Abstract
The anterior olfactory nucleus (AON), a component of the main olfactory system, is a cortical region that processes olfactory information and acts as a relay between the main olfactory bulbs and higher brain regions such as the piriform cortex. Utilizing a transgenic rat in which an enhanced green fluorescent protein reporter gene is expressed in vasopressin neurones (eGFP-vasopressin), we have discovered a population of vasopressin neurones in the AON. These vasopressin neurones co-express vasopressin V1 receptors. They also co-express GABA and calbinin-D28k indicating that they are neurochemically different from the newly described vasopressin neurons in the main olfactory bulb. We utilized the immediate early gene product, early growth response protein 1 (Egr-1), to examine the functional role of these vasopressin neurons in processing social and non-social odours in the AON. Exposure of adult rats to a conspecific juvenile or a heterospecific predator odour leads to increases in Egr-1 expression in the AON in a subregion specific manner. However, only exposure to a juvenile increases Egr-1 expression in AON vasopressin neurons. These data suggest that vasopressin neurones in the AON may be selectively involved in the coding of social odour information.
Introduction
Complex social behaviour in vertebrates, from sexual behaviour to the maintenance of social hierarchies, involves individual recognition. In rodents, recognition is primarily facilitated by olfactory information (Brennan & Kendrick, 2006; Sanchez-Andrade & Kendrick, 2009). Vasopressin modulates social recognition at the level of the olfactory bulbs (Dluzen et al. 1998a; Tobin et al. 2010) and brain regions such as the lateral septum (Dantzer et al. 1988; Bielsky et al. 2005), and, in turn, regulates social behaviours such as aggression (Ferris & Potegal, 1988; Blanchard et al. 2005), pair-bonding (Winslow et al. 1993), and parental behaviour (Parker & Lee, 2001; Bosch et al. 2010). Recent stuhave also linked vasopressin signalling to human social behaviour (Coccaro et al. 1998; Thompson et al. 2004; Bachner-Melman et al. 2005; Thompson et al. 2006; Knafo et al. 2008; Walum et al. 2008; Meyer-Lindenberg et al. 2009), as well as neurological disorders such as autism (Kim et al. 2002; Wassink et al. 2004; Yirmiya et al. 2006; Meyer-Lindenberg et al. 2009).
The AON, an olfactory cortex located just caudal to the olfactory bulbs, acts as a relay between the main olfactory bulbs and higher brain regions such as the piriform cortex, and between the left and right AONs (Haberly & Price, 1978a,b; Mohedano-Moriano et al. 2005; Illig & Eudy, 2009). The AON is segregated into five different subdivisions, each with topographically relevant connections and differential expression of neurochemicals and receptors (reviewed in Brunjes et al. 2005). The behavioural relevance of the AON has been only rudimentarily explored. The dorsal, lateral and posteroventral subdivisions of the rat AON (AONd, AONl, and AONpv respectively) all express the protein products of immediate early genes following exposure to predator odour (Staples et al. 2005, 2008a,b), with the AONl potentially playing a role in olfactory recognition (Staples et al. 2008a). Immediate early gene expression in the AON also increases in anoestrous female ewes in the presence of a ram or ram's fleece (Gelez & Fabre-Nys, 2006), suggesting that this region is also involved in the processing of social odour information in other species. Vasopressin V1 receptor binding (Vallet et al. 1995; Schorscher-Petcu et al. 2009) and V1a receptor mRNA expression (Szot et al. 1994) occur in the anterior olfactory nucleus (AON), suggesting that vasopressin signalling in this area may also be important in the processing of social stimuli.
We have discovered a new population of vasopressin neurones distributed across multiple subdivisions of the AON by utilizing a transgenic rat line in which an enhanced green fluorescent protein reporter gene is expressed specifically in vasopressin cells (eGFP-vasopressin) (Ueta et al. 2005). We have characterised these neurons based on numerous other chemical markers. As the rostral migratory stream, by which new neurones migrate into the olfactory bulb, lies within the AON, we have also utilized bromodeoxyuridine (BrDU) to determine if these vasopressin neurones, as well as those previously identified in the olfactory bulb, are continually produced in the adult rat. Finally, in view of the established role of vasopressin in social recognition, we have quantified Egr-1 expression in these neurones in adult rats following exposure to social and non-social odours.
Methods
Experimental animals
All procedures were carried out in accordance with guidelines defined by the UK Home Office and all efforts were made to minimize the numbers of rats used (Drummond, 2009). Adult wild-type or homozygous eGFP-vasopressin Sprague-Dawley rats were housed in same sex groups prior to experimental manipulation.
Tissue preparation
Rats were deeply anaesthetised with pentobarbital (1–2 ml i.p.) and transcardially perfused with 200 ml of 0.9% saline and heparin (5000 U ml−1) followed by 300 ml of 4% paraformaldehyde in 0.1 m phosphate buffer (PB, pH 7.4) or 4% paraformaldehyde + 0.2% glutaraldehyde in 0.1 m PB (only for tissue treated with either GABA or glutamate antibodies). Brains were removed and cryoprotected at 4°C in 4% paraformaldehyde and 15% sucrose in 0.1 m PB for 24 h followed by incubation in 30% sucrose in 0.1 m PB for 48 h. Brains were then frozen on dry ice and stored at −70°C. Sections were cut with a freezing microtome at 40 μm (for immunofluorescence) or 52 μm (for immunohistochemistry).
Immunofluorescence – characterization of vasopressin neurones in AON
Immunohistochemical protocols were based on Tobin et al. (2010). Horizontal sections from six eGFP-vasopressin rats were distributed so that representative sections (dorsal, medial and ventral) from at least three rats were contained in each well exposed to each combination of primary antibodies. Free-floating sections to be incubated with biotinylated secondaries were placed in 0.001% avidin (Sigma-Aldrich, UK) in 0.1 m PB for 30 min, rinsed in 0.1 m PB, then placed in 0.001% biotin (Sigma-Aldrich) in 0.1 m PB for 30 min. All sections were then washed in 0.1 m PB and incubated for 60 min at room temperature in a blocking buffer consisting of 10% normal serum (matching host animal of secondary antibody) + 0.2% Triton X-100 in 0.1 m PB (PB-T). Sections were then incubated in either a rabbit anti-eGFP polyclonal antibody (1:1000; AB3080, Millipore, Hertfordshire, UK) or mouse anti-eGFP monoclonal antibody (1:1000; MAB3580 Millipore, Hertfordshire, UK) and one other primary antibody (mouse anti-calbindin D-28K: 1:500, 300, Swant, Bellinzona, Switzerland; mouse anti-calretinin: 1:500, 6B3, Swant; mouse anti-GABA: 1:500, 3D5, Swant; mouse anti-glutamate: 1:5000, 2D7, Swant; mouse anti-vasopressin: 1:1000, PS41, Prof. H. Gainer, NIH, Bethesda, MD; goat anti-vasopressin receptor V1a: 1:50, Sc-1896, Santa Cruz Biotechnology, Heidelberg, Germany; rabbit anti-vasopressin receptor V1b: 1:500, 905-750-100, Cambridge Bioscience Ltd, Cambridge, UK; mouse anti-oxytocin 1:1000, PS38, Prof. H. Gainer; rabbit anti-oxytocin: 1:100, PC226L, Merk Chemicals Ltd, Nottingham, UK) first for 60 min at room temperature then 48 h at 4°C. After washing in 0.1 m PB, sections were incubated for 60 min at room temperature with an Alexa 488 labelled secondary antibody (1:500, Invitrogen Ltd, Paisley, UK) to label eGFP-vasopressin neurones and either a biotylated goat anti-rabbit (1:500, BA-1000), horse anti-mouse (1:500, BA-2001), or horse anti-goat (1:500, BA-9500, Vector Laboratories Ltd, Peterborough, UK), or Alexa 568 labelled secondary antibody (1:500, Invitrogen Ltd, Paisley, UK) to label the other antigen of interest. Sections incubated with the biotinylated secondary antibody were washed and incubated for 60 min in Alexa 568–streptavidin conjugate (1:500; S11226, Invitrogen). All primary and secondary antibodies were diluted in blocking buffer. Sections were mounted using a Mowiol 4–88 based (Calbiochem, USA) mounting medium, supplemented with 2.5% DABCO (Sigma-Aldrich). eGFP immunoreactivity was not detectable in wild-type rats (data not shown) or when the primary antibody was omitted. Information regarding antibody specificity was provided by suppliers, but in addition we have tested the V1a and V1b antibodies in pituitary sections and, in agreement with previous studies, found no V1a, but V1b immunoreactivity in a subset of anterior pituitary cells (data not shown). In addition, we tested a rabbit polyclonal antibody to vasopressin (PC234L, Calbiochem, Merck Chemicals) and found the same pattern of immunoreactivity, i.e. immunoreactivity co-localised with PS41 and eGFP. Fluorescence signals were acquired using a Zeiss LSM510 Axiovert confocal laser scanning microscope equipped with argon–krypton lasers. Signals were acquired at 1024 × 1024 pixels, using a Zeiss Plan NeoFLUAR 1.4 NA ×63 oil-immersion objective. Emissions for Alexa 488 and Alexa 568 were obtained consecutively to avoid channel cross-talk. Images were captured with Zeiss Laser Scanning Systems LSM510 software and cropped and labelled in Adobe Photoshop or Illustrator. The pars externa and pars principalis (dorsal and lateral sub-regions) from at least three sections per primary antibody combination were examined to quantify numbers of eGFP-vasopressin cells positive or negative for calbindin-D-28K, calretinin, GABA, glutamate, vasopressin receptors V1a and V1b, vasopressin or oxytocin.
Immunohistochemistry – characterization of vasopressin neurones in AON
Sections from wild-type adult female rats were rinsed in 0.1 m PB, incubated in 1% BSA + 3% normal horse serum (NHS) in PB-T for 30 min, incubated in a mouse anti-vasopressin primary antibody (1:1000 in 0.1 m PB-T + 3% NHS + 1% bovine serum albumin (BSA); PS41, donated by Prof. H. Gainer) for 60 min at room temperature, then at 4°C for 36 h. Following PB-T washes, endogenous peroxidase was blocked with 0.3% H2O2 in 0.1 m PB for 20 min, then sections were washed in PB before being incubated in a biotinylated horse anti-mouse secondary antibody for 60 min (1:500 in 0.1 m PB-T + 1% BSA; BA-9500, Vector Laboratories). Sections were then washed in 0.1 m PB, incubated in 0.05 m acetate buffer for 10 min, incubated with R.T.U. horseradish-peroxidase-strepavidin for 30 min (Vector Laboratories), rinsed in 0.05 m acetate buffer, and visualized with diaminobenzidine (DAB) with nickel sulphate (adapted from Shu et al. 1988). The visualization reaction was terminated with 0.05 m acetate buffer and repeated washes of 0.1 m PB. Sections were then mounted, dehydrated through a graded series of ethanols, counterstained with cresyl violet (1.25 g cresyl violet acetate and 0.75 ml glacial acetic acid per 250 ml of dH20), and coverslipped.
BrDU injections
Three groups of eGFP-vasopressin rats (n= 7 per group) received daily injections of bromodeoxyuridine (BrdU; i.p., 50 mg kg−1), a marker of cell division. Group 1 received BrdU for 4 days and were killed 14 days later, Group 2 received BrdU for 4 days and were killed 30 days later, and Group 3 received BrdU for 6 days and were killed 70 days later. All animals were transcardially perfused and brains were processed as described under Tissue preparation above.
Immunofluorescence – BrDU
The BrDU labelling protocol was based on Winner et al. (2002). Briefly, sections were incubated in 0.2% H2O2 in Tris-buffered saline (TBS: 0.15 m NaCl, 0.1 m Tris-HCl, pH 7.5) for 30 min, then incubated in 50% formamide/2× SSC (SSC: 0.3 m NaCl, 0.03 m sodium citrate) at 65°C for 2 h, and washed in 2× SSC for 5 min. Sections were then incubated in 2 n HCl at 37°C for 30 min followed by a 2 × 5 min neutralisation wash in 0.1 m borate buffer (pH 8.5). Sections were then washed in TBS + 0.25% Triton X-100 (TBS-T) + 5% NHS for 30 min, followed by incubation with a mouse anti-BrdU monoclonal antibody (1:100 in TBS-T + 5% NHS; no. 11170376001, Roche, Mannheim, Germany) for 70 h at 4°C. Sections were then washed in TBS 3 × 5 min and incubated in a horse anti-mouse secondary conjugated to Texas Red (1:200; TI-2000, Vector Laboratories) in TBS-T with 5% NHS for 2 h at room temperature. Sections were then incubated in 0.1 m TBS-T + 5% normal goat serum (NGS) for 30 min, followed by a rabbit anti-eGFP polyclonal antibody (1:1000; AB3080, Chemicon, USA) + 5% NGS for 70 h at 4°C. Sections were then rinsed in 0.1 m TBS-T and incubated in a goat anti-rabbit secondary antibody conjugated to fluorescein (1:200; FI-2000, Vector Laboratories) in 0.1 m TBS-T with 5% NGS for 2 h at room temperature. Sections were mounted with ProLong Gold Antifade Reagent (Invitrogen, USA), and coverslipped.
Three to four brain sections for each animal were examined for BrDU/eGFP-vasopressin co-localization on a Leica DMR light microscope equipped with fluorescence (Leica Microsystems, Wetzlar, Germany) under ×20, ×40 and ×100 objectives. Multiple images were captured in areas of the MOB and AON for each section where both signals were present in the same field of view. In addition, areas of interest were examined on a Leica TCS-NT confocal microscope (Leica Microsystems) under both ×40 and ×63 objectives, Z-stacks were acquired, red and green channels were obtained serially, and orthogonal photomicrographs were examined.
Odour stimulus test
Social odour tests were modified from Engelmann et al. (1995). Adult male (n= 14) and female (n= 14) rats were moved to single experimental cages 2 h prior to testing. Rats were presented with one of the following: (1) a novel juvenile rat for 4 min (n= 10), (2) a combination of non-social odours (mixed 1:1 in diethyl phthalate, 25 μl total volume, Sigma-Aldrich): (R)-(–)-carvone (Merck, Ismaning, Germany), isoamylacetate, allylcaproate, anethole (all Sigma-Aldrich) on a cotton bud placed into an air stream produced by a silent computer cooling fan and directed to the head of the animal for 4 min (n= 10), or (3) no olfactory stimulus (cage moved as in other trials, but no stimulus provided – i.e. cage disturbance, n= 8). It has been previously shown that such non-social odours stimulate c-fos protein synthesis in several brain areas (Richter et al. 2005). Rats were transcardially perfused 70 min after the initiation of the test and tissue was processed for eGFP-vasopressin + Egr-1 immunohistochemistry as outlined below.
Predator odour test
Predator odour tests were modified from Staples et al. (2008b). Adult male (n= 14) and female (n= 14) rats were moved to single experimental cages 12 h prior to testing. Rats were presented with a 20 cm section of cat collar: (1) worn by a male domestic outdoor cat for 28 days (n= 10), or (2) impregnated with 30 μl of trimethylthiazoline (TMT; PheroTech Inc., Delta, BC, Canada), a constituent of fox faeces (n= 8), or (3) impregnated with a combination of non-social odours (mixed 1:100 in diethyl phthalate, otherwise as described above) for 60 min (n= 10). Rats had access to the collar piece during the course of the experiment. Rats were transcardially perfused 70 min after the initiation of the test and tissue was processed for eGFP-vasopressin + Egr-1 immunohistochemistry as outlined below.
Immunohistochemistry – eGFP-vasopressin + Egr-1
Immunohistochemical protocols were based on Meddle et al. (2007). Sections were rinsed in 0.1 m PB with and without 0.2% Triton X-100 (PB-T), and incubated in 0.3% H2O2 in 0.1 m PB to block endogenous peroxidase activity. Sections were then blocked in 3% NGS in 0.1 m PBT for 30 min and incubated in a rabbit anti-Egr-1 polyclonal antibody (1:1000; SC-189 Santa Cruz Biotechnology, Santa Cruz, CA, USA) in 0.1 m PB-T + 3% NGS for 48 h at 4°C. This antibody recognizes the C-terminus of human Egr-1, has been utilized successfully in rats, and immunoreactivity in a Western blot is completely absent in A-431 cells treated with a specific an Egr-1 siRNA (SC-189 product datasheet; Santa Cruz Biotechnology). Egr-1 is a relatively ubiquitous zinc finger transcription factor involved in recognition memory and used as a marker of neuronal activation (Bozon et al. 2003; Knapska & Kaczmarek, 2004). Sections were then rinsed in 0.1 m PB-T and incubated in a goat anti-rabbit secondary antibody (1:500 in 0.1 m PB-T + 3% NGS, BA-1000, Vector Laboratories) for 1 h at room temperature. Egr-1 was visualized using a Vectastain Elite ABC Kit (PK-6101; Vector Laboratories), followed by a 5 min rinse in 0.1 m sodium acetate buffer, and incubation in 0.025% DAB with nickel sulphate, ammonium chloride and 0.03% H2O2 in 0.1 m sodium acetate. Sections were then washed in 0.1 m PB-T, blocked in 3% NHS in 0.1 m PB-T and then incubated in a mouse anti-eGFP monoclonal antibody (1:1000; MAB3580, Millipore, Billerica, MA, USA) for 48 h at 4°C. Sections were then washed in 0.1 m PB-T and incubated in a horse anti-mouse secondary antibody (1:500 in 0.1 m PB-T + 3% NHS, BA-2001, Vector Laboratories) for 1 h at room temperature. eGFP was visualized with a Vectastain Elite ABC Kit (PK-6102; Vector Laboratories), followed by incubation in 0.025% DAB with 0.03% H2O2 in 0.1 m PB. Sections were mounted on gelatine-subbed slides, dehydrated in a graded ethanol series, and coverslipped using DPX (Sigma-Aldrich). eGFP immunoreactivity was not detectable in wild-type rats (data not shown) or when the primary antibody was omitted.
The AON and its subdivisions were identified on a Leica DMR light microscope under a ×5 objective using multiple rodent brain atlases (König & Klippel, 1963; Paxinos & Watson, 1998) and Brunjes et al. (2005). As the subdivisions of the AON change shape across the sagittal plane, and because the number of vasopressin neurons decreases medially within each subdivision, all cell counts were distributed evenly across the sagittal extent of each subdivision (Fig. 3A–F). It was not possible to delineate the pars ventroposterior (AONpv) and pars lateralis (AONl) in the sagittal plane, so these regions are collectively referred to as AONl. Egr-1-immunoreactivity was restricted to cell nuclei (black staining), while eGFP-vasopressin immunoreactivity was visualised as brown cytosolic staining (Fig. 3G and H). Total Egr-1 immunoreactivity was assessed in photos acquired on a Leica DMR light microscope under a ×20 objective, and captured with a Leica DFC490 digital camera and Leica Application Suite version 2.8.1 software. Areas within each photograph were sampled for total Egr-1 immunoreactivity utilizing sampling rectangles covering a majority of each respective AON subdivision, with the size of each sampling rectangle consistent in size and placement for each subdivision. Brown eGFP staining was filtered out utilizing the Thresholdour plugin (G. Landini; available from http://www.dentistry.bham.ac.uk/landinig/software) for ImageJ (National Institutes of Health, Bethesda, MD, USA). Photos were then transformed into black and white images and the total area covered by black (i.e. Egr-1 immunoreactivity) was calculated utilizing the same programs. Ratios of the number cells immunoreactive for both Egr-1 and eGFP-vasopressin to the total number of cells immunoreactive for eGFP-vasopressin were calculated, and are represented as activated:total in all graphs. Cell counts were conducted under a ×20 objective on a Leica DMR light microscope by one observer who was blind to treatment. Images were captured using a Leica DFC490 digital camera and Leica Application Suite version 2.8.1 software and were cropped and labelled in Adobe Photoshop or Illustrator. Overview photos of the AON (Fig. 3A–C) were stitched together from multiple adjoining images using PTGui Pro version 8.22 (New House Internet Services B.V., The Netherlands).
Figure 3. Immediate early gene expression (Egr-1) in vasopressin cells in the anterior olfactory nucleus following odour exposure.
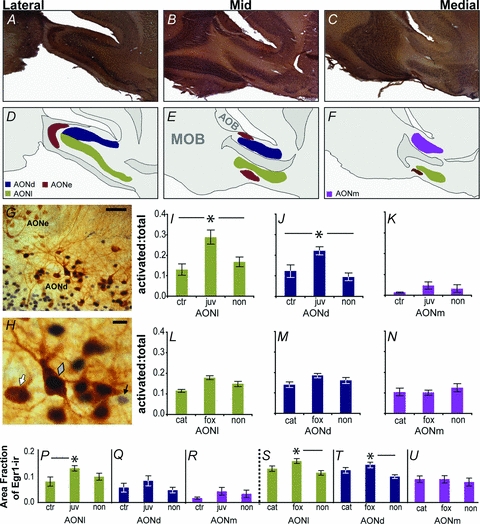
The AON is subdivided into the pars externa (AONe) and pars principalis. The pars principalis is composed of three subdivisions, the pars dorsalis (AONd), pars lateralis (AONl), and pars medialis (AONm) (A–C). Diagrammatic representations of the cellular layers of AON subdivisions are indicative of a typical adult male or female eGFP-vasopressin rat (D–F; MOB, main olfactory bulb; AOB, accessory olfactory bulb). Vasopressin cells were most regularly located along the superficial edge of the deep cellular layer of the pars principalis (G; scale bar = 50 μm). An eGFP-vasopressin+/Egr-1−(white arrow), a double labelled (grey diamond), and an eGFP-vasopressin−/Egr-1+ cell (black arrow) are shown (H; scale bar = 10 μm). Exposure to a conspecific juvenile increased the ratio of vasopressin cells expressing the Egr-1 protein over the total number of vasopressin cells (i.e. activated: total) in AONl (I) and AONd (J), but not AONm (K) as compared to both cage agitation (ctr) and non-social odour (non) controls. Exposure to heterospecific predator odours (cat, fox) did not alter the ratio of vasopressin cells expressing the Egr-1 protein over the total number of vasopressin cells as compared to non-social odour controls in AONl (L), AONd (M), or AONm (N). Exposure to a juvenile increases overall Egr-1 expression in the AONl (P), but not AONd (Q) or AONm (R). Exposure to fox odour increases Egr-1 expression in the AONl (S) and AONd (T), but not AONm (U), over non-social odour controls. Egr-1 expression was widespread in the AON, and is represented as the total area fraction covered by Egr-1 immunoreactivity within a sampled area within an AON subdivision. Asterisks denote significant differences in post hoc analyses.
Total vasopressin cell counts in the AON
The total number of vasopressin cells in the AON was estimated according to the following methodology. The number of eGFP-vasopressin cells were counted in a subset of 52 μm sections across animals in the predator odour study. Sections were divided into sagittal regions indicative of the lateral to medial aspects of this brain area. The average number of eGFP-vasopressin cells in each sagittal region was multiplied by the total number of sections typical for that region. Cell counts were Abercrombie corrected to increase precision (Guillery, 2002).
Statistical analysis
Data that were normally distributed with homogeneous variance across treatment groups are represented with mean ± standard error of the mean measurements. Data were natural log transformed (ln transform) or ranked to achieve normality or homogeneous variance as necessary. Total Egr-1 immunoreactivity (area fraction) and ratios of the number of Egr-1 immunoreactive cells and eGFP-vasopressin immunoreactive cells to the total number of eGFP-vasopressin immunoreactive cells were compared with two factor ANOVA, with treatment and sex as factors. For the odour stimulus test, relevant post hoc pairwise Holm–Sidak comparisons were conducted. As we demonstrated that the non-social odour cocktail does not increase the ratio of Egr-1 expressing eGFP-vasopressin to total eGFP-vasopressin cells in any subdivision, for the predator odour test, relevant post hoc Holm–Sidak comparisons were made against the non-social odour control only. As neither an effect of sex nor a treatment × sex interaction was detected in any analysis, males and females were combined in all figures. Sex differences in the number of eGFP-vasopressin neurones were assessed with two factor ANOVA, with treatment and sex as factors. Comparisons of cells showing co-expression of neurochemical markers were made by comparing the percentages of immunopositive cells in each region counted in at least three sections from three rats. Statistical comparison was made using Kruskal–Wallis one-way ANOVA on ranks. Data reported in the text represent the overall percentage in each region for all sections and cell numbers counted.
Results
Characterization of eGFP-vasopressin neurones in the AON
There are approximately 2240 ± 104 vasopressin cells in the pars externa and 3780 ± 142 cells in the pars principalis of each AON; the latter excludes some vasopressin cells that appear continuous with those within the pars principalis, but would normally be considered part of the piriform cortex. In all areas of the AON, every cell that was immunoreactive for eGFP was also immunoreactive for vasopressin in eGFP-vasopressin rats (Fig. 1B–D, AONe: n= 352 cells examined; AONd: n= 454; AONl: n= 482; AONe: n= 352; AONd: n= 454; AONl: n= 482), and the distribution and number of vasopressin immunoreactive cells in wild-type rats matches that seen in eGFP-vasopressin transgenics (Fig. 1A). eGFP-vasopressin cells are located in both the pars externa (AONe) and all subdivisions of the pars principalis, primarily in the superficial half of the deep cellular zone (Figs 1A, 3A–H). We found no sex differences in the number of eGFP-vasopressin cells per 52 μm section in AONl (males 285 ± 18, females 258 ± 13; F0.05,2,22= 1.876, P= 0.185), AONd (males 140 ± 11, females 134 ± 9; F0.05,2,22= 0.347, P= 0.562), AONm (males 85 ± 8, females 80 ± 7; F0.05,2,22= 0.279, P= 0.603), or AONe (males 196 ± 7, females 167 ± 14; F0.05,2,22= 1.663, P= 0.211). There was no treatment effect or significant sex × treatment interaction on the number of eGFP-vasopressin neurons for any AON subdivision (data not shown).
Figure 1. Vasopressin neurones in the AON.
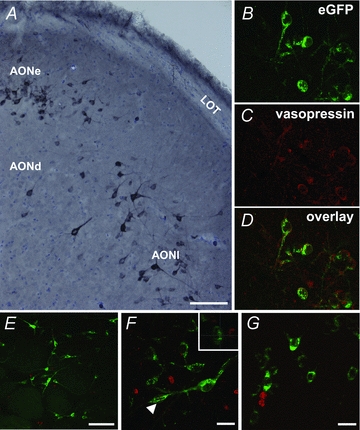
Vasopressin neurones are found in all AON subdivisions in wild-type Sprague–Dawley rats (A; scale bar = 100 μm). eGFP-immunoreactivity mirrors vasopressin immunoreactivity in eGFP-vasopressin transgenic rats (B–D– AONl). BrDU labelling in the AON was typically found around the rostral migratory tract, while eGFP-vasopressin cells in the pars principalis were most often distributed in the superficial aspect of the deep cellular layer (Layer II). Vasopressin neurones were not co-localised with BrDU in the olfactory bulb (E and F– glomerular layer of the MOB; scale bar = 100 μm and 20 μm, respectively; F– arrow shows location of orthogonal view in the boxed inset) or in any region of the AON (G– lateral AONl; scale bar = 20 μm), suggesting that vasopressin cells in the AON are not simply newly born neurones.
Although extensive BrDU labelling was detected in the olfactory bulb and along the rostral migratory tract, no vasopressin cells were labelled with BrDU, 14, 30, or 70 days post-injection, in the olfactory bulb (Fig. 1E and F). BrDU and eGFP-vasopressin immunoreactive cells were both found at the intersection of the glomerular and external plexiform layers of the main and accessory olfactory bulbs. In the AON, BrDU labelled cells (14 days: 132.9 ± 7.5; 30 days 126.1 ± 5.1; 70 days: 131.2 ± 5.0, mean three sections/seven animals per group) were typically located in and around the rostral migratory tract through the pars principalis, while eGFP-vasopressin cells were most often found in the superficial aspect of the deep cellular layer (Layer II). Extensive examination by confocal microscopy never demonstrated any co-localization of these two signals in the AON (0 of 7884 BrDU positive cells counted were eGFP-vasopressin positive). This suggests that these vasopressin cells are not newly born migratory neurones.
Most eGFP-vasopressin cells in the AON were also immunoreactive for V1a receptors (Fig. 2A–C), in all subdivisions (AONe: 100%, n= 259 cells; AONd: 97%, n= 194 cells; AONl: 98%, n= 253). All eGFP-immunoreactive cells in the AON express V1b receptors (Fig. 2D–F; AONe: n= 596 cells; AONd: n= 432 cells; AONl: n= 513). The eGFP-vasopressin cells in the AON also all expressed calbindin-D28k (Fig. 2G–I, AONe: n= 171; AONd:, n= 454; AONl: n= 397), but none expressed calretinin (Fig. 2J–L, AONe: n= 408; AONd: n= 264; AONl: n= 331). In the AON, eGFP-vasopressin cells were all immunoreactive for GABA (Fig. 2M–O, AONe: n= 162; AONd: n= 204; AONl: n= 280), and none were immunoreactive for glutamate (Fig. 2P–Q, AONe: n= 183; AONd: n= 128; AONl: n= 81). Not all V1b, calbindin-D28k or GABA positive cells in the AON are immunoreactive for eGFP. No immunoreactivity to oxytocin was detected in any region of the AON (data not shown).
Figure 2. Chemical characterization of vasopressin neurones in the AON.
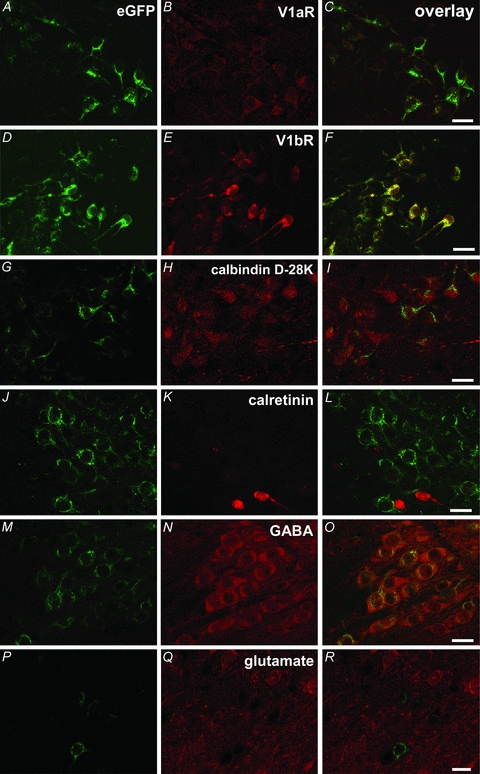
Some vasopressin cells in the AON co-express V1aR (A–C) and all co-express V1bR (D–F). Vasopressin neurones in the AON express calbindin-D28k (G–I), but not calretinin (J–L) and are GABAergic (M–O), but not glutamatergic (P–R). Scale bars = 20 μm.
Odour stimulus test
The AON is an olfactory cortex located just caudal to the olfactory bulbs (Fig. 3A–F). Egr-1 and eGFP-vasopressin immunoreactivity and co-localization of these signals were easily discernible in the pars externa and all subdivisions of the pars principalis as black nuclear and brown cytosolic staining, respectively (Fig. 3G–H). Egr-1 immunoreactivity was significantly different across treatments in AONl (F0.05,2,20= 3.528, P < 0.05). Post hoc tests after the two factor ANOVA showed no pairwise differences between groups; however, as there was no effect of sex (F0.05,2,20= 0.000, P= 0.987) or a sex × treatment interaction (F0.05,2,20= 0.527, P= 0.598), a post hoc analysis following a one way ANOVA with the sexes lumped together showed that levels were higher in animals presented with a novel juvenile rat versus animals in control group (Fig. 3P). Egr-1 immunoreactivity did not vary by treatment (F0.05,2,20= 1.184, P= 0.326) or sex (F0.05,2,20= 0.174, P= 0.681), nor was there a sex × treatment interaction (F0.05,2,20= 0.156, P= 0.857) in AONd (Fig. 3Q). Egr-1 immunoreactivity did not vary by treatment (F0.05,2,20= 0.855, P= 0.440) or sex (F0.05,2,20= 0.278, P= 0.604), nor was there a sex × treatment interaction (F0.05,2,20= 0.692, P= 0.512) in AONm (Fig. 3R).
The ratio of Egr-1 expressing eGFP-vasopressin to total eGFP-vasopressin cells was significantly different across treatments in AONl (F0.05,2,22= 7.368, P < 0.005; Fig. 3I). Post hoc tests showed that more vasopressin cells expressed Egr-1 in AONl after exposure to a novel juvenile rat than either a non-social odour or cage disturbance. There was no effect of sex (F0.05,1,22= 1.135, P= 0.298) nor a significant sex × treatment interaction (F0.05,2,22= 0.107, P= 0.899) on the ratio of Egr-1 expressing eGFP-vasopressin to total eGFP-vasopressin cells in AONl. The ratio of Egr-1 expressing eGFP-vasopressin to total eGFP-vasopressin cells was significantly different across treatments in AONd (F0.05,2,22= 8.504, P < 0.005; Fig. 3J). Post hoc tests showed that more vasopressin cells expressed Egr-1 in AONd after exposure to a novel juvenile rat than either a non-social odour or cage disturbance. There was no effect of sex (F0.05,1,22= 0.230, P= 0.636) nor a significant sex × treatment interaction (F0.05,2,22= 0.318, P= 0.731) on the ratio of Egr-1 expressing eGFP-vasopressin to total eGFP-vasopressin cells in AONd. There was no treatment effect (F0.05,2,18= 1.908, P= 0.177), sex difference (F0.05,1,18= 2.050, P= 0.169), or significant sex × treatment interaction effect (F0.05,2,18= 2.706, P= 0.094) on the ratio of Egr-1 expressing eGFP-vasopressin to total eGFP-vasopressin cells in AONm (Fig. 3K). There was no treatment effect (ln transform; F0.05,2,22= 2.679, P= 0.091), sex difference (ln transform; F0.05,1,22= 0.582, P= 0.454), or significant sex × treatment interaction effect (ln transform; F0.05,2,22= 0.448, P= 0.644) on the ratio of Egr-1 expressing eGFP-vasopressin to total eGFP-vasopressin cells in AONe.
Predator odour test
Egr-1 immunoreactivity was significantly different across treatments in AONl (F0.05,2,22= 5.029, P < 0.05; Fig. 3S). Post hoc tests showed a greater increase in animals exposed to a fox versus non-social odour. There was no effect of sex (F0.05,2,22= 0.357, P= 0.556) or a sex × treatment interaction (F0.05,2,22= 1.202, P= 0.319) in AONl. Egr-1 immunoreactivity was significantly different across treatments in AONd (F0.05,2,22= 4.827, P < 0.05; Fig. 3T). Post hoc tests showed a greater increase in animals exposed to a fox versus non-social odour. There was no effect of sex (F0.05,2,22= 0.939, P= 0.343) or a sex × treatment interaction (F0.05,2,22= 0.278, P= 0.760) in AONd. Egr-1 immunoreactivity did not vary by treatment (F0.05,2,22= 0.226, P= 0.799) or sex (F0.05,2,22= 0.000, P= 0.989), nor was there a sex × treatment interaction (F0.05,2,22= 1.853, P= 0.180) in AONm (Fig. 3U).
The ratio of Egr-1 expressing eGFP-vasopressin to total eGFP-vasopressin cells was significantly different across treatments in AONl (F0.05,2,22= 8.709, P < 0.005; Fig. 3L), but post hoc tests showed that neither cat nor fox odour treatments was significantly different from non-social odour controls. There was no effect of sex (F0.05,1,22= 0.109, P= 0.745) nor a significant sex × treatment interaction (F0.05,2,22= 1.776, P= 0.193) on the ratio of Egr-1 expressing eGFP-vasopressin to total eGFP-vasopressin cells in AONl. There was no treatment effect (F0.05,2,22= 2.468, P= 0.108), sex difference (F0.05,1,22= 0.144, P= 0.708), or significant sex × treatment interaction effect (F0.05,2,22= 0.516, P= 0.604) on the ratio of Egr-1 expressing eGFP-vasopressin to total eGFP-vasopressin cells in AONd (Fig. 3M). There was no treatment effect (F0.05,2,22= 0.544, P= 0.588), sex difference (F0.05,1,22= 0.315, P= 0.580), or significant sex × treatment interaction effect (F0.05,2,22= 1.175, P= 0.328) on the ratio of Egr-1 expressing eGFP-vasopressin to total eGFP-vasopressin cells in AONm (Fig. 3N). There was no treatment effect (ranked; F0.05,2,22= 1.218, P= 0.315), sex difference (ranked; F0.05,1,22= 0.763, P= 0.392), or significant sex × treatment interaction effect (ranked; F0.05,2,22= 2.078, P= 0.149) on the ratio of Egr-1 expressing eGFP-vasopressin to total eGFP-vasopressin cells in AONe. A Kruskal–Wallis one-way analysis of variance on ranks with treatment as the factor further verified that there were no treatment effects in AONe (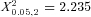 , P= 0.327).
, P= 0.327).
Discussion
Here, we have described a previously undiscovered population of vasopressin neurones in the AON, a cortical region where odour stimuli are integrated (Lei et al. 2006). These cells are widely distributed across both the pars externa and in all subdivisions of the pars principalis. We have recently shown a novel population of vasopressin neurones in the external plexiform and glomerular layer of the main and accessory olfactory bulb. However, the vasopressin neurones in the AON differ from those in the olfactory bulb in their neurochemical characteristics. Vasopressin neurones in the olfactory bulb do not express GABA, calbindin-D28k or calretinin, but co-express glutamate (Tobin et al. 2010). Vasopressin neurones in the AON co-express calbindin-D28k and GABA, but not calretinin and glutamate. We found no sex differences in the number of vasopressin cells in the AON, differentiating it from other brain regions where such differences are well established, such as in the bed nucleus of the stria terminalis (De Vries & al-Shamma, 1990).
As neurones newly born in the subventricular zone in the adult rat migrate to the main and accessory olfactory bulbs via the rostral migratory stream (Lledo et al. 2008), a fibre tract that runs through the AON, it is possible that vasopressin neurones in the AON are new neurones destined for the olfactory bulb. However, while injections of BrDU, a marker of cell division, yielded a high level of incorporation in olfactory bulb neurones and in the rostral migratory stream, there was no incorporation in vasopressin neurones in the main or accessory olfactory bulbs or the AON. This suggests that vasopressin neurones in the AON are not simply migrating to the olfactory bulbs, and that vasopressin neurones in the olfactory bulb and AON are not replaced in the adult rat.
Although the axonal targets of the AON have been well characterized, including the main olfactory bulb, piriform cortex and contralateral AON itself (Haberly & Price, 1978a,b; Mohedano-Moriano et al. 2005; Illig & Eudy, 2009), the targets of the vasopressin neurones in the AON are not yet known. Recent work suggests that the somato-dendritic release of neuropeptides, including vasopressin, in the brain is at least as relevant as release from axonal terminals (Ludwig et al. 2002; Engelmann et al. 2004; Leng & Ludwig, 2008). Considering such release, as well as the presence of bilateral AON axonal connections, AON-produced vasopressin would be predicted to exert some of its effects locally. We have shown V1a receptor expression in the AON, confirming results from previous receptor binding (Vallet et al. 1995; Schorscher-Petcu et al. 2009) and mRNA (Szot et al. 1994) studies. We have also shown V1b receptor immunoreactivity for the first time in the AON, although it has been described in both the olfactory tubercle and piriform cortex previously (Hernando et al. 2001). All vasopressin cells in the AON express V1b receptor and some express V1a receptor, which suggests that these cells might be sensitive to their own signal. Thus, vasopressin may provide a direct feedback to its neurones of origin in the AON.
We have shown that expression of the immediate early gene product, Egr-1, is increased in a subdivision-specific pattern in the AON in rats exposed to either conspecific or heterospecific social odours. Although changes in immediate early gene expression have not been previously examined in rats exposed to conspecific juveniles, our results are consistent with reports showing that exposure to predator odours leads to such increases in subdivisions of the AON (Staples et al. 2005, 2008b). These and other studies examined the expression of Fos, the protein product of the immediate early gene c-fos. Preliminary studies in our lab showed a general paucity of immunoreactivity for Fos in AON relative to that for Egr-1 (data not shown). By contrast, Egr-1 expression in the AON is extremely widespread. Egr-1 is also involved in memory, being necessary for late phase LTP in the dentate gyrus and associated with impaired object recognition when knocked out in mice (Bozon et al. 2003). It was therefore a logical choice for neuronal activation studies investigating brain regions involved in the formation of social odour memory.
We have demonstrated that exposure to a conspecific, but not heterospecific, social odour leads to increased Egr-1 protein expression in vasopressin neurones in the AON. It is important to note that the conspecific juvenile stimulus used in this study differed from the other stimuli in that a live animal, rather than just an odour mixture, was presented. It is therefore possible that other cues, such as visual or auditory, may have affected our results. The olfactory system interacts with other sensory systems (Wesson & Wilson, 2010), and future studies should address whether multiple sensory modalities might feed back on olfactory-specific brain regions, such as AON.
Our results are consistent with previous studies documenting the role of olfactory vasopressin in social recognition and the modulation of social behaviour (Dluzen et al. 1998a,b;). Classically, social odour cues are thought to be processed via the accessory olfactory system. The AON is largely considered to be part of the main olfactory system, but recent studies suggest that social odour cues, including non-volatile pheromonal signals, may be processed by both the main and the accessory olfactory bulb (Spehr et al. 2006; Keller et al. 2009), with active compounds able to elicit neuronal activity in both regions in mice (Brennan & Zufall, 2006). There is also some evidence that social cues may also be processed through the main olfactory system in rats. For example, social buffering of fear responses is eliminated in main olfactory bulb lesioned male rats (Kiyokawa et al. 2009).
The AON lies at a nexus of regions that process olfactory information, exchanging information with both the piriform cortex and the main olfactory bulb. If vasopressin produced in the AON acts locally in the processing of social odour cues, it may serve as a quick reference site for conspecific social recognition. When an animal is exposed to a novel odour combination, for example from a conspecific juvenile, a predictable topographic pattern of neuronal activation emerges in the olfactory bulb (Uchida et al. 2000). During a subsequent exposure to the same animal, the combination of its relevant odours would be predicted to create a similar activation pattern. It is thought that the AON mediates the first order central integration of odour information from spatially diffuse bulbar signals prior to subsequent central processing (Haberly, 2001). It is therefore in the perfect position for a quick comparison of a new and remembered social odour combination. In the present study, we have shown that an initial encounter with a novel juvenile rat leads to an increase in Egr-1 in vasopressin neurones in the AON, which may be indicative of vasopressin release. Similar to the hypothesized function of vasopressin in the olfactory bulb (Tobin et al. 2010), vasopressin in the AON may act to filter out recognized odour information, and thereby facilitate the remembrance of conspecifics. This would be advantageous as it would prevent an animal from expending energy engaging in unnecessary olfactory investigation. Filtering at the level of both the olfactory bulb and the AON may facilitate discrimination of subtle odour combinations likely to be required for individual recognition. That a response in vasopressin neurones is seen after exposure to a conspecific but not a predator odour is not surprising, as we would predict responses to a predator odour would be more stereotyped and perhaps less likely to be modulated by peptidergic signalling.
If vasopressin produced in the AON is also released from axon terminals into the main olfactory bulb, it may act there to further modulate social recognition. Vasopressin administered bilaterally into the olfactory bulb lengthens the time that male rats can recognize a conspecific juvenile upon re-exposure in a social discrimination test (Dluzen et al. 1998a). Both a general vasopressin V1 receptor antagonist and a V1a receptor siRNA administered bilaterally into the olfactory bulbs disrupt social recognition in the adult rat (Tobin et al. 2010). As the olfactory bulb produces its own vasopressin intrinsically (Tobin et al. 2010), additional release from the AON may represent a higher order fine tuning of the recognition responses mediated at this level.
Recently, there have been a wealth of studies linking vasopressin signalling to human behaviour, including altruism (Knafo et al. 2008), aggression (Coccaro et al. 1998), social cognition (Thompson et al. 2004, 2006), personality and creativity (Bachner-Melman et al. 2005; Meyer-Lindenberg et al. 2009), pair-bonding (Walum et al. 2008), and autism (Wassink et al. 2004; Yirmiya et al. 2006). Comparisons of human and rodent olfaction are often difficult, given that rodents rely heavily on a functional accessory olfactory system that humans do not possess. The AON, however, is part of the main olfactory system and is present in humans. It is one of the first sites of neural pathology in Parkinson's disease (Lerner & Bagic, 2008), a malady primarily known for its debilitating effects on motor skills, but one that also disrupts social cognition and emotional recognition (Suzuki et al. 2006; Kawamura & Koyama, 2007). Furthermore, intranasal administration of vasopressin has significant effects on human social cognition (Thompson et al. 2004, 2006), which suggests that vasopressin signalling in olfactory regions may be of eventual clinical importance.
We have discovered a new population of vasopressin neurons in the AON and demonstrated that the activity of these cells is linked to the processing of social odour information in a region-specific manner. Specifically, vasopressin neurones in the AONl and AONd, but not the AONm, of the pars principalis show increased activity after exposure to a novel juvenile rat versus a non-social odour stimulus, while heterospecific predator odours do not activate these neurons above control levels. Future studies should address the connectivity of these cells, as well as the ability of other contextually relevant conspecific odours (e.g. odour of a sexually receptive female, rival adult male) to alter the activity of vasopressin neurones in the AON.
Acknowledgments
The authors would like to thank Jenifer Rodrigues and Hitoshi Suzuki for technical assistance, Professor Gareth Leng (Edinburgh) for critical reading of the manuscript, and Trudi Gillespie from the IMPACT imaging facility at the University of Edinburgh for assistance with confocal microscopy. Work was supported by grants from the BBSRC (M.L. and S.L.M.), British Council Academic Research Collaboration (M.L. and S.M.) and Deutsche Forschungsgemeinschaft and the German Academic Exchange Service (M.E.).
Glossary
Abbreviations
- AON
anterior olfactory nucleus
- AONl
pars lateralis
- AONd
pars dorsalis
- AONm
pars medialis
- AONe
pars externa
- BrDU
bromodeoxyuridine
- eGFP
enhanced green fluorescent protein
- Egr-1
early growth response protein 1
Author contributions
Experiments were performed in the laboratories of M.L. and S.M. D.W., M.E. and M.L. conducted the BrDU experiment. D.W. quantified and analysed the odour stimulus study and conducted the predator odour study. V.T. completed the chemical characterization studies and the Nissl/vasopressin immunohistochemistry (IHC). J.N., V.B. and S.M. performed the behaviour tests and IHC for the odour stimulus study. A.D. analysed overall Egr-1 expression in the behavioural studies. D.W., M.E., S.M. and M.L. designed the experiments and wrote and revised the paper. All authors approved the final version of the manuscript.
References
- Bachner-Melman R, Dina C, Zohar AH, Constantini N, Lerer E, Hoch S, Sella S, Nemanov L, Gritsenko I, Lichtenberg P, Granot R, Ebstein RP. AVPR1a and SLC6A4 gene polymorphisms are associated with creative dance performance. PLoS Genet. 2005;1:e42. doi: 10.1371/journal.pgen.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Griebel G, Farrokhi C, Markham C, Yang M, Blanchard DC. AVP V1b selective antagonist SSR149415 blocks aggressive behaviors in hamsters. Pharmacol Biochem Behav. 2005;80:189–194. doi: 10.1016/j.pbb.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Pfortsch J, Beiderbeck DI, Landgraf R, Neumann ID. Maternal behaviour is associated with vasopressin release in the medial preoptic area and bed nucleus of the stria terminalis in the rat. J Neuroendocrinol. 2010;22:420–429. doi: 10.1111/j.1365-2826.2010.01984.x. [DOI] [PubMed] [Google Scholar]
- Bozon B, Kelly A, Josselyn SA, Silva AJ, Davis S, Laroche S. MAPK, CREB and zif268 are all required for the consolidation of recognition memory. Philos Trans R Soc Lond B Biol Sci. 2003;358:805–814. doi: 10.1098/rstb.2002.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA, Kendrick KM. Mammalian social odours: attraction and individual recognition. Philos Trans R Soc Lond B Biol Sci. 2006;361:2061–2078. doi: 10.1098/rstb.2006.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA, Zufall F. Pheromonal communication in vertebrates. Nature. 2006;444:308–315. doi: 10.1038/nature05404. [DOI] [PubMed] [Google Scholar]
- Brunjes PC, Illig KR, Meyer EA. A field guide to the anterior olfactory nucleus (cortex) Brain Res Rev. 2005;50:305–335. doi: 10.1016/j.brainresrev.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ, Hauger RL, Cooper TB, Ferris CF. Cerebrospinal fluid vasopressin levels: correlates with aggression and serotonin function in personality-disordered subjects. Arch Gen Psychiatry. 1998;55:708–714. doi: 10.1001/archpsyc.55.8.708. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Koob GF, Bluthe RM, Le Moal M. Septal vasopressin modulates social memory in male rats. Brain Res. 1988;457:143–147. doi: 10.1016/0006-8993(88)90066-2. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, al-Shamma HA. Sex differences in hormonal responses of vasopressin pathways in the rat brain. J Neurobiol. 1990;21:686–693. doi: 10.1002/neu.480210503. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Muraoka S, Engelmann M, Landgraf R. The effects of infusion of arginine vasopressin, oxytocin, or their antagonists into the olfactory bulb upon social recognition responses in male rats. Peptides. 1998a;19:999–1005. doi: 10.1016/s0196-9781(98)00047-3. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Muraoka S, Landgraf R. Olfactory bulb norepinephrine depletion abolishes vasopressin and oxytocin preservation of social recognition responses in rats. Neurosci Lett. 1998b;254:161–164. doi: 10.1016/s0304-3940(98)00691-0. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front Neuroendocrinol. 2004;25:132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Landgraf R. Social discrimination procedure: an alternative method to investigate juvenile recognition abilities in rats. Physiol Behav. 1995;58:315–321. doi: 10.1016/0031-9384(95)00053-l. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Potegal M. Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters. Physiol Behav. 1988;44:235–239. doi: 10.1016/0031-9384(88)90144-8. [DOI] [PubMed] [Google Scholar]
- Gelez H, Fabre-Nys C. Neural pathways involved in the endocrine response of anestrous ewes to the male or its odor. Neuroscience. 2006;140:791–800. doi: 10.1016/j.neuroscience.2006.02.066. [DOI] [PubMed] [Google Scholar]
- Guillery RW. On counting and counting errors. J Comp Neurol. 2002;447:1–7. doi: 10.1002/cne.10221. [DOI] [PubMed] [Google Scholar]
- Haberly LB. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Senses. 2001;26:551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- Haberly LB, Price JL. Association and commissural fiber systems of the olfactory cortex of the rat. I. Systems originating in the piriform cortex and adjacent areas. J Comp Neurol. 1978a;178:711–740. doi: 10.1002/cne.901780408. [DOI] [PubMed] [Google Scholar]
- Haberly LB, Price JL. Association and commissural fiber systems of the olfactory cortex of the rat. II. Systems originating in the olfactory peduncle. J Comp Neurol. 1978b;181:781–807. doi: 10.1002/cne.901810407. [DOI] [PubMed] [Google Scholar]
- Hernando F, Schoots O, Lolait SJ, Burbach JP. Immunohistochemical localization of the vasopressin V1b receptor in the rat brain and pituitary gland: anatomical support for its involvement in the central effects of vasopressin. Endocrinology. 2001;142:1659–1668. doi: 10.1210/endo.142.4.8067. [DOI] [PubMed] [Google Scholar]
- Illig KR, Eudy JD. Contralateral projections of the rat anterior olfactory nucleus. J Comp Neurol. 2009;512:115–123. doi: 10.1002/cne.21900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M, Koyama S. Social cognitive impairment in Parkinson's disease. J Neurol. 2007;254(Suppl 4):IV/49–IV/53. [Google Scholar]
- Keller M, Baum MJ, Brock O, Brennan PA, Bakker J. The main and the accessory olfactory systems interact in the control of mate recognition and sexual behavior. Behav Brain Res. 2009;200:268–276. doi: 10.1016/j.bbr.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Young LJ, Gonen D, Veenstra-VanderWeele J, Courchesne R, Courchesne E, Lord C, Leventhal BL, Cook EH, Jr, Insel TR. Transmission disequilibrium testing of arginine vasopressin receptor 1A (AVPR1A) polymorphisms in autism. Mol Psychiatry. 2002;7:503–507. doi: 10.1038/sj.mp.4001125. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y, Takeuchi Y, Nishihara M, Mori Y. Main olfactory system mediates social buffering of conditioned fear responses in male rats. Eur J Neurosci. 2009;29:777–785. doi: 10.1111/j.1460-9568.2009.06618.x. [DOI] [PubMed] [Google Scholar]
- Knafo A, Israel S, Darvasi A, Bachner-Melman R, Uzefovsky F, Cohen L, Feldman E, Lerer E, Laiba E, Raz Y, Nemanov L, Gritsenko I, Dina C, Agam G, Dean B, Bornstein G, Ebstein RP. Individual differences in allocation of funds in the dictator game associated with length of the arginine vasopressin 1a receptor RS3 promoter region and correlation between RS3 length and hippocampal mRNA. Genes Brain Behav. 2008;7:266–275. doi: 10.1111/j.1601-183X.2007.00341.x. [DOI] [PubMed] [Google Scholar]
- Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog Neurobiol. 2004;74:183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- König JFR, Klippel RA. The Rat Brain: A Stereotaxic Atlas of the Forebrain and Lower Parts of the Brain Stem. Baltimore, MD: The Williams & Wilkins Company; 1963. [Google Scholar]
- Lei H, Mooney R, Katz LC. Synaptic integration of olfactory information in mouse anterior olfactory nucleus. J Neurosci. 2006;26:12023–12032. doi: 10.1523/JNEUROSCI.2598-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Ludwig M. Neurotransmitters and peptides: whispered secrets and public announcements. J Physiol. 2008;586:5625–5632. doi: 10.1113/jphysiol.2008.159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner A, Bagic A. Olfactory pathogenesis of idiopathic Parkinson disease revisited. Mov Disord. 2008;23:1076–1084. doi: 10.1002/mds.22066. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Merkle FT, Alvarez-Buylla A. Origin and function of olfactory bulb interneuron diversity. Trends Neurosci. 2008;31:392–400. doi: 10.1016/j.tins.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Sabatier N, Bull PM, Landgraf R, Dayanithi G, Leng G. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature. 2002;418:85–89. doi: 10.1038/nature00822. [DOI] [PubMed] [Google Scholar]
- Meddle SL, Bishop VR, Gkoumassi E, van Leeuwen FW, Douglas AJ. Dynamic changes in oxytocin receptor expression and activation at parturition in the rat brain. Endocrinology. 2007;148:5095–5104. doi: 10.1210/en.2007-0615. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kolachana B, Gold B, Olsh A, Nicodemus KK, Mattay V, Dean M, Weinberger DR. Genetic variants in AVPR1A linked to autism predict amygdala activation and personality traits in healthy humans. Mol Psychiatry. 2009;14:968–975. doi: 10.1038/mp.2008.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohedano-Moriano A, Martinez-Marcos A, Munoz M, Arroyo-Jimenez MM, Marcos P, Artacho-Perula E, Blaizot X, Insausti R. Reciprocal connections between olfactory structures and the cortex of the rostral superior temporal sulcus in the Macaca fascicularis monkey. Eur J Neurosci. 2005;22:2503–2518. doi: 10.1111/j.1460-9568.2005.04443.x. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Lee TM. Central vasopressin administration regulates the onset of facultative paternal behavior in Microtus pennsylvanicus (meadow voles) Horm Behav. 2001;39:285–294. doi: 10.1006/hbeh.2001.1655. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Richter K, Wolf G, Engelmann M. Social recognition memory requires two stages of protein synthesis in mice. Learn Mem. 2005;12:407–413. doi: 10.1101/lm.97505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Andrade G, Kendrick KM. The main olfactory system and social learning in mammals. Behav Brain Res. 2009;200:323–335. doi: 10.1016/j.bbr.2008.12.021. [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Dupre A, Tribollet E. Distribution of vasopressin and oxytocin binding sites in the brain and upper spinal cord of the common marmoset. Neurosci Lett. 2009;461:217–222. doi: 10.1016/j.neulet.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Shu SY, Ju G, Fan LZ. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett. 1988;85:169–171. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- Spehr M, Kelliher KR, Li XH, Boehm T, Leinders-Zufall T, Zufall F. Essential role of the main olfactory system in social recognition of major histocompatibility complex peptide ligands. J Neurosci. 2006;26:1961–1970. doi: 10.1523/JNEUROSCI.4939-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples LG, Hunt GE, Cornish JL, McGregor IS. Neural activation during cat odor-induced conditioned fear and ‘trial 2’ fear in rats. Neurosci Biobehav Rev. 2005;29:1265–1277. doi: 10.1016/j.neubiorev.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Staples LG, Hunt GE, van Nieuwenhuijzen PS, McGregor IS. Rats discriminate individual cats by their odor: possible involvement of the accessory olfactory system. Neurosci Biobehav Rev. 2008a;32:1209–1217. doi: 10.1016/j.neubiorev.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Staples LG, McGregor IS, Apfelbach R, Hunt GE. Cat odor, but not trimethylthiazoline (fox odor), activates accessory olfactory and defense-related brain regions in rats. Neuroscience. 2008b;151:937–947. doi: 10.1016/j.neuroscience.2007.11.039. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Hoshino T, Shigemasu K, Kawamura M. Disgust-specific impairment of facial expression recognition in Parkinson's disease. Brain. 2006;129:707–717. doi: 10.1093/brain/awl011. [DOI] [PubMed] [Google Scholar]
- Szot P, Bale TL, Dorsa DM. Distribution of messenger RNA for the vasopressin V1a receptor in the CNS of male and female rats. Mol Brain Res. 1994;24:1–10. doi: 10.1016/0169-328x(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Thompson R, Gupta S, Miller K, Mills S, Orr S. The effects of vasopressin on human facial responses related to social communication. Psychoneuroendocrinology. 2004;29:35–48. doi: 10.1016/s0306-4530(02)00133-6. [DOI] [PubMed] [Google Scholar]
- Thompson RR, George K, Walton JC, Orr SP, Benson J. Sex-specific influences of vasopressin on human social communication. Proc Natl Acad Sci U S A. 2006;103:7889–7894. doi: 10.1073/pnas.0600406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin VA, Hashimoto H, Wacker DW, Takayanagi Y, Langnaese K, Caquineau C, Noack J, Landgraf R, Onaka T, Leng G, Meddle SL, Engelmann M, Ludwig M. An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature. 2010;464:413–417. doi: 10.1038/nature08826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Takahashi YK, Tanifuji M, Mori K. Odor maps in the mammalian olfactory bulb: domain organization and odorant structural features. Nat Neurosci. 2000;3:1035–1043. doi: 10.1038/79857. [DOI] [PubMed] [Google Scholar]
- Ueta Y, Fujihara H, Serino R, Dayanithi G, Ozawa H, Matsuda K, Kawata M, Yamada J, Ueno S, Fukuda A, Murphy D. Transgenic expression of enhanced green fluorescent protein enables direct visualization for physiological studies of vasopressin neurons and isolated nerve terminals of the rat. Endocrinology. 2005;146:406–413. doi: 10.1210/en.2004-0830. [DOI] [PubMed] [Google Scholar]
- Vallet P, Bouras C, Barberis C, Dreifuss JJ, Dubois-Dauphin M. Vasopressin binding in the cerebral cortex of the Mongolian gerbil is reduced by transient cerebral ischemia. J Comp Neurol. 1995;362:223–232. doi: 10.1002/cne.903620206. [DOI] [PubMed] [Google Scholar]
- Walum H, Westberg L, Henningsson S, Neiderhiser JM, Reiss D, Igl W, Ganiban JM, Spotts EL, Pedersen NL, Eriksson E, Lichtenstein P. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proc Natl Acad Sci U S A. 2008;105:14153–14156. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassink TH, Piven J, Vieland VJ, Pietila J, Goedken RJ, Folstein SE, Sheffield VC. Examination of AVPR1a as an autism susceptibility gene. Mol Psychiatry. 2004;9:968–972. doi: 10.1038/sj.mp.4001503. [DOI] [PubMed] [Google Scholar]
- Wesson DW, Wilson DA. Smelling sounds: Olfacoty-auditory sensory convergence in the olfactory tubercle. J Neurosci. 2010;20:3013–3021. doi: 10.1523/JNEUROSCI.6003-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, Cooper-Kuhn CM, Aigner R, Winkler J, Kuhn HG. Long-term survival and cell death of newly generated neurons in the adult rat olfactory bulb. Eur J Neurosci. 2002;16:1681–1689. doi: 10.1046/j.1460-9568.2002.02238.x. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Yirmiya N, Rosenberg C, Levi S, Salomon S, Shulman C, Nemanov L, Dina C, Ebstein RP. Association between the arginine vasopressin 1a receptor (AVPR1a) gene and autism in a family-based study: mediation by socialization skills. Mol Psychiatry. 2006;11:488–494. doi: 10.1038/sj.mp.4001812. [DOI] [PubMed] [Google Scholar]


