Abstract
Acoustic plus electric (electric-acoustic) speech processing has been successful in highlighting the important role of articulation information in consonant recognition in those adults that have profound high-frequency hearing loss at frequencies greater than 1500 Hz and less than 60% discrimination scores. Eighty-seven subjects were enrolled in an adult Hybrid multicenter Food and Drug Administration clinical trial. Immediate hearing preservation was accomplished in 85/87 subjects. Over time (3 months to 5 years), some hearing preservation was maintained in 91% of the group. Combined electric-acoustic processing enabled most of this group of volunteers to gain improved speech understanding, compared to their preoperative hearing, with bilateral hearing aids. Most have preservation of low-frequency acoustic hearing within 15 dB of their preoperative pure tone levels. Those with greater losses (> 30 dB) also benefited from the combination of electric-acoustic speech processing. Postoperatively, in the electric-acoustic processing condition, loss of low-frequency hearing did not correlate with improvements in speech perception scores in quiet. Sixteen subjects were identified as poor performers in that they did not achieve a significant improvement through electric-acoustic processing. A multiple regression analysis determined that 91% of the variance in the poorly performing group can be explained by the preoperative speech recognition score and duration of deafness. Signal-to-noise ratios for speech understanding in noise improved more than 9 dB in some individuals in the electric-acoustic processing condition. The relation between speech understanding in noise thresholds and residual low-frequency acoustic hearing is significant (r = 0.62; p < 0.05). The data suggest that, in general, the advantages gained for speech recognition in noise by preserving residual hearing exist, unless the hearing loss approaches profound levels. Preservation of residual low-frequency hearing should be considered when expanding candidate selection criteria for standard cochlear implants. Duration of profound high-frequency hearing loss appears to be an important variable when determining selection criteria for the Hybrid implant.
Keywords: Short electrode, Electric-acoustic stimulation, Sensorineural, Hearing loss, Hearing preservation
Introduction
Combining residual acoustic hearing with electric speech processing is an emerging new strategy for those individuals who are struggling with poor speech perception, even when using state of the art amplification systems. These hearing-impaired individuals have lost most of their high-frequency hearing, but have too much residual hearing and speech perception to qualify for conventional cochlear implants. Our cochlear implant research group [Gantz and Turner, 2003, 2004; Gantz et al., 2005, 2006; Gfeller et al., 2006; Reiss et al., 2007; Turner et al., 2004; Turner and Gantz, 2004, 2005], as well as others [Von Ilberg et al., 1999; Gstoettner et al., 2004; Fraysse et al., 2006], have been exploring methods of implanting the inner ear with electrodes that preserve residual low-frequency hearing for the purpose of combining acoustic and electric speech processing.
The most common type of sensorineural hearing loss is a loss of hearing sensitivity that increases as frequency increases. This configuration of hearing loss can be linked to many different causes, including aging, noise exposure and treatment with ototoxic drugs. In the typical high-frequency sensorineural hearing loss, the damage occurs primarily in the basal end of the cochlea, either to the hair cells themselves or, in the case of aging, apparently also to the endocochlear potential that stimulates the hair cells [Schmiedt et al., 2002]. When the hearing loss exceeds approximately 60–80 dB, this damage to the hair cells begins to affect not only the more susceptible outer hair cells, but also results in missing inner hair cells [Liberman and Dodds, 1984]. These inner hair cells are the sensory receptor cells that are responsible for sending signals to the central auditory system; thus, in the case of severe-to-profound hearing loss, information can no longer be transmitted to the brain for the cochlear regions involved. Conventional amplification of the high-frequency regions with hearing aids is often not effective for these reasons [Hogan and Turner, 1998].
One way to improve perception of high-frequency speech sounds is to combine electric speech processing through a modified cochlear implant with residual low-frequency acoustic hearing. Our team has been exploring delivery of high-frequency signals using an electrode that is limited to the basal portion of the cochlea in order to restore hearing sensitivity for high-frequency sounds [Gantz and Turner, 2003, 2004; Gantz et al., 2005, 2006; Gfeller et al., 2006; Reiss et al., 2007; Turner et al., 2004; Turner and Gantz, 2004, 2005]. This is the basis for the Hybrid or ‘short-electrode’ option developed at the University of Iowa in cooperation with the Cochlear Corporation.
The Iowa/Nucleus 10-mm Hybrid cochlear implant has been implanted under a Food and Drug Administration (FDA) clinical trial IDE (investigational device exemption) in the USA since 2000. A version of this device has been under development since 1995, with initial implantations taking place in 1999. Enrollment in the clinical trial is now closed, and collection of clinical trial data is in process. This report will provide updated data on hearing preservation, preliminary data on speech perception in quiet and noise, and multiple regression analysis of subject biographical data that impacted speech perception performance in a group of volunteers that are participating in an FDA clinical trial of the Iowa/Nucleus 10-mm Hybrid implant.
Methods
Eighty-seven adults with severe to profound hearing loss above 2000 Hz were enrolled in an FDA clinical trial in the USA and implanted with the Iowa/Nucleus 10-mm Hybrid implant. The poorer hearing ear received the device. Candidates could have preoperative acoustic-only consonant-nucleus-consonant (CNC) word scores of between 10 and 60%. The contralateral ear could have up to 80% CNC word scores. Subjects were enrolled and implanted at 13 multicenter sites, and the study was conducted according to the guidelines for the protection of human subjects as set forth by the Institutional Review Boards for the individual study sites.
The 10-mm Iowa/Nucleus Hybrid electrode was designed to be minimally invasive and only enter the descending basal turn of the scala tympani. This short intracochlear electrode has a reduced diameter of 0.2 × 0.4 mm, as compared to standard cochlear implants. Six electrodes (channels) are located in the distal 6 mm of the electrode. The device is placed through a 0.5-mm cochleostomy located anteriorly and caudal to the round window annulus, using care to preserve residual low-frequency hearing [Gantz et al., 2005].
All patients’ processors were programmed with a standard CIS (ACE with the number of maxima = number of channels) type processing strategy, with frequency maps chosen to begin at the upper frequency cutoff of useful residual hearing in the implanted ear, typically resulting in frequency ranges of 688–7938 or 1063–7938 Hz of speech information allocated to the 6 electrodes. ‘Useful’ acoustic hearing was defined as frequencies with thresholds better than 90 dB hearing level (HL) and provided with acoustic amplification.
Preoperatively, all patients used broadband hearing aids bilaterally on a daily basis, and were tested using speech perception measures with bilateral hearing aids. At candidacy evaluation, if a patient presented with no amplification or an unsatisfactory fitting (e.g. not meeting fitting targets) a minimum 2-week hearing aid trial was initiated with optimally fitted amplification prior to baseline testing. Amplification characteristics were determined based on targets derived from the National Acoustics Laboratories (NAL) hearing aid fitting procedure [Byrne et al., 1990; Dillon, 1999]. Postoperatively, patients continued to use a hearing aid in the implanted ear, with amplification provided up to the cutoff of useful residual hearing. The amplification characteristics applied to the low frequencies were also based on targets derived from the NAL hearing aid fitting procedure. Broadband amplification was applied to the contralateral ear for all subjects, again based on the NAL hearing aid fitting procedure. Adjustments were made to amplification postoperatively, per NAL guidelines, only if necessary (e.g. to compensate for changes in acoustic hearing).
Two subjects preferred to use their natural low-frequency hearing in both ears, rather than hearing aids, with the Hybrid implant (both had mild to moderate hearing thresholds up to 750 Hz in both ears) and were tested in this listening mode. These 2 subjects were still considered to be using combined electric and bilateral acoustic hearing. Two subjects (1 with profound low-frequency hearing loss and 1 with complete loss) did not use amplification in the implanted ear and were tested with the Hybrid implant alone and contralateral amplification (the bimodal mode). An additional subject with profound loss of hearing in the implanted ear likely did not receive benefit from and did not use amplification on a daily basis in that ear, but was tested with amplification in both ears with the Hybrid implant. All other subjects were tested postoperatively using amplification in both ears with the Hybrid implant (the combined mode). In cases where a subject lost sufficient hearing so that amplification in the implanted ear was not beneficial, the implant processor map was programmed to correspond with the upper frequency cutoff of the contralateral ear as well as a broadband map. Testing proceeded with the patient’s preferred map, which in all cases was the map with the abbreviated frequency allocation.
Standard pure tone audiometric testing to document changes in residual acoustic hearing was performed preoperatively, and postoperatively at 1, 3, 6 and 12 months, and at yearly intervals. For the purposes of documenting pre- to postoperative changes in hearing, mean low-frequency thresholds were calculated over the range 125, 250, 500, 750 and 1000 Hz and compared pre- vs. postoperatively in both the implanted ear and the contralateral unimplanted ear (though only data for the implanted ear are presented herein). This permitted any change associated with the patient’s ongoing disease process to be factored out of any changes observed in the implanted ear. Speech discrimination in quiet and in noise were measured using the CNC recorded word test [Peterson and Lehiste, 1962] and the BKB Speech-in-Noise test (BKB-SIN; Etymotic Research, Elk Grove Village, Ill., USA; 2005) under 2 conditions: (1) preoperatively with the hearing aid(s) alone; (2) with the hearing aid(s) plus Hybrid implant (combined mode), 3, 6, 9 and 12 months postoperatively. The speech scores obtained by the subjects using hearing aids preoperatively were compared to the most recent postoperative scores obtained by the subjects using the Hybrid implant with hearing aids.
A subgroup of 27 subjects from the multicenter study with 12 months’ or more experience were tested at the University of Iowa using spondee recognition in multitalker babble [Turner, 2004].
The data set presented here represents the data collected as of November 2007.
Results
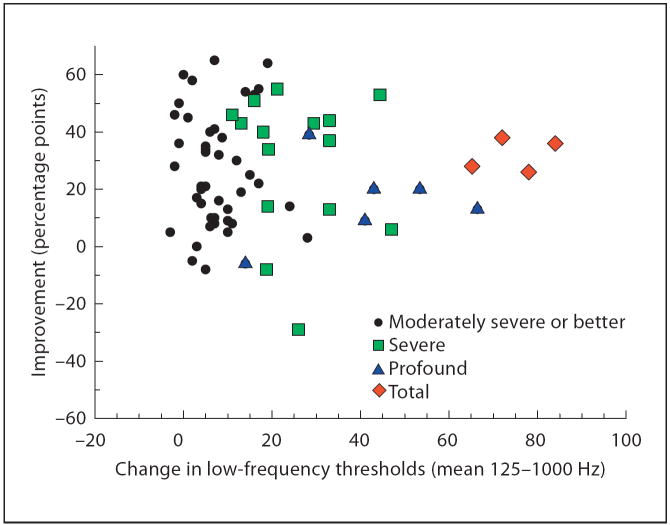
Audiometrically measurable low-frequency hearing was maintained at implantation and documented at the 1-month postoperative initial programming session in 85 of 87 (98%) subjects. Total loss of hearing occurred in 2 cases within the first month after surgery. Total hearing loss occurred in 6 additional subjects between 3 and 24 months after activation resulting in some level of preservation of hearing in 79 of 87 (91%) subjects. Some progressive neurosensory hearing loss was evident in the FDA clinical trial population. At the subjects’ most recent evaluation, 3–36 months, 30% exhibited greater than 30 dB mean low-frequency threshold shifts compared to preoperative thresholds. The impact of acoustic hearing loss on performance in the combined mode (acoustic hearing both ears + Hybrid implant) was evaluated by comparing the change in the mean low-frequency pure tone acoustic audiometric thresholds after implantation and the improvement in speech perception in quiet (the percentage point difference in CNC word scores in the combined mode postoperatively versus 2 hearing aids preoperatively). The results of this comparison are seen in figure 1. The 9- to 12-month milestone had been reached by 68 of the 87 subjects when these comparisons were made. A Pearson correlation test yielded no significant correlation between the amount of residual hearing lost after implantation and the improvement in CNC word scores (ρ = −0.013, p = 0.92). It appears that some loss of acoustic hearing can be tolerated by this subject population with no significant negative impact on the performance as measured by CNC word scores in the combined mode condition. In most cases, where substantial acoustic hearing was lost, the contribution of the electric stimulation more than compensated for this decrease in residual hearing.
Fig. 1.
Change in low-frequency thresholds compared to percentage point improvement in CNC word scores in the combined mode (9- and 12-month follow-ups combined; n = 68). Changes were measured in reference to preoperative low-frequency thresholds and preoperative CNC word scores with 2 hearing aids. Different colors and symbols indicate the degree of postoperative low-frequency hearing loss in the implanted ear. Two subjects, with mild to moderate hearing thresholds up to 750 Hz in both ears, preferred to use their natural low-frequency hearing in both ears, rather than hearing aids, with the Hybrid implant and were tested in this listening mode. These 2 subjects were still considered to be using combined electric and acoustic hearing.
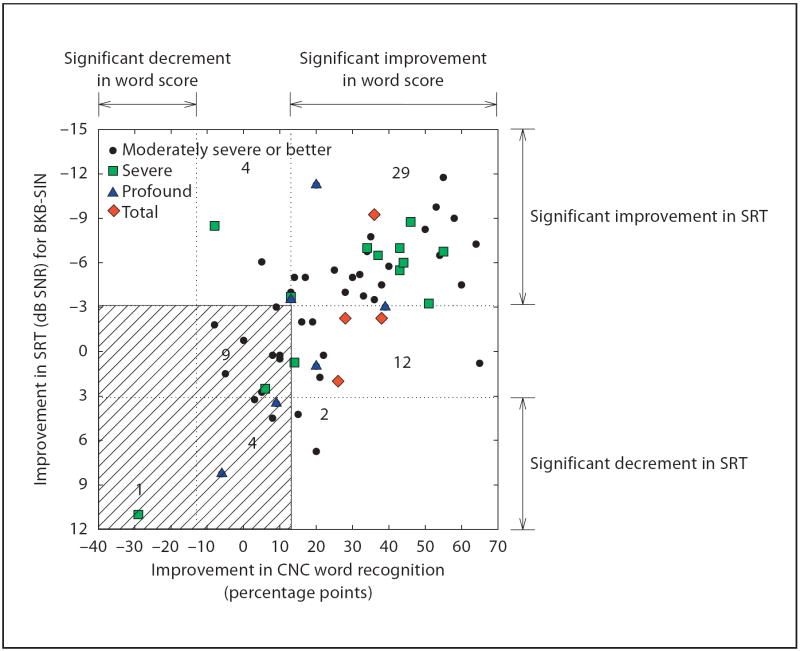
As discussed previously, 2 possible types of improvements in speech understanding have been noted in previous reports on the Iowa Hybrid device; the first is an improvement in speech understanding in quiet, the second is an improved ability to understand speech presented in a background noise. Group data for improvements in CNC words in the combined mode and changes in speech reception threshold [SRT; in dB signal-to-noise ratio (SNR)] based on the results of the BKB-SIN test for 61 subjects are shown in figure 2. Note that BKB-SIN SRTs were not measured for 7 of the 68 subjects shown in figure 1 . Improvements in either word score or speech reception threshold occurred in 45/61 (74%). Improvements in both scores were observed in 29/61 (48%). Of more concern, however, were subjects who did not show any significant enhancement with the combination of acoustic and electric processing (14 subjects in shaded area of fig. 2) or a decline on 1 of the 2 speech measures (2 subjects in lower right quadrant of fig. 2).
Fig. 2.
Improvement in CNC word recognition and speech reception thresholds for the BKB-SIN test for 61 subjects who have 9–12 months experience with their implant in the combined mode for both test measures (n = 61). Different colors and symbols indicate the degree of postoperative low-frequency hearing loss in the implanted ear. Points that fall within vertical dotted lines indicate no significant pre- to postoperative changes on the CNC word test, based on binomial (95%) comparisons. Points that fall within horizontal dotted lines indicate no significant pre- to postoperative changes for the BKB-SIN test, based on the critical difference (±3.1 dB) for the test as administered (BKB-SIN Test Manual, 2005). Seven of the subjects shown in figure 1 were not assessed on the BKB-SIN test and not included in this figure.
Multiple regression analyses on CNC scores based on demographic data were performed to find out what factors influence the CNC scores up to 12 months. Furthermore, we wished to see what influenced those who improved after surgery and those who did not improve. The outcome variable for the regression analysis was postoperative CNC score for the 68 subjects, as shown in figure 1. As potential predictor variables, we examined a combination of preoperative CNC score, age at onset of deafness, age at implantation, duration of deafness from onset to implantation, and an indicator variable of postsurgical acoustic hearing loss being greater than 30 dB.
Model selection is a statistical term for finding the best subset of important predictors in a multiple regression analysis. AIC [Akaike, 1973] is a penalized likelihood measure that assesses whether a fitted model offers an optimal balance between goodness of fit and parsimony. Ideally, large values of the criterion will correspond to candidate models which are either too simplistic to accommodate the data or unnecessarily complex. All possible models were fit and the lowest AIC score yields the subset of variables with the best model fit on CNC postoperative score. The model with the lowest AIC included preoperative CNC score and duration of deafness (F = 13.36, p < 0.0001). Twenty-nine percent of the variance (R2) can be explained by examining preoperative score and duration of deafness for these 68 people. Thus, in conjunction with the CNC preoperative score, the duration variable alone was a better predictor of postoperative CNC score than using age at implantation or age of onset for hearing loss or both. The CNC score before the operation (β = 0.56, p < 0.01) and the duration of time between age of onset of hearing loss and age implanted (β = −0.52, p < 0.01) were both significant predictors of postoperative CNC score. Approximately every 2 percentage point increase in preoperative score was indicative of a 1% increase in postoperative score, and for every 2 years of duration of deafness we expect the postoperative score to decrease by almost 1%, with the intercept at 55.14%.
In an effort to learn more about good and poor performers, we included performance in the model as an indicator variable. One approach is to consider 2 separate regression models, 1 for good performers and 1 for poor performers. A mathematically equivalent approach is to include the performance indicator variable in the earlier multiple regression model as well as all potential interactions. For simplicity and easier interpretations, we chose the former. The poor performers were 15 subjects with less than 10% improvement in CNC word recognition and the good performers were the other 53 subjects. The same regression model used on the full data set was used on this subset of people. For the poor performers, 91% of the variance can be explained by preoperative score and duration of deafness in the high frequencies. For the good performers, 33% of the variance can be explained by those variables.
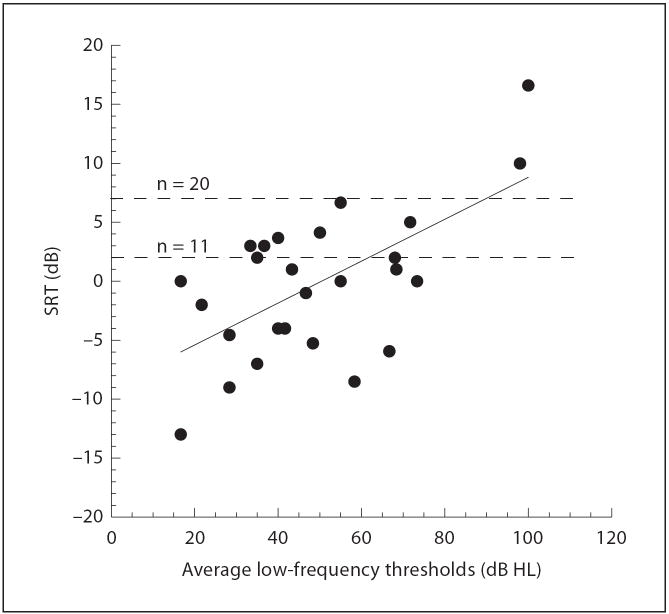
Hearing in noise is another significant advantage for the Hybrid population compared to the standard long-electrode cochlear implant population. A subgroup of 27 subjects with 12 months or more experience were tested at the University of Iowa and evaluated using spondee recognition in multitalker babble (fig. 3). In figure 3, the filled circles represent the SRT values for each of the 27 Hybrid subjects plotted as a function of their pure tone acoustic thresholds (averages of 125, 250 and 500 Hz). These data include 3 subjects whose thresholds shifted more than 30 dB. The relation between thresholds and SRT is significant (r = 0.62; p < 0.05). There are 2 dashed horizontal lines in the graph as well. The upper represents the mean SRT value (+6.7 dB) for a large group of long-electrode patients (n = 20); the lower line represents the mean SRT value (+ 1.9 dB) for a select matched group of top-performing long-electrode patients (n = 11) whose consonant recognition in quiet scores were matched to the Hybrid group. These data suggest that, on average, the advantage for speech recognition in noise of preserving residual hearing (as compared to a standard long-electrode implantation) exists unless the low-frequency postoperative hearing levels approach profound levels.
Fig. 3.
SRT for the recognition of spondees in competing talkers as a function of the low-frequency average pure tone thresholds. The 2 dashed lines at the top represent the average long-electrode SRT values for the large unselected group (n = 20) and the smaller matched group (n = 11).
Discussion
The Iowa/Nucleus Hybrid FDA clinical trial completed enrollment with 87 adult subjects. Preservation of some acoustic hearing 1 month following implantation was found in 98% of subjects. Six additional subjects lost all acoustic hearing between 3 and 60 months. Twenty-five percent (18/72; fig. 1) of subjects experienced more than 30 dB of hearing loss 3–12 months following implantation. It is interesting that many in this group did not call to report any significant change in hearing, in contrast to a group of hearing subjects who were seen for sudden hearing loss syndrome. Some reported that they needed to turn up the gain in their hearing aids, but did not experience a loss of acoustic speech discrimination. Importantly, however, for this group it appears that loss of more than 30 dB in pure tone threshold has not had a significant impact on speech perception in quiet in the combined mode (hearing aids both ears + Hybrid) as it does not predict a poor outcome, as seen in figure 1 (the Pearson correlation test did not demonstrate a correlation between threshold shift and CNC word score). It could be argued, however, that the speech understanding scores for those who lose acoustic hearing would likely be better if they had not experienced additional pure tone threshold loss.
The speech recognition (in quiet backgrounds) performance for Hybrid users has been reported in previous publications [Gantz et al., 2005, 2006]. There are several ways to view the success of the Hybrid cochlear implant strategy. One view of the Hybrid device’s success comes from comparing the ipsilateral acoustic-alone speech recognition performance (implant speech processor turned off, contralateral ear plugged and/or ear muffed) to the performance obtained when the electric stimulation is added ipsilaterally (Hybrid mode, implant plus hearing aid). Most subjects used a hearing aid in the implanted ear, depending on their degree of hearing loss. In Reiss et al. [2007], we reported the most recent results of such a comparison using /aCa/ consonant speech materials. The mean acoustic-alone scores were 44% correct after 12 months of implant use. The addition of electric stimulation increased this score to 58%; after 24 months, the score increased to 62%. The improvements across patients ranged from 0 to nearly 60%.
An alternative method of looking at the benefits of the Hybrid implant is to use a more ‘real-world’ comparison in which patients are tested when listening with both ears (the implanted ear and the contralateral acoustic-only ear). In these tests [Reiss et al., 2007], the speech materials were CNC words and the results only included patients implanted and tested at Iowa. Again, some subjects wore a hearing aid in the implanted ear (and also sometimes in the contralateral ear) depending on their degree of hearing loss. The mean preoperative bilateral acoustic-only score was 35%. At 12 months after implantation, their mean score was 74% with the Hybrid contribution added, and at 24 months of implant experience their scores remained constant at 73%. The range of improvements across patients was from 8 to nearly 70% and depended on their preoperative acoustic hearing tested with binaural hearing aids.
Improved speech perception in quiet occured for most of the FDA clinical trial group. The statistical model presented in the results section provides some insight into selection criteria variables that impact the performance with a 10-mm Hybrid implant. The predictive performance model takes into account the preoperative speech perception score using the CNC word test and duration of high-frequency hearing loss. Certainly, the postoperative speech perception score is highly related to the preoperative score (r = 0.39, p < 0.001). The percentage of variance explained is not particularly high at 15% since it considers both those who did well and poor performers, which should be considered as different populations. Still, after adjusting for preoperative score, the duration explains about 8.5% of the remaining overall variation, which is statistically significant (p < 0.02). It is important to consider preoperative score in such a model because subjects who score well on their preoperative CNC test do not have as much room to improve as someone who scores poorly. In other words, a 10% increase might be a good outcome for a preoperative CNC score of 80%, yet a 10% increase might only be a mediocre outcome for a preoperative CNC score of 30%. The multiple regression analysis takes this information into account by adjusting for the subjects’ preoperative CNC score. After adjusting for the preoperative score, we found that duration of deafness is still highly significant and negatively associated with improvement in speech perception, and should be considered at least a partial factor determining the poor performance of the group of 15 subjects. Increasing the Hybrid subject pool will determine if this preliminary model will be useful clinically. Duration of hearing loss is a known factor accounting for significant variance, and has a negative impact on performance with standard cochlear implants [Gantz et al., 1993; Rubinstein et al., 1999]. It was surprising to see the same impact on the Hybrid population, but it is reasonable to assume that loss of ganglion cells over the duration of severe-to-profound high-frequency hearing loss in the basal region of the cochlea contributes to the inability of this group to benefit from electric stimulation. Subjects with a long duration of high-frequency hearing loss (possibly more than 30 years) might require a longer electrode to access residual neuronal elements. Duration and age at implantation are associated with and also have a negative impact on the use of the Hybrid implant. Older adults might not be able to integrate the acoustic and electric information as a result of cognitive issues. The effects of age and cognitive function on use of the Hybrid implant have not been studied, but should be in the future.
Standard cochlear implants have done an excellent job of providing improved speech understanding in quiet; however, their performance in noise is quite poor. Better frequency resolution improves hearing in noise, as has been shown by Qin and Oxenham [2003]. Turner et al. [2004] demonstrated, using simulated Hybrid processing of speech presented to normal-hearing subjects, that high-frequency electric stimulation along with acoustic low-frequency hearing (as compared to full speech range electric stimulation alone) had the potential to provide a significant advantage for understanding speech in background noise, particularly when the competing signal was other talkers. The Turner et al. [2004] findings demonstrate that improving frequency resolution in only the low-frequency speech region can show this advantage. Turner et al. [2004] also tested 3 Hybrid patients using speech in backgrounds. When those 3 subjects were compared to a group of long-electrode patients (whose speech scores in quiet were matched to the Hybrid subjects), a significant advantage was also observed. The subgroup of 27 Hybrid subjects reported here provide further evidence that preservation of low-frequency acoustic hearing is important for hearing in noise. Furthermore, the better the preservation of low-frequency hearing, the better the performance in multitalker background noise for this group of individuals, although as long as residual hearing can be preserved to levels no poorer than a severe loss, a benefit is observed when compared to traditional long-electrode implants.
Acknowledgments
Supported (in part) by research grants R01 DC000377 and 2 P50 DC00242 from the National Institutes on Deafness and Other Communication Disorders, National Institutes of Health; grant RR00059 from the General Clinical Research Centers Program, NCRR, National Institutes of Health; Iowa Lions Sight and Hearing Foundation; and the Cochlear Corporation for developing a cochlear implant to our specifications and providing initial devices at no cost.
Footnotes
Disclosure Statement Dr. Gantz is a consultant to Cochlear Corporation.
References
- Akaikie H. Information theory as an extension of the maximum likelihood principle. In: Akaikie H, Petrov VN, Csaki F, et al., editors. Second International Symposium on Information Theory; Budapest. Akademia Kiado; 1973. pp. 267–281. [Google Scholar]
- ►.Byrne D, Parkinson A, Newall P. Hearing aid gain and frequency response requirements for the severely/profoundly hearing-impaired. Ear Hear. 1990;11:40–49. doi: 10.1097/00003446-199002000-00009. [DOI] [PubMed] [Google Scholar]
- Dillon H. NAL-NL1: A new prescriptive fitting procedure for non-linear hearing aids. Hear J. 1999;52:10–16. [Google Scholar]
- ►.Fraysse B, Ramos A, Sterkers O, et al. Residual hearing conservation and electro-acoustic stimulation with the Nucleus 24 Contour Advance cochlear implant. Otol Neurotol. 2006;27:624–633. doi: 10.1097/01.mao.0000226289.04048.0f. [DOI] [PubMed] [Google Scholar]
- ►.Gantz BJ, Turner CW. Combining acoustic and electrical hearing. Laryngoscope. 2003;113:1726–1730. doi: 10.1097/00005537-200310000-00012. [DOI] [PubMed] [Google Scholar]
- ►.Gantz BJ, Turner CW. Combining acoustic and electric speech processing: Iowa/Nucleus Hybrid implant. Acta Otolaryngologica. 2004;24:344–347. doi: 10.1080/00016480410016423. [DOI] [PubMed] [Google Scholar]
- ►.Gantz BJ, Turner CW, Gfeller KE. Acoustic plus electric speech processing: results of a multi-center clinical trial of the Iowa/Nucleus Hybrid implant. Audiol Neurootol. 2006;11(suppl 1):63–68. doi: 10.1159/000095616. [DOI] [PubMed] [Google Scholar]
- ►.Gantz BJ, Turner CW, Gfeller KE, Lowder MW. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. Laryngoscope. 2005;115:796–802. doi: 10.1097/01.MLG.0000157695.07536.D2. [DOI] [PubMed] [Google Scholar]
- ►.Gantz BJ, Woodworth GG, Knutson JF, Abbas PJ, Tyler RS. Multivariate predictors of audiological success with cochlear implants. Ann Otol Rhinol Laryngol. 1993;48:153–167. doi: 10.1177/000348949310201201. [DOI] [PubMed] [Google Scholar]
- ►.Gfeller K, Olszewski C, Turner CW, Gantz B. Music perception with cochlear implants and residual hearing. Audiol Neurootol. 2006;11(suppl 1):12–15. doi: 10.1159/000095608. [DOI] [PubMed] [Google Scholar]
- ►.Gstoettner W, Kiefer J, Baumgartner W, Pok S, Peters S, Adunka O. Hearing preservation in cochlear implantation for electric acoustic stimulation. Acta Otolaryngologica. 2004;124:348–352. doi: 10.1080/00016480410016432. [DOI] [PubMed] [Google Scholar]
- ►.Hogan CA, Turner CW. High-frequency audibility: benefits for hearing-impaired listeners. J Acoust Soc Am. 1998;104:432–441. doi: 10.1121/1.423247. [DOI] [PubMed] [Google Scholar]
- ►.Liberman MC, Dodds LW. Single-neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves. Hear Res. 1984;6:55–74. doi: 10.1016/0378-5955(84)90025-x. [DOI] [PubMed] [Google Scholar]
- ►.Peterson FE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Dis. 1962;27:62–70. doi: 10.1044/jshd.2701.62. [DOI] [PubMed] [Google Scholar]
- ►.Qin MK, Oxenham AJ. Effects of simulated cochlear implant processing on speech reception in fluctuating maskers. J Acoust Soc Am. 2003;114:446–454. doi: 10.1121/1.1579009. [DOI] [PubMed] [Google Scholar]
- ►.Reiss LA, Turner CW, Erenberg SR, Gantz B. Changes in pitch with a cochlear implant over time. J Assoc Res Otol. 2007;8:241–257. doi: 10.1007/s10162-007-0077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ►.Rubinstein JT, Parkinson WS, Tyler RS, Gantz BJ. Residual speech recognition and cochlear implant performance: effects of implantation criteria. Am J Otol. 1999;20:445–452. [PubMed] [Google Scholar]
- ►.Schmiedt RA, Lang H, Okamura HO, Schulte BA. Effects of furosemide applied chronically to the round window: a model of metabolic presbyacusis. J NeuroSci. 2002;22:9643–9650. doi: 10.1523/JNEUROSCI.22-21-09643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ►.Turner CW, Gantz BJ. Preservation of residual acoustic hearing in cochlear implantation. Int Congr Ser. 2004;1273:243–246. [Google Scholar]
- ►.Turner CW, Gantz BJ. Combined acoustic and electric hearing for severe high-frequency hearing loss. Audiol Today. 2005;17:14–15. [Google Scholar]
- ►.Turner CW, Gantz BJ, Vidal C, Behrens A. Speech recognition in noise for cochlear implant listeners: benefits of residual acoustic hearing. J Acoust Soc Am. 2004;115:1729–1735. doi: 10.1121/1.1687425. [DOI] [PubMed] [Google Scholar]
- ►.Von Ilberg C, Keifer J, Tillein J, et al. Electro-acoustic stimulation of the auditory system. Ann Otol Rhinol Laryngol. 1999;61:334–340. doi: 10.1159/000027695. [DOI] [PubMed] [Google Scholar]