Abstract
The Saccharomyces cerevisiae genes TOR1 and TOR2 were originally identified by mutations that confer resistance to the immunosuppressant rapamycin. TOR2 was previously shown to encode an essential 282-kDa phosphatidylinositol kinase (PI kinase) homologue. The TOR1 gene product is also a large (281 kDa) PI kinase homologue, with 67% identity to TOR2. TOR1 is not essential, but a TOR1 TOR2 double disruption uniquely confers a cell cycle (G1) arrest as does exposure to rapamycin; disruption of TOR2 alone is lethal but does not cause a cell cycle arrest. TOR1-TOR2 and TOR2-TOR1 hybrids indicate that carboxy-terminal domains of TOR1 and TOR2 containing a lipid kinase sequence motif are interchangeable and therefore functionally equivalent; the other portions of TOR1 and TOR2 are not interchangeable. The TOR1-1 and TOR2-1 mutations, which confer rapamycin resistance, alter the same potential protein kinase C site in the respective protein's lipid kinase domain. Thus, TOR1 and TOR2 are likely similar but not identical, rapamycin-sensitive PI kinases possibly regulated by phosphorylation. TOR1 and TOR2 may be components of a novel signal transduction pathway controlling progression through G1.
Full text
PDF



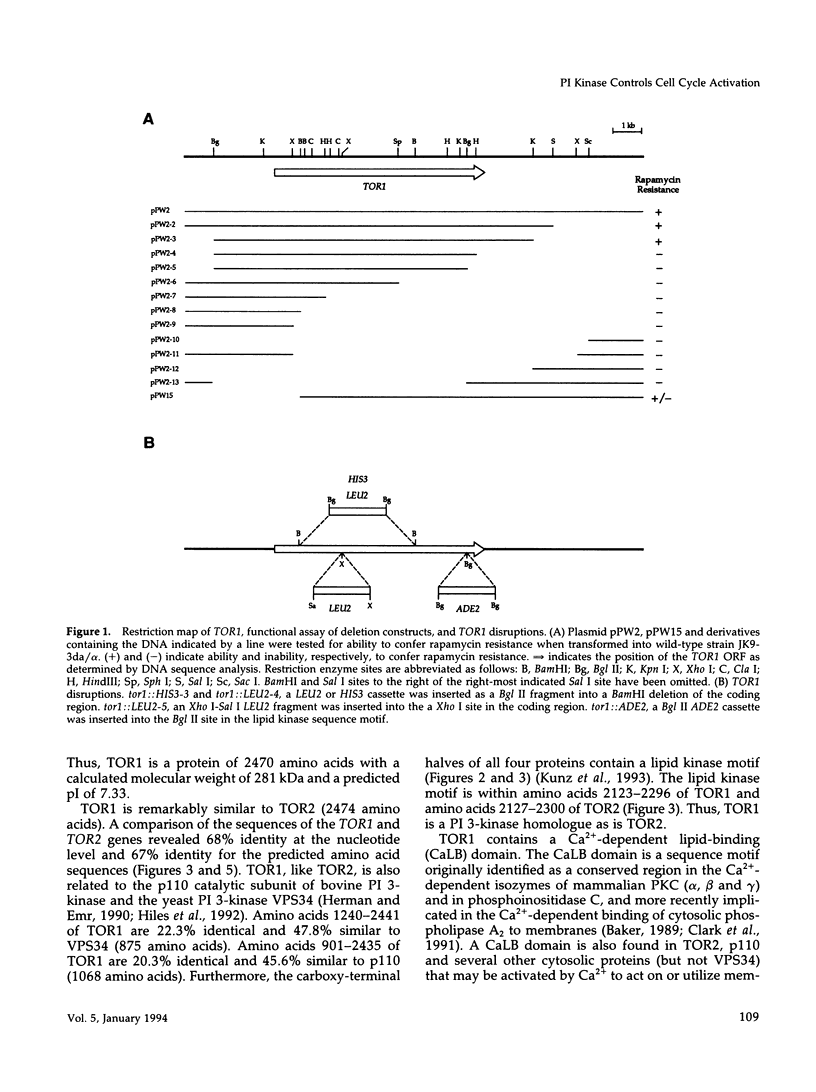
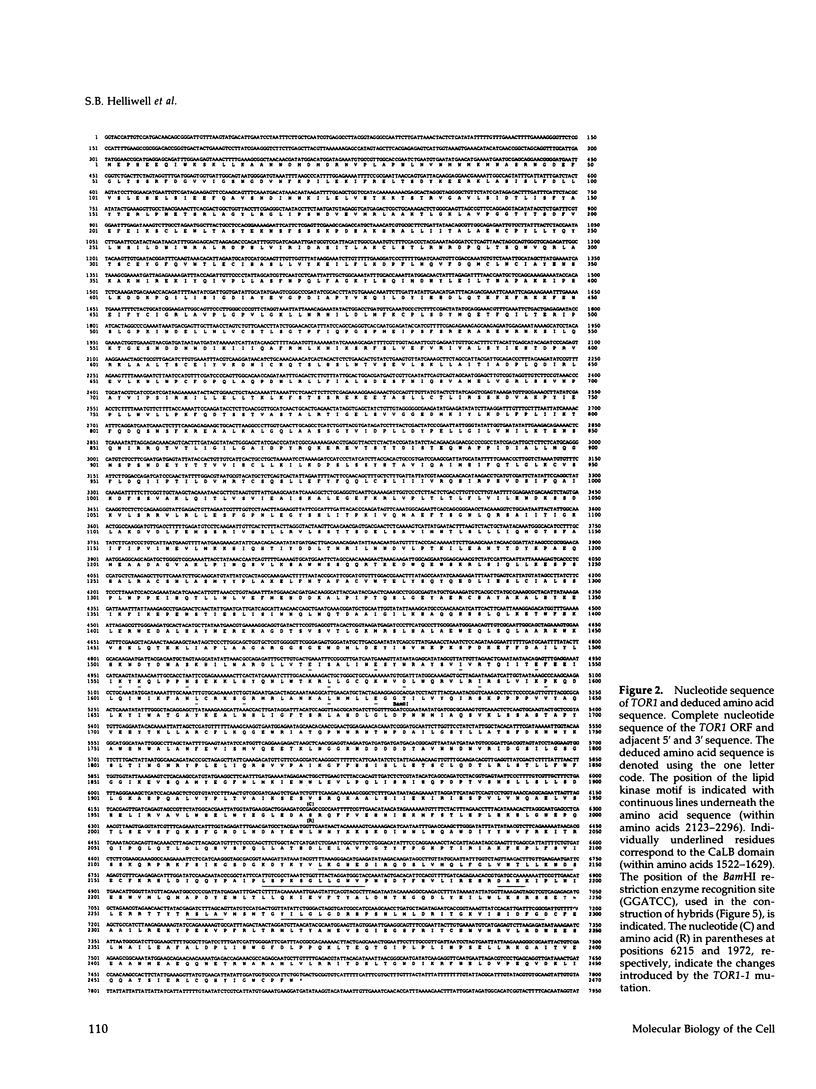

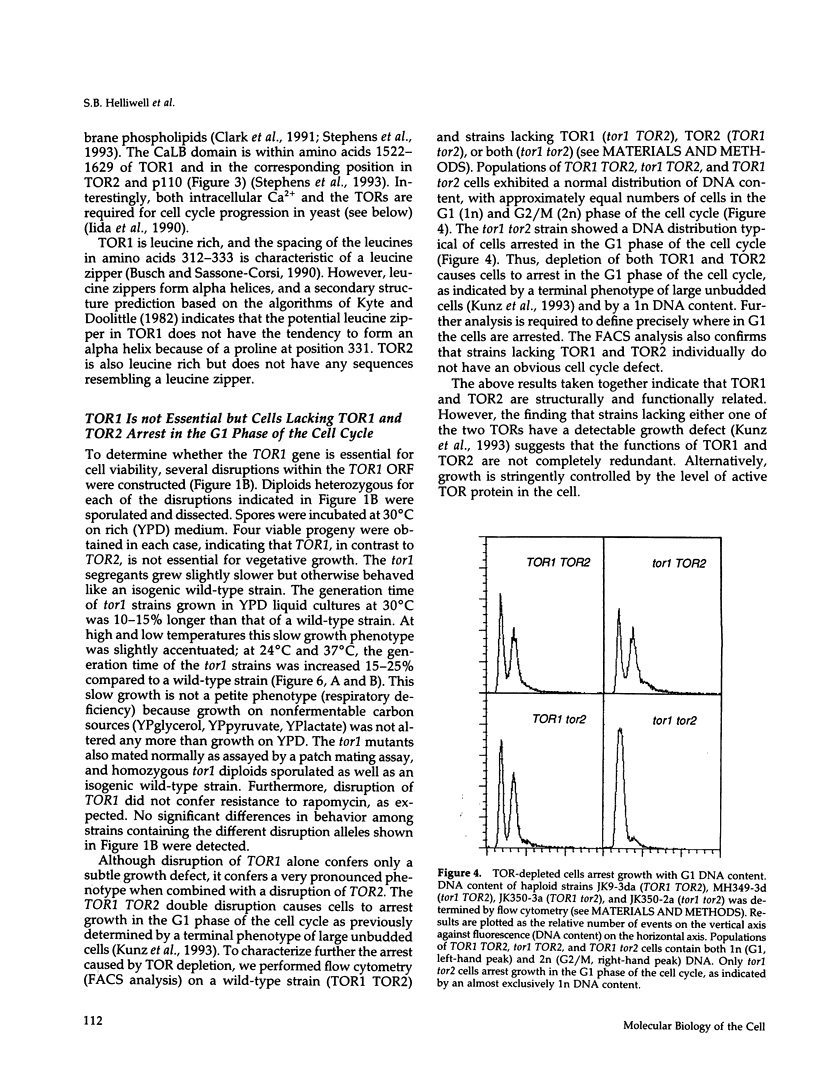
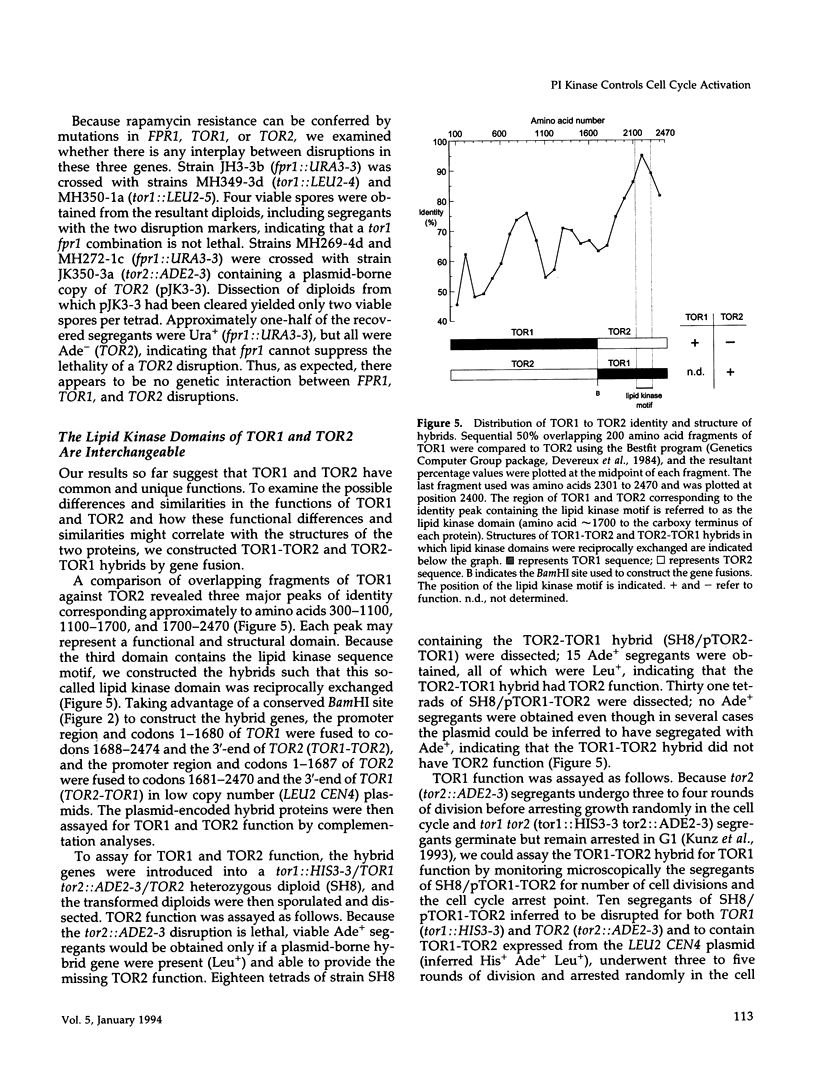





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auger K. R., Serunian L. A., Soltoff S. P., Libby P., Cantley L. C. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 1989 Apr 7;57(1):167–175. doi: 10.1016/0092-8674(89)90182-7. [DOI] [PubMed] [Google Scholar]
- Baker M. E. Similarity between phospholipase C and the regulatory domain of protein kinase C. Mol Cell Endocrinol. 1989 Jan;61(1):129–131. doi: 10.1016/0303-7207(89)90197-4. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Busch S. J., Sassone-Corsi P. Dimers, leucine zippers and DNA-binding domains. Trends Genet. 1990 Feb;6(2):36–40. doi: 10.1016/0168-9525(90)90071-d. [DOI] [PubMed] [Google Scholar]
- Buxeda R. J., Nickels J. T., Jr, Belunis C. J., Carman G. M. Phosphatidylinositol 4-kinase from Saccharomyces cerevisiae. Kinetic analysis using Triton X-100/phosphatidylinositol-mixed micelles. J Biol Chem. 1991 Jul 25;266(21):13859–13865. [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Carpenter C. L., Auger K. R., Duckworth B. C., Hou W. M., Schaffhausen B., Cantley L. C. A tightly associated serine/threonine protein kinase regulates phosphoinositide 3-kinase activity. Mol Cell Biol. 1993 Mar;13(3):1657–1665. doi: 10.1128/mcb.13.3.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C. L., Cantley L. C. Phosphoinositide kinases. Biochemistry. 1990 Dec 25;29(51):11147–11156. doi: 10.1021/bi00503a001. [DOI] [PubMed] [Google Scholar]
- Carpenter C. L., Duckworth B. C., Auger K. R., Cohen B., Schaffhausen B. S., Cantley L. C. Purification and characterization of phosphoinositide 3-kinase from rat liver. J Biol Chem. 1990 Nov 15;265(32):19704–19711. [PubMed] [Google Scholar]
- Clark J. D., Lin L. L., Kriz R. W., Ramesha C. S., Sultzman L. A., Lin A. Y., Milona N., Knopf J. L. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991 Jun 14;65(6):1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- Dahl C., Biemann H. P., Dahl J. A protein kinase antigenically related to pp60v-src possibly involved in yeast cell cycle control: positive in vivo regulation by sterol. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4012–4016. doi: 10.1073/pnas.84.12.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E. S., Becker A., Heitman J., Hall M. N., Brennan M. B. A yeast cyclophilin gene essential for lactate metabolism at high temperature. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11169–11173. doi: 10.1073/pnas.89.23.11169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan C. A., Thorner J. Purification and characterization of a soluble phosphatidylinositol 4-kinase from the yeast Saccharomyces cerevisiae. J Biol Chem. 1992 Nov 25;267(33):24117–24125. [PubMed] [Google Scholar]
- Foor F., Parent S. A., Morin N., Dahl A. M., Ramadan N., Chrebet G., Bostian K. A., Nielsen J. B. Calcineurin mediates inhibition by FK506 and cyclosporin of recovery from alpha-factor arrest in yeast. Nature. 1992 Dec 17;360(6405):682–684. doi: 10.1038/360682a0. [DOI] [PubMed] [Google Scholar]
- Gietz R. D., Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988 Dec 30;74(2):527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- Hawkins P. T., Stephens L. R., Piggott J. R. Analysis of inositol metabolites produced by Saccharomyces cerevisiae in response to glucose stimulation. J Biol Chem. 1993 Feb 15;268(5):3374–3383. [PubMed] [Google Scholar]
- Heitman J., Movva N. R., Hall M. N. Proline isomerases at the crossroads of protein folding, signal transduction, and immunosuppression. New Biol. 1992 May;4(5):448–460. [PubMed] [Google Scholar]
- Heitman J., Movva N. R., Hall M. N. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991 Aug 23;253(5022):905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- Heitman J., Movva N. R., Hiestand P. C., Hall M. N. FK 506-binding protein proline rotamase is a target for the immunosuppressive agent FK 506 in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1948–1952. doi: 10.1073/pnas.88.5.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman P. K., Emr S. D. Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Dec;10(12):6742–6754. doi: 10.1128/mcb.10.12.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiles I. D., Otsu M., Volinia S., Fry M. J., Gout I., Dhand R., Panayotou G., Ruiz-Larrea F., Thompson A., Totty N. F. Phosphatidylinositol 3-kinase: structure and expression of the 110 kd catalytic subunit. Cell. 1992 Aug 7;70(3):419–429. doi: 10.1016/0092-8674(92)90166-a. [DOI] [PubMed] [Google Scholar]
- Hoffman C. S., Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57(2-3):267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Hou W. M., Zhang Z. L., Tai H. H. Purification and characterization of phosphatidylinositol kinase from porcine liver microsomes. Biochim Biophys Acta. 1988 Mar 4;959(1):67–75. [PubMed] [Google Scholar]
- Iida H., Sakaguchi S., Yagawa Y., Anraku Y. Cell cycle control by Ca2+ in Saccharomyces cerevisiae. J Biol Chem. 1990 Dec 5;265(34):21216–21222. [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh W. M., Klippel A., Escobedo J. A., Williams L. T. Modification of the 85-kilodalton subunit of phosphatidylinositol-3 kinase in platelet-derived growth factor-stimulated cells. Mol Cell Biol. 1992 Aug;12(8):3415–3424. doi: 10.1128/mcb.12.8.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto A., Nishiyama K., Nakanishi H., Uratsuji Y., Nomura H., Takeyama Y., Nishizuka Y. Studies on the phosphorylation of myelin basic protein by protein kinase C and adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1985 Oct 15;260(23):12492–12499. [PubMed] [Google Scholar]
- Kunz J., Hall M. N. Cyclosporin A, FK506 and rapamycin: more than just immunosuppression. Trends Biochem Sci. 1993 Sep;18(9):334–338. doi: 10.1016/0968-0004(93)90069-y. [DOI] [PubMed] [Google Scholar]
- Kunz J., Henriquez R., Schneider U., Deuter-Reinhard M., Movva N. R., Hall M. N. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell. 1993 May 7;73(3):585–596. doi: 10.1016/0092-8674(93)90144-f. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Levin D. E., Bartlett-Heubusch E. Mutants in the S. cerevisiae PKC1 gene display a cell cycle-specific osmotic stability defect. J Cell Biol. 1992 Mar;116(5):1221–1229. doi: 10.1083/jcb.116.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. E., Fields F. O., Kunisawa R., Bishop J. M., Thorner J. A candidate protein kinase C gene, PKC1, is required for the S. cerevisiae cell cycle. Cell. 1990 Jul 27;62(2):213–224. doi: 10.1016/0092-8674(90)90360-q. [DOI] [PubMed] [Google Scholar]
- Lips D. L., Majerus P. W., Gorga F. R., Young A. T., Benjamin T. L. Phosphatidylinositol 3-phosphate is present in normal and transformed fibroblasts and is resistant to hydrolysis by bovine brain phospholipase C II. J Biol Chem. 1989 May 25;264(15):8759–8763. [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Nakanishi H., Brewer K. A., Exton J. H. Activation of the zeta isozyme of protein kinase C by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1993 Jan 5;268(1):13–16. [PubMed] [Google Scholar]
- Nickels J. T., Jr, Buxeda R. J., Carman G. M. Purification, characterization, and kinetic analysis of a 55-kDa form of phosphatidylinositol 4-kinase from Saccharomyces cerevisiae. J Biol Chem. 1992 Aug 15;267(23):16297–16304. [PubMed] [Google Scholar]
- Otsu M., Hiles I., Gout I., Fry M. J., Ruiz-Larrea F., Panayotou G., Thompson A., Dhand R., Hsuan J., Totty N. Characterization of two 85 kd proteins that associate with receptor tyrosine kinases, middle-T/pp60c-src complexes, and PI3-kinase. Cell. 1991 Apr 5;65(1):91–104. doi: 10.1016/0092-8674(91)90411-q. [DOI] [PubMed] [Google Scholar]
- Pawson T., Schlessingert J. SH2 and SH3 domains. Curr Biol. 1993 Jul 1;3(7):434–442. doi: 10.1016/0960-9822(93)90350-w. [DOI] [PubMed] [Google Scholar]
- Philippsen P., Stotz A., Scherf C. DNA of Saccharomyces cerevisiae. Methods Enzymol. 1991;194:169–182. doi: 10.1016/0076-6879(91)94014-4. [DOI] [PubMed] [Google Scholar]
- Porter F. D., Li Y. S., Deuel T. F. Purification and characterization of a phosphatidylinositol 4-kinase from bovine uteri. J Biol Chem. 1988 Jun 25;263(18):8989–8995. [PubMed] [Google Scholar]
- Reif K., Gout I., Waterfield M. D., Cantrell D. A. Divergent regulation of phosphatidylinositol 3-kinase P85 alpha and P85 beta isoforms upon T cell activation. J Biol Chem. 1993 May 25;268(15):10780–10788. [PubMed] [Google Scholar]
- Riedel H., Parissenti A. M., Hansen H., Su L., Shieh H. L. Stimulation of calcium uptake in Saccharomyces cerevisiae by bovine protein kinase C alpha. J Biol Chem. 1993 Feb 15;268(5):3456–3462. [PubMed] [Google Scholar]
- Rothstein R. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 1991;194:281–301. doi: 10.1016/0076-6879(91)94022-5. [DOI] [PubMed] [Google Scholar]
- Schomerus C., Küntzel H. CDC25-dependent induction of inositol 1,4,5-trisphosphate and diacylglycerol in Saccharomyces cerevisiae by nitrogen. FEBS Lett. 1992 Aug 3;307(3):249–252. doi: 10.1016/0014-5793(92)80688-d. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L., Crabtree G. R. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992 Apr;13(4):136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L. Immunophilin-sensitive protein phosphatase action in cell signaling pathways. Cell. 1992 Aug 7;70(3):365–368. doi: 10.1016/0092-8674(92)90158-9. [DOI] [PubMed] [Google Scholar]
- Schu P. V., Takegawa K., Fry M. J., Stack J. H., Waterfield M. D., Emr S. D. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993 Apr 2;260(5104):88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- Serunian L. A., Haber M. T., Fukui T., Kim J. W., Rhee S. G., Lowenstein J. M., Cantley L. C. Polyphosphoinositides produced by phosphatidylinositol 3-kinase are poor substrates for phospholipases C from rat liver and bovine brain. J Biol Chem. 1989 Oct 25;264(30):17809–17815. [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Sigal N. H., Dumont F. J. Cyclosporin A, FK-506, and rapamycin: pharmacologic probes of lymphocyte signal transduction. Annu Rev Immunol. 1992;10:519–560. doi: 10.1146/annurev.iy.10.040192.002511. [DOI] [PubMed] [Google Scholar]
- Simon A. J., Milner Y., Saville S. P., Dvir A., Mochly-Rosen D., Orr E. The identification and purification of a mammalian-like protein kinase C in the yeast Saccharomyces cerevisiae. Proc Biol Sci. 1991 Feb 22;243(1307):165–171. doi: 10.1098/rspb.1991.0027. [DOI] [PubMed] [Google Scholar]
- Stack J. H., Herman P. K., Schu P. V., Emr S. D. A membrane-associated complex containing the Vps15 protein kinase and the Vps34 PI 3-kinase is essential for protein sorting to the yeast lysosome-like vacuole. EMBO J. 1993 May;12(5):2195–2204. doi: 10.1002/j.1460-2075.1993.tb05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L. R., Jackson T. R., Hawkins P. T. Agonist-stimulated synthesis of phosphatidylinositol(3,4,5)-trisphosphate: a new intracellular signalling system? Biochim Biophys Acta. 1993 Oct 7;1179(1):27–75. doi: 10.1016/0167-4889(93)90072-w. [DOI] [PubMed] [Google Scholar]
- Stephens L., Hawkins P. T., Downes C. P. Metabolic and structural evidence for the existence of a third species of polyphosphoinositide in cells: D-phosphatidyl-myo-inositol 3-phosphate. Biochem J. 1989 Apr 1;259(1):267–276. doi: 10.1042/bj2590267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno I., Fukami K., Kato H., Takenawa T., Ishikawa T. Essential role for phosphatidylinositol 4,5-bisphosphate in yeast cell proliferation. Nature. 1988 May 12;333(6169):188–190. doi: 10.1038/333188a0. [DOI] [PubMed] [Google Scholar]
- Valius M., Kazlauskas A. Phospholipase C-gamma 1 and phosphatidylinositol 3 kinase are the downstream mediators of the PDGF receptor's mitogenic signal. Cell. 1993 Apr 23;73(2):321–334. doi: 10.1016/0092-8674(93)90232-f. [DOI] [PubMed] [Google Scholar]
- Walker D. H., Dougherty N., Pike L. J. Purification and characterization of a phosphatidylinositol kinase from A431 cells. Biochemistry. 1988 Aug 23;27(17):6504–6511. doi: 10.1021/bi00417a046. [DOI] [PubMed] [Google Scholar]
- Whitman M., Downes C. P., Keeler M., Keller T., Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 1988 Apr 14;332(6165):644–646. doi: 10.1038/332644a0. [DOI] [PubMed] [Google Scholar]
- Wiederrecht G., Brizuela L., Elliston K., Sigal N. H., Siekierka J. J. FKB1 encodes a nonessential FK 506-binding protein in Saccharomyces cerevisiae and contains regions suggesting homology to the cyclophilins. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1029–1033. doi: 10.1073/pnas.88.3.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett J. R., Gould K. L., Hunter T. Substrate specificity of protein kinase C. Use of synthetic peptides corresponding to physiological sites as probes for substrate recognition requirements. Eur J Biochem. 1986 Nov 17;161(1):177–184. doi: 10.1111/j.1432-1033.1986.tb10139.x. [DOI] [PubMed] [Google Scholar]




