Abstract
The aim of this study was to find out the optimum combination of electroporation (EP) and reverse iontophoresis (RI) on noninvasive and transdermal determination of blood uric acid level in humans. EP is the use of high-voltage electric pulse to create nano-channels on the stratum corneum, temporarily and reversibly. RI is the use of small current to facilitate both charged and uncharged molecule transportation across the skin. It is believed that the combination of these two techniques has additional benefits on the molecules’ extraction across the human skin. In vitro studies using porcine skin and diffusion cell have indicated that the optimum mode for transdermal uric acid extraction is the combination of RI with symmetrical biphasic direct current (current density = 0.3 mA/cm2; phase duration = 180 s) and EP with 10 pulses per second (voltage = 100 V/cm2; pulse width = 1 ms). This optimum mode was applied to six human subjects. Uric acid was successfully extracted through the subjects’ skin into the collection solution. A good correlation (r2 = 0.88) between the subject’s blood uric acid level and uric acid concentrations in collection solutions was observed. The results suggest that it may be possible to noninvasively and transdermally determine blood uric acid levels.
Keywords: Reverse iontophoresis, electroporation, uric acid, monitoring, noninvasive, transdermal
Introduction
Gout is one of the most common forms of arthritis,1 affecting more than 700,000 adults in the UK2 and nearly three million adults in the USA,3 and accounting for almost four million outpatient visits every year,4 with a substantial economic burden.5 Excessive amounts of uric acid, known as hyperuricemia, is one of most frequent metabolism disturbances. Hyperuricemia and high mean serum uric acid concentrations as risk factors for gout have been well documented in the past decades.6 Gouty arthritis, a rapid onset of joint inflammation, is a complication of hyperuricemia and is precipitated by deposits of uric acid crystals in the synovial fluid and synovial lining. Recent research has highlighted that patients with hyperuricemia and gout are at increased risk of morbidity and mortality related to cardiovascular disease.7 Therefore, blood uric acid monitoring is very important.
The routine method to determine blood uric acid level is achieved by blood sampling. However, this method is invasive, painful, and inconvenient. To the best of our knowledge, there is no noninvasive method to determine the blood uric acid level. Therefore, reverse iontophoresis (RI) and electroporation (EP), noninvasive techniques, were used in this study to transdermally and noninvasively extract uric acid in humans.
RI refers to the passage of a low level of current through the skin to promote the transport of both charged and neutral molecules.8 The main mechanisms that contribute to RI are the electro migration of charged species to the electrode of opposite polarity, electroosmosis of neutral molecules to the cathode or anode, or a combination of both. RI across the skin has been investigated as a noninvasive method for clinical monitoring.9 Its first clinical application was the transdermal extraction of glucose.10 EP is the use of an intense electric pulse to make the cell membrane transiently porous and then permeable to exogenous molecules present in the surrounding media.11,12 EP is widely used for the loading of cells with drugs13,14 and the delivery of drugs into the cells of living tissues.15,16 Recently, EP has been used for transcutaneous sampling of molecules, like glucose.17 Several papers have reported the use of combined RI and EP to enhance the effect of extracting metabolites,18,19 but no papers have been found for the use of combined RI and EP for the extraction of uric acid and this was the novelty of this study. The aim of this study was to find out the optimum combination of EP and RI on noninvasive and transdermal determination of blood uric acid level in humans.
Materials and methods
Reagents and solutions
All reagents used in this study were commercially available and used without further purification: phosphate buffered saline and uric acid were purchased from Sigma Chemical Company (St. Louis, MO) and uric acid assay kit (Catalog number: K608–100) from BioVision (Mountain View, CA). De-ionized water, purified by a Millipore Milli-Q UFplus System (Bedford, MA) was used to prepare all solutions.
Equipment
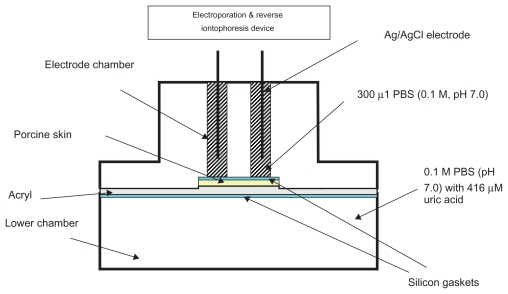
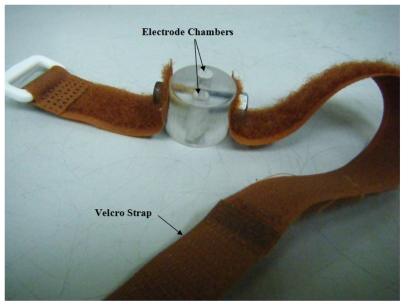
The diffusion cell for in vitro studies was the same as previously described (Figure 1).20 The diffusion cell for human studies was newly-designed in this study, with a 5 mm diameter for each electrode chamber and the two electrode chambers were 11 mm apart (Figure 2). RI and EP devices were also newly-designed in this study. The RI device has the accuracy of ± 0.01 μA on delivering current and ±0.01 ms on timing. The EP device has the accuracy of ±0.1 V on delivering voltage and ± 0.01 ms on timing. A Bio-Rad 680 microplate reader (Bio-Rad, Hemel Hempstead, UK) was used for all colorimetric analysis.
Figure 1.
Diffusion cell for in vitro studies.
Figure 2.
Diffusion cell (diameter = 27 mm) for human studies. Each electrode chamber had a diameter of 5 mm and they were 11 mm apart.
Preparation of silver–silver chloride electrodes
Silver–silver chloride (Ag/AgCl) electrodes were used in both in vitro and human studies. The Ag/AgCl electrodes were prepared by chloridizing 99.99% pure silver wire, 1 mm in diameter and 25 mm in length (Aldrich Chemical Company Inc, Milwaukee, WI), immersed in 0.1 M HCl solution (Pt-cathode) for 90 minutes at an applied current of 314 μA.
In vitro studies
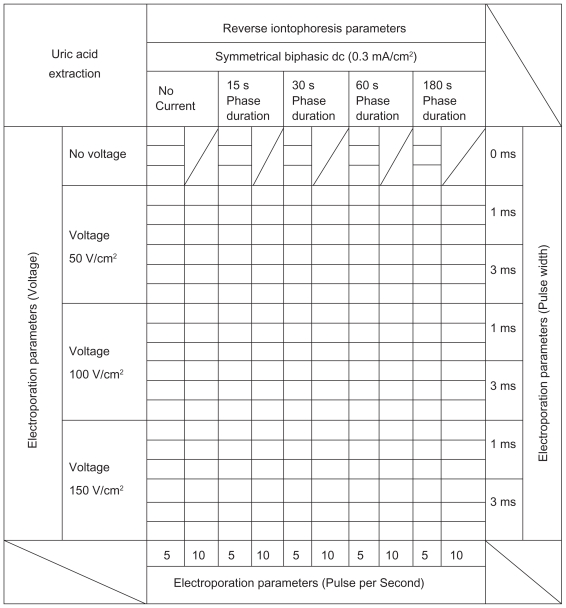
All experiments were conducted using diffusion cells, in which both electrode chambers were located on the same surface side of a porcine skin (250 μm) obtained by dermatome. Both the electrode chambers and the lower chamber of the diffusion cell were filled with 0.1 M phosphate buffer (pH 7.0) while the lower chamber additionally contained 416 μM uric acid. Each electrode chamber contained a Ag/AgCl electrode. The surface area of the porcine skin exposed to the electrode in each chamber was 0.2 cm2 and the electrode chambers were 11 mm apart. Different modes of EP and RI (Table 1 and Figure 3) were applied via the Ag/AgCl electrodes. The entire content of the electrode chambers were removed at the end of the experiment to determine the amount of uric acid extracted.
Table 1.
Experimental protocol for the in vitro studies
Notes: One hundred ninety five different combinations of reverse iontophoresis and electroporation parameters were selected for the in vitro transdermal uric acid extraction, in order to determine the optimum combination for uric acid extraction.
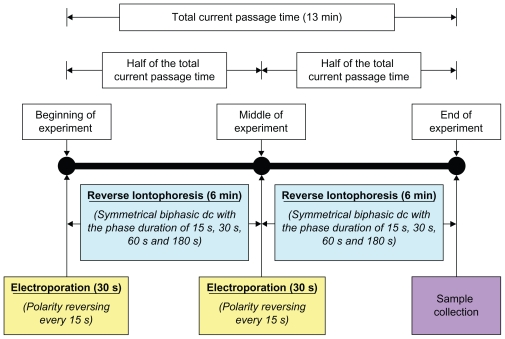
Figure 3.
Experimental protocol showing the time sequence for the application of electroporation, reverse iontophoresis, and sample collection.
Human studies
Six subjects (four men and two women; mean age 45 ± 13 years) were recruited in this study. The study was approved by The Institutional Review Board of National Chi Nan University. Informed consent was obtained from each subject before the experiment.
The areas of skin of the subject’s outer forearm where diffusion cell was to be located were prepared by briskly rubbing the areas for 6–8 s with alcohol preparation pads to remove dry skin, oils, and other contaminants. The areas were then allowed to dry thoroughly. Once the diffusion cell and Ag/AgCl electrodes were fixed in position onto the human forearm, 300 μl phosphate buffer (0.1 M; pH 7.0) was added into each electrode chamber of the diffusion cell. Then, the optimum combination of RI (symmetrical biphasic direct current (dc) with the current density of 0.3 mA/cm2 and the phase duration of 180 s) and EP (100 V/cm2 electric pulse with the pulse width of 1 ms and 10 pulses per second) was applied via the Ag/AgCl electrodes to the human skin. At the end of the experiment, phosphate buffer was pipetted from the electrode chambers and stored in microcentrifuge tubes at 4°C for later quantification of uric acid. The subject’s blood uric acid level was also measured before experiments at the central laboratory of the Puli Christian Hospital.
Determination of the extracted uric acid
Colorimetric assay was used to determine the amount of uric acid extracted through the porcine or human skin during the in vitro and human studies. An excellent linear relationship (r2 > 0.9) between uric acid concentrations and its relative absorbance was found, allowing the uric acid concentration to be calculated simply by linear regression.
Statistical analysis
One-way ANOVA was used to determine whether there were significant differences between EP parameters within a RI parameter, and between RI parameters within an EP parameter for the uric acid extraction. Post hoc comparisons were made with independent samples t-tests. A linear regression test was used to determine the relationship between the subject’s blood uric acid level and uric acid concentrations in collection solutions. All statistical analyses were carried out using SPSS software (SPSS Inc., Chicago, IL), with the level of statistical significance set at P < 0.05.
Results and discussion
In vitro studies
In this study, 195 combinations of RI and EP were chosen for the transdermal uric acid extraction in order to find out the optimum combination of EP and RI on noninvasive and transdermal determination of blood uric acid level in humans.
Uric acid is a negatively-charged molecule and its molecular weight is about 168 Da. Because of its small size and negative charge, uric acid was expected to be transdermally extractable. Electromigration was expected to be the dominant physical factor for the uric acid extraction.
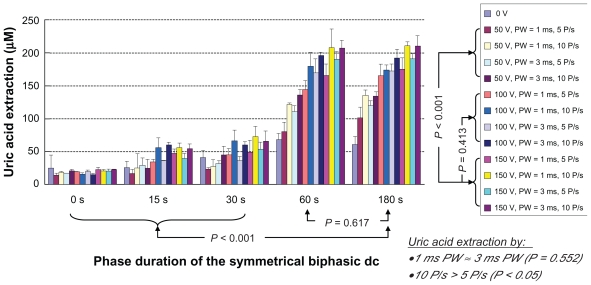
As shown in Figure 4, the application of RI, EP, or the combination of both could facilitate a higher uric acid extraction as compared to diffusion alone. It was found that the uric acid extraction increased as the phase duration of the symmetrical biphasic dc (PDSBdc) increased. At 50 V/cm2, 100 V/cm2, and 150 V/cm2 EP, uric acid extraction using the 180 s PDSBdc was found to be significantly higher (P < 0.001 in all cases) than that of protocols utilizing 0 s, 15 s, and 30 s PDSBdc conditions. Although an independent samples t-test revealed no significant difference (P = 0.617) between uric acid extraction using the 60 s and 180 s PDSBdc, it was observed that the longer the PDSBdc, the better the uric acid extraction was. Therefore, 180 s PDSBdc was recommended as the optimum setting. On the other hand, the combination of RI and EP could facilitate a higher uric acid extraction as compared to RI or EP alone. This was because EP could create reversible nanochannels on the skin10,11 and this could further facilitate the uric acid extraction as compared with RI alone. It was also found that the higher the voltage of the EP, the better the uric acid extraction was. This meant that 150 V/cm2 EP was better than 50 V/cm2 EP (P < 0.001 in all cases of 15 s, 30 s, 60 s, and 180 s PDSBdc), as 150 V/cm2 EP had relatively more energy to effectively create more nanochannels. On the other hand, there was no significant difference (P = 0.413) between the 100 V/cm2 EP and 150 V/cm2 EP on the uric acid extraction. 100 V/cm2 EP was therefore recommended as the optimum setting. Also, no significant difference (P = 0.552) was found between 1 ms and 3 ms pulse width of the EP on the uric acid extraction. As such, a 1 ms pulse width of the EP was recommended as the optimum setting because 1 ms pulse width was commonly used in clinic. Moreover, EP with 10 pulses per second facilitated significantly more uric acid extraction (P < 0.05) as compared with EP with 5 pulses per second. Therefore, EP with 10 pulses per second was recommended as the optimum setting. In a nutshell, the optimum combination of RI and EP on uric acid extraction was as follows:
Figure 4.
In vitro studies of the transdermal and noninvasive extraction of uric acid by different combinations of reverse iontophoresis (RI) and electroporation (EP). For the RI setting, it was a symmetrical biphasic dc with the current density of 0.3 mA/cm2. The legend shows the electroporation setting where PW and P/s are the pulse width and pulse per second of the electroporation, respectively.
RI: symmetrical biphasic dc with a current density of 0.3 mA/cm2 and a phase duration of 180 s
EP: 100 V/cm2 electric pulse with a pulse width of 1 ms and 10 pulses per second.
Human studies
No erythema was observed on any subject’s skin after the application of RI and EP. In addition, no subject reported pain or even unpleasant feeling during or after the experiment. Therefore, this human study demonstrated that RI and EP could provide a safe, painless, and non invasive extraction of uric acid in humans.
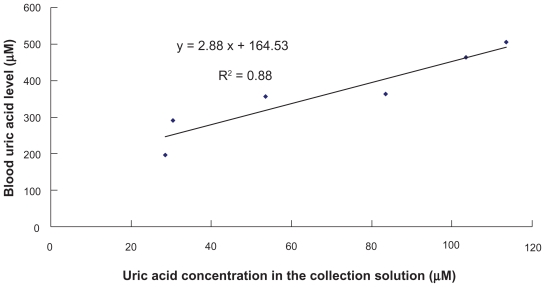
In this human study, the combination of RI and EP significantly promoted transdermal uric acid extraction, but there are no other human studies for data comparison. As shown in Figure 5, a good correlation (r2 = 0.88) between the subject’s blood uric acid level and uric acid concentrations in collection solutions was observed. This meant that the subject’s blood uric acid level could be predicted by the uric acid concentrations in collection solutions using the linear regression equation. Therefore, a transdermal and noninvasive method for blood uric acid monitoring was established.
Figure 5.
Comparison of real blood uric acid levels of subjects and uric acid concentrations in the collection solution after the application of the optimum combination of reverse iontophoresis and electroporation.
Despite promising early results, the correlation between the subject’s blood uric acid level and uric acid concentration in the collection solution was still not too high. Hence, it is necessary to test this method using larger subject groups in order to achieve a better correlation between the subject’s blood uric acid level and uric acid concentration in the collection solution. On the other hand, subjects with a large difference of blood uric acid level are expected to improve the correlation as well.
Conclusion
Uric acid could be transdermally and noninvasively extracted by the combination of RI and EP. The optimum mode was the combination of the RI with symmetrical biphasic dc (current density = 0.3 mA/cm2; phase duration = 180 s) and EP with 10 pulses per second (voltage = 100V/cm2; pulse width = 1 ms). A good correlation between the subject’s blood uric acid level and uric acid concentrations in collection solutions was observed. Therefore, a transdermal and noninvasive method for blood uric acid monitoring was established.
Acknowledgments
The authors would like to thank the six subjects who participated in this study. This work was supported by grants (98A032) from the National Chi Nan University and Puli Christian Hospital, Taichung, Taiwan, Republic of China. This work was also partially supported by grants (NSC 99–2221–E–260–004– and NSC 98–2221–E–260–024–MY3) from the National Science Council, Taiwan, Republic of China.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Choi HK, Mount DB, Reginato AM. Pathogenesis of gout. Ann Intern Med. 2005;143:499–516. doi: 10.7326/0003-4819-143-7-200510040-00009. [DOI] [PubMed] [Google Scholar]
- 2.Mikuls TR, Farrar JT, Bilker WB, Fernandes S, Schumacher HR, Saag KG. Gout epidemiology: results from the UK General Practice Research Database, 1990–1999. Ann Rheum Dis. 2005;64:267–272. doi: 10.1136/ard.2004.024091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawrence RC, Felson DT, Helmick CG. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krishnam E, Lienesch D, Kwoh CK. Gout in ambulatory care setting in the United States. J Rheumatol. 2008;35:498–501. [PubMed] [Google Scholar]
- 5.Wu EQ, Patel PA, Yu AP. Disease-related and all-cause health care costs of elderly patients with gout. J Manag Care Pharm. 2008;14:164–175. doi: 10.18553/jmcp.2008.14.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glynn RJ, Campion EW, Silbert EJ. Trends in serum uric acid levels 1961–1980. Arthritis Rheum. 1983;26:87–93. doi: 10.1002/art.1780260115. [DOI] [PubMed] [Google Scholar]
- 7.Edwards NL. The role of hyperuricemia and gout in kidney and cardiovascular disease. Cleve Clin J Med. 2008;75(Suppl 5):S13–S16. doi: 10.3949/ccjm.75.suppl_5.s13. [DOI] [PubMed] [Google Scholar]
- 8.Merino V, Kalia YN, Guy RH. Transdermal therapy and diagnosis by iontophoresis. Trends Biotechnol. 1997;15:288–290. doi: 10.1016/S0167-7799(97)01069-X. [DOI] [PubMed] [Google Scholar]
- 9.Leboulanger B, Guy RH, Delgado-Charro MB. Reverse iontophoresis for non-invasive transdermal monitoring. Physiol Meas. 2004;25:R35–50. doi: 10.1088/0967-3334/25/3/r01. [DOI] [PubMed] [Google Scholar]
- 10.Guy RH. A sweeter life for diabetics. Nat Med. 1995;1:1132–1133. doi: 10.1038/nm1195-1132. [DOI] [PubMed] [Google Scholar]
- 11.Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse lyoma cells by electroporation in high electric field. EMBO J. 1982;7:841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang DC, Chassy BM, Saunders JA, Sowers AE, editors. Guide to Electroporation and Electrofusion. San Diego, CA: Academic Press Inc; 1992. [Google Scholar]
- 13.Zimmermann U, Vienken J, Pilwat G. Development of drug carrier systems: Electrical field induced effects in cell membranes. Bioelectrochem Bioenerg. 1980;7:553–574. [Google Scholar]
- 14.Sixou S, Teissié J. Specific electropermeabilization of leucocytes in a blood sample and application to large volumes of cells. Biochem Biophys Acta. 1990;1028:154–160. doi: 10.1016/0005-2736(90)90149-i. [DOI] [PubMed] [Google Scholar]
- 15.Okino M, Mohri H. Effects of a high-voltage electrical impulse and an anticancer drug on in vivo growing tumors. Jpn J Cancer Res. 1987;78:1319–1321. [PubMed] [Google Scholar]
- 16.Mir LM, Orlowski S, Belehradek J, Jr, Paoletti C. In vivo potentiation of the bleomycin cytotoxicity by local electric pulses. Eur J Cancer. 1991;27:68–72. doi: 10.1016/0277-5379(91)90064-k. [DOI] [PubMed] [Google Scholar]
- 17.Srinivasa Murthy S, Siva Ram, Kiran V, Mathur S, Narasimha Murthy S. Noninvasive transcutaneous sampling of glucose by electroporation. J Diabetes Sci Technol. 2008;2:250–254. doi: 10.1177/193229680800200213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jadoul A, Bouwstra J, Preat V. Effect of iontophoresis and electroporation on the stratum corneum. Adv Drug Deliv Rev. 1999;35:89–105. doi: 10.1016/s0169-409x(98)00065-9. [DOI] [PubMed] [Google Scholar]
- 19.Tokumoto S, Higo N, Sugibayashi K. Effect of electroporation and pH on the iontophoresis transdermal delivery of human insulin. Int J Pharm. 2006;326:13–19. doi: 10.1016/j.ijpharm.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Ching CTS, Connolly P. Reverse iontophoresis: A non-invasive technique for measuring blood lactate level. Sensor Actuat B-Chem. 2008;129:352–358. [Google Scholar]