Abstract
OBJECTIVE:
To describe presenting symptoms and signs according to age group in a cohort of 243 patients with tuberous sclerosis complex (TSC) and identify earlier symptoms and signs that did not lead to immediate diagnosis.
PATIENTS AND METHODS:
We performed a retrospective chart review for 278 patients with TSC who were examined at Children's Hospital Boston in Massachusetts and at the Herscot Center for Tuberous Sclerosis Complex, Massachusetts General Hospital. The presenting symptom or sign was the first symptom or sign to cause suspicion for TSC and lead to diagnosis. Missed symptoms or signs were those that were documented in the patient's chart but did not immediately lead to diagnosis.
RESULTS:
There were 243 patients for whom there were sufficient data for inclusion in this study. Patients were diagnosed with TSC at ages ranging from birth to 73 years. The average age at diagnosis was 7.5 years. Of the patients, 81% were diagnosed before the age of 10. Diagnosis during adolescence and adulthood was not uncommon. The most common presenting symptoms and signs included new onset of seizures, history of seizures, infantile spasms, family history of TSC, cardiac rhabdomyomas, and hypopigmented macules. Of the patients, 39% reported missed symptoms or signs of TSC, most commonly seizures (including infantile spasms) and dermatologic features.
CONCLUSIONS:
Many patients had symptoms or signs of TSC that did not lead to immediate diagnosis. Clinicians should be aware of the myriad potential presenting symptoms and signs of TSC. Early diagnosis may reduce morbidity and mortality.
Keywords: tuberous sclerosis complex, diagnosis, epilepsy, seizures, neurocutaneous disorders
WHAT'S KNOWN ON THIS SUBJECT:
Tuberous sclerosis complex (TSC) is an autosomal dominant neurocutaneous disorder that is clinically diagnosed. Diagnosis of TSC may be difficult because no single symptom is present in all patients, and none are absolutely pathognomonic.
WHAT THIS STUDY ADDS:
Presenting symptoms and signs of TSC according to age group, missed symptoms, and signs that did not lead to diagnosis are described. Early diagnosis may reduce morbidity and mortality in patients with TSC.
Tuberous sclerosis complex (TSC) is an autosomal dominant, multisystem disorder characterized by the formation of hamartomas in multiple organ systems, most commonly the brain, skin, kidney, and eye.1 Incidence of TSC is estimated to be 1 in 6000.2,3 TSC can be caused by mutations in either of 2 tumor-suppressor genes, TSC1 or TSC2,4 although mutations cannot be identified in ∼15% of cases. In up to two-thirds of patients there is no parental history of TSC, which signifies a high rate of spontaneous mutation.5 Phenotype is highly variable, even among patients with identical mutations.6
Diagnosis of TSC may be difficult because no single symptom is present in all patients, and none are absolutely pathognomonic.7 Currently, clinical criteria defined by the Tuberous Sclerosis Consensus Conference in 1998 are used to diagnose TSC (Table 1).8 Definite TSC is diagnosed when either 2 major features or 1 major and 2 minor features are present.
TABLE 1.
Diagnostic Criteria for TSC
| Major features |
| Facial angiofibroma or forehead plaque |
| Nontraumatic ungula or periungual fibroma |
| Hypopigmented macules (>3) |
| Shagreen patch (connective tissue nevus) |
| Multiple retinal nodular hamartomas |
| Cortical tubera |
| Subependymal nodule |
| SGCT |
| Cardiac rhabdomyomas, single or multiple |
| Lymphangioleiomyomatosisb |
| Renal angiomyolipomab |
| Minor features |
| Multiple randomly distributed pits in dental enamel |
| Hamartomatous rectal polypsc |
| Bone cystsd |
| Cerebral white matter migration linesa,d |
| Gingival fibromas |
| Nonrenal hamartomac |
| Retinal achromic patch |
| Confetti skin lesions |
| Multiple renal cystsc |
Definite TSC indicates either 2 major features or 1 major feature plus 2 minor features; probable TSC, 1 major plus 1 minor feature; suspect TSC, either 1 major feature or 2 or more minor features.
When cerebral cortical dysplasia and cerebral white-matter migration tracts occur together, they should be counted as 1 rather than 2 features of TSC.
When both lymphangioleiomyomatosis and renal angiomyolipomas are present, other features of TSC should be present before a definite diagnosis is assigned.
Histologic confirmation is suggested.
Radiographic confirmation is sufficient.
Adapted from Roach ES, Gomez MR, Northrup H. J Child Neurol. 1998;13(12):624–628.
Neurologic manifestations of TSC were first described by D. M. Bourneville in 1880, and were later associated with clinical signs by H. Vogt in 1908. Vogt described what is commonly known as the “classic triad” of symptoms in TSC: seizures; mental retardation; and adenoma sebaceum (angiofibromas).9 However, studies have demonstrated that the triad occurs in only 29% of patients with TSC, and 6% lack all 3 symptoms.10
Some presentations of TSC, such as neonatal infantile spasms with hypopigmented macules, are widely recognizable and frequently lead to diagnosis.11 However, some features of TSC remain entirely asymptomatic,7 such as bone lesions; whereas others appear, grow, and regress over time,12,13 such as cardiac rhabdomyomas and renal cysts, which reduce the likelihood of detection and diagnosis. This is particularly important in mildly affected children because many cerebral and dermatologic features of TSC may not be present or readily apparent in early life.11 Many patients with TSC are not diagnosed until adulthood. It is not uncommon for a parent to be diagnosed only after the birth of a more severely affected child.14
Manifestations of TSC, including seizures,15 subependymal giant cell tumor (SGCT),16 renal failure,17 and lymphangioleiomyomatosis14 can contribute to morbidity and mortality. However, many of these health risks can be minimized by early diagnosis of TSC, lifelong monitoring, and proactive treatment.10
We examined age at diagnosis, presenting symptoms, and missed earlier signs in 243 patients with TSC. Our aim was to elucidate the role of diagnosis in the natural history of TSC.
PATIENTS AND METHODS
We performed a retrospective chart review of 278 patients examined by Dr Elizabeth A. Thiele at Children's Hospital Boston between January 1999 and January 2002 and at the Carol and James Herscot Center for Tuberous Sclerosis Complex at Massachusetts General Hospital between January 2002 and March 2008.
Patients receive complete clinical evaluation during the initial visit at the Herscot Center unless the results of recent outside studies are available. Clinical evaluation includes brain and abdominal imaging, ophthalmologic examination, echocardiogram and ECG, and detailed skin examination.
Approximately half of the patients seen at the Herscot Center are adults, and not all meet clinical criteria for TSC. Although the Herscot Center is managed by a pediatric neurologist, referred patients with suspected TSC may have any symptoms or signs of TSC. Therefore, patients with TSC who were seen at the Herscot Center are representative of all patients with TSC. Patients were excluded from the study if there were no available data for age at diagnosis or presenting symptoms or signs.
The presenting symptom or sign was defined as the first symptom or sign to cause suspicion for TSC and lead to diagnosis. If there were 2 or more simultaneous presenting symptoms or signs, they were all counted. Infantile spasms, new onset of seizures, and a history of seizures were differentiated as separate presenting symptoms. History of seizures was defined as seizure onset occurring at least 6 months before TSC diagnosis. Earlier missed symptoms or signs were defined as symptoms or signs of TSC that were documented in the patient's chart but did not immediately lead to diagnosis. Presenting symptoms and signs and missed earlier symptoms and signs included both diagnostic criteria for TSC and other features associated with TSC (family history of TSC, developmental delay, polycystic kidney disorder, fat-poor angiomyolipoma or renal cell carcinoma, and multifocal micronodular pneumocyte hyperplasia) seen in our patient population.
Data were supplemented by telephone interview of patients and caregivers from June to August 2007. Genetic mutational analysis was conducted by Massachusetts General Hospital and Athena Diagnostics Inc (Worcester, MA). This study was granted permission from the hospital's institutional review board.
RESULTS
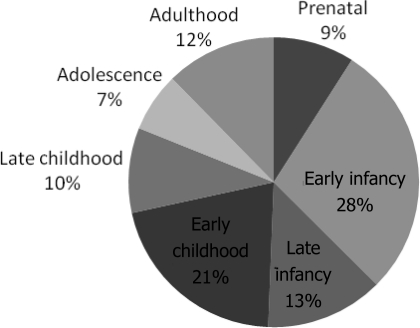
There were 243 records that contained information sufficient for inclusion. Presenting symptoms and signs varied with age (Table 2). Patients were diagnosed with TSC at various stages of life (Fig 1). Patients' age at diagnosis ranged from birth to 73 years. The average age at diagnosis was 7.5 years, and median was 1 year. Patients were most likely to be diagnosed during the first 6 months of life. There were 197 (81%) patients diagnosed before the age of 10. Diagnosis during adolescence and adulthood was not uncommon.
TABLE 2.
Presenting Symptoms and Signs of TSC According to Age Group
| Patients, N | FH, n (%)a | New sz, n (%)b | Sz, n (%)c | IS, n (%)d | CT, n (%)e | SGCT, n (%) | DD, n (%)f | SEN, n (%)g | HPM, n (%)h | AF, n (%)i | PUF, n (%)j | GF, n (%)k | SP, n (%)l | CR, n (%)m | AML, n (%)n | PKD, n (%)o | RC, n (%)p | Fat-Poor AML or RCC, n (%)q | LAM, n (%)r | MMPH, n (%)s | RH, n (%)t | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prenatal | 22 | 3 (14) | 2 (9) | — | — | 1 (5) | 1 (5) | — | — | — | — | — | — | — | 19 (86) | — | — | — | — | — | — | — |
| Early infancy | 69 | 7 (10) | 29 (42) | — | 23 (33) | 2 (3) | — | — | 1 (1) | 4 (6) | — | — | 1 (1) | — | 8 (12) | — | 1 (1) | — | — | — | — | 1 (1) |
| Late infancy | 32 | 1 (3) | 20 (63) | 1 (3) | 8 (25) | 1 (3) | — | — | — | 6 (19) | — | — | — | — | — | — | — | 1 (3) | — | — | — | — |
| Early childhood | 51 | 4 (8) | 27 (53) | 12 (24) | 1 (2) | 1 (2) | — | 1 (2) | — | 8 (16) | 3 (6) | — | — | — | 3 (6) | — | — | — | — | — | — | 1 (2) |
| Late childhood | 23 | 6 (26) | 6 (26) | 6 (26) | — | 1 (4) | 1 (4) | 1 (4) | — | 6 (26) | 3 (13) | — | — | 1 (4) | — | — | — | — | — | — | — | 1 (4) |
| Adolescence | 16 | 1 (6) | 5 (31) | 2 (13) | — | 2 (13) | 2 (13) | — | 1 (6) | — | 5 (31) | — | — | — | — | 2 (13) | — | — | — | — | — | — |
| Adulthood | 30 | 11 (37) | 1 (3) | 4 (13) | — | 2 (7) | 1 (3) | 2 (7) | 1 (3) | — | 5 (17) | 4 (13) | — | — | — | 5 (17) | — | — | 1 (3) | 1 (3) | 1 (3) | — |
| Total | 243 | 33 (14) | 90 (37) | 25 (10) | 32 (13) | 10 (4) | 5 (2) | 4 (2) | 3 (1) | 24 (10) | 16 (7) | 4 (2) | 1 (0.4) | 1 (0.4) | 30 (12) | 7 (3) | 1 (0.4) | 1 (0.4) | 1 (0.4) | 1 (0.4) | 1 (0.4) | 3 (1) |
Numbers in the presenting-symptom columns will not add up to the total numbers of patients in each age group because many patients had more than 1 presenting symptom, and all presenting symptoms were counted.
Family history of TSC or family with symptoms of TSC.
New onset of seizures (not including infantile spasms).
History of seizures (not including infantile spasms).
Infantile spasms.
Cortical tuber(s).
Developmental delay.
Subependymal nodule(s).
Hypopigmented macules.
Facial angiofibromas.
Periungal fibroma(s).
Gingival fibroma(s).
Shagreen patch.
Cardiac rhabdomyoma(s).
Angiomyolipoma(s).
Polycystic kidney disease.
Renal cyst(s).
Fat-poor renal angiomyolipoma(s) or renal cell carcinoma.
Lymphangioleiomyomatosis.
Multifocal micronodular pneumocyte hyperplasia.
Retinal hamartoma(s).
FIGURE 1.
Age at diagnosis of TSC. Prenatal is defined as 0 to 1 week; early infancy, 1 week to 6 months; late infancy, 6 months to 1 year; early childhood, 1 to 5 years; late childhood, 6 to 10 years; adolescence, 11 to 20 years; and adulthood, 21 years and older.
Genotype
There were 199 (82%) patients who underwent genetic mutational analysis, and 198 results were available for inclusion in this study (Table 3). Patients with a TSC2 mutation were diagnosed, on average, 9 years before those with TSC1 mutations, and 11 years before those without identifiable mutations. Presenting symptoms and signs varied by genotype (Table 4).
TABLE 3.
Age at Diagnosis of TSC According to Genotype
| Mutation | N | Age at Diagnosis, y |
|||||
|---|---|---|---|---|---|---|---|
| Mean (SE) | Median | Minimum | Maximum | 25th Percentile | 75th Percentile | ||
| TSC1 | 51 | 12.6 (2.3) | 4.5 | 0 | 63 | 2 | 15 |
| TSC2 | 115 | 3.5 (0.8) | 0.5 | 0 | 73 | 0.2 | 1.8 |
| None identified | 32 | 15.5 (3.3) | 4.75 | 0 | 58 | 0.6 | 35 |
| Results pending | 1 | 0.1 | — | — | — | — | — |
| Total tested | 199 | — | — | — | — | — | — |
| Not tested | 44 | 6.4 (2.3) | 0.9 | 0 | 58.4 | 0.5 | 5.8 |
TABLE 4.
Presenting Symptoms and Signs of TSC According to Genotype
| Patients, N | FH, n (%)a | New sz, n (%)b | Sz, n (%)c | IS, n (%)d | CT, n (%)e | SGCT, n (%) | DD, n (%)f | SEN, n (%)g | HPM, n (%)h | AF, n (%)i | PUF, n (%)j | GF, n (%)k | SP, n (%)l | CR, n (%)m | AML, n (%)n | PKD, n (%)o | RC, n (%)p | Fat-Poor AML or RCC, n (%)q | LAM, n (%)r | MMPH, n (%)s | RH, n (%)t | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TSC1 | 51 | 17 (33) | 21 (41) | 9 (18) | 2 (4) | 2 (4) | 1 (2) | — | 2 (4) | 8 (16) | 1 (2) | — | — | — | 3 (6) | — | — | — | — | — | 1 (2) | — |
| TSC2 | 115 | 15 (13) | 42 (37) | 7 (6) | 20 (17) | 4 (3) | 2 (2) | 3 (3) | 1 (1) | 9 (8) | 9 (8) | — | 1 (1) | — | 20 (17) | 2 (2) | 1 (1) | — | — | — | — | 1 (1) |
| None identified | 32 | — | 7 (22) | 4 (13) | 3 (9) | 3 (9) | 1 (3) | — | — | 2 (6) | 2 (6) | 2 (6) | — | — | 5 (16) | 5 (16) | — | — | 1 (3) | 1 (3) | — | 1 (3) |
| Results pending | 1 | — | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Total tested | 199 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Not tested | 44 | 1 (2) | 19 (43) | 5 (11) | 7 (16) | 1 (2) | 1 (2) | 1 (2) | — | 5 (11) | 4 (9) | 2 (5) | — | 1 (2) | 2 (5) | — | — | 1 (2) | — | — | — | 1 (2) |
Numbers in presenting symptom columns will not add up to the total numbers of patients in each genotype group because many patients had more than 1 presenting symptom, and all presenting symptoms were counted.
Family history of TSC or family with symptoms of TSC.
New onset of seizures (not including infantile spasms).
History of seizures (not including infantile spasms).
Infantile spasms.
Cortical tuber(s).
Developmental delay.
Subependymal nodule(s).
Hypopigmented macules.
Facial angiofibromas.
Periungal fibroma(s).
Gingival fibroma(s).
Shagreen patch.
Cardiac rhabdomyoma(s).
Renal angiomyolipoma(s).
Polycystic kidney disease.
Renal cyst(s).
Fat-poor renal angiomyolipoma(s) or renal cell carcinoma.
Lymphangioleiomyomatosis.
Multifocal micronodular pneumocyte hyperplasia.
Retinal hamartoma(s).
Presenting Symptoms
Prenatal: 0 to 1 Week
There were 22 patients (9%) diagnosed during the first week of life; 17 showed signs of TSC in utero, but none satisfied criteria for diagnosis until after delivery. Patients diagnosed during the first week of life were most likely to present with 1 or more cardiac rhabdomyomas, most of which were asymptomatic. There were 16 who had cardiac rhabdomyomas first detected by prenatal ultrasound between 17 and 33 weeks; 2 were observed in the setting of fetal arrhythmia. Prenatal imaging revealed a SGCT in 1 patient, and cortical tubers in another with a known family history of TSC.
Five infants seemed normal during gestation but presented with symptoms of TSC shortly after birth. One presented with seizures in the first hour of life. Three presented with symptoms associated with cardiac rhabdomyomas within 3 days. One was asymptomatic, but underwent diagnostic evaluation at birth because of a family history of TSC.
Early Infancy: 1 Week to 6 Months
There were 69 patients (28%) diagnosed within the first 6 months of life, most of whom presented with a new onset of seizures or infantile spasms. One patient had partial onset of seizures before onset of infantile spasms. Several patients underwent diagnostic evaluation because of family history of TSC.
Cardiac rhabdomyomas and hypopigmented macules also led to diagnosis. More unusual presentations included cases of polycystic kidney disease, gingival fibromas, and symptomatic retinal hamartoma. Cortical tubers and subependymal nodules were detected in a 4-month-old girl after her parents observed left hemineglect.
Late Infancy: 6 Months to 1 Year
There were 32 patients (13%) diagnosed in the second 6 months of life. Most common presenting symptoms and signs included new onset of seizures, infantile spasms, and hypopigmented macules. Two presented with partial onset of seizures and infantile spasms. Two presented with new onset of seizures and hypopigmented macules. One presented with a family history of TSC, hypopigmented macules, and new onset of seizures.
Cases of earlier missed symptoms or signs included 3 with suspected developmental delay, 2 with hypopigmented macules, 3 with history of infantile spasms, and 1 whose seizures were noted only in hindsight. In 2 cases of infantile spasms and 3 cases of seizures, TSC diagnosis was delayed for undocumented reasons. In another case of onset of infantile spasms at the age of 3 months, the spasms were initially thought to be behavioral or related to milk formula. TSC was not diagnosed until the age of 9 months because of hypopigmented macules and calcifications seen on brain MRI. One infant was treated pharmacologically for hypertension during the neonatal period before renal cysts were detected at the age of 5 months.
Early Childhood: 1 to 5 Years
There were 51 patients (21%) diagnosed in early childhood. New onset of seizures was the most common presenting symptom, leading to diagnosis in 27 cases, including 1 in which seizures were observed during a sleep study for night terrors. History of seizures led to diagnosis in 12 children, including 1 with a history of infantile spasms and partial onset of seizures. Dermatologic features were also common presenting signs.
In 7 children, developmental delay was observed by parents or physicians before TSC diagnosis. Four children had previously noted cardiac rhabdomyomas. In 3, rhabdomyomas were observed prenatally or at birth, but those children did not initially meet sufficient criteria for diagnosis. In the fourth, cardiac rhabdomyoma was seen on a chest radiograph at the age of 2 in the setting of an upper respiratory infection, but it is unclear why diagnosis of TSC was delayed for another 2.5 years.
Late Childhood: 6 to 10 Years
There were 23 patients (10%) diagnosed in late childhood, most commonly presenting with new onset of seizures, history of seizures, family history of TSC, or hypopigmented macules. It was not uncommon for patients to present with angiofibromas. One child was diagnosed with TSC after presenting with strabismus caused by enlarging SGCT. Asymptomatic retinal hamartoma, observed on routine eye examination, led to diagnosis in another.
Cases of earlier missed symptoms or signs included 6 patients with developmental delay and 1 with infantile spasms. One child with developmental delay was diagnosed with cerebral palsy until the appearance of dermatologic features at the age of 8.
Adolescence: 11 to 20 Years
There were 16 patients (7%) diagnosed in adolescence. The most common presenting symptoms and signs were new onset of seizures and angiofibromas. In 1 case, seizures were first observed in the setting of psychiatric hospitalization. In 3 patients, neurologic manifestations of TSC were seen on MRI during evaluation for other neurologic symptoms: headaches; hemiparesis; and increased intracranial pressure.
Missed earlier symptoms and signs were not uncommon in this age group. One patient had seizures until the age of 5 and was not diagnosed with TSC until epilepsy reemerged at the age of 17. In another, cortical tubers and subependymal nodules were noted on MRI in the setting of seizures, but he was not evaluated for TSC until his mother was diagnosed 5 years later. Two patients reported a history of migraines before diagnosis.
Adulthood: 21 Years and Older
There were 30 patients (12%) diagnosed in adulthood. They were most likely to present for diagnostic evaluation because of newly diagnosed family members, or a known family history of TSC.
Only 7 adults were diagnosed at the first presentation of TSC symptoms or signs. The remaining 23 had symptoms or signs years before being diagnosed (Table 5). Four had family histories of known or suspected TSC. Eight had received previous treatment for dermatologic and/or renal symptoms. Three had MRIs that revealed neurologic manifestations consistent with TSC during evaluation for other symptoms. Of the 23 patients with missed earlier symptoms or signs, only 5 reported that the possibility of TSC had been mentioned by a clinician.
TABLE 5.
Missed Earlier Symptoms and Signs of TSC in Patients Diagnosed in Adulthood (N = 30)
| Patient | FHa | Szb | ISc | CTd | SENe | SGCT | AFf | HPMg | PUFh | SPi | FPj | AMLk | RCl | LAMm | MMPHn | RHo | DPp | STq | Total Missed Symptoms/Signs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | — | — | — | — | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — | 1 |
| B | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 0 |
| C | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 0 |
| D | 1 | 1 | — | — | — | — | — | — | — | — | 1 | — | — | — | — | — | — | — | 3 |
| E | — | 1 | — | — | — | — | — | — | — | — | — | 1 | — | — | — | — | — | — | 2 |
| F | 1 | — | — | — | — | — | — | — | — | 1 | — | 1 | 1 | — | — | — | — | 1 | 5 |
| G | — | — | — | — | — | — | — | — | 1 | — | — | — | — | — | — | — | — | — | 1 |
| H | — | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 |
| I | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 |
| J | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 0 |
| K | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 0 |
| L | — | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 |
| M | 1 | — | — | — | — | — | — | — | — | 1 | — | — | — | — | — | — | — | — | 2 |
| N | 1 | 1 | — | — | — | — | — | 1 | — | — | — | — | — | — | — | 1 | — | — | 4 |
| O | 1 | 1 | — | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 3 |
| P | — | — | — | — | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — | 1 |
| Q | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 0 |
| R | — | — | — | — | — | — | — | — | — | — | — | 1 | — | 1 | — | — | — | — | 2 |
| S | 1 | — | — | 1 | 1 | — | — | — | 1 | — | — | — | — | — | — | — | — | — | 4 |
| T | — | — | — | — | — | 1 | — | — | — | — | — | — | — | — | 1 | — | — | — | 2 |
| U | — | 1 | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 2 |
| V | — | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 |
| W | — | 1 | — | — | — | — | 1 | 1 | — | — | — | — | — | — | — | — | — | — | 3 |
| X | — | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 |
| Y | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 0 |
| Z | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 |
| AA | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 |
| BB | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 | — | 1 |
| CC | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 0 |
| DD | — | 1 | — | — | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — | 2 |
| Total | 9 | 11 | 1 | 2 | 1 | 1 | 4 | 2 | 2 | 2 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 |
“1” indicates presence of symptom or sign.
Family history of TSC or family with symptoms of TSC.
Seizures (not including infantile spasms).
Infantile spasms.
Cortical tuber(s).
Subependymal nodule(s).
Facial angiofibromas.
Hypopigmented macules.
Periungal fibroma(s).
Shagreen patch.
Forehead plaque.
Renal angiomyolipoma(s).
Renal cyst(s).
Lymphangioleiomyomatosis.
Multifocal micronodular pneumocyte hyperplasia.
Retinal hamartoma(s).
Dental pits.
Supraventricular trachycardia.
Of these patients, 11 had a history of seizures, including 1 with both infantile spasms and seizures, but seizure history raised suspicion for TSC in only 2. One patient with a history of seizures in early childhood was diagnosed with TSC after seizures reemerged at the age of 21. Three with no previous seizures were diagnosed when onset occurred in adulthood. The latest observed onset of seizures was in a 45-year-old woman who had no other symptomatic or cutaneous manifestations.
Earlier Missed Symptoms or Signs
There were 95 patients (39%) in which earlier-reported manifestations of TSC did not lead to diagnosis. Of these, 24 had 2 or more symptoms or signs.
Seizures (including new onset, history of seizures, and infantile spasms) were the most commonly missed earlier symptom of TSC and were noted in 46 (19%) patients. In these cases, seizures were treated for periods of time that ranged from 6 weeks to 36 years before TSC diagnosis.
Infantile spasms preceded TSC diagnosis in 15 (6%) cases. In 9, spasms were diagnosed at onset. In 1, spasms were not recognized until 4 months after onset. In 5, spasms were not initially diagnosed, but were retrospectively characterized as such years later.
Dermatologic features did not lead to TSC diagnosis in 22 (9%) patients and were frequently present with other signs of TSC. Developmental delay had been noted in 16 of these patients, 1 of whom was diagnosed with cerebral palsy until the appearance of angiofibromas in late childhood. Other missed earlier signs included cardiac rhabdomyoma, renal angiomyolipoma, family history of TSC, SGCT, and multifocal micronodular pneumocyte hyperplasia.
DISCUSSION
Genetically tested patients with a TSC2 mutation were diagnosed, on average, 9 years before patients with a TSC1 mutation and 11 years before those with no mutation identified (NMI). Earlier diagnosis of patients with TSC2 mutation may have been a result of the associated phenotype, which generally includes a greater number and severity of symptoms than phenotypes associated with TSC1 mutation and NMI.4,18 Patients with a TSC2 mutation were more likely to present with infantile spasms, developmental delay, or angiofibromas than were patients with a TSC1 mutation or NMI. Patients with a TSC1 mutation were more likely to present with a family history of TSC or hypopigmented macules than were patients with TSC2 mutation or NMI, and were less likely to present with cardiac rhabdomyomas. Patients with NMI were more likely to present with renal angiomyolipomas than those with identified mutations. The only case of presentation with lymphangioleiomyomatosis was a patient with NMI.
Disease-causing mutations have been identified in patients with TSC before appearance of symptoms. Diagnostic criteria of TSC should be revised to include disease-causing mutations.19 Genetic testing can facilitate early diagnosis, especially in patients with a family history of TSC. However, because mutations cannot be identified in ∼15% of TSC cases,5 lack of identifiable mutation should not preclude additional investigation for TSC. Patients with NMI may be more likely to have certain symptoms, such as lymphangioleiomyomatosis,20 which requires careful monitoring.
A previous study of 73 patients with TSC demonstrated that 25% were not diagnosed after first appearance of symptoms.21 In this study, 95 records (39%) reported earlier symptoms or signs that were missed. Seizures were the most commonly missed symptom, and were noted in 19% of patients.
Although seizures were the most commonly missed symptom, seizures (other than infantile spasms) were also the most common presenting symptom (47%). This finding is slightly lower than that of a previous study,18 but the authors did not differentiate between seizures and infantile spasms. Both studies suggest that seizures are the most common presenting symptom of TSC.22 Patients with TSC have an 84% lifetime risk of seizures, which typically begin early in life15 and may change in semiology with age.11,23 Prenatal seizures15 and onset of epilepsy in adolescence18 and adulthood15 have been reported in TSC. In this study, prenatal seizures occurred in 1 patient. Newly emergent seizures occurred in patients as old as 45. Patients with TSC and refractory seizures are at higher risk for cognitive impairment,15,24 and early and effective treatment of seizures may improve prognosis.20
Infantile spasms are a common presenting symptom of TSC,25 and 13% of patients in this study were diagnosed because of their emergence. Infantile spasms are generalized myoclonic seizures typically characterized by hypsarrhythmia on EEG. They are seen in one-third to two-thirds of children with TSC.11,20 In some series, 20% to 25% of cases of infantile spasms are associated with TSC.26 Infantile spasms may precede, follow, or coexist with other types of seizures, and often begin between 4 and 6 months of age.19 The earliest case of infantile spasms in this study was diagnosed at the age of 2 months, but parents reported onset at a few weeks after birth. The latest reported onset was at 10 months. Infantile spasms are a risk factor for poor cognitive development in TSC.27 Immediate identification and treatment are necessary to reduce seizure frequency28 and risk of cognitive impairment.29
Cardiac rhabdomyomas were a presenting sign in 12% of all patients, and in 30% of patients who were diagnosed before the age of 6 months. Prenatal cardiac rhabdomyomas are frequently the first detectable manifestation of TSC,30 and were found in all patients diagnosed during the first week of life. The 3 patients who presented with rhabdomyomas (either prenatally or at birth) but did not initially meet diagnostic criteria demonstrate the importance of continued monitoring in children with suspected TSC. A comprehensive care plan can address the potential emergence of more serious complications, such as seizures.
Dermatologic features of TSC can be easily recognizable, and are present in 96% of patients with TSC.31 In this study, dermatologic features were common presenting signs, but were also missed in 9% of patients. Presentation of dermatologic features is age-dependent. Hypopigmented macules, which may serve as first evidence of TSC,32 were a common presenting sign in infants and young children. Angiofibromas and periungual fibromas were more likely to lead to diagnosis in adolescence and adulthood. Angiofibromas do not typically appear before the age of 3 or 4,7 but may grow rapidly during puberty.30
Family history of TSC often played a role in the diagnosis of our patients. However, because approximately two-thirds of cases of TSC arise spontaneously,5 a negative family history should not preclude additional investigation for TSC.
It is recommended that patients with epilepsy, developmental delay, or a learning disability are investigated for TSC in the absence of other known etiology because of the high number of patients with TSC presenting with these symptoms.18 Our results support this recommendation.
This study is limited because data were collected retrospectively; therefore, some information may be incomplete. Our findings of earlier missed symptoms or signs of TSC may represent an underestimate, as earlier symptoms or signs may not be well documented.
CONCLUSIONS
It is not uncommon for patients with TSC to have symptoms or signs that do not lead to immediate diagnosis. In some cases, diagnosis is delayed for prolonged periods of time. Symptoms and signs of TSC may be diagnosed incorrectly, or incorrectly lead to diagnosis of other disorder or illness. Clinicians, including child and adult neurologists, dermatologists, nephrologists, and cardiologists, should be aware of the myriad potential presenting symptoms and signs of TSC. Early diagnosis is important for thorough clinical and radiologic evaluation, continuous monitoring of symptoms, family planning, genetic counseling, and reduction of morbidity and mortality.
Ongoing clinical trials of the drug rapamycin have demonstrated efficacy in reducing symptoms of TSC.10 Early diagnosis could be especially beneficial to patients if preventive treatment becomes available.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke grant P01 NS024279.
We thank Leigh Horne-Mebel for assistance in record completion, Linda Connors for support, and the Herscot Center for tuberous sclerosis complex.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
- TSC
- tuberous sclerosis complex
- SGCT
- subependymal giant cell tumor
- NMI
- no mutation identified
REFERENCES
- 1. Gomez MR. Definition and criteria for diagnosis. In: Gomez MR, Sampson JR, Whittemore VH, eds. Tuberous Sclerosis Complex: Developmental Perspectives in Psychiatry. 3rd ed Oxford, United Kingdom: Oxford University Press; 1999:10–23 [Google Scholar]
- 2. Young J, Povey S. The genetic basis of tuberous sclerosis. Mol Med Today. 1998;4(7):313–319 [DOI] [PubMed] [Google Scholar]
- 3. Franz DN. Non-neurologic manifestations of tuberous sclerosis complex. J Child Neurol. 2004;19(9):690–698 [DOI] [PubMed] [Google Scholar]
- 4. Dabora SL, Józwiak S, Franz DN, et al. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet. 2001;68(1):64–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sancak O, Nellist M, Goedbloed M, et al. Mutational analysis of the TSC1 and TSC2 genes in a diagnostic setting: genotype–phenotype correlations and comparison of diagnostic DNA techniques in Tuberous Sclerosis Complex. Eur J Hum Genet. 2005;13(6):731–741 [DOI] [PubMed] [Google Scholar]
- 6. Rok P, Kasprzyk-Obara J, Domańska-Pakieła D, Józwiak S. Clinical symptoms of tuberous sclerosis complex in patients with an identical TSC2 mutation. Med Sci Monit. 2005;11(5):CR230–CR234 [PubMed] [Google Scholar]
- 7. Roach ES, Sparagana SP. Diagnosis of tuberous sclerosis complex. J Child Neurol. 2004;19(9):643–649 [DOI] [PubMed] [Google Scholar]
- 8. Roach ES, Gomez MR, Northrup H. Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. J Child Neurol. 1998;13(12):624–628 [DOI] [PubMed] [Google Scholar]
- 9. Curatolo P. Historical background. In: Curatolo P, ed. Tuberous Sclerosis Complex: From Basic Science to Clinical Phenotypes. London, United Kingdom: Mac Keith Press; 2003:1–10 [Google Scholar]
- 10. Schwartz RA, Fernández G, Kotulska K, Józwiak S. Tuberous sclerosis complex: advances in diagnosis, genetics, and management. J Am Acad Dermatol. 2007;57(2):189–202 [DOI] [PubMed] [Google Scholar]
- 11. Józwiak S, Schwartz RA, Janniger CK, Bielicka-Cymerman J. Usefulness of diagnostic criteria of tuberous sclerosis complex in pediatric patients. J Child Neurol. 2000;15(10):652–659 [DOI] [PubMed] [Google Scholar]
- 12. Smythe JF, Dyck JD, Smallhorn JF, Freedom RM. Natural history of cardiac rhabdomyoma in infancy and childhood. Am J Cardiol. 1990;66(17):1247–1249 [DOI] [PubMed] [Google Scholar]
- 13. Cohen MM, Pollock-BarZiv S, Johnson SR. Emerging clinical picture of lymphangioleiomyomatosis. Thorax. 2005;60(10):875–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cassidy SB, Pagon RA, Pepin M, Blumhagen JD. Family studies in tuberous sclerosis: evaluation of apparently unaffected parents. JAMA. 1983;249(10):1302–1304 [PubMed] [Google Scholar]
- 15. Gomez MR. Natural history of cerebral tuberous sclerosis. In: Gomez MR, Sampson JR, Whittemore VH, eds. Tuberous Sclerosis Complex: Developmental Perspectives in Psychiatry. 3rd ed Oxford, United Kingdom: Oxford University Press; 1999:29–46 [Google Scholar]
- 16. Chong DY, Hirunwiwatkul P, McKeever PE, Trobe JD. Papilledema in obstructive hydrocephalus caused by giant cell astrocytoma of tuberous sclerosis. J Neuroophthalmol. 2007;27(1):50–54 [DOI] [PubMed] [Google Scholar]
- 17. Clarke A, Hancock E, Kingswood C, Osborne JP. End-stage renal failure in adults with the tuberous sclerosis complex. Nephrol Dial Transplant. 1999;14(4):988–991 [DOI] [PubMed] [Google Scholar]
- 18. Józwiak S, Schwartz RA. Dermatological and stomatological manifestations. In: Curatolo P, ed. Tuberous Sclerosis Complex: From Basic Science to Clinical Phenotypes. London, United Kingdom: Mac Keith Press; 2003:136–169 [Google Scholar]
- 19. Vail EA, Rakowski SK, Numis AL, Thiele EA. Role of mutational analysis in diagnosis of tuberous sclerosis complex. Clin Genet. 2009;75(3):282–285 [DOI] [PubMed] [Google Scholar]
- 20. Muzykewicz DA, Sharma A, Muse V, Numis AL, Rajagopal J, Thiele EA. TSC1 and TSC2 mutations in patients with lymphangioleiomyomatosis and tuberous sclerosis complex. J Med Genet. 2009;46(7):465–468 [DOI] [PubMed] [Google Scholar]
- 21. Devlin LA, Shepherd CH, Crawford H, Morrison PJ. Tuberous sclerosis complex: clinical features, diagnosis, and prevalence within Northern Ireland. Dev Med Child Neurol. 2006;48(6):495–499 [DOI] [PubMed] [Google Scholar]
- 22. Thiele EA. Managing epilepsy in tuberous sclerosis complex. J Child Neurol. 2004;19(9):680–686 [DOI] [PubMed] [Google Scholar]
- 23. Curatolo P, Seri S. Seizures. In: Curatolo P, ed. Tuberous Sclerosis Complex: From Basic Science to Clinical Phenotypes. London, United Kingdom: Mac Keith Press; 2003:46–76 [Google Scholar]
- 24. Winterkorn EB, Pulsifer MB, Thiele EA. Cognitive prognosis of patients with tuberous sclerosis complex. Neurology. 2007;68(1):62–64 [DOI] [PubMed] [Google Scholar]
- 25. Pampiglione G, Pugh E. Letter: Infantile spasms and subsequent appearance of tuberous sclerosis syndrome. Lancet. 1975;2(7943):1046. [DOI] [PubMed] [Google Scholar]
- 26. Yeung RS. Tuberous sclerosis as an underlying basis for infantile spasm. Int Rev Neurobiol. 2002;49:315–332 [DOI] [PubMed] [Google Scholar]
- 27. Józwiak S, Goodman M, Lamm SH. Poor mental development in patients with tuberous sclerosis complex: clinical risk factors. Arch Neurol. 1998;55(3):379–384 [DOI] [PubMed] [Google Scholar]
- 28. Husain AM, Foley CM, Legido A, et al. West syndrome in tuberous sclerosis complex. Pediatr Neurol. 2000;23(3):233–235 [DOI] [PubMed] [Google Scholar]
- 29. Goh S, Kwiatkowski DJ, Dorer DJ, Thiele EA. Infantile spasms and intellectual outcomes in children with tuberous sclerosis complex. Neurology. 2005;65(2):235–238 [DOI] [PubMed] [Google Scholar]
- 30. Giacoia GP. Fetal rhabdomyoma: a prenatal echocardiographic marker of tuberous sclerosis. Am J Perinatol. 1992;9(2):111–114 [DOI] [PubMed] [Google Scholar]
- 31. Webb DW, Clarke A, Fryer A, Osborne J. The cutaneous manifestations of tuberous sclerosis: a population study. Br J Dermatol. 1996;135(1):1–5 [PubMed] [Google Scholar]
- 32. Józwiak S, Schwartz RA. Dermatological and stomatological manifestations. In: Curatolo P, ed. Tuberous Sclerosis Complex: From Basic Science to Clinical Phenotypes. London, United Kingdom: Mac Keith Press; 2003:136–169 [Google Scholar]