Abstract
Background
While adverse rearing is thought to alter threat responding, the effects on appetitive behavior remains minimally explored. This study examines the effects that early-life emotional adversity has on response to both threatening and appetitive stimuli in juvenile rhesus monkeys.
Methods
Twenty-four, two year old monkeys with differential rearing histories were tested for fear-potentiated startle responding and consumption of an artificially sweetened solution.
Results
Relative to monkeys reared under typical conditions, monkeys removed from their mothers at birth and reared with peers demonstrated both increases in reward responding, as evidenced by greater consumption of a palatable solution in a free choice test, and increased threat responding, as evidenced by enhanced fear potentiated startle responding.
Conclusions
Findings suggest that early rearing impacts juvenile manifestations of both appetitive and aversive emotional systems. Results are discussed in the context of development, anxiety, depression and substance abuse.
Keywords: Development, Anxiety, Depression, Hedonic, Fear
Adverse early-life emotional experiences heighten risk for adult mood disorders [1]. Animal models suggest that these associations reflect the effects of early environment on organization of threat related brain circuits [2, 3]. Although there is some indication of changes in reward related neurobiology as well [1, 4], relatively few studies have focused on the relationship between early adversity and response to reward, and among those that have conflicting data emerge [4–10]. This is an important issue as developmental organization of both appetitive and aversive emotional systems likely contribute to the pathophysiology of mood disorders. In the present study we examined responses to threatening and rewarding stimuli in juvenile rhesus monkeys who underwent peer (PR) or mother (MR) rearing in early life.
Materials and Methods
Subjects and Rearing
All procedures were approved by the NIH Animal Care and Use Committee. Twenty-four male monkeys from three annual birth cohorts were randomly assigned to Peer (PR) or Mother (MR) rearing conditions at birth. These procedures have been detailed elsewhere (see supplements 1 and 2 for rationale and details). Briefly PR monkeys were removed from their mothers at birth, cared for by humans for several weeks and then housed with peers until they were approximately 8 months old. In contrast MR monkeys remained in an indoor-outdoor semi-natural environment with multiple generations of other monkeys. Both groups were then housed together in a large pen for several months and then moved to indoor pair housing with a like reared cage-mate in a room with other pair housed monkeys. Animals received standard laboratory care which included ad libitum access to water and daily feeding.
Aspartame Testing
Aspartame preference testing began when animals were between 18-24 months of age. Testing was conducted over 3–4 consecutive days in the early to mid afternoon. On the day of testing water bottles were removed from the home cage 1 hour prior to testing. Monkey pairs were then separated from their cage-mates with the insertion of an opaque divider and each monkey had two bottles attached to their side of the home cage. One bottle contained 800 ml of 0.37% aspartame (w:w). The other bottle was a standard water bottle and contained 800 ml tap water and was attached on the opposite side of the cage. After 60 minutes experimenters returned and measured amount of each solution consumed. Although we attempted to get three consecutive days of testing on all animals technical problems resulted in the need for an additional day of testing in some animals. In addition one PR subject repeatedly knocked both bottles off the cage. We were not able to obtain reliable data for this subject.
Startle Testing
The startle protocol has been described in detail previously [11, 12]. All startle was measured inside a sound attenuating, lightproof chamber, equipped with a primate chair, an accelerometer connected to an amplifier (Endevco, San Juan Capistrano, CA). Fear potentiated startle (FPS) was assessed in two daily sessions consisting of 40 startle probes of three intensities (95dB, 105dB, and 115dB) delivered through audio speakers. Startle sessions were initiated in a darkened startle chamber and half of the probes occurred in the dark. The other half occurred after overhead lights inside the chamber were illuminated (CS+). On the first and last trials, and two other randomly determined trials overhead light illumination inside the chamber terminated in the delivery of a 500 ms burst of compressed air to the face. Thus four of the lights-on startle trials were learning trials and the remaining 36 of the startle probes occurred in the absence of the aversive air blasts.
Results
Aspartame Consumption
A repeated measures ANOVA revealed a significant rearing-group-by-choice interaction, F (1, 21) = 5.33, p <.05, indicating a greater preference for the aspartame solution than water in the PR group (see Fig One). Main effects for solution choice F (1, 21) = 13.08, p <.01, and for test day F (2, 42) = 6.94, p <.01, indicated greater consumption of aspartame solution than water and more overall consumption across test days in both groups, respectively. The three-way group by choice by test day interaction was not significant indicating group differences in solution choice did not vary significantly across the testing days.
Figure One.
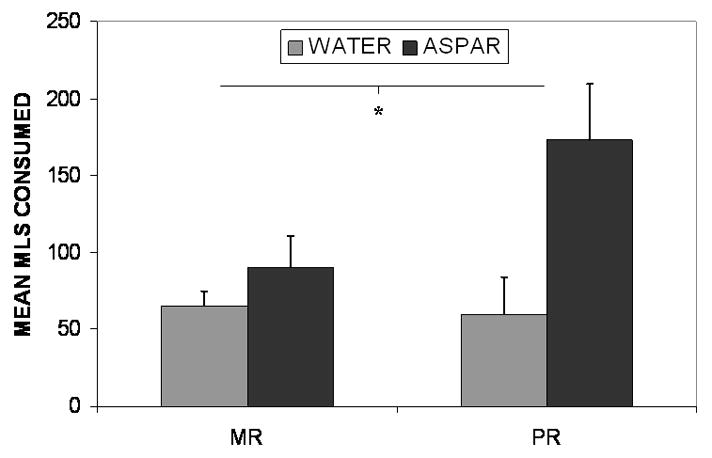
Figure One depicts the mean (+S.E.M.) milliliters of water (gray bars) and an aspartame sweetened solution (black bars) consumed by mother reared (MR) and peer reared (PR) monkeys across three one-hour tests in the home cage.
Follow-up ANOVAs were conducted on the aspartame and water consumption separately.The aspartame ANOVA revealed a significant main effect for rearing group F (1, 21) = 5.27, p <.05 and a significant effect of test day F (2, 42) = 4.34, p<.05. Analysis of water consumption revealed no significant differences.
Fear Potentiated Startle
Previous work with this protocol found an amygdala-dependent fear potentiation of startle develops across testing/conditioning days and varies with startle probe intensity [11, 12]. Accordingly, startle was measured over two testing days and employed three probe intensities.
All startle responses were subject to a four factor repeated-measures ANOVA with rearing group, illumination condition, probe intensity, and test day as factors. This analysis revealed the hypothesized four-way interaction F (2, 21) = 5.75, p =.01 (see Fig Two). Post-hoc testing revealed that the four way interaction reflected group-by light-interactions for each of the two highest intensity stimuli on the second test day: 105dB F (1, 22) = 4.82, p<.05; 115db; F (1, 22) = 6.86, p <.05. This result suggests that PR animals, relative to their MR counterparts, acquire a greater startle response when tested in a threatening environment, at relatively strong startle-elicitation intensities. A significant group-by-stimulus intensity-by-light interaction was also found across both test days F (2, 21) = 5.83, p <.01 indicating a greater FPS response in the PR group across testing days, again, at strong intensities; and an overall effect of group (PR>MR) collapsed across day, stimulus intensity and light condition, F (1, 22) = 4.81, p <.05 was found, indicating overall higher startle responses in the PR group.
Figure Two.
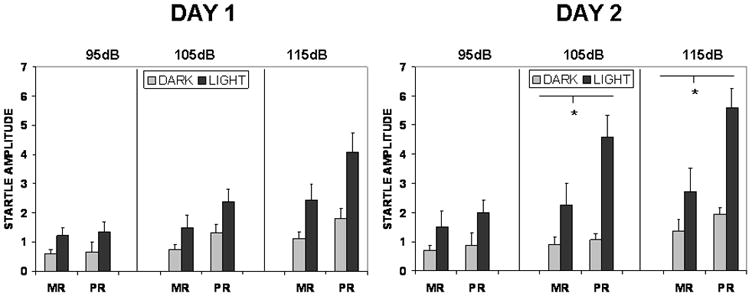
Figure Two depicts the mean (+S.E.M.) startle response of mother reared and peer reared monkeys across two testing days. Data are displayed for three startle intensities presented in either a darkened (black) or illuminated (gray) chamber. Overhead lights were associated with an aversive blast of compressed air. Significantly greater startle responses occurred in the light than in the dark for the PR than the MR monkeys at the two highest probe intensities on the second test day.
Discussion
Results demonstrate that an adverse rearing environment may increase responding to both threatening and rewarding stimuli. The FPS finding is consistent with many studies linking early adversity to enhanced threat responding; while the aspartame findings indicate that the same manipulation created an enhanced response to an appetitive stimulus. It is unclear at this point whether the enhanced reward consumption is a reflection of increased “wanting” or increased “liking” in the traditional conception of reward, although the enhanced consumption suggests it is not limited to “wanting” alone.
At a mechanistic level the reward-aversion relationship likely indicates altered processes in structures such as amygdala or striatum that have overlapping functional roles in appetitive and aversive responding [13, 14]. Alternatively a heightened response to rewarding stimuli may not represent any functional alteration in reward circuitry per se but may be a behavioral palliative for the negative emotions that are experienced to a greater degree in adversely reared animals [15].
The reward interpretation should be viewed somewhat cautiously because it is the response to a single stimulus and is contradicted by some [4, 8, 9, 16], but not all [5–7], similar studies. Moreover, between-group differences in nutrition or various aspects of sensory processing also could influence the observed patterns of responding. However, while we do not know the extent to which the potentiated consumption extends to other sensory modalities, it is unlikely to merely reflect unique responding to aspartame as we have tested a subset of these same monkeys with a sucrose solution and found a similar response profile (see supplement 3). Interestingly, these data are also consistent with three recently published human studies in anxious or behaviorally-inhibited adolescents that displayed an enhanced neuronal response to positive affective stimuli [17–19].
Variable results across prior nonhuman primate studies might reflect study-related differences in developmental state at testing. Risk factors such as early life adversity or inhibited temperament may produce early, non-specific hyper-emotionality, which becomes increasingly valence-specific with maturity. It is noteworthy in the present context that a previously published study reported a reduction in sucrose consumption in adult monkeys who had undergone early life adverse rearing [8]. Taken together, current and prior work suggests that the early developmental period may be a sensitive period for the initial organization of affective systems, which then may be further shaped by experiences across development. For example, several reports have found that exposure to substances of abuse or to stressful stimuli in immature individuals can have profoundly different long-term effects than exposure of those same stimuli in maturity [20].
In sum, early developmental experience clearly influences the behavioral response to negatively-valenced stimuli. Under some conditions it also appears to influence the response to positively-valenced stimuli. Understanding the manner in which positive affect, negative affect and development interact emerges as one of the important challenges for understanding mood disorders.
Supplementary Material
Acknowledgments
This research was supported entirely by the Intramural Research Program of the NIH, NIMH. The authors are indebted to the contributions of the animal care and veterinary staff of the NIH Animal Research facility.
Footnotes
Financial Disclosures
The authors have no conflicts of interest to report.
References
- 1.Gilmer WS, McKinney WT. Early experience and depressive disorders: human and non-human primate studies. J Affect Disord. 2003;75(2):97–113. doi: 10.1016/s0165-0327(03)00046-6. [DOI] [PubMed] [Google Scholar]
- 2.Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci. 2005;7(2):103–23. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nemeroff CB. Early-Life Adversity, CRF Dysregulation, and Vulnerability to Mood and Anxiety Disorders. Psychopharmacol Bull. 2004;38(Suppl 1):14–20. [PubMed] [Google Scholar]
- 4.Matthews K, Robbins TW. Early experience as a determinant of adult behavioural responses to reward: the effects of repeated maternal separation in the rat. Neurosci Biobehav Rev. 2003;27(1–2):45–55. doi: 10.1016/s0149-7634(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 5.Higley JD, et al. Nonhuman primate model of alcohol abuse: effects of early experience, personality, and stress on alcohol consumption. Proc Natl Acad Sci U S A. 1991;88(16):7261–5. doi: 10.1073/pnas.88.16.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lomanowska AM, et al. Artificial rearing alters the response of rats to natural and drug-mediated rewards. Dev Psychobiol. 2006;48(4):301–14. doi: 10.1002/dev.20139. [DOI] [PubMed] [Google Scholar]
- 7.Michaels CC, Holtzman SG. Neonatal stress and litter composition alter sucrose intake in both rat dam and offspring. Physiol Behav. 2006;89(5):735–41. doi: 10.1016/j.physbeh.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Paul IA, English JA, Halaris A. Sucrose and quinine intake by maternally-deprived and control rhesus monkeys. Behav Brain Res. 2000;112(1–2):127–34. doi: 10.1016/s0166-4328(00)00173-x. [DOI] [PubMed] [Google Scholar]
- 9.Pryce CR, et al. Deprivation of parenting disrupts development of homeostatic and reward systems in marmoset monkey offspring. Biol Psychiatry. 2004;56(2):72–9. doi: 10.1016/j.biopsych.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Ruedi-Bettschen D, et al. Early deprivation under specific conditions leads to reduced interest in reward in adulthood in Wistar rats. Behav Brain Res. 2005;156(2):297–310. doi: 10.1016/j.bbr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Antoniadis EA, et al. Role of the primate amygdala in fear-potentiated startle: effects of chronic lesions in the rhesus monkey. J Neurosci. 2007;27(28):7386–96. doi: 10.1523/JNEUROSCI.5643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winslow JT, Parr LA, Davis M. Acoustic startle, prepulse inhibition, and fear-potentiated startle measured in rhesus monkeys. Biol Psychiatry. 2002;51(11):859–66. doi: 10.1016/s0006-3223(02)01345-8. [DOI] [PubMed] [Google Scholar]
- 13.Belova MA, et al. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55(6):970–84. doi: 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds SM, Berridge KC. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nat Neurosci. 2008;11(4):423–5. doi: 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volkow ND. The reality of comorbidity: depression and drug abuse. Biol Psychiatry. 2004;56(10):714–7. doi: 10.1016/j.biopsych.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Ruedi-Bettschen D, et al. Early deprivation leads to altered behavioural, autonomic and endocrine responses to environmental challenge in adult Fischer rats. Eur J Neurosci. 2006;24(10):2879–93. doi: 10.1111/j.1460-9568.2006.05158.x. [DOI] [PubMed] [Google Scholar]
- 17.Bar-Haim Y, et al. Neural correlates of reward processing in adolescents with a history of shyness and inhibited temperament. Psychological Science. 2009 doi: 10.1111/j.1467-9280.2009.02401.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forbes EE, et al. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. J Child Psychol Psychiatry. 2006;47(10):1031–40. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guyer AE, et al. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. J Neurosci. 2006;26(24):6399–405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


