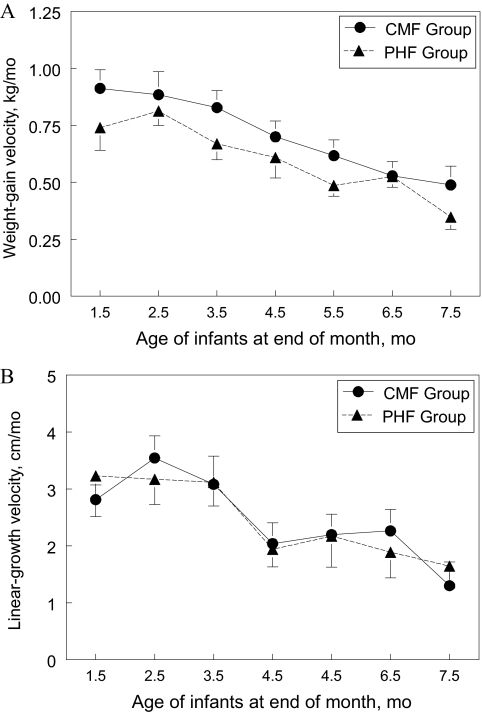
Figure 1 presents weight-for-age, length-for-age, and weight-for-length z score trajectories calculated from the WHO growth standards from birth to 7.5 months. At study entry, group z scores did not differ for weight-for-age, weight-for-length, or length-for-age. However, PHF-fed infants had significantly lower z scores for weight-for-age from 3.5 to 7.5 months of age (F1,42 = 4.72; P < .05) and for weight-for-length from 2.5 to 7.5 months of age (F1,42 = 8.52; P < .01) compared with CMF-fed infants (Fig 1). In addition, from 0.5 to 7.5 months of age, the PHF-fed group had significantly less z-score change for weight-for-age (F1,409 = 20.00; P < .0001) and weight-for-length (F1,409 = 7.08; P < .01) than did CMF-fed infants. In contrast, the groups did not significantly differ in length-for-age z scores across the study period (P = .86). All of these findings were unchanged when we used the Centers for Disease Control and Prevention–based growth-referenced z scores and when we excluded the 3 infants who also received breast milk during the first 2 months of the study. Figure 2A shows that, overall, infants fed PHF had a slower weight-gain velocity compared with infants fed CMF (F1,44 = 6.60; P < .05), whereas Fig 2B illustrates that these groups of infants did not differ in linear-growth velocity.
FIGURE 1.
Infant weight-for-age (A), weight-for-length (B), and length-for-age (C) z-score trajectories from birth to 7.5 months according to formula group. z scores were calculated by using the WHO growth standards. The arrow (↓) indicates the age at which infants were randomly assigned to either CMF or PHF. Infants randomly assigned to PHF had significantly lower weight-for-age and weight-for-length z scores and significantly less weight-for-age and weight-for-length z score change than did infants randomly assigned to CMF. No effect of formula was found for length-for-age z scores. A z score of 0 is considered normative, and z score-tracking is a clinical indicator of normative growth.32 a Groups differed significantly at P < .05 in the posthoc comparison.
FIGURE 2.
A, Infant weight-gain velocity, calculated as weight change (in kilograms) divided by age change (in days) between assessments (which were ∼1 month apart), from 1.5 to 7.5 months for infants randomly assigned to either CMF or PHF. B, Infant linear-growth velocity, calculated as length change (in centimeters) divided by age change (in days) between assessments (which were ∼1 month apart), from 1.5 to 7.5 months for infants randomly assigned to either CMF or PHF. x-axis values represent the end point for the monthly linear-growth velocity calculation (eg, 1.5 months represents the length change between the 0.5- and 1.5-month visits). Infants randomly assigned to PHF had significantly slower overall weight-gain velocity (F1,44 = 6.60; P < .05) (A) but no difference in linear-growth velocity (F1,44 = 0.02; P = .89) (B) across the study period than did infants randomly assigned to CMF.
Objective Monthly Assessments of Infant Intake and Acceptance of the Formulas
There was a main effect of group on infants' intake of the formulas during the monthly assessment (F1,52 = 3.96; P = .05). From 1.5 to 7.5 months of age, PHF-fed infants consumed less formula to satiation during the monthly assessment than did CMF-fed infants (Table 2). However, they did not differ in length of feeding (CMF: 11.2 ± 5.9 minutes; PHF: 11.9 ± 6.1 minutes) (P = .86) or mothers' ratings of their infant's liking of the formulas (CMF: 8.4 ± 1.1; PHF: 8.3 ± 1.5) (P = .63).
DISCUSSION
Beginning 2 months after random assignment of the formulas, growth trajectories for infants fed PHF differed significantly from those for infants fed CMF on the basis of weight-for-length z scores. Weight-for-age, length-for-age, and weight-for-length z scores of 0 are considered normative, and z-score-tracking (or, alternatively, percentile-tracking) is used as an indication of healthy, normative growth.31 Weight-for-age and weight-for-length z scores for the PHF-fed group tracked close to 0 for most of the study period, which indicated that they were gaining weight at a normal rate, whereas those for the CMF-fed group were consistently above 0 and increased across time, which indicated accelerated rates of weight gain. These trajectory differences were supported by analyses of growth velocities, which indicated that CMF-fed infants had a significantly greater weight-gain velocity than PHF-fed infants across the first 7 months of life. These findings are similar to previous findings that infants fed CMF exhibit accelerated weight gain during infancy when compared with breastfed infants.32,33 Length-for-age z scores and linear-growth velocity did not differ between groups, which indicated that growth differences were attributable to differences in gains in weight, not length, across the study period. Overall, our findings for slower weight gain for healthy infants randomly assigned to be fed a PHF compared with those randomly assigned to be fed a CMF are consistent with previous research on infants who had a family history of atopic disease, many of whom also were breastfed for the first few months of life.17,18
PHF-fed infants consumed less formula than did CMF-fed infants during monthly laboratory-based, infant-led feeding sessions.25 This finding is also consistent with previous, shorter-term (0.5- to 3-month) studies of healthy 1- to 3-month-old infants, which also revealed, on the basis of feeding diaries kept by mothers, that infants randomly assigned to feed with PHFs had lower daily intakes than did infants randomly assigned to feed CMF.34–36 These effects of being fed PHF on infant weight gain, at least during the first 8 months of life, did not seem to be mediated by the introduction of solid foods into the infant's diet. Such null effects of solid-food introduction on formula-fed infants' weight gain are consistent with previous studies.18,37 In addition, the age at which infants in the current study began eating solids, although younger than current recommendations,38 is consistent with findings in national studies.39
What can explain these substantial differences in both intake and weight gain related to the type of formula consumed? We present several, not mutually exclusive, hypotheses. First, the sensory characteristics of the formulas may have differentially influenced the infants' feeding behaviors. Infants may dislike the taste of PHF and consequently consume less, thereby gaining weight more slowly. To adults, PHFs have a distinctive, unpleasant flavor (taste and odor) because the hydrolysis results in high levels of free amino acids and small peptides, which taste sour, bitter, and savory and emit unpleasant sulfur-based odors.11,40,41 However, the evidence that negative sensory properties of food or beverages alone can result in decreased growth during infancy is weak or nonexistent. For example, in animal models, total food intake and the growth efficiency of weanling rats was not affected by feeding a solid-food diet adulterated with aversive flavors.42 Moreover, introducing PHF to infants during the first 3 months of life renders this formula highly palatable and accepted throughout infancy.27 The infants in our study were introduced to PHF during this sensitive period; they were fed PHF to satiation, and their mothers perceived that they enjoyed the formula during the course of the 7-month study. Taken together, all of these findings strongly suggest that the lower intakes and differences in growth were not attributable to rejection of the formula on the basis of its negative flavor characteristics.
Second, as has been observed in animal-model studies and studies on older children and adults,43–46 the higher protein content of the PHF may have made this formula more satiating than CMF for infants. PHF and CMF are isocaloric but differ in their protein (or protein equivalent47) content in that PHF is 35% higher in protein than CMF.20,21 However, this explanation is contradictory to data obtained from recent clinical trials in which infants who were fed CMFs that were high in protein consumed more formula and gained more weight than infants who consumed lower-protein milk formulas, even when controlling for energy intake.37,48 Specifically, Koletzko et al37 randomly assigned healthy newborns to be fed, during the first year of life, isocaloric CMF and follow-on formulas with low (1.8 and 2.2 g of protein per 100 kcal, respectively) or high (2.9 and 4.4 g of protein per 100 kcal, respectively) protein content (as a reference, the CMF and PHF used in or study contained 2.1 and 2.8 g of protein equivalent per 100 kcal, respectively20,21). Infants who consumed the higher-protein CMF and follow-on formula had higher weight-for-age z scores than did infants who consumed the lower-protein CMF. Thus, absolute difference in the protein equivalencies of the 2 formulas in our study, in and of themselves, was unlikely to be solely responsible for the relative differences in intake, body weight, and weight gain between the 2 groups.
The third and most parsimonious hypothesis, given the present findings, is that the form in which the amino acids are delivered to the infants, rather than overall amount of amino acids consumed (via total protein content), was responsible for the differences in infant intake and growth. Specifically, the amino acids in PHF predominantly are free amino acids, which means that they are not contained within intact proteins, whereas little of the amino acid content of CMF is in free form (A.K.V., A. San Gabriel, DVM MS, M. Hirota, BS, and J.A.M., unpublished data, 2010). This difference that may have important implications in nutrient absorption and metabolism.13–16 Differential intake and growth patterns may result from the ability of free amino acids to stimulate sensory receptors in the oral cavity and/or gastrointestinal tract,12 which, in turn, may serve as key signals for intake regulation and satiation.49,50
Previous research14,15 has shown that protein hydrolysates stimulate a cascade of satiation signals. Specifically, receptor mechanisms in the gut release cholecystokinin in response to protein hydrolysates,51 which provides a mechanistic pathway for associations among protein hydrolysate ingestion, cholecystokinin release, and satiation.14,15,50–53 In addition, receptor mechanisms for certain amino acids, such as free glutamate, are present in the gut, which, in turn, signal the presence of ingested protein12 through stimulation of the vagus nerve.54 The vagus, a principal transmitter of food-related messages from the gastrointestinal mucosa to the central nervous system, seems to be the primary pathway that conveys gut glutamate information to the brain as minimal plasma glutamate passes the blood-brain barrier.55 Thus, the detection of protein hydrolysates, in general, or specific free amino acids or small peptides in the gut after feeding may serve as satiation signals and stimulate earlier meal termination for infants who consume PHFs. Alternatively, stimulation of gut receptors by free amino acids (eg, glutamate) may stimulate an increase in energy expenditure, which, in turn, contributes to slower weight gain over time.56 We caution, however, that several studies have documented that infants who were fed PHF have significantly higher serum free amino acids compared with infants fed breast milk or CMF.57–60 The consequence of the higher serum amino acids is still unclear, but they may signal an inefficient use of nutrients, which also could have contributed to the slower growth rates among PHF-fed infants. Furthermore, it is unclear whether there are components in CMF that contribute to overfeeding by infants.
CONCLUSIONS
Our results show that the compositional differences between CMF and PHF result in differential weight-gain trajectories. Additional research into the exact mechanisms underlying these differences is needed both for practical concerns of optimizing infant feeding and for theoretical concerns focusing on understanding mechanisms underlying hunger and satiation. Longer-term effects of hydrolyzed protein diets, which are relatively new in the human food supply and are growing in use, also need to be investigated. Because dietary and nutritional programming can have long-term consequences in terms of later development of obesity, diabetes, and other diseases,61 it is imperative that we learn more about the long-term consequences of the early growth differences caused by environmental triggers, such as those associated with infant formulas, and how and why they differ from breastfeeding, which is the optimal mode of feeding.
ACKNOWLEDGMENTS
This project was supported by grant R01HD37119 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH). We acknowledge the expert technical assistance of Laura Lukasewycz, Sara Castor, Lauren Yourshaw, and Myrline Gillot, a recipient of a special supplement from this NIH grant under the Research Supplements to Promote Diversity in Health-Related Research Program. Dr Ventura is a postdoctoral trainee on NIH grant T32-DC00014.
We thank Dr Virginia Stallings for valuable comments on an earlier version of the manuscript, Dr Daniel Tomé for insightful discussions, and Mead Johnson Nutrionals for supplying the formulas.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00994747).
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
- CMF
- cow-milk formula
- PHF
- protein hydrolysate formula
- WHO
- World Health Organization
REFERENCES
- 1. Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331(7522):929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chomtho S, Wells JC, Davies PS, Lucas A, Fewtrell MS. Early growth and body composition in infancy. Adv Exp Med Biol. 2009;646:165–168 [DOI] [PubMed] [Google Scholar]
- 3. Ekelund U, Ong KK, Linne Y, et al. Association of weight gain in infancy and early childhood with metabolic risk in young adults. J Clin Endocrinol Metab. 2007;92(1):98–103 [DOI] [PubMed] [Google Scholar]
- 4. Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23(6 suppl):588S–595S [DOI] [PubMed] [Google Scholar]
- 5. Lucas A. The developmental origins of adult health and well-being. Adv Exp Med Biol. 2005;569:13–15 [DOI] [PubMed] [Google Scholar]
- 6. Armstrong J, Reilly JJ. Breastfeeding and lowering the risk of childhood obesity. Lancet. 2002;359(9322):2003–2004 [DOI] [PubMed] [Google Scholar]
- 7. Burke V, Beilin LJ, Simmer K, et al. Breastfeeding and overweight: longitudinal analysis in an Australian birth cohort. J Pediatr. 2005;147(1):56–61 [DOI] [PubMed] [Google Scholar]
- 8. Grummer-Strawn LM, Mei Z. Does breastfeeding protect against pediatric overweight? Analysis of longitudinal data from the Centers for Disease Control and Prevention Pediatric Nutrition Surveillance System. Pediatrics. 2004;113(2). Available at: www.pediatrics.org/cgi/content/full/113/2/e81 [DOI] [PubMed] [Google Scholar]
- 9. Kramer MS. Do breast-feeding and delayed introduction of solid foods protect against subsequent obesity? J Pediatr. 1981;98(6):883–887 [DOI] [PubMed] [Google Scholar]
- 10. American Academy of Pediatrics, Committee on Nutrition Hypoallergenic infant formulas. Pediatrics. 2000;106(2 pt 1). Available at: www.pediatrics.org/cgi/content/full/106/2/346 [PubMed] [Google Scholar]
- 11. Cook DA, Sarett HP. Design of Infant Formulas for Meeting Normal and Special Need. Pediatric Nutrition: Infant Feeding, Deficiencies, Disease. New York, NY: Marcel Dekker, Inc; 1982 [Google Scholar]
- 12. San Gabriel A, Maekawa T, Uneyama H, Yoshie S, Torii K. mGluR1 in the fundic glands of rat stomach. FEBS Lett. 2007;581(6):1119–1123 [DOI] [PubMed] [Google Scholar]
- 13. Koopman R, Crombach N, Gijsen AP, et al. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am J Clin Nutr. 2009;90(1):106–115 [DOI] [PubMed] [Google Scholar]
- 14. Diepvens K, Haberer D, Westerterp-Plantenga M. Different proteins and biopeptides differently affect satiety and anorexigenic/orexigenic hormones in healthy humans. Int J Obes (Lond). 2008;32(3):510–518 [DOI] [PubMed] [Google Scholar]
- 15. Foltz M, Ansems P, Schwarz J, Tasker MC, Lourbakos A, Gerhardt CC. Protein hydrolysates induce CCK release from enteroendocrine cells and act as partial agonists of the CCK1 receptor. J Agric Food Chem. 2008;56(3):837–843 [DOI] [PubMed] [Google Scholar]
- 16. Keohane PP, Grimble GK, Brown B, Spiller RC, Silk DB. Influence of protein composition and hydrolysis method on intestinal absorption of protein in man. Gut. 1985;26(9):907–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roche AF, Guo S, Siervogel RM, Khamis HJ, Chandra RK. Growth comparison of breast-fed and formula-fed infants. Can J Public Health. 1993;84(2):132–135 [PubMed] [Google Scholar]
- 18. Rzehak P, Sausenthaler S, Koletzko S, et al. German Infant Nutritional Intervention Study Group. Short- and long-term effects of feeding hydrolyzed protein infant formulas on growth at < or = 6 y of age: results from the German Infant Nutritional Intervention Study. Am J Clin Nutr. 2009;89(6):1846–1856 [DOI] [PubMed] [Google Scholar]
- 19. Isolauri E, Sutas Y, Salo MK, Isosomppi R, Kaila M. Elimination diet in cow's milk allergy: risk for impaired growth in young children. J Pediatr. 1998;132(6):1004–1009 [DOI] [PubMed] [Google Scholar]
- 20. Mead Johnson Nutrition Health Care Professional Resource Center: Nutramigen Product Information. Available at: www.mjn.com/app/iwp/hcp2/content2.do?dm=mj&id=/HCP_Home2/ProductInformation/hcpProducts/hcpInfants/hcpNutramigen&iwpst=MJN&Is=O&csred=1&r=3449135060 Accessed January 10, 2010
- 21. Mead Johnson Nutrition Health Care Professional Resource Center: Enfamil Product Information. Available at: www.mjn.com/app/iwp/hcp2/content2.do?dm=mj&id=/HCP_Home2/ProductInformation/hcpProducts/hcpInfants/hcpEnfamilLIPIL&iwpst=MJN&Is=O&csred=1&r=3449135119 Accessed January 10, 2010
- 22. Agostoni C, Carratu B, Boniglia C, Riva E, Sanzini E. Free amino acid content in standard infant formulas: comparison with human milk. J Am Coll Nutr. 2000;19(4):434–438 [DOI] [PubMed] [Google Scholar]
- 23. Lee YH. Food-processing approaches to altering allergenic potential of milk-based formula. J Pediatr. 1992;121(5 pt 2):S47–S50 [DOI] [PubMed] [Google Scholar]
- 24. Seidman DS, Slater PE, Ever-Hadani P, Gale R. Accuracy of mothers' recall of birthweight and gestational age. Br J Obstet Gynaecol. 1987;94(8):731–735 [DOI] [PubMed] [Google Scholar]
- 25. Mennella JA, Beauchamp GK. Developmental changes in the acceptance of protein hydrolysate formula. J Dev Behav Pediatr. 1996;17(6):386–391 [DOI] [PubMed] [Google Scholar]
- 26. Mennella JA, Griffin CE, Beauchamp GK. Flavor programming during infancy. Pediatrics. 2004;113(4)840–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27 [PubMed] [Google Scholar]
- 28. Burnham N, Ittenbach RF, Stallings VA, et al. Genetic factors are important determinants of impaired growth after infant cardiac surgery. J Thorac Cardiovasc Surg. 140(1):144–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singer JD. Using SAS proc mixed to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat. 1998;23(4):323–355 [Google Scholar]
- 30. Singer JD, Willett JB. Applied Longitudinal Data Analysis. New York, NY: Oxford University Press; 2003 [Google Scholar]
- 31. Cole TJ. Measuring normal growth and growth variance in infancy and childhood. In: Birch LL, Dietz W, Eds. Eating Behaviors of the Young Child: Prenatal and Postnatal Influences on Healthy Eating. Elk Grove Village, IL: American Academy of Pediatrics; 2008:17–31 [Google Scholar]
- 32. Dewey KG, Heinig MJ, Nommsen LA, Peerson JM, Lönnerdal B. Growth of breast-fed and formula-fed infants from 0 to 18 months: the DARLING Study. Pediatrics. 1992;89(6 pt 1):1035–1041 [PubMed] [Google Scholar]
- 33. Kramer MS, Guo T, Platt RW, et al. Feeding effects on growth during infancy. J Pediatr. 2004;145(5):600–605 [DOI] [PubMed] [Google Scholar]
- 34. Hyams JS, Treem WR, Etienne NL, et al. Effect of infant formula on stool characteristics of young infants. Pediatrics. 1995;95(1):50–54 [PubMed] [Google Scholar]
- 35. Hauser B, Keymolen K, Blecker U, et al. A comparative evaluation of whey hydrolysate and whey-predominant formulas: how well do infants accept and tolerate them? Clin Pediatr (Phila). 1993;32(7):433–437 [DOI] [PubMed] [Google Scholar]
- 36. Vandenplas Y, Hauser B, Blecker U, et al. The nutritional value of a whey hydrolysate formula compared with a whey-predominant formula in healthy infants. J Pediatr Gastroenterol Nutr. 1993;17(1):92–96 [DOI] [PubMed] [Google Scholar]
- 37. Koletzko B, von Kries R, Closa R, et al. Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr. 2009;89(6):1836–1845 [DOI] [PubMed] [Google Scholar]
- 38. American Academy of Pediatrics, Section on Breastfeeding Breastfeeding and the use of human milk. Pediatrics. 2005;115(2):496–506 [DOI] [PubMed] [Google Scholar]
- 39. Briefel RR, Reidy K, Karwe V, Devaney B. Feeding infants and toddlers study: improvements needed in meeting infant feeding recommendations. J Am Diet Assoc. 2004;104(suppl 1):s31–s37 [DOI] [PubMed] [Google Scholar]
- 40. Mennella JA, Beauchamp GK. Understanding the origin of flavor preferences. Chem Senses. 2005;30(suppl 1):i242–i243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schiffman SS, Dackis C. Taste of nutrients: amino acids, vitamins, and fatty acids. Percept Psychophys. 1975;17(2):140–146 [Google Scholar]
- 42. Naim M, Brand JG, Kare MR, Kaufmann NA, Kratz CM. Effects of unpalatable diets and food restriction on feed efficiency in growing rats. Physiol Behav. 1980;25(5):609–614 [DOI] [PubMed] [Google Scholar]
- 43. Johnson J, Vickers Z. Effects of flavor and macronutrient composition of food servings on liking, hunger and subsequent intake. Appetite. 1993;21(1):25–39 [DOI] [PubMed] [Google Scholar]
- 44. Poppitt SD, McCormack D, Buffenstein R. Short-term effects of macronutrient preloads on appetite and energy intake in lean women. Physiol Behav. 1998;64(3):279–285 [DOI] [PubMed] [Google Scholar]
- 45. Rolls BJ, Hetherington M, Burley VJ. The specificity of satiety: the influence of foods of different macronutrient content on the development of satiety. Physiol Behav. 1988;43(2):145–153 [DOI] [PubMed] [Google Scholar]
- 46. Westerterp-Plantenga MS, Rolland V, Wilson SA, Westerterp KR. Satiety related to 24 h diet-induced thermogenesis during high protein/carbohydrate vs high fat diets measured in a respiration chamber. Eur J Clin Nutr. 1999;53(6):495–502 [DOI] [PubMed] [Google Scholar]
- 47. Koletzko B, Baker S, Cleghorn G, et al. Global standard for the composition of infant formula: recommendations of an ESPGHAN coordinated international expert group. J Pediatr Gastroenterol Nutr. 2005;41(5):584–599 [DOI] [PubMed] [Google Scholar]
- 48. Schulze KF, Stefanski M, Masterson J, et al. Energy expenditure, energy balance, and composition of weight gain in low birth weight infants fed diets of different protein and energy content. J Pediatr. 1987;110(5):753–759 [DOI] [PubMed] [Google Scholar]
- 49. Viarouge C, Even P, Rougeot C, Nicolaidis S. Effects on metabolic and hormonal parameters of monosodium glutamate (umami taste) ingestion in the rat. Physiol Behav. 1991;49(5):1013–1018 [DOI] [PubMed] [Google Scholar]
- 50. Viarouge C, Caulliez R, Nicolaidis S. Umami taste of monosodium glutamate enhances the thermic effect of food and affects the respiratory quotient in the rat. Physiol Behav. 1992;52(5):879–884 [DOI] [PubMed] [Google Scholar]
- 51. Choi S, Lee M, Shiu AL, Yo SJ, Aponte GW. Identification of a protein hydrolysate responsive G protein-coupled receptor in enterocytes. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G98–G112 [DOI] [PubMed] [Google Scholar]
- 52. Choi S, Lee M, Shiu AL, Yo SJ, Hallden G, Aponte GW. GPR93 activation by protein hydrolysate induces CCK transcription and secretion in STC-1 cells. Am J Physiol Gastrointest Liver Physiol. 2007;292(5):G1366–G1375 [DOI] [PubMed] [Google Scholar]
- 53. Weller A. The ontogeny of postingestive inhibitory stimuli: examining the role of CCK. Dev Psychobiol. 2006;48(5):368–379 [DOI] [PubMed] [Google Scholar]
- 54. Uneyama H, Niijima A, San Gabriel A, Torii K. Luminal amino acid sensing in the rat gastric mucosa. Am J Physiol Gastrointest Liver Physiol. 2006;291(6):G1163–G1170 [DOI] [PubMed] [Google Scholar]
- 55. Tsurugizawa T, Uematsu A, Nakamura E, et al. Mechanisms of neural response to gastrointestinal nutritive stimuli: the gut-brain axis. Gastroenterology. 2009;137(1):262–273 [DOI] [PubMed] [Google Scholar]
- 56. Kondoh T, Torii K. MSG intake suppresses weight gain, fat deposition, and plasma leptin levels in male Sprague-Dawley rats. Physiol Behav. 2008;95(1–2):135–144 [DOI] [PubMed] [Google Scholar]
- 57. Decsi T, Veitl V, Szasz M, Pinter Z, Mehes K. Plasma amino acid concentrations in healthy, full-term infants fed hydrolysate infant formula. J Pediatr Gastroenterol Nutr. 1996;22(1):62–67 [DOI] [PubMed] [Google Scholar]
- 58. Hernell O, Lonnerdal B. Nutritional evaluation of protein hydrolysate formulas in healthy term infants: plasma amino acids, hematology, and trace elements. Am J Clin Nutr. 2003;78(2):296–301 [DOI] [PubMed] [Google Scholar]
- 59. Giovannini M, Agostoni C, Fiocchi A, Bellu R, Trojan S, Riva E. Antigen-reduced infant formulas versus human milk: growth and metabolic parameters in the first 6 months of life. J Am Coll Nutr. 1994;13(4):357–363 [DOI] [PubMed] [Google Scholar]
- 60. Rigo J, Salle BL, Picaud JC, Putet G, Senterre J. Nutritional evaluation of protein hydrolysate formulas. Eur J Clin Nutr. 1995;49(suppl 1):S26–S38 [PubMed] [Google Scholar]
- 61. Gluckman PD, Hanson MA. Developmental and epigenetic pathways to obesity: an evolutionary-developmental perspective. Int J Obes (Lond). 2008;32(suppl 7):S62–S71 [DOI] [PubMed] [Google Scholar]