Abstract
Glutamate receptors are major excitatory receptors in the brain. Recent findings have established auxiliary subunits of glutamate receptors as critical modulators of synaptic transmission, synaptic plasticity and neurological disorder. The elucidation of the molecular rules governing glutamate receptors and subunits will improve our understanding of synapses and of neural-circuit regulation in the brain.
1. Introduction
The brain is the primary coordinator of animal behavior. Neural circuits, which are composed of billions of neurons, are the functional units of the central nervous system (CNS). Neurons communicate with each other at synapses. Neurotransmitters released from the presynaptic terminal of one neuron act on neurotransmitter receptors at the postsynaptic membranes of another neuron to induce changes in membrane potential or to activate signaling cascades. This newly generated information at postsynaptic sites travels through dendrites and axons to presynaptic terminals and to adjacent neurons via synaptic transmission. This network of connections organizes neural circuits of the CNS. Therefore, the elucidation of the rules of synaptic transmission and of the changes in neuronal membrane potentials will allow us to generate blueprints of functional neural circuits to enhance our understanding of the brain.
Glutamate receptors
There are two types of synapse in the brain: excitatory and inhibitory synapses. Excitatory synapses, where neurotransmitters induce depolarization of postsynaptic membranes, utilize glutamate as a major neurotransmitter in the vertebrate brain. In contrast, inhibitory synapses utilize GABA and glycine as major inhibitory neurotransmitters in the vertebrate brain. At excitatory synapses, glutamate released from presynaptic terminals binds to glutamate receptors, which are classified as ionotropic or metabotropic glutamate receptors. Ionotropic glutamate receptors are further classified pharmacologically as AMPA-, NMDA-, and kainate-sensitive glutamate receptors. Postsynaptic membranes contain all three ionotropic glutamate receptors and each receptor plays distinct roles in the brain. NMDA- and kainate-type receptors play roles in synaptic plasticity or slower transmission (10–100 ms), whereas AMPA receptors (AMPARs) play dominant roles in fast synaptic transmission (faster than 10 ms) to induce membrane depolarization after glutamate binding. Therefore, fast synaptic transmission is determined by channel activity and the number of AMPARs at synapses. In this review, we will discuss recent progress in the research of the role of ionotropic glutamate receptors and their auxiliary subunits in the control of synaptic transmission.
2. AMPA-type glutamate receptors and the transmembrane AMPAR regulatory protein (TARP) auxiliary subunit
AMPARs play major roles in fast synaptic transmission. Four subunits of AMPARs (GluA1–4) assemble as a tetramer. AMPAR tetramers can function as glutamate-gated ion channels. However, native AMPAR complexes comprise transmembrane AMPAR regulatory proteins (TARPs) as AMPAR auxiliary subunits to modulate channel activity and the trafficking of AMPARs.
TARP genes and proteins
The prototypical TARP stargazin/γ-2 was identified as the causative gene in the spontaneous mutant mouse stargazer, which shows ataxia and absence epilepsy, and is considered to be a calcium channel γ subunit because of its 23% sequence homology with the γ-1 auxiliary subunit of the 1,4-dihydropyridine (DHP)-sensitive calcium channel from skeletal muscle (FIGURE 1A) (40). Eight γ-1 homologous proteins (γ-1–8) were identified from a genomic database (6, 7, 14, 35). Among the γ-1 homologous proteins, six proteins modulate AMPAR activity and are termed transmembrane AMPAR regulatory proteins (TARPs) (FIGURE 1A) (32, 33, 79). TARPs are tetramembrane-spanning proteins (FIGURE 1B) and each of the TARP isoforms is expressed distinctly in the brain (25, 35, 79). TARPs are classified into two classes according to their distinct functions in AMPAR modulation (discussed later). Class I TARPs include stargazin/γ-2, γ-3, γ-4, and γ-8, which all contain a typical PDZ domain-binding motif (−TTPV) at their C terminus. In contrast, class II TARPs include γ-5 and γ-7, which both contain an atypical PDZ-binding motif (−S/TTPC) at their C terminus (FIGURE 1B). TARPs are well conserved among species, including vertebrates and invertebrates. Mammalian and C. elegans TARPs (STG-1 and 2) share low homology; however, both are tetramembrane-spanning proteins and modulate AMPAR functions (91-93). In addition, TARPs share homology with claudin, which plays roles in the formation of tight junctions, presumably as an adhesion molecule (86) (FIGURE 1A). Therefore, TARPs may function as claudin-like cell adhesion molecules (64, 81). However, synapses lacking TARPs show normal synaptic morphology (8); thus, TARPs may require specific circumstances to function as adhesion molecules.
FIGURE 1. TARP structure and phylogenetic tree of TARP-related proteins.
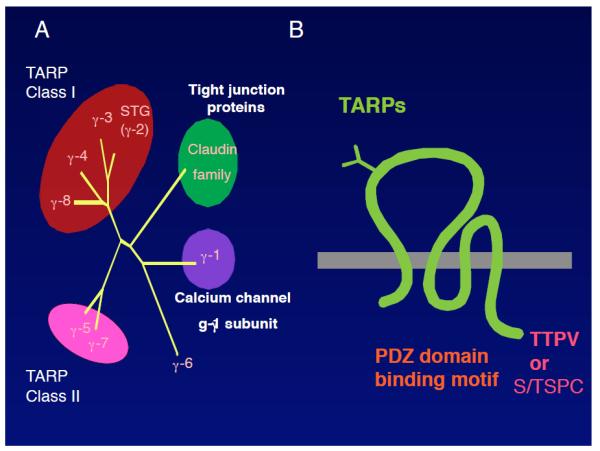
A. γ-1 is a calcium channel gamma subunit (CACNG-1). Subsequently, eight homologous genes were identified that were termed γ-1–8. Among the eight γ-1 homologous proteins, six proteins modulate AMPAR activity and were classified as class I and class II TARPs, functionally. Class I TARPs comprise stargazin/γ-2, γ-3, γ-4, and γ-8 and Class II TARPs include γ-5 and γ-7. The roles of γ-6 remain unclear. B. TARPs are tetramembrane-spanning proteins that contain typical (−TTPV) and atypical (−S/TTPC) binding motifs for the PDZ domain in their C terminus.
Interaction of TARPs with AMPARs
Immunopurification of the TARP complex from the brain identified all AMPAR subunits (GluA1–4) as major interactors (23, 80). Purification of the native AMPAR complex identified TARPs as major binding proteins (23, 57). Furthermore, Blue-Native PAGE analysis of the TARP and AMPAR complexes revealed that all stargazin/γ-2 interact with AMPARs and that most AMPARs interact with TARPs in the cerebellum (89). These results established TARPs as major components of the AMPAR complex in the brain.
Where do TARPs interact with AMPARs in neurons?
The total levels of AMPAR are decreased in the cerebellum of stargazin/γ-2 disrupted mice and in the hippocampus of γ-8 knockout mice, where each TARP isoform is expressed as a major TARP (24, 67, 79). Furthermore, the ratio of EndoH-sensitive immature to EndoH-resistant mature AMPAR is increased in both mouse models (67, 79). Interestingly, the expression of ER chaperones, BiP/GRp78, is increased in stargazin/γ-2 disrupted mice, as part of the AMPAR unfolded protein response. Therefore, TARPs are likely to interact with AMPARs at the ER (88).
Interaction domains between TARPs and AMPARs
Interaction domains remain unclear, probably because of the difficulty in handling two transmembrane proteins. Single-particle analysis, which revealed the structure of the native AMPA receptor complex with and without TARPs at a 40 Å resolution, suggests that the transmembrane domains could act as interaction domains (57, 58). The determination of the atomic structure of the complex is necessary to determine the precise mechanism of this interaction. As described later, TARPs modulate the pharmacology of AMPARs. The difference between the pharmacology of AMPARs alone (TARPless AMPARs) and AMPARs with TARP (TARPin AMPARs) suggests that TARPs could assume variable stoichiometry (0/2/4) on AMPARs in neurons (71).
3. AMPAR trafficking and synaptic localization
Excitatory synapses in the vertebrate brain show two characteristic features: an electron-dense area beneath postsynaptic sites, the so-called postsynaptic density (PSD), and the use of glutamate as a major excitatory neurotransmitter. The molecular link between the PSD and glutamate receptors has been studied extensively. In a series of studies, a PDZ domain-containing protein, PSD-95, was identified as a major component of the PSD (12). In addition, overexpression of PSD-95 in neurons increases AMPAR activity at synapses, as shown by the increase in the levels of AMPAR and in excitatory postsynaptic currents (EPSCs) (4, 20, 21, 59, 68). However, PSD-95 cannot interact directly with AMPARs, which suggests that an additional molecule links PSD-95 to AMPARs. TARP, which binds to both AMPARs and PSD-95, was identified as such a molecule.
TARP-mediated AMPAR trafficking
Cerebellar granule cells express stargazin/γ-2 as the sole TARP. The stargazer mouse does not exhibit AMPAR activity at cerebellar mossy fiber/granule cell synapses (8, 28). Interestingly, overexpression of full-length stargazin/γ-2 in primary cerebellar granule cell cultures from stargazer mice restores both synaptic and surface AMPAR activity, whereas overexpression of stargazin/γ-2 lacking the C-terminal PDZ domain-binding motif (four amino acids, −TTPV) restored surface, but not synaptic, AMPAR activity (8). This result indicates that stargazin/γ-2 modulates AMPAR activity via two distinct mechanisms, i.e., TARPs regulates the surface expression of AMPARs and the C-terminal PDZ-binding motif of TARPs (−TTPV) controls the synaptic localization of AMPARs (FIGURE 2B, 2A).
FIGURE 2. Functional domains of TARPs and AMPARs.
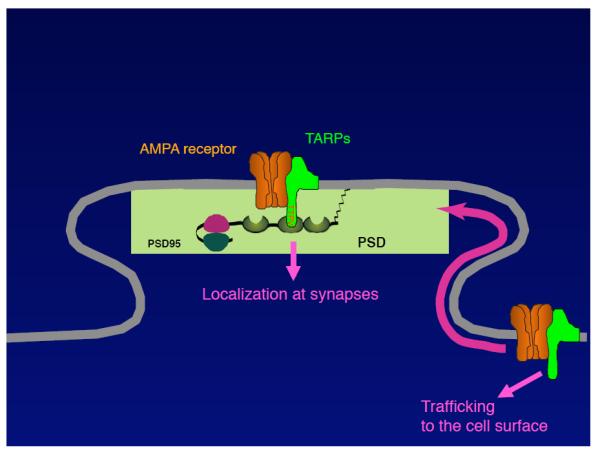
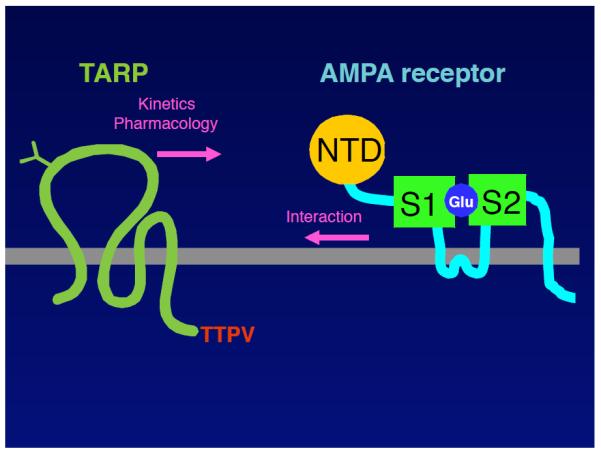
A. The cytoplasmic domain of TARPs is necessary and sufficient for the surface expression of AMPARs. The C-terminal PDZ-binding motif is necessary for the synaptic localization of the AMPAR/TARP complex. The interaction domains of TARPs with AMPARs remain unclear. B. Extensive structure/function studies revealed that the first extracellular loop of TARPs is necessary and sufficient for the modulation of the channel properties of AMPARs. Furthermore, mutations in the ligand-binding domain of AMPARs prohibit TARP interaction with AMPARs.
In contrast, the γ-8 knockout mouse exhibits a 90% reduction in surface AMPAR activity, but only a 30% reduction in AMPAR-mediated EPSCs in hippocampal pyramidal cells (67). The milder deficit in AMPAR-mediated synaptic transmission in γ-8 knockout mice compared with stargazer mice could be due to redundancy by other TARPs in the hippocampus, because all class I TARPs are expressed in hippocampal pyramidal cells (25, 79). In support of this, γ-8 and stargazin/γ-2 double-knockout mice exhibit a more severe reduction (50%) in synaptic transmission (67). Other possibilities to explain the difference in the extent of reduction in synaptic transmission between stargazer and γ-8 knockout mice could be the differences in the expression of AMPAR in distinct brain regions or in the expression of TARP subunits. For instance, TARP-dependent AMPAR trafficking is dominant in the cerebellum, but not in the hippocampus, and TARPless AMPARs may be localized at synapses in the hippocampus. Because mice carrying disruption of three class I TARPs (stargazin/γ-2, γ-3, and γ-8) exhibit lethality at postnatal day 0 (49), it is difficult to study adult mice carrying disruption of all six TARP isoforms. Conditional targeting disruption is required to examine this possibility. Alternatively, stargazin/γ-2 and γ-8 are preferentially targeted to synapses and extrasynapses, respectively. In support of this assumption, biochemical fractionation showed that stargazin/γ-2 is more abundant in the synaptic fraction, whereas γ-8 is more abundant in the extrasynaptic fraction (30).
TARP interactors
TARPs interact with PSD-95-like MAGUKs (8, 18). Compensatory mutations in both the PDZ domain 1 of PSD-95 and in the C-terminal PDZ domain-binding motif of stargazin/γ-2 increase AMPAR-mediated EPSCs, whereas mutation in only one of these proteins does not (69). Furthermore, TARP interaction with PSD-95 slows AMPAR diffusion at the cell surface (1). These results suggest that TARPs interact directly with PSD-95 to control synaptic AMPARs. Other TARP interactors have also been reported. PDZ domain-containing proteins (OMP25, MUPP1, PIST, and MAGI2) and non-PDZ-containing proteins (light chain 2 of the microtubule associate protein (LC2)) were identified, in addition to PSD-95-like MAGUKs (17-19, 31). It would be important to examine the distinct roles of each of these interactors in the regulation of the TARP/AMPAR complex.
4. TARPs modulate the channel properties and pharmacology of AMPARs
Synaptic strength is determined by the number and channel properties of AMPARs at synapses. TARPs modulate not only the trafficking, but also the channel properties of AMPARs. Xenopus laevis oocytes are widely used as a system to evaluate receptor activity. Glutamate-evoked currents and AMPAR surface expression in oocytes coinjected with GluA1 and Stargazin/γ-2 cRNAs are significantly larger than those evoked by GluA1 alone (9, 80, 94). Furthermore, TARPs increase glutamate-evoked currents about four times more than they increase the surface expression of AMPARs, which suggests that TARPs increase both the trafficking and the individual channel activity of AMPARs (77).
TARPs slow the decay kinetics of AMPARs
AMPARs open their channel pore after glutamate binding, which is followed by closing of the channel pore after glutamate removal (deactivation) or with glutamate binding (desensitization). During synaptic transmission, the decay of AMPAR-mediated EPSCs is determined by deactivation and desensitization. TARPs slow both the deactivation and the desensitization processes in heterologous cells (65, 77, 87). Furthermore, γ-4 and γ-8 slow the decay kinetics of AMPARs to a greater extent than do γ-2 and γ-3 in heterologous cells and at synapses (11, 37, 54, 76). Single-channel analysis revealed that TARPs increase AMPAR open channel probability by increasing burst length without changing open-dwell time, with no effect on conductance (77). This result indicates that TARPs accelerate the gating of AMPARs (77). Importantly, the decay of AMPAR-mediated EPSCs is controlled by TARPs, as overexpression of a dominant-negative form of TARP in neurons accelerated the decay of AMPAR-mediated EPSCs (77). In addition, TARPs render AMPARs more inwardly rectifying channels (11, 72, 73). Mutations in the channel pore of AMPARs changed the magnitude of TARP modulation of AMPAR activity (36). These observations suggest that TARPs may change the molecular environment surrounding the AMPAR-channel pore.
TARPs modulate the pharmacology of AMPARs
Native AMPARs respond to kainate more than to glutamate, whereas AMPARs expressed in heterologous cells respond to glutamate more than to kainate. Interestingly, AMPARs coexpressed with TARP respond to kainate more than to glutamate, which suggests that native AMPARs contain TARPs (11, 39, 54, 71, 76-78, 87). TARPs also modulate the efficacy of AMPAR potentiators, e.g., cyclothiazide or PEPA, which slow the desensitization and deactivation of AMPARs (82, 95). Importantly, cyclothiazide and TARPs shows additive effects on AMPAR activity, indicating that cyclothiazide and TARPs modulate AMPAR activity via distinct mechanisms, which is consistent with the fact that TARPs accelerate gating and cyclothiazide slows entry into desensitization. TARPs also modulate the sensitivity to AMPAR antagonists. Surprisingly, TARPs convert the AMPAR competitive antagonists CNQX and DNQX into partial agonists (38, 52). Furthermore, TARPs change the potency of the non-competitive AMPAR inhibitor GYKI53655 (15). These results suggest that inclusion of TARP in AMPARs is necessary for future drug screening.
Structural and functional analyses of TARPs and AMPARs
Extensive structure/function studies revealed that the first extracellular loop of TARPs is necessary and sufficient for the modulation of the channel properties of AMPARs (FIGURE 2A) (77, 87). Furthermore, mutations in the ligand-binding domain of AMPAR prohibit TARP interaction with AMPARs (83), which indicates that the first extracellular loop of TARPs may interact directly with the ligand-binding domain of AMPAR to modulate its channel properties. In addition to the extracellular domain, the cytoplasmic domain of TARPs was recently suggested to be involved in the modulation of channel properties (2, 53); however, its mechanism remains unclear. In contrast, chimeric and deletion studies showed that the cytoplasmic domain of TARPs is necessary and sufficient for the surface expression of AMPARs (3, 77). The C-terminal PDZ-binding motif is necessary for the synaptic localization of the AMPAR/TARP complex, as described above (1, 8, 69). Interestingly, AMPAR and TARPs accumulate in the axons of mice carrying a disruption of the ß subunit of AP-4, which is an adaptor protein for protein sorting, via the interaction between AP-4 and the cytoplasmic domain of TARPs (48).
5. Regulation of AMPAR activity by TARPs
One of the intrinsic features of AMPAR is that neuronal activity modulates synaptic AMPAR activity. Two mechanisms have been proposed for the TARP-mediated dynamic regulation of AMPAR activity.
TARP phosphorylation regulates AMPAR activity at synapses
Neuronal activity increases calcium influx through NMDA-type glutamate receptor (NMDAR) to activate calcium-dependent kinases, which is followed by the increase in AMPARs at synapses (FIGURE 3) (16, 43, 45-47). However, the substrate of calcium-dependent kinases is unknown. TARPs are highly phosphorylated at the PSD (84). TARP phosphorylation is bidirectionally regulated by PKC and CaMKII under NMDAR activity in neurons (30, 84). Furthermore, TARP carrying replacement mutations of its phosphorylated serine residues to aspartic acid (constitutive phospho-mimic) were generated and overexpression of phospho-mimic stargazin/γ-2 increases AMPAR-mediated EPSCs specifically in neurons (34, 77). These results indicate that TARP phosphorylation is a regulator of synaptic AMPAR activity and may be a substrate for NMDAR-mediated synaptic plasticity (FIGURE 3). In support of this, neuronal activity phosphorylated TARPs and PSD-95 better than other proteins in glutamate receptor complex (85).
FIGURE 3. A model for the regulation of AMPAR localization.
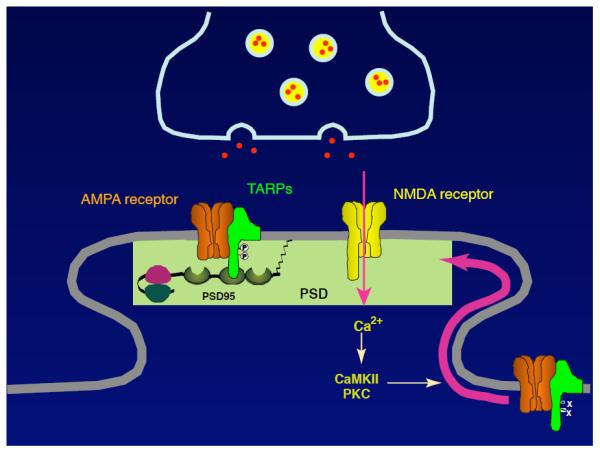
Neuronal activity increases calcium influx through NMDAR to activate calcium-dependent kinases, which is followed by an increase in AMPARs at synapses. TARPs are highly phosphorylated at the PSD. TARP is a substrate of PKC and CaMKII in vitro. These observations indicate that TARP phosphorylation is a regulator of synaptic AMPAR activity and may be a substrate for NMDAR-mediated synaptic plasticity.
TARPs are phosphorylated at nine serine residues in their cytoplasmic domain. It is not known how many TARP phosphorylation sites are required for the regulation of synaptic AMPAR activity. Interestingly, these phosphorylated residues are located within a short consecutive region and the total negative charge of this short stretch changes in a gradient manner. If the total negative charge is the mediator of synaptic AMPAR activity, the short stretch containing the nine phosphorylated serine residues could serve as a molecular rheostat for synaptic AMPAR activity. In addition to the nine phosphorylated serine residues within this short stretch, TARPs are phosphorylated at the threonine residue in the C-terminal PDZ-binding motif. Moreover, the TARP mutant that is phosphorylated at this threonine residue, which can be phosphorylated by PKA, does not interact with PSD-95. In addition to regulation by calcium-dependent kinases, the localization of the AMPAR/TARP complex may be regulated by PKA (10, 13).
Dynamic interaction between TARP and AMPAR
TARPs interact with AMPARs and modulate the trafficking and channel properties of AMPARs. Glutamate-induced AMPAR desensitization could induce partial or complete dissociation of AMPARs from TARPs (55, 80).
In some neurons, AMPARs show a bell-shaped dose-response curve, where the amplitude of the steady-state current declines at glutamate concentrations above 100 μM. The mechanism underlying this observation is not completely understood, as AMPAR expression in heterologous cells does not exhibit a bell-shaped dose response (27, 66, 90). However, AMPARs coexpressed with TARPs show such a response. Interestingly, an AMPAR–TARP covalently linked tandem protein (TARPed AMPAR) shows similar channel properties to AMPARs coexpressed with TARPs; however, TARPed AMPAR does not show a bell-shaped dose response (55). In addition, cyclothiazide blocks the reduction of AMPAR currents at higher glutamate concentrations (55). These results indicate that glutamate induces desensitization of the AMPARs that interact with TARPs and that, subsequently, TARPs dissociate from AMPARs to reduce AMPAR activity via loss of TARP modulation. Interestingly, AMPAR desensitization regulates synaptic AMPAR distribution (29). This mechanism could be due to TARP–AMPAR dissociation, although it remains unclear whether AMPARs dissociate from TARPs completely or partially after AMPAR desensitization in a short time scale (10–50 ms). In contrast, relatively long exposure of AMPA (over a few minutes) induces complete dissociation of AMPAR from TARPs, which subsequently induces the internalization of AMPAR, but not of TARPs, within a few minutes (80). The regulation of the TARP–AMPAR interaction allows diverse responses of AMPARs in a glutamate concentration- and exposure-time-dependent manner.
6. Neurological aspects
Ataxia and absence epilepsy
TARPs play multiple roles in AMPAR modulation in normal conditions, but also in disease conditions. The stargazer mouse is a spontaneous mutant mouse that exhibits ataxia and absence epilepsy (61). Although the ataxic phenotypes could be explained by loss of AMPAR activity in the cerebellum (28), the absence epilepsy phenotype is the opposite of what one would expect from the loss of AMPAR activity, because epilepsy is in general caused by synchronized and enhanced neural activity. One possible explanation for the absence epilepsy observed in the stargazer mouse is the disinhibition of interneurons. Stargazin/γ-2 is also expressed in interneurons (79) and strong reduction of AMPAR activity is observed in interneurons of TARP knockout mice (50). Thus, loss of stargazin/γ-2 could cause loss of AMPAR in some unidentified interneurons, which would lead to loss of inhibition of inhibitory neurons, i.e., hyperexcitation of neural activity and induction of absence epilepsy. Mice in which both stargazin/γ-2 and γ-4 have been disrupted shows progression of absence epilepsy compared to mice disrupting stargazin/γ-2 alone (41). Because each TARP functions in a redundant fashion in mice (51), the detailed analysis of TARP-isoform expression could lead to the identification of the neurons that cause these phenotypes.
Excitotoxicity
Kainate is a natural toxin from a type of seaweed and induces seizures and neurotoxicity in humans and other animals. High-affinity kainate receptors are believed to be involved in kainate-induced phenomena. Kainate receptor (GluK2) knockout mice show an altered threshold for kainate-induced seizures and some gliosis (56). Interestingly, kainate-induced cell loss in the hippocampus is reduced in γ-8 knockout mice, whereas kainate-induced seizure was not altered (78). The difference in kainate-induced neurotoxicity is probably due to a loss of kainate sensitivity by AMPARs via the absence of TARPs in the AMPAR complex. This could mean that TARPs may be involved in excitotoxicity in neurons.
TARPs as potential drug targets
TARPs may be potential drug targets for the potentiation or suppression of AMPAR activity at synapses. AMPAR potentiators (AMPAkines) enhance cognitive function and are currently being investigated as a potential treatment for a variety of neurological disorders, including schizophrenia, Alzheimer’s disease, and Parkinson’s disease (42, 44, 62, 74). Each AMPAkine has a specific preference for the flip or flop AMPAR splicing isoform (22, 63), although in the presence of stargazin/γ-2, these potentiators can act on either isoform (82). One drawback of AMPAkine treatment is the fact that AMPARs are ubiquitously expressed, which makes it difficult to target AMPARs in specific regions of the brain. Drugs that alter the stability of the AMPAR/TARP complex could, in principle, up- or downregulate AMPAR activity via TARP modulation of channel activity, and TARP isoform-specific drugs would allow the efficient targeting of specific brain regions (25, 79). Therefore, targeting TARPs may be an effective method for regulating AMPAR activity and synaptic strength.
7. Other transmembrane auxiliary subunits of glutamate receptors
TARPs are auxiliary subunits of AMPARs; however, extensive studies have been performed to identify other subunits of ionotropic glutamate receptors, which resulted in the identification of several proteins as candidate subunits (not yet fully confirmed) (FIGURE 4).
FIGURE 4. Auxiliary and accessory subunits of ionotropic glutamate receptors.
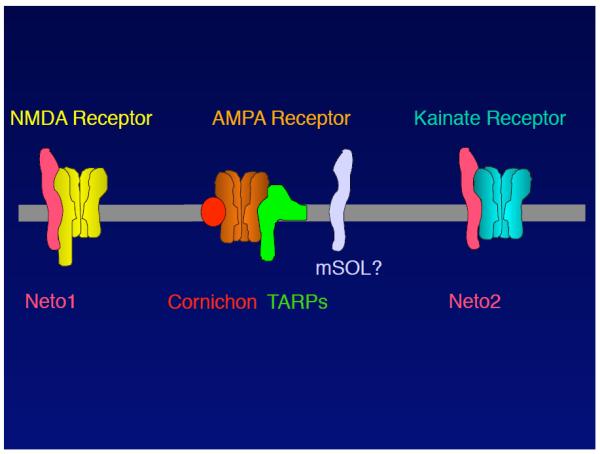
Ionotropic glutamate receptors are pharmacologically classified as AMPA-, NMDA-, and kainate-sensitive glutamate receptors. Several transmembrane subunits have been proposed to date, but remain unconfirmed. The NMDAR complex comprises Neto1, which is a CUB domain-containing protein. The AMPAR complex comprises TARPs and cornichon. Notably, it remains unclear whether AMPAR/TARP/cornichon form a tripartite complex. The C. elegans AMPAR complex comprises SOL-1, which is a CUB domain-containing protein. Therefore, mammalian AMPAR complexes may comprise a mammalian homolog of SOL-1 (mSOL). The kainate receptor complex comprises Neto2, which is a CUB domain-containing protein. The high sequence homology between Neto1 and 2 raises the question of whether Neto1 and 2 act as auxiliary subunits for both NMDA and kainate receptors.
AMPARs
A high-throughput proteomics approach identified cornichon (CNIH2 and 3) as a novel auxiliary subunit of AMPARs (FIGURE 4) (70). Cornichon is a trimembrane-spanning protein that modulates the channel properties and surface expression of AMPAR in heterologous cells (70). The precise localization of the AMPAR/CNIH complex remains unclear. Because Drosophila cornichon acts as a cargo receptor for ER export (5), cornichon may promote the exit of AMPAR from the ER. Further studies are required to elucidate this mechanism.
C. elegans AMPAR (GLR-1) contains another auxiliary subunit (SOL-1), which was identified in a genetic screening as a suppressor of lurcher, which AMPAR carrying Lurcher mutation is a constitutive active form of AMPAR (98). SOL-1 is a single-transmembrane protein that contains four CUB domains in its extracellular domain. In heterologous cells and C. elegans, SOL-1 slows the decay kinetics of GLR-1 AMPAR (97). Interestingly, C. elegans AMPAR (GLR-1), TARP (STG-1 and 2), and SOL-1 form a tripartite complex that exhibits distinct channel properties (91, 92). This result suggests the existence of an unidentified mammalian SOL-1 homolog (FIGURE 4).
NMDA receptors
The neuronal protein Neto1 was identified as a retina-specific protein; subsequently, other splicing isoforms of Neto1 expressed in the brain were identified (75). Neto1 is a CUB domain-containing protein. Two other CUB domain-containing proteins, SOL-1 and LEV-10, were identified as subunits of the worm glutamate receptor (GLR-1) and of the worm acetylcholine receptor, respectively (26, 98). Neto1, a protein with uknown function, is a vertebrate protein containing two CUB domains that shared highest homology with CUB domains in SOL-1 and LEV-10. Neto1 was examined as a subunit of ion channels, NMDARs. Neto1 knockout mice exhibit NMDAR-mediated synaptic plasticity and impairment in learning tasks (60). These results propose Neto1 as a novel subunit of NMDARs (FIGURE 4).
Kainate receptors
The channel properties of native and recombinant kainate receptors are significantly different. A proteomics approach identified Neto2 as a kainate receptor-binding protein (96). Neto2 modulates the channel properties of kainate receptors in heterologous cells (96). In contrast, kainate receptors modulate the surface expression of Neto2 in heterologous cells and in neurons (96). Furthermore, overexpression of Neto2 increases the frequency of kainate receptor (KAR)-mediated events and slows the decay kinetics of KAR-mediated EPSCs (96). These results propose Neto2 as a subunit of kainate receptors (FIGURE 4). Interestingly, Neto2 shares very high homology with Neto1 identified as an auxiliary subunit of NMDARs. It is important to examine possible dual roles of Neto1 and 2 as auxiliary subunits of both KAR and NMDAR, simultaneously.
8. Concluding remarks
Recent extensive studies established TARPs as auxiliary subunits of AMPARs in the brain. TARPs interact with AMPARs and modulate AMPAR channel gating, channel pharmacology, and trafficking to the cell surface and to synapses. Several questions remain unanswered in this field, including “do all AMPAR complexes in the brain contain TARPs?”, “how does neuronal activity modulate AMPAR activity?”, and “what is the role of TARPs in neurological disorders and how should these disorders be treated?” These mechanisms should be revealed in the future. Furthermore, growing knowledge of the auxiliary and accessory subunits of ionotropic glutamate receptors will shed light on the fundamental rules that govern excitatory synaptic transmission.
Table 1.
TARPs modulate AMPAR functions
| Function | TARP modulation | TARP domains involved in each modulation |
|---|---|---|
| AMPAR Interaction |
Interaction | No report Note, AMPAR desensitization induces TARP dissociation |
| Channel properties |
EPSC Decay kinetics (Synaptic transmission) |
Extracellular domain, TARP subfamily (γ-2/3 and γ-4/8) |
| Decay kinetics (deactivation/desensitization) |
Extracellular domain, Cytoplasmic domain, TARP subfamily (γ-2/3 and γ-4/8) |
|
| AMPAR open probability (Accelerating gating) |
No report | |
| Channel rectification | No report | |
| KA efficacy | Extracellular domain, Cytoplasmic domain, TARP subfamily (γ-2/3 and γ-4/8) |
|
| AMPA potentiator | No report (AMPAR splicing isoform, flip/flop) |
|
| Trafficking | Convert AMPAR antagonists into partial agonists |
No report |
| Synaptic localization | C-terminal PDZ binding domain (−TTPV) |
|
| TARP Phosphorylation | ||
| Surface expression | TARP cytoplasmic domain |
Acknowledgement
I thank members of the Tomita lab for comments. S.T. is supported by NIH.
References
- 1.Bats C, Groc L, Choquet D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron. 2007;53:719–734. doi: 10.1016/j.neuron.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 2.Bedoukian MA, Weeks AM, Partin KM. Different domains of the AMPA receptor direct stargazin-mediated trafficking and stargazin-mediated modulation of kinetics. J Biol Chem. 2006;281:23908–23921. doi: 10.1074/jbc.M600679200. [DOI] [PubMed] [Google Scholar]
- 3.Bedoukian MA, Whitesell JD, Peterson EJ, Clay CM, Partin KM. The stargazin C terminus encodes an intrinsic and transferable membrane sorting signal. J Biol Chem. 2008;283:1597–1600. doi: 10.1074/jbc.M708141200. [DOI] [PubMed] [Google Scholar]
- 4.Beique JC, Andrade R. PSD-95 regulates synaptic transmission and plasticity in rat cerebral cortex. J Physiol. 2003;546:859–867. doi: 10.1113/jphysiol.2002.031369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bokel C, Dass S, Wilsch-Brauninger M, Roth S. Drosophila Cornichon acts as cargo receptor for ER export of the TGFalpha-like growth factor Gurken. Development. 2006;133:459–470. doi: 10.1242/dev.02219. [DOI] [PubMed] [Google Scholar]
- 6.Burgess DL, Davis CF, Gefrides LA, Noebels JL. Identification of three novel Ca(2+) channel gamma subunit genes reveals molecular diversification by tandem and chromosome duplication. Genome Res. 1999;9:1204–1213. doi: 10.1101/gr.9.12.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgess DL, Gefrides LA, Foreman PJ, Noebels JL. A cluster of three novel Ca2+ channel gamma subunit genes on chromosome 19q13.4: evolution and expression profile of the gamma subunit gene family. Genomics. 2001;71:339–350. doi: 10.1006/geno.2000.6440. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, El-Husseini A, Tomita S, Bredt DS, Nicoll RA. Stargazin differentially controls the trafficking of alpha-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate and kainate receptors. Mol Pharmacol. 2003;64:703–706. doi: 10.1124/mol.64.3.703. [DOI] [PubMed] [Google Scholar]
- 10.Chetkovich DM, Chen L, Stocker TJ, Nicoll RA, Bredt DS. Phosphorylation of the postsynaptic density-95 (PSD-95)/discs large/zona occludens-1 binding site of stargazin regulates binding to PSD-95 and synaptic targeting of AMPA receptors. J Neurosci. 2002;22:5791–5796. doi: 10.1523/JNEUROSCI.22-14-05791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho CH, St-Gelais F, Zhang W, Tomita S, Howe JR. Two Families of TARP Isoforms that Have Distinct Effects on the Kinetic Properties of AMPA Receptors and Synaptic Currents. Neuron. 2007;55:890–904. doi: 10.1016/j.neuron.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Cho KO, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- 13.Choi J, Ko J, Park E, Lee JR, Yoon J, Lim S, Kim E. Phosphorylation of stargazin by protein kinase A regulates its interaction with PSD-95. J Biol Chem. 2002;277:12359–12363. doi: 10.1074/jbc.M200528200. [DOI] [PubMed] [Google Scholar]
- 14.Chu PJ, Robertson HM, Best PM. Calcium channel gamma subunits provide insights into the evolution of this gene family. Gene. 2001;280:37–48. doi: 10.1016/s0378-1119(01)00738-7. [DOI] [PubMed] [Google Scholar]
- 15.Cokic B, Stein V. Stargazin modulates AMPA receptor antagonism. Neuropharmacology. 2008;54:1062–1070. doi: 10.1016/j.neuropharm.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- 17.Cuadra AE, Kuo SH, Kawasaki Y, Bredt DS, Chetkovich DM. AMPA receptor synaptic targeting regulated by stargazin interactions with the Golgi-resident PDZ protein nPIST. J Neurosci. 2004;24:7491–7502. doi: 10.1523/JNEUROSCI.1255-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dakoji S, Tomita S, Karimzadegan S, Nicoll RA, Bredt DS. Interaction of transmembrane AMPA receptor regulatory proteins with multiple membrane associated guanylate kinases. Neuropharmacology. 2003;45:849–856. doi: 10.1016/s0028-3908(03)00267-3. [DOI] [PubMed] [Google Scholar]
- 19.Deng F, Price MG, Davis CF, Mori M, Burgess DL. Stargazin and other transmembrane AMPA receptor regulating proteins interact with synaptic scaffolding protein MAGI-2 in brain. J Neurosci. 2006;26:7875–7884. doi: 10.1523/JNEUROSCI.1851-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- 21.Elias GM, Funke L, Stein V, Grant SG, Bredt DS, Nicoll RA. Synapse-Specific and Developmentally Regulated Targeting of AMPA Receptors by a Family of MAGUK Scaffolding Proteins. Neuron. 2006;52:307–320. doi: 10.1016/j.neuron.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Fleck MW, Bahring R, Patneau DK, Mayer ML. AMPA receptor heterogeneity in rat hippocampal neurons revealed by differential sensitivity to cyclothiazide. Journal of Neurophysiology. 1996;75:2322–2333. doi: 10.1152/jn.1996.75.6.2322. [DOI] [PubMed] [Google Scholar]
- 23.Fukata Y, Tzingounis AV, Trinidad JC, Fukata M, Burlingame AL, Nicoll RA, Bredt DS. Molecular constituents of neuronal AMPA receptors. J Cell Biol. 2005;169:399–404. doi: 10.1083/jcb.200501121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukaya M, Tsujita M, Yamazaki M, Kushiya E, Abe K, Natsume R, Kano M, Kamiya H, Watanabe M, Sakimura K. Abundant distribution of TARP gamma-8 in synaptic and extrasynaptic surface of hippocampal neurons and its major role in AMPA receptor expression on spines and dendrites. Eur J Neurosci. 2006;24:2177–2190. doi: 10.1111/j.1460-9568.2006.05081.x. [DOI] [PubMed] [Google Scholar]
- 25.Fukaya M, Yamazaki M, Sakimura K, Watanabe M. Spatial diversity in gene expression for VDCCgamma subunit family in developing and adult mouse brains. Neurosci Res. 2005;53:376–383. doi: 10.1016/j.neures.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Gally C, Eimer S, Richmond JE, Bessereau JL. A transmembrane protein required for acetylcholine receptor clustering in Caenorhabditis elegans. Nature. 2004;431:578–582. doi: 10.1038/nature02893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geoffroy M, Lambolez B, Audinat E, Hamon B, Crepel F, Rossier J, Kado RT. Reduction of desensitization of a glutamate ionotropic receptor by antagonists. Mol Pharmacol. 1991;39:587–591. [PubMed] [Google Scholar]
- 28.Hashimoto K, Fukaya M, Qiao X, Sakimura K, Watanabe M, Kano M. Impairment of AMPA receptor function in cerebellar granule cells of ataxic mutant mouse stargazer. J Neurosci. 1999;19:6027–6036. doi: 10.1523/JNEUROSCI.19-14-06027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heine M, Groc L, Frischknecht R, Beique JC, Lounis B, Rumbaugh G, Huganir RL, Cognet L, Choquet D. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science. 2008;320:201–205. doi: 10.1126/science.1152089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inamura M, Itakura M, Okamoto H, Hoka S, Mizoguchi A, Fukazawa Y, Shigemoto R, Yamamori S, Takahashi M. Differential localization and regulation of stargazin-like protein, gamma-8 and stargazin in the plasma membrane of hippocampal and cortical neurons. Neurosci Res. 2006;55:45–53. doi: 10.1016/j.neures.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Ives JH, Fung S, Tiwari P, Payne HL, Thompson CL. Microtubule-associated protein light chain 2 is a stargazin-AMPA receptor complex-interacting protein in vivo. J Biol Chem. 2004;279:31002–31009. doi: 10.1074/jbc.M402214200. [DOI] [PubMed] [Google Scholar]
- 32.Kato AS, Siuda ER, Nisenbaum ES, Bredt DS. AMPA receptor subunit-specific regulation by a distinct family of type II TARPs. Neuron. 2008;59:986–996. doi: 10.1016/j.neuron.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 33.Kato AS, Zhou W, Milstein AD, Knierman MD, Siuda ER, Dotzlaf JE, Yu H, Hale JE, Nisenbaum ES, Nicoll RA, Bredt DS. New transmembrane AMPA receptor regulatory protein isoform, gamma-7, differentially regulates AMPA receptors. J Neurosci. 2007;27:4969–4977. doi: 10.1523/JNEUROSCI.5561-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kessels HW, Kopec CD, Klein ME, Malinow R. Roles of stargazin and phosphorylation in the control of AMPA receptor subcellular distribution. Nat Neurosci. 2009;12:888–896. doi: 10.1038/nn.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klugbauer N, Dai S, Specht V, Lacinová L, Marais E, Bohn G, Hofmann F. A family of gamma-like calcium channel subunits. Febs Letters. 2000;470:189–197. doi: 10.1016/s0014-5793(00)01306-5. [DOI] [PubMed] [Google Scholar]
- 36.Korber C, Werner M, Hoffmann J, Sager C, Tietze M, Schmid SM, Kott S, Hollmann M. Stargazin interaction with alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptors is critically dependent on the amino acid at the narrow constriction of the ion channel. J Biol Chem. 2007;282:18758–18766. doi: 10.1074/jbc.M611182200. [DOI] [PubMed] [Google Scholar]
- 37.Korber C, Werner M, Kott S, Ma ZL, Hollmann M. The transmembrane AMPA receptor regulatory protein gamma 4 is a more effective modulator of AMPA receptor function than stargazin (gamma 2) J Neurosci. 2007;27:8442–8447. doi: 10.1523/JNEUROSCI.0424-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kott S, Sager C, Tapken D, Werner M, Hollmann M. Comparative analysis of the pharmacology of GluR1 in complex with transmembrane AMPA receptor regulatory proteins gamma2, gamma3, gamma4, and gamma8. Neuroscience. 2009;158:78–88. doi: 10.1016/j.neuroscience.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 39.Kott S, Werner M, Korber C, Hollmann M. Electrophysiological properties of AMPA receptors are differentially modulated depending on the associated member of the TARP family. J Neurosci. 2007;27:3780–3789. doi: 10.1523/JNEUROSCI.4185-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Letts VA, Felix R, Biddlecome GH, Arikkath J, Mahaffey CL, Valenzuela A, Bartlett FS, 2nd, Mori Y, Campbell KP, Frankel WN. The mouse stargazer gene encodes a neuronal Ca2+-channel gamma subunit. Nat Genet. 1998;19:340–347. doi: 10.1038/1228. [DOI] [PubMed] [Google Scholar]
- 41.Letts VA, Mahaffey CL, Beyer B, Frankel WN. A targeted mutation in Cacng4 exacerbates spike-wave seizures in stargazer (Cacng2) mice. Proc Natl Acad Sci U S A. 2005;102:2123–2128. doi: 10.1073/pnas.0409527102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X, Witkin JM, Need AB, Skolnick P. Enhancement of antidepressant potency by a potentiator of AMPA receptors. Cell Mol Neurobiol. 2003;23:419–430. doi: 10.1023/A:1023648923447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lisman J. Long-term potentiation: outstanding questions and attempted synthesis. Philos Trans R Soc Lond B Biol Sci. 2003;358:829–842. doi: 10.1098/rstb.2002.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lynch G, Granger R, Ambros-Ingerson J, Davis CM, Kessler M, Schehr R. Evidence that a positive modulator of AMPA-type glutamate receptors improves delayed recall in aged humans. Exp Neurol. 1997;145:89–92. doi: 10.1006/exnr.1997.6447. [DOI] [PubMed] [Google Scholar]
- 45.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 47.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 48.Matsuda S, Miura E, Matsuda K, Kakegawa W, Kohda K, Watanabe M, Yuzaki M. Accumulation of AMPA receptors in autophagosomes in neuronal axons lacking adaptor protein AP-4. Neuron. 2008;57:730–745. doi: 10.1016/j.neuron.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 49.Menuz K, Kerchner GA, O’Brien JL, Nicoll RA. Critical role for TARPs in early development despite broad functional redundancy. Neuropharmacology. 2009;56:22–29. doi: 10.1016/j.neuropharm.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Menuz K, Nicoll RA. Loss of inhibitory neuron AMPA receptors contributes to ataxia and epilepsy in stargazer mice. J Neurosci. 2008;28:10599–10603. doi: 10.1523/JNEUROSCI.2732-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Menuz K, O’Brien JL, Karmizadegan S, Bredt DS, Nicoll RA. TARP redundancy is critical for maintaining AMPA receptor function. J Neurosci. 2008;28:8740–8746. doi: 10.1523/JNEUROSCI.1319-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Menuz K, Stroud RM, Nicoll RA, Hays FA. TARP Auxiliary Subunits Switch AMPA Receptor Antagonists into Partial Agonists. Science. 2007;318:815–817. doi: 10.1126/science.1146317. [DOI] [PubMed] [Google Scholar]
- 53.Milstein AD, Nicoll RA. TARP modulation of synaptic AMPA receptor trafficking and gating depends on multiple intracellular domains. Proc Natl Acad Sci U S A. 2009;106:11348–11351. doi: 10.1073/pnas.0905570106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Milstein AD, Zhou W, Karimzadegan S, Bredt DS, Nicoll RA. TARP Subtypes Differentially and Dose-Dependently Control Synaptic AMPA Receptor Gating. Neuron. 2007;55:905–918. doi: 10.1016/j.neuron.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morimoto-Tomita M, Zhang W, Straub C, Cho CH, Kim KS, Howe JR, Tomita S. Autoinactivation of neuronal AMPA receptors via glutamate-regulated TARP interaction. Neuron. 2009;61:101–112. doi: 10.1016/j.neuron.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mulle C, Sailer A, Perez-Otano I, Dickinson-Anson H, Castillo PE, Bureau I, Maron C, Gage FH, Mann JR, Bettler B, Heinemann SF. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature. 1998;392:601–605. doi: 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- 57.Nakagawa T, Cheng Y, Ramm E, Sheng M, Walz T. Structure and different conformational states of native AMPA receptor complexes. Nature. 2005;433:545–549. doi: 10.1038/nature03328. [DOI] [PubMed] [Google Scholar]
- 58.Nakagawa T, Cheng Y, Sheng M, Walz T. Three-dimensional structure of an AMPA receptor without associated stargazin/TARP proteins. Biol Chem. 2006;387:179–187. doi: 10.1515/BC.2006.024. [DOI] [PubMed] [Google Scholar]
- 59.Nakagawa T, Futai K, Lashuel HA, Lo I, Okamoto K, Walz T, Hayashi Y, Sheng M. Quaternary structure, protein dynamics, and synaptic function of SAP97 controlled by L27 domain interactions. Neuron. 2004;44:453–467. doi: 10.1016/j.neuron.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 60.Ng D, Pitcher GM, Szilard RK, Sertie A, Kanisek M, Clapcote SJ, Lipina T, Kalia LV, Joo D, McKerlie C, Cortez M, Roder JC, Salter MW, McInnes RR. Neto1 is a novel CUB-domain NMDA receptor-interacting protein required for synaptic plasticity and learning. PLoS Biol. 2009;7:e41. doi: 10.1371/journal.pbio.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noebels JL, Qiao X, Bronson RT, Spencer C, Davisson MT. Stargazer: a new neurological mutant on chromosome 15 in the mouse with prolonged cortical seizures. Epilepsy Res. 1990;7:129–135. doi: 10.1016/0920-1211(90)90098-g. [DOI] [PubMed] [Google Scholar]
- 62.O’Neill MJ, Murray TK, Whalley K, Ward MA, Hicks CA, Woodhouse S, Osborne DJ, Skolnick P. Neurotrophic actions of the novel AMPA receptor potentiator, LY404187, in rodent models of Parkinson’s disease. Eur J Pharmacol. 2004;486:163–174. doi: 10.1016/j.ejphar.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 63.Partin KM, Bowie D, Mayer ML. Structural determinants of allosteric regulation in alternatively spliced AMPA receptors. Neuron. 1995;14:833–843. doi: 10.1016/0896-6273(95)90227-9. [DOI] [PubMed] [Google Scholar]
- 64.Price MG, Davis CF, Deng F, Burgess DL. The AMPA receptor trafficking regulator stargazin is related to the Claudin family of proteins by its ability to mediate cell-cell adhesion. J Biol Chem. 2005 doi: 10.1074/jbc.M500623200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Priel A, Kolleker A, Ayalon G, Gillor M, Osten P, Stern-Bach Y. Stargazin reduces desensitization and slows deactivation of the AMPA-type glutamate receptors. J Neurosci. 2005;25:2682–2686. doi: 10.1523/JNEUROSCI.4834-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raman IM, Trussell LO. The kinetics of the response to glutamate and kainate in neurons of the avian cochlear nucleus. Neuron. 1992;9:173–186. doi: 10.1016/0896-6273(92)90232-3. [DOI] [PubMed] [Google Scholar]
- 67.Rouach N, Byrd K, Petralia RS, Elias GM, Adesnik H, Tomita S, Karimzadegan S, Kealey C, Bredt DS, Nicoll RA. TARP gamma-8 controls hippocampal AMPA receptor number, distribution and synaptic plasticity. Nat Neurosci. 2005;8:1525–1533. doi: 10.1038/nn1551. [DOI] [PubMed] [Google Scholar]
- 68.Schluter OM, Xu W, Malenka RC. Alternative N-terminal domains of PSD-95 and SAP97 govern activity-dependent regulation of synaptic AMPA receptor function. Neuron. 2006;51:99–111. doi: 10.1016/j.neuron.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 69.Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci U S A. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwenk J, Harmel N, Zolles G, Bildl W, Kulik A, Heimrich B, Chisaka O, Jonas P, Schulte U, Fakler B, Klocker N. Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science. 2009;323:1313–1319. doi: 10.1126/science.1167852. [DOI] [PubMed] [Google Scholar]
- 71.Shi Y, Lu W, Milstein AD, Nicoll RA. The stoichiometry of AMPA receptors and TARPs varies by neuronal cell type. Neuron. 2009;62:633–640. doi: 10.1016/j.neuron.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soto D, Coombs ID, Kelly L, Farrant M, Cull-Candy SG. Stargazin attenuates intracellular polyamine block of calcium-permeable AMPA receptors. Nat Neurosci. 2007;10:1260–1267. doi: 10.1038/nn1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Soto D, Coombs ID, Renzi M, Zonouzi M, Farrant M, Cull-Candy SG. Selective regulation of long-form calcium-permeable AMPA receptors by an atypical TARP, gamma-5. Nat Neurosci. 2009;12:277–285. doi: 10.1038/nn.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Staubli U, Rogers G, Lynch G. Facilitation of glutamate receptors enhances memory. Proc Natl Acad Sci U S A. 1994;91:777–781. doi: 10.1073/pnas.91.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stohr H, Berger C, Frohlich S, Weber BH. A novel gene encoding a putative transmembrane protein with two extracellular CUB domains and a low-density lipoprotein class A module: isolation of alternatively spliced isoforms in retina and brain. Gene. 2002;286:223–231. doi: 10.1016/s0378-1119(02)00438-9. [DOI] [PubMed] [Google Scholar]
- 76.Suzuki E, Kessler M, Arai AC. The fast kinetics of AMPA GluR3 receptors is selectively modulated by the TARPs gamma 4 and gamma 8. Mol Cell Neurosci. 2008;38:117–123. doi: 10.1016/j.mcn.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 77.Tomita S, Adesnik H, Sekiguchi M, Zhang W, Wada K, Howe JR, Nicoll RA, Bredt DS. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature. 2005;435:1052–1058. doi: 10.1038/nature03624. [DOI] [PubMed] [Google Scholar]
- 78.Tomita S, Byrd RK, Rouach N, Bellone C, Venegas A, O’Brien JL, Kim KS, Olsen O, Nicoll RA, Bredt DS. AMPA receptors and stargazin-like transmembrane AMPA receptor-regulatory proteins mediate hippocampal kainate neurotoxicity. Proc Natl Acad Sci U S A. 2007;104:18784–18788. doi: 10.1073/pnas.0708970104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J Cell Biol. 2003;161:805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tomita S, Fukata M, Nicoll RA, Bredt DS. Dynamic interaction of stargazin-like TARPs with cycling AMPA receptors at synapses. Science. 2004;303:1508–1511. doi: 10.1126/science.1090262. [DOI] [PubMed] [Google Scholar]
- 81.Tomita S, Nicoll RA, Bredt DS. PDZ protein interactions regulating glutamate receptor function and plasticity. J Cell Biol. 2001;153:F19–24. doi: 10.1083/jcb.153.5.f19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tomita S, Sekiguchi M, Wada K, Nicoll RA, Bredt DS. Stargazin controls the pharmacology of AMPA receptor potentiators. Proc Natl Acad Sci U S A. 2006;103:10064–10067. doi: 10.1073/pnas.0603128103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tomita S, Shenoy A, Fukata Y, Nicoll RA, Bredt DS. Stargazin interacts functionally with the AMPA receptor glutamate-binding module. Neuropharmacology. 2007;52:87–91. doi: 10.1016/j.neuropharm.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 84.Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS. Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron. 2005;45:269–277. doi: 10.1016/j.neuron.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 85.Tsui J, Malenka RC. Substrate localization creates specificity in calcium/calmodulin-dependent protein kinase II signaling at synapses. J Biol Chem. 2006;281:13794–13804. doi: 10.1074/jbc.M600966200. [DOI] [PubMed] [Google Scholar]
- 86.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 87.Turetsky D, Garringer E, Patneau DK. Stargazin modulates native AMPA receptor functional properties by two distinct mechanisms. J Neurosci. 2005;25:7438–7448. doi: 10.1523/JNEUROSCI.1108-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vandenberghe W, Nicoll RA, Bredt DS. Interaction with the unfolded protein response reveals a role for stargazin in biosynthetic AMPA receptor transport. J Neurosci. 2005;25:1095–1102. doi: 10.1523/JNEUROSCI.3568-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vandenberghe W, Nicoll RA, Bredt DS. Stargazin is an AMPA receptor auxiliary subunit. Proc Natl Acad Sci U S A. 2005;102:485–490. doi: 10.1073/pnas.0408269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vlachova V, Vyklicky L, Vyklicky L, Jr., Vyskocil F. The action of excitatory amino acids on chick spinal cord neurones in culture. J Physiol. 1987;386:425–438. doi: 10.1113/jphysiol.1987.sp016542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Walker CS, Brockie PJ, Madsen DM, Francis MM, Zheng Y, Koduri S, Mellem JE, Strutz-Seebohm N, Maricq AV. Reconstitution of invertebrate glutamate receptor function depends on stargazin-like proteins. Proc Natl Acad Sci U S A. 2006;103:10781–10786. doi: 10.1073/pnas.0604482103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Walker CS, Francis MM, Brockie PJ, Madsen DM, Zheng Y, Maricq AV. Conserved SOL-1 proteins regulate ionotropic glutamate receptor desensitization. Proc Natl Acad Sci U S A. 2006;103:10787–10792. doi: 10.1073/pnas.0604520103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang R, Walker CS, Brockie PJ, Francis MM, Mellem JE, Madsen DM, Maricq AV. Evolutionary conserved role for TARPs in the gating of glutamate receptors and tuning of synaptic function. Neuron. 2008;59:997–1008. doi: 10.1016/j.neuron.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yamazaki M, Ohno-Shosaku T, Fukaya M, Kano M, Watanabe M, Sakimura K. A novel action of stargazin as an enhancer of AMPA receptor activity. Neurosci Res. 2004;50:369–374. doi: 10.1016/j.neures.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 95.Zhang W, Robert A, Vogensen SB, Howe JR. The relationship between agonist potency and AMPA receptor kinetics. Biophys J. 2006;91:1336–1346. doi: 10.1529/biophysj.106.084426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang W, St-Gelais F, Grabner CP, Trinidad JC, Sumioka A, Morimoto-Tomita M, Kim KS, Straub C, Burlingame AL, Howe JR, Tomita S. A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron. 2009;61:385–396. doi: 10.1016/j.neuron.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zheng Y, Brockie PJ, Mellem JE, Madsen DM, Walker CS, Francis MM, Maricq AV. SOL-1 is an auxiliary subunit that modulates the gating of GLR-1 glutamate receptors in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2006;103:1100–1105. doi: 10.1073/pnas.0504612103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zheng Y, Mellem JE, Brockie PJ, Madsen DM, Maricq AV. SOL-1 is a CUB-domain protein required for GLR-1 glutamate receptor function in C. elegans. Nature. 2004;427:451–457. doi: 10.1038/nature02244. [DOI] [PubMed] [Google Scholar]


