Abstract
In human and non-human primates, higher form vision matures substantially later than spatial acuity and contrast sensitivity, as revealed by performance on such tasks as figure-ground segregation and contour integration. Our goal was to understand whether delayed maturation on these tasks was intrinsically form-dependent or, rather, related to the nature of spatial integration necessary for extracting task-relevant cues. We used an intermediate-level form task that did not call for extensive spatial integration. We trained monkeys (6–201 weeks) to discriminate the orientation of pattern modulation in a two-alternative forced choice paradigm. We presented two families of form patterns, defined by texture or contrast variations, and luminance-defined patterns for comparison. Infant monkeys could discriminate texture- and contrast-defined form as early as 6 weeks; sensitivity improved up to 40 weeks. Surprisingly, sensitivity for texture- and contrast-defined form matured earlier than for luminance-defined form. These results suggest that intermediate-level form vision develops in concert with basic spatial vision rather than following sequentially. Comparison with earlier results reveals that different aspects of form vision develop over different time courses, with processes that depend on comparing local image content maturing earlier than those requiring “global” linking of multiple visual elements across a larger spatial extent.
Keywords: visual development, form vision, texture sensitivity, first-order, second-order, contrast sensitivity, macaque
Introduction
Visual form cues range in complexity and spatial extent. Form vision is therefore limited by the processes that mediate the detection and integration of signals over space. The time course over which these mechanisms develop gives an indication of their underlying neural substrates, making it of interest to examine behavioral sensitivity to different form cues throughout development. Basic spatial vision is established early; infant monkeys can detect sinusoidal luminance gratings at birth and reach adult levels of acuity and contrast sensitivity by the end of the first postnatal year (Boothe, Kiorpes, Williams, & Teller, 1988; Kiorpes, 1992). When tested with complex form cues, however, infant monkeys show delayed visual maturation. They are unable to extract global structure in Glass pattern displays before 12 weeks (Kiorpes & Movshon, 2003) and fail to detect extended contours composed of collinear Gabor patches before 20 weeks (Kiorpes & Bassin, 2003). These complex aspects of form vision show an extended developmental time course, continuing over the first 18–24 months after birth.
Why do different aspects of form vision develop at different rates? The answer may be related to stimulus content and task structure. For example, simple spatial resolution and vernier acuity, which mature along similar time courses during the first postnatal year (Kiorpes, 1992), may be mediated by “local” mechanisms sensitive to changes in luminance or contrast across small image regions and may therefore depend on the output of linear filters or channels on the scale of the receptive field of a neuron in primary visual cortex (e.g., Parker & Hawken, 1985). Contour integration and Glass pattern discrimination, which continue to develop for 2 years or more, must be mediated by mechanisms that integrate information over a large number of spatially dispersed visual elements; such tasks depend on the output of many filters or receptive fields at different positions in visual space. The degree to which form signals must be integrated to extract task-relevant information may therefore account for the different developmental trajectories. If so, discrimination performance on a form task of intermediate complexity, for example, one that depends on the output of several nearby channels, might develop at an intermediate rate between the two extremes of basic acuity and global form perception.
To test this idea, we trained developing macaque monkeys on form discrimination tasks which did not require extensive integration of signals over space, but which also required some non-local visual analysis. We used two families of second-order form stimuli: texture-defined form, based on a prior study of second-order texture perception in adult humans (Landy & Oruç, 2002), and contrast-defined form like that used by Solomon and Sperling (1995). These stimuli have two advantages. First, they have a well-defined set of parameters that can be manipulated systematically for psychophysical testing (modulation depth and spatial frequency). Human psychophysical data using these stimuli showed that subjects could perform discriminations over a wide range of stimulus parameters, indicating the salience of these cues and the robustness of texture segregation in this task (Landy & Oruç, 2002; Larsson, Landy, & Heeger, 2006). Second, the relevant cues in these stimuli can be extracted by relatively simple spatial mechanisms, as exemplified by the “filter–rectify–filter” (FRF) model of texture analysis (reviewed in Landy & Graham, 2004). In this model, a visual pattern is first analyzed by a bank of linear filters sensitive to luminance contrast; such mechanisms are known to be functional early in development. The output of this first filtering stage is then rectified and pooled by a second filtering stage consisting of a linear filter of larger scale (lower spatial frequency). The resulting “second-order” filter is sensitive to modulations of orientation or contrast across its spatial extent and can be used to detect and discriminate the orientation of texture boundaries by comparing image content in neighboring subregions.
Texture patterns have been used in several studies of human visual development. Recordings of visual-evoked potentials in infants showed significant signals driven by global texture structure at 2–5 months (Arcand et al., 2007; Norcia et al., 2005). Using visual preference testing, infants as young as 3 months have been shown to detect texture patches defined by differences in the orientation of line elements, element size, or contrast embedded in larger homogeneous displays (Atkinson & Braddick, 1992; Rieth & Sireteanu, 1994). Preference for these displays increases gradually over the course of infancy and childhood and depends to some degree on stimulus configuration, context, and element density (Sireteanu & Rieth, 1992; Sireteanu, Encke, & Bachert, 2005). Lewis, Kingdon, Ellemberg, and Maurer (2007) tested the ability of 5-year-old children to discriminate the orientation of contrast-modulated second-order gratings. The children had no difficulty discriminating the orientation of the patterns although their thresholds were significantly higher than those of the adults (18–25 years). Due to differences in stimulus design and testing conditions across these studies and to the limited data available between infancy and age 5 years, the developmental trajectory for texture sensitivity in humans remains incompletely defined.
To assess the full developmental profile of second-order form sensitivity, we tested macaque monkeys longitudinally and cross-sectionally through development and directly compared visual texture sensitivity to luminance contrast sensitivity. Infant monkeys were able to discriminate the orientation of texture-defined form as early as 6 weeks, and their behavioral sensitivity was adult-like around 40 weeks. This was significantly earlier than they could perform more global form tasks. Interestingly, sensitivity to both texture- and contrast-defined form matured earlier than sensitivity to luminance-defined form. Our results suggest that mechanisms subserving texture analysis develop in parallel with—or are the same as—the mechanisms of basic spatial vision. Texture cues can therefore support visual discriminations in relatively young infants. The late development of performance on global form tasks does not reflect general limitations of infant form vision but may instead depend on the spatial extent over which signals must be integrated to derive task-relevant form information.
Methods
Subjects
We tested 18 visually normal pig-tailed macaque monkeys (Macaca nemestrina), ranging in age at the time of testing from 6 to 201 weeks. Of these, 12 were tested longitudinally 2–4 times throughout development at a median interval of 33 weeks; 6 were tested cross-sectionally. All animals were hand-raised from infancy in our primate nursery. The visual environment was enriched with a wide variety of appropriate visual and tactile stimuli. The animals were also given daily opportunities for interaction with other monkeys and humans. Animal care and testing protocols were approved by the New York University UAWC and conformed to the NIH Guide for the Care and Use of Laboratory Animals.
Visual stimuli
Stimuli were presented on a 21-inch Eizo FlexScan FX-E8 color display monitor at a refresh rate of 100 Hz. The display was driven by a Dell Optiflex GX1 computer via a VSG2/3 graphics card (Cambridge Research Systems, Cambridge, UK). Animals viewed the display at a distance of 50 cm (young infants) or 100 cm (older animals). Stimuli subtended 15.4° or 7.7° of visual angle at these distances and were presented to the left and right of screen center at an eccentricity of 11.6° or 5.8°, respectively.
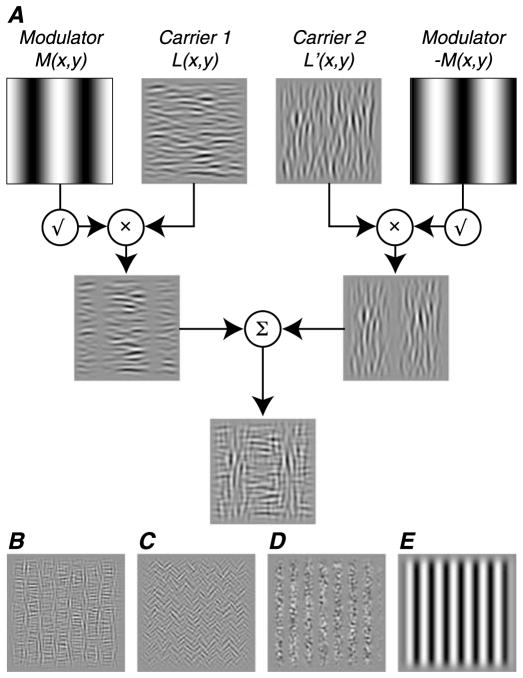
We presented two families of second-order patterns, which were used previously in human psychophysical and imaging studies (Landy & Oruç, 2002; Larrson et al., 2006; Solomon & Sperling, 1995). In the first family (“texture-defined”), form cues were defined by differences in the orientation content of neighboring image subregions, resulting in a texture boundary (Figure 1A, adapted from Landy & Oruç, 2002). The stimuli were created by combining two orthogonal 2-D noise patterns (carriers 1 and 2), each of which was amplitude-modulated by a sinusoidal grating (modulators M and −M). Before combination, a square root operation was applied to each modulator to maintain constant contrast energy across the final stimulus image. We generated two versions: one from horizontal and vertical carriers (Figure 1B), the other from right- and left-oblique carriers (Figure 1C). In the second family (“contrast-defined”), form cues were defined by differences in contrast in neighboring image subregions; these were created by amplitude modulation of an isotropic 2-D noise pattern (Figure 1D). For both stimulus families, space-average luminance was constant across the image, making them second-order by definition; the form cues could not be detected by linear filters. The modulator envelope, which could be vertical or horizontal, defined the orientation of the relevant form cue used to perform the discrimination task. For comparison, we also presented “first-order” gratings, which use luminance-defined form to measure luminance contrast sensitivity (Figure 1E).
Figure 1.
Stimulus composition. (A) Texture-defined form stimuli were created by periodic modulation of two orthogonal noise patterns (carriers 1 and 2). Each carrier pattern was multiplied by a low spatial frequency grating (modulators M and −M). A square root operation was applied to each modulator to ensure that contrast energy did not vary across the final image. The resulting contrast-modulated carrier patterns were combined additively to produce the final image (adapted from Landy & Oruç, 2002). (B) Stimulus composed of vertical and horizontal carriers. (C) Stimulus composed of right- and left-oblique carriers. (D) Contrast-defined form stimulus created by sinusoidal contrast modulation of an isotropic noise carrier pattern. (E) Control luminance-defined stimulus.
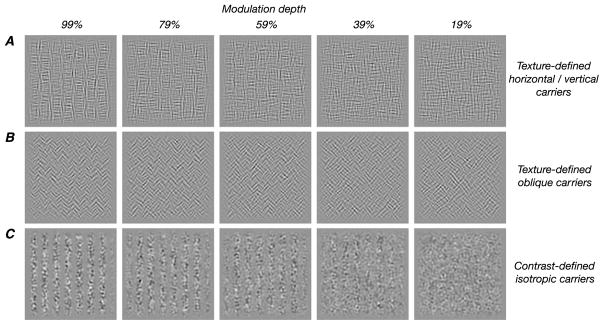
For texture stimuli, we varied two parameters: the amplitude of the modulator, which controlled the strength of the form cue (modulation depth 9–99%), and the spatial frequency of the modulator envelope (0.38–3.76 c/deg). Figure 2 shows example stimuli at 5 modulation depths (99%, 79%, 59%, 39%, and 19%). Orientation discriminations based on stimuli with 99% modulation depth were comparatively easy; those with 19% were difficult. The center frequency of the carrier was at least one octave higher than the modulator frequency to minimize the impact of first-order spectral cues. The combinations of modulator and carrier spatial frequencies are shown in Table 1.
Figure 2.
Texture modulation depth. Example stimuli showing the effect of varying the depth of texture modulation. Columns from left to right show stimuli of decreasing modulation depth (99%, 79%, 59%, 39%, and 19%). (A) Texture-defined form stimuli composed of horizontal/vertical carrier patterns (created as shown in Figure 1). (B) Texture-defined form stimuli composed of right/left oblique carrier patterns. (C) Contrast-defined form stimuli created by sinusoidal contrast modulation of an isotropic noise carrier pattern.
Table 1.
Combinations of modulator and carrier spatial frequencies used in our texture patterns.
| Modulator (cpd) | Carrier center (cpd) |
|---|---|
| 0.47 | 1.88 |
| 0.94 | 3.76 |
| 1.88 | 3.76 |
| 3.77 | 7.54 |
For first-order patterns, we varied luminance contrast (0.15–99 %) and spatial frequency (0.19–7.54 c/deg).
Two-alternative forced choice task
We used standard operant conditioning techniques to measure psychophysical thresholds. Training and testing procedures have been described in detail previously (Kiorpes, Kiper, & Movshon, 1993). We trained animals to discriminate the orientation of pattern modulation in a spatial two-alternative forced choice paradigm. The primary second-order task was to discriminate vertical from horizontal patterns as a function of modulation depth (texture- or contrast-defined). The comparison first-order task was to discriminate vertical from horizontal patterns as a function of luminance contrast. In each case, subjects indicated which of two patches contained the vertical pattern. We have previously shown that monkeys as young as 6 weeks can easily perform the first-order orientation discrimination (Hall-Haro & Kiorpes, 2008).
Animals viewed the stimuli for a minimum of 500 ms, after which they were free to respond; maximum stimulus duration was 3 s. Subjects indicated their choices either by making a saccade to the correct target (young macaques, 6–12 weeks) or by pulling one of two grab bars located within their reach. Monkeys received liquid rewards for correct discriminations; error trials were signaled by a tone and a brief time-out period. Subjects were first trained to perform the orientation discrimination task with luminance-defined form. Most subjects quickly generalized to texture- and contrast-defined form discriminations. Only a few animals needed additional training with the second-order stimuli.
Data analysis
Behavioral thresholds
We used the method of constant stimuli to estimate thresholds based on a minimum of 75 trials per stimulus condition, with 3–5 conditions per modulator spatial frequency. Threshold values corresponding to 75% correct performance and standard errors of estimate were obtained by Probit analysis of the log-transformed data sets (Finney, 1971), using a maximum likelihood technique.
Function bandwidths
We fit texture modulation sensitivity data (inverse of threshold at each tested spatial frequency) two ways: with a double-exponential function, commonly used for contrast sensitivity data (Kiorpes et al., 1993; Wilson & Bergen, 1979), and with a straight line. We compared the goodness of fit of the two models by calculating their normalized χ2 error. For most subjects, linear fits had lower errors and were therefore better descriptions of the data. We fit the luminance contrast sensitivity data with the same double-exponential function.
Population developmental trends
To characterize the developmental trajectories of different form tasks, we fit a Michaelis–Menten function to peak sensitivity data as follows:
| (1) |
where S is the fit sensitivity, Smax is the peak sensitivity, A is the subjects’ age in weeks, C is the criterion age at which sensitivity reached half its maximum value, and e is the fit exponent. For cases where the model fit did not reach a clear plateau, we computed an analogous quantity for C that was restricted to be within the range of the observed data. To compute confidence intervals for these fits, we bootstrapped the data (1000 iterations). For each of the three data sets (behavioral sensitivity to texture-, contrast-, and luminance-defined form), we resampled the data points with replacement, fitting the data on each iteration and extracting the parameter C (half-max age). From this analysis, we calculated the 68% confidence intervals (±1 standard deviation) of the bootstrap estimates. To evaluate the significance of differences between two data sets, we drew 1000 estimates of C from each and compared their magnitudes with a binary decision rule (i.e., does the value of C for the first data set exceed that for the second?). We repeated this process 10,000 times and computed the probability that the observed differences between fits for different stimulus types could have occurred by chance. We adopted a criterion of P ≤ 0.05 for significance.
Results
Development of sensitivity to texture-defined form
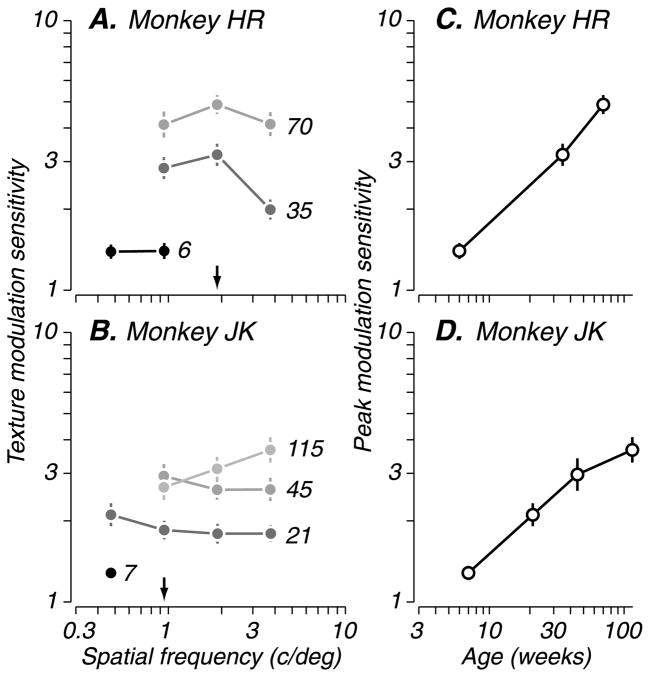
First, we describe the results for texture-defined form patterns composed of vertical and horizontal carriers (see Figures 1A and 1B). We varied modulation depth (as illustrated in Figure 2) and spatial frequency, measuring threshold at different ages. The performance of two monkeys tested longitudinally is shown in Figures 3A and 3B, where texture modulation sensitivity (inverse of threshold) is plotted as a function of spatial frequency; test age in weeks is indicated next to each data set. At the youngest ages tested (black circles), modulation sensitivity was poor, and the animals could only perform the task at low modulator spatial frequencies, reflecting limited spatial resolution (arrows mark failures to perform the task at the indicated spatial frequency at that age). Nevertheless, we found that infant monkeys as young as 6–7 weeks could discriminate modulator orientation in this task, with above-criterion performance. We also tested three younger infants (4–5 weeks) with a preferential looking paradigm and found that they oriented more frequently toward a texture pattern containing vertical modulation structure compared to a homogeneous control target that lacked structure (data not shown). Thus, monkeys were able to detect texture-defined form as early as 4 weeks and could use it as a basis for orientation discrimination by 6 weeks. This is substantially earlier than they are able to perform global form tasks such as contour integration and Glass pattern discrimination (Kiorpes & Bassin, 2003; Kiorpes & Movshon, 2003). This result suggests that the youngest infants tested have access to mid-level form cues that are not global, in the sense that they do not require extensive integration over space. For all longitudinally tested subjects, modulation sensitivity and spatial resolution improved gradually with age (gray circles), despite some individual variation in the developmental trends. To track performance improvements with development, we plotted peak modulation sensitivity—that is, the best sensitivity over the range of modulator spatial frequencies tested—as a function of age. For the same subjects, peak sensitivity (Figures 3C and 3D; open circles) improved steadily, reflecting the gradual maturation of the mechanisms mediating visual texture analysis.
Figure 3.
Development of sensitivity to texture-defined form. Behavioral sensitivity of two longitudinally tested subjects is shown. (A and B) Texture modulation sensitivity (inverse of threshold, filled circles) as a function of modulator spatial frequency. Data from a given age are coded in grayscale (young ages in black; older ages in lighter shades); subjects’ age in weeks is indicated alongside each curve. Arrows indicate the modulator spatial frequency at which the youngest infants failed to perform the task; vertical lines represent standard errors of the mean estimates. (C and D) For the same subjects in panels A and B, peak sensitivity (open circles; best sensitivity for each curve) is plotted for each age tested. Sensitivity for texture-defined form improved gradually with age, as did spatial resolution.
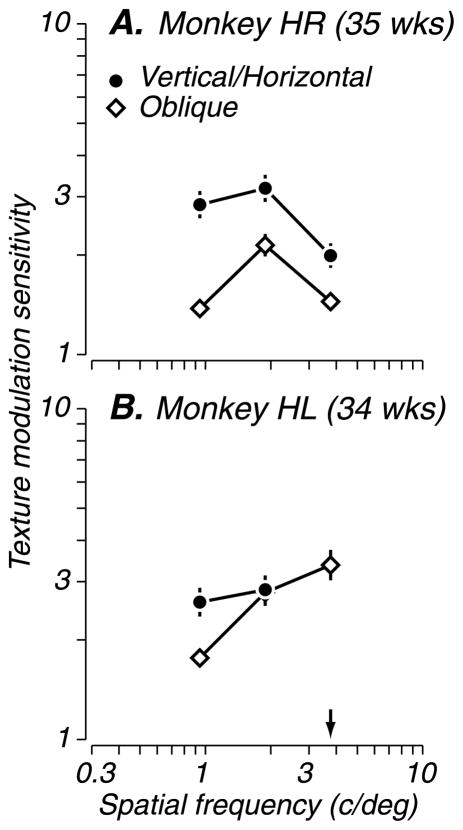
We next asked whether the particular choice of carrier orientations affected discrimination performance. We tested four monkeys (27–36 weeks) with patterns composed of right- and left-oblique carriers (see Figure 1C), in addition to the standard vertical and horizontal carriers. The performance of two example subjects is shown in Figure 4. Subject HR had higher sensitivity for patterns made of vertical/horizontal carriers (circles) than oblique carriers (diamonds). Subject HL showed similar peak sensitivity for both types of patterns but could resolve higher modulator spatial frequencies with the oblique carriers. Two other subjects (not shown) generally showed better performance with vertical and horizontal carriers. Thus, there was a moderate superiority for vertical/horizontal carriers, suggesting easier texture segregation for those stimuli. Interestingly, human psychophysical studies of texture-segregation have also showed that performance was enhanced when one of the carrier patterns was oriented parallel to the texture-defined boundary (Sutter & Hwang, 1999; Wolfson & Landy, 1995; but see Dakin & Mareschal, 2000). Because animals demonstrated above-criterion performance with both carrier combinations, however, the particular choice of carrier orientations did not qualitatively alter texture discrimination performance.
Figure 4.
Effect of carrier orientation on texture modulation sensitivity. Performance of two subjects tested with patterns composed of vertical/horizontal carriers (filled circles) and right/left oblique carriers (open diamonds). Subject HR showed higher modulation sensitivity for stimuli composed of vertical/horizontal carriers. Subject HL showed higher resolution for stimuli composed of oblique carriers. The arrow indicates a modulator spatial frequency at which HL could perform the task with oblique carriers but not vertical/horizontal carriers. Vertical lines represent standard errors of the mean estimates. Overall, carrier orientation had little influence on performance in this orientation discrimination task.
Development of sensitivity to contrast-defined form
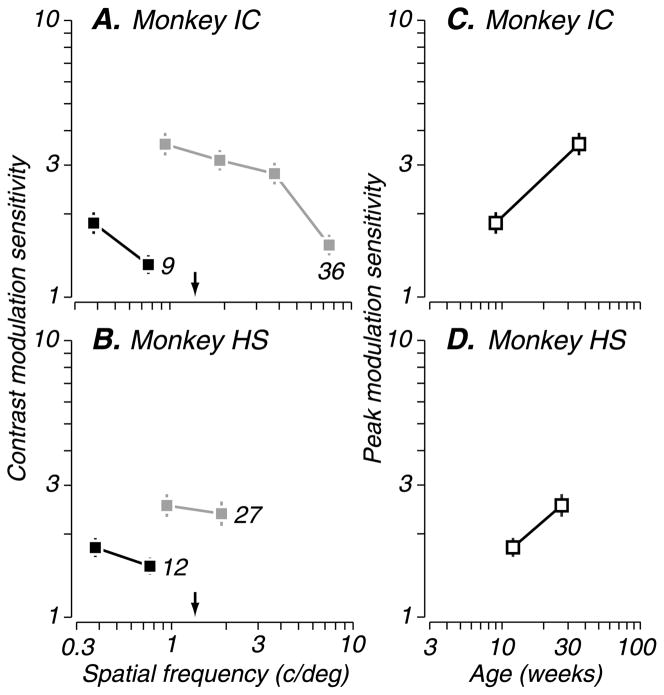
We now turn to the results for contrast-defined form (Figure 1D). Again, we varied modulation depth and spatial frequency, measuring thresholds at each age tested. The performance of two longitudinally tested subjects is shown in Figures 5A and 5B. As observed for texture-defined form, subjects had lower contrast modulation sensitivity and limited spatial resolution at the youngest ages (black squares) compared to older ages (gray squares). Again, we tracked performance changes by plotting peak modulation sensitivity across ages (Figures 5C and 5D) and found that sensitivity improved with age similarly to texture-defined form sensitivity. Thus, the qualitative results for both families of second-order patterns were remarkably similar.
Figure 5.
Development of sensitivity to contrast-defined form. Behavioral sensitivity of two longitudinally tested subjects (same conventions as Figure 3). (A and B) Contrast modulation sensitivity as a function of modulator spatial frequency, at each age tested (filled squares). Arrows indicate the modulator spatial frequency at which the youngest infants failed to perform the task; vertical lines represent standard errors of the mean estimates. (C and D) Peak sensitivity (open squares) for each curve in panel A at both ages tested. Sensitivity for contrast-defined form improved with age, as did spatial resolution.
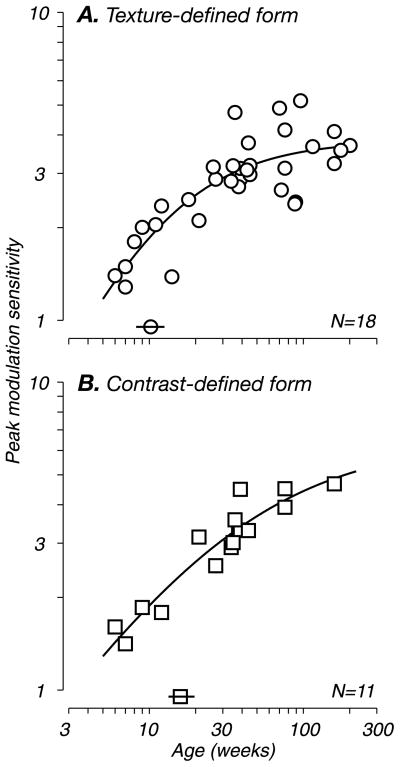
Developmental time course of behavioral sensitivity
To compare the developmental time courses, we plot peak modulation sensitivity for both second-order discrimination tasks across all subjects and ages in Figure 6. Data for texture-defined form (Figure 6A, circles) and for contrast-defined form (Figure 6B, squares) are shown separately for comparison. Note that because we tested many of these subjects longitudinally, each plot contains more data points than the indicated population size (N is the number of monkeys). For both texture types, discrimination ability as measured by peak modulation sensitivity improved steadily during the first 40 weeks, reaching a plateau thereafter. We characterized the developmental trends quantitatively by fitting a descriptive function (solid lines, see Methods) and extracting relevant fit parameters. Based on these population data, we found that performance on the second-order discrimination tasks reached half of maximum—adult—levels at 10 and 17 weeks for texture- and contrast-defined form, respectively (isolated symbols with horizontal bars indicate the half-maximum values and 68% confidence intervals respectively, see Methods). The small difference between the time courses for the two tasks was not statistically significant in a bootstrap analysis (p = 0.15). This is not unexpected, as many models of second-order vision assume a common cue-invariant second-order process for both texture- and contrast-defined patterns.
Figure 6.
Developmental time course of modulation sensitivity. Peak modulation sensitivity for all subjects is plotted as a function of age. (A) Data for texture-defined form (circles). (B) Data for contrast-defined form (squares). Solid lines represent fits to the data (see Methods). Isolated symbols intersected by horizontal bars (above the abscissa) indicate the age at which modulation sensitivity reached half of adult levels (10 and 17 weeks for A and B, respectively); horizontal bars through each indicate 68% confidence intervals (±1 SD) from the bootstrap analysis (see Methods). N indicates the number of monkeys tested. Because many of these subjects were tested longitudinally, each plot contains more data points than the indicated population size.
Comparing sensitivity to first-order and second-order form
To relate the development of second-order form sensitivity to basic luminance contrast sensitivity, we tested the same monkeys on an identical orientation discrimination task with luminance-defined patterns. Again, the task was to discriminate vertically oriented targets from horizontally oriented comparison stimuli. We measured threshold by varying grating contrast and spatial frequency. Then for each subject, we extracted peak contrast sensitivity at each age tested.
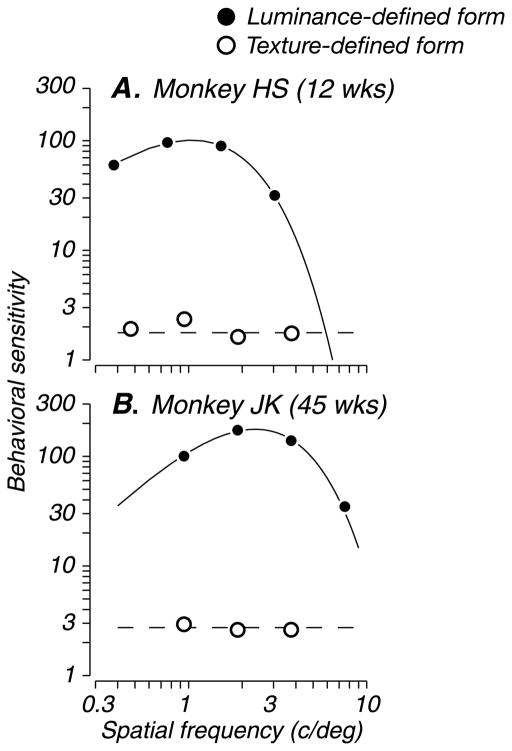
First, we compared the bandwidth properties of modulation sensitivity for texture- and luminance-defined form. Figures 7A and 7B, shows behavioral sensitivity of two subjects (12 and 45 weeks) as a function of spatial frequency, for both discrimination tasks. Whereas first-order contrast sensitivity functions (filled circles) were band-pass filtered, second-order modulation sensitivity functions (open circles) were flat over the range of modulator spatial frequencies tested. Lines through the data points represent a fitted double-exponential function for luminance-defined form and a fitted line for texture-defined form (solid and dashed lines, respectively). We fit texture sensitivity profiles both ways and compared their goodness of fit; the linear fit was a better description of the data, with lower normalized χ2 errors. Thus, unlike sensitivity to luminance-defined form, texture sensitivity profiles were not band-pass filtered, suggesting again that first- and second-order stimuli are processed by different mechanisms. This finding is consistent with human psychophysical studies conducted using the same patterns (Landy & Oruç, 2002), confirming the suitability of this animal model for studies of texture perception.
Figure 7.
Bandwidth of sensitivity to first- and second-order patterns. Behavioral performance of two example subjects. Sensitivity to luminance-defined form (filled circles) and texture-defined form (open circles) are shown as a function of grating and modulator spatial frequency, respectively. For both subjects (12 and 45 weeks), performance on the first-order discrimination task was band-pass filtered and was well fit by a double-exponential function (solid lines, see Methods). Performance on the second-order discrimination task was flat over the range of spatial frequencies tested and well fit by a line (dashed lines).
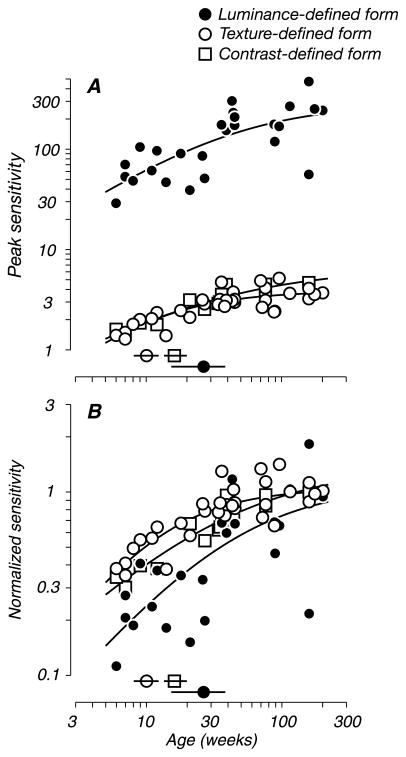
Figure 8A shows the developmental trajectory for peak sensitivity for luminance-defined form (filled circles) alongside the analogously measured trends for texture-and contrast-defined form (open circles and squares). As expected, grating sensitivity improved gradually with age, reflecting the maturation of contrast and spatial mechanisms. Peak sensitivity for first-order luminance gratings was substantially higher than for both second-order pattern types at all ages. However, the absolute sensitivity cannot easily be compared directly; it is the relative rate of development that is of interest. To compare performance on all three tasks across the population, we quantified the development of peak sensitivity as described earlier (see Figure 6). We found that sensitivity for luminance-defined form reached half-maximum at 27 weeks (black symbol with horizontal bar indicates the half-maximum and 68% confidence interval, respectively). This value was consistent with our previous studies (Kiorpes, 1992; Stavros & Kiorpes, 2008). Recall however that second-order form sensitivity reached this criterion by 10 and 17 weeks for texture- and contrast-defined form, respectively (open symbols with horizontal bars). To demonstrate the comparison further, we normalized all three developmental trends to adult performance levels for each task (age ≥100 weeks; Figure 8B). Again, second-order form sensitivity reached adult levels earlier than first-order sensitivity; performance with texture-defined form (open circles) was the first to reach maturity. The difference between the developmental time courses for sensitivity to luminance- and texture-defined form was statistically significant by a bootstrap analysis (p = 0.02), while the sensitivity profiles for luminance- and contrast-defined form did not differ (p = 0.07). These results show that basic spatial vision need not be fully mature to successfully support discriminations based on comparisons of low-level features across neighboring image subregions.
Figure 8.
Relative development of sensitivity to first- and second-order patterns. (A) Peak sensitivity as a function of age for each stimulus type tested; data are shown together for luminance-defined form (filled circles), for texture-defined form (open circles), and contrast-defined form (open squares). (B) The same data as in panel A, normalized to adult sensitivity levels (average of animals ≥100 weeks) for each stimulus type. Same conventions as in panel A. Solid lines represent fits to the data. Isolated symbols intersected by horizontal bars (above the abscissa) indicate the age at which modulation sensitivity reached half of adult levels; horizontal bars through each indicate 68% confidence intervals (±1 SD) from the bootstrap analysis (see Methods). Both second-order discrimination tasks had similar peak sensitivities and developmental trends (10 and 17 weeks). Peak sensitivity for the first-order discrimination task was always higher than for second-order tasks but developed at a slower rate (27 weeks).
Discussion
Developmental studies in human and non-human primates suggest that form vision is restricted in infancy and is slower to develop than basic spatial and temporal vision. We wondered whether these reported limitations were general to form vision or more specifically linked to the nature of spatial integration required to extract form information in different tasks. To address this, we tested animals during visual development on a form task in which the cues were accessible to relatively simple mechanisms that compare the local content of neighboring image subregions. Our results suggest that the late development of performance on some complex form tasks does not reflect general limitations of infant form vision but may instead depend on the spatial extent over which signals must be integrated to derive task-relevant form information.
In the present study, we found that infant monkeys could perform a second-order texture discrimination task as early as 6 weeks postnatal, achieving half-adult levels of behavioral sensitivity by 10 weeks for texture-defined form and 17 weeks for contrast-defined form. This is considerably earlier than the development of global form discrimination, based on tasks that require spatial integration such as contour integration (Kiorpes & Bassin, 2003) and Glass pattern discrimination (Kiorpes & Movshon, 2003). Monkeys are unable to perform these integrative tasks until after 12 weeks postnatal, and sensitivity does not reach half-adult levels until 37 and 47 weeks, respectively (Price, Kiorpes, & Movshon, 2004). Differences in the maturation rates of these tasks may be related to the amount of spatial integration necessary to extract task-relevant form information. Complex tasks, such as contour integration and Glass pattern discrimination, require visual analysis mechanisms that link information over large regions of visual space to achieve a global percept; such mechanisms apparently develop slowly. Our results suggest, however, that young infants have access to form cues of intermediate complexity very early in visual development, much earlier than global shape mechanisms are functional.
Studies of development of texture sensitivity in human infants suggest that visually evoked potentials and visual preference for texture boundaries and organization can be detected as early as 3–4 months (Arcand et al., 2007; Atkinson & Braddick, 1992; Lewis et al., 2007; Norcia et al., 2005; Rieth & Sireteanu, 1994; Sireteanu & Rieth, 1992; Sireteanu et al., 2005). However, depending on the actual stimulus configuration, significant preferences are sometimes not shown until much later in childhood, and performance continues to improve into the teenage years (Rieth & Sireteanu, 1994). More global form processing seems to emerge later, again depending on the nature of the stimuli and the task (Braddick & Atkinson, 2007; Kovács, Kozma, Fehér, & Benedek, 1999; Lewis et al., 2007). Our data in monkeys reveal for the first time a full developmental trajectory for visual texture sensitivity at a local scale.
We do not know exactly what stimulus cues the animals use to support their discrimination performance. The canonical view of texture perception is that specific visual mechanisms signal boundaries between regions that differ in texture (Landy & Graham, 2004). We assume that performance at all ages is based on similar mechanisms. It is possible in principle for different mechanisms to act at different ages, but there is no hint of this in the data. For example, in agreement with adult human psychophysical studies conducted using the same texture patterns (Landy & Oruç, 2002), we found little effect of modulator spatial frequency on performance. Texture modulation sensitivity functions were flat over the range of modulator frequencies tested. We could not test texture-modulation frequencies higher than 4 c/deg because the required carrier spatial frequencies risked falling beyond the resolution limit of infant monkeys. But as in adult human subjects tested over a wider range of modulator frequencies, we found no sign of a high spatial frequency falloff of the kind seen in luminance contrast sensitivity functions. It appears that in developing monkeys—as in adult humans—the mechanisms that detect the spatial structure of orientation and contrast modulation are essentially untuned for spatial frequency.
The texture-defined form patterns contained both horizontal and vertical structure; correct discrimination required the animal to determine the orientation of the modulator that controlled the contrast of the carrier elements. The cues for detecting contrast-modulated form were similar, consistent with our finding that the developmental profiles for these two types of second-order patterns were similar. This similarity suggests that it is unlikely that the monkeys relied on idiosyncratic local features (such as the “T”-like structure found near texture transitions). Such cues would have been more salient at higher texture-modulation frequencies when the boundary transitions were abrupt, yet we found no difference in performance with modulator frequency.
Interestingly, behavioral sensitivity to second-order patterns—both texture- and contrast-defined form—matured earlier than luminance contrast sensitivity, which reached half-adult levels at 27 weeks. One possibility is that these two kinds of form cue are processed by different visual mechanisms, as suggested by some psychophysical literature on second-order vision in humans (Landy & Graham, 2004). The standard psychophysical model for how second-order cues are extracted is the filter–rectify–filter (FRF) model (reviewed in Landy & Graham, 2004). In this model, the output of a bank of linear luminance-sensitive filters operating on local image regions is rectified and pooled by a second stage linear filter of larger spatial scale. This second-order filter signals variations in orientation or contrast across neighboring image subregions, providing the cues necessary for performance in our discrimination task. Viewed from a developmental perspective, the FRF framework would seem to require that luminance-sensitive mechanisms in the first stage of the model be developmentally functional to support the detection and discrimination of texture by the second stage. Our prior and present studies have established that first-order channels are present and functional—although immature—in young infant macaques (Kiorpes, 1992; Kiorpes & Movshon, 1998; Stavros & Kiorpes, 2008). Our finding that infant monkeys as young as 6 weeks could discriminate the orientation of texture-defined forms therefore suggests that mechanisms supporting second-order vision are also present and functional relatively early, earlier than first-order mechanisms mature. Recall, however, that the late phase of the development of luminance contrast sensitivity is due to improved processing of information about low contrast targets. Our texture-defined and contrast-defined forms were made out of high-contrast elements, so the processing machinery required to extract information about their orientations could mature well before the end of the course of contrast sensitivity development, giving rise to the unexpected reversal of developmental sequence that we found.
It is often tacitly assumed that the first stage of the FRF model resides in striate cortex (V1), while the second stage reflects processing in extrastriate areas V2 and beyond. Physiological studies of neuronal function in early visual areas in infant macaques find that receptive field properties and neural luminance contrast sensitivity mature quite early, reaching adult levels by 8–16 weeks (Kiorpes & Movshon, 2004; Zheng et al., 2007). Thus, although the neural substrates are available quite early to support adult levels of visual processing in V1 and V2, behaviorally measured sensitivity to luminance-defined form matures slowly, suggesting that these early visual areas do not set an important limit on the development of contrast sensitivity in particular and that it may not be reasonable to presume a direct link between the maturation of these areas and visual performance. And while our study was grounded in the FRF framework, texture analysis might be mediated by modulating the activity of first-order channels, for example, through the action of tuned surround suppression mechanisms, rather than by specialized higher order channels. Suppressive interactions between V1 receptive field centers and surrounds may be sufficient to signal texture cues, particularly if the surrounds are spatially inhomogeneous. Such mechanisms, which are known to exist in V1, have been shown to confer some neuronal selectivity for the orientation and spatial frequency of contrast modulation (Tanaka & Ohzawa, 2009).
Little is known about the neural correlates of texture perception in primates. While there is physiological evidence for selective second-order responses in cat area 18 (Mareschal & Baker, 1998a, 1998b; Song & Baker, 2007; Zhou & Baker, 1994), recent data from our laboratory show little evidence for this selectivity in the primate homolog of area 18, extrastriate area V2 of the macaque monkey (El-Shamayleh, 2009; El-Shamayleh & Movshon, 2006). Most V2 neurons respond selectively to the orientation of luminance-defined form but not texture-defined form, suggesting that primate V2 is not the locus of the second-order channels proposed by the FRF model. The results of our recordings are consistent with human functional imaging data collected using the same texture stimuli in which an adaptation protocol was used to localize selective responses to texture cues (Larsson et al., 2006). Adaptation effects were found to be modest in early visual cortex (V1 and V2) and increased gradually along the ventral stream pathway; the strongest adaptation was found in high-level visual areas in the ventral occipital cortex. Other evidence from experiments in monkeys is consistent with the idea that V4 and higher areas are important for texture segmentation (De Weerd, Desimone, & Ungerleider, 1996; Huxlin, Saunders, Marchionini, Pham, & Merigan, 2000; Merigan, 2000). Together, these results imply that successful performance on our task at the youngest ages (6–7 weeks) may depend in part on functional high-level extrastriate areas such as V4. As these areas continue to develop during the first postnatal year (Batardière et al., 2002; Condé, Lund, & Lewis, 1996; Rodman, Scalaidhe, & Gross, 1993; for a review, see Kennedy & Burkhalter, 2004; also Guillery, 2005), so does texture sensitivity.
Conclusions
Infant macaques have access to texture-defined form cues early in visual development and, surprisingly, their sensitivity to visual texture modulation matures before basic spatial vision is fully adult. Texture segregation is an important perceptual capacity that contributes to many intermediate aspects of form vision, including the segmentation of objects from their backgrounds. In contrast, sensitivity to more complex form tasks that involve global shape detection and discrimination appears to develop much later, perhaps because it requires more extensive integration of visual features across space. Thus, different aspects of form vision develop at different rates; the developmental trajectory of a given form task is linked to the nature of spatial integration required to extract task-relevant cues. Differences in the development of different visual form tasks seems to reflect a cascade of visual functions, which may reflect the gradual maturation of ventral extrastriate areas underlying form vision.
Acknowledgments
This work was supported by NIH grant EY05864 to L. Kiorpes, EY 2017 to J. A. Movshon and NIH grant RR00166 to the Washington National Primate Center. We thank Norma Graham, Michael Landy, and Luke Hallum for helpful discussions; Michael Gorman and Alexander Gavlin for assistance with animal care and testing; and Chao Tang for assistance with experimental software.
Footnotes
Commercial relationships: none.
Contributor Information
Yasmine El-Shamayleh, Center for Neural Science, New York University, New York, NY, USA.
J. Anthony Movshon, Center for Neural Science, New York University, New York, NY, USA.
Lynne Kiorpes, Center for Neural Science, New York University, New York, NY, USA.
References
- Arcand C, Tremblay E, Vannasing P, Ouimet C, Roy MS, Fallaha N, et al. Development of visual texture segregation during the first year of life: A high-density electrophysiological study. Experimental Brain Research. 2007;180:263–272. doi: 10.1007/s00221-007-0854-y. [DOI] [PubMed] [Google Scholar]
- Atkinson J, Braddick O. Visual segmentation of oriented textures by infants. Behavioural Brain Research. 1992;49:123–131. doi: 10.1016/s0166-4328(05)80202-5. [DOI] [PubMed] [Google Scholar]
- Batardière A, Barone P, Knoblauch K, Giroud P, Berland M, Dumans AM, et al. Early specification of the hierarchical organization of visual cortical areas in the macaque monkey. Cerebral Cortex. 2002;12:453–465. doi: 10.1093/cercor/12.5.453. [DOI] [PubMed] [Google Scholar]
- Boothe RG, Kiorpes L, Williams RA, Teller DY. Operant measurements of contrast sensitivity in infant macaque monkeys during normal development. Vision Research. 1988;28:387–396. doi: 10.1016/0042-6989(88)90181-2. [DOI] [PubMed] [Google Scholar]
- Braddick O, Atkinson J. Development of brain mechanisms for visual global processing and object segmentation. Progress in Brain Research. 2007;164:151–168. doi: 10.1016/S0079-6123(07)64008-4. [DOI] [PubMed] [Google Scholar]
- Condé F, Lund JS, Lewis DA. The hierarchical development of monkey visual cortical regions as revealed by the maturation of parvalbumin-immunoreactive neurons. Brain Research: Developmental Brain Research. 1996;96:261–276. doi: 10.1016/0165-3806(96)00126-5. [DOI] [PubMed] [Google Scholar]
- Dakin SC, Mareschal I. Sensitivity to contrast modulation depends on carrier spatial frequency and orientation. Vision Research. 2000;40:311–329. doi: 10.1016/s0042-6989(99)00179-0. [DOI] [PubMed] [Google Scholar]
- De Weerd P, Desimone R, Ungerleider LG. Cue-dependent deficits in grating orientation discrimination after V4 lesions in macaques. Visual Neuroscience. 1996;13:529–538. doi: 10.1017/s0952523800008208. [DOI] [PubMed] [Google Scholar]
- El-Shamayleh Y. Doctoral dissertation. Center for Neural Science, New York University; New York: 2009. Neuronal and behavioral responses to visual form. [Google Scholar]
- El-Shamayleh Y, Movshon JA. Responses to texture-defined forms in macaque V2 neurons. Society for Neuroscience Abstracts; Neuroscience Meeting Planner; San Diego, CA: Society for Neuroscience; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney DJ. Probit analysis. Cambridge, England: Cambridge University Press; 1971. [Google Scholar]
- Guillery RW. Is postnatal neocortical maturation hierarchical? Trends in Neurosciences. 2005;28:12–517. doi: 10.1016/j.tins.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hall-Haro C, Kiorpes L. Normal development of pattern motion sensitivity in macaque monkeys. Visual Neuroscience. 2008;25:675–684. doi: 10.1017/S0952523808080802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxlin KR, Saunders RC, Marchionini D, Pham HA, Merigan WH. Perceptual deficits after lesions of inferotemporal cortex in macaques. Cerebral Cortex. 2000;10:671–683. doi: 10.1093/cercor/10.7.671. [DOI] [PubMed] [Google Scholar]
- Kennedy H, Burkhalter A. Ontogenesis of cortical connectivity. In: Chalupa LM, Werner JS, editors. The visual neurosciences. Cambridge, MA: MIT Press; 2004. pp. 146–158. [Google Scholar]
- Kiorpes L. Development of vernier acuity and grating acuity in normally reared monkeys. Visual Neuroscience. 1992;9:243–251. doi: 10.1017/s0952523800010658. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Bassin SA. Development of contour integration in macaque monkeys. Visual Neuroscience. 2003;20:567–575. doi: 10.1017/s0952523803205101. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Kiper DC, Movshon JA. Contrast sensitivity and vernier acuity in amblyopic monkeys. Vision Research. 1993;33:2301–2311. doi: 10.1016/0042-6989(93)90107-8. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Movshon JA. Peripheral and central factors limiting the development of contrast sensitivity in macaque monkeys. Vision Research. 1998;38:61–70. doi: 10.1016/s0042-6989(97)00155-7. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Movshon JA. Differential development of form and motion perception in monkeys. Paper presented at the third annual meeting of the Vision Sciences Society; Sarasota, FL. 2003. [Google Scholar]
- Kiorpes L, Movshon JA. Neural limitations on visual development in primates. In: Chalupa LM, Werner JS, editors. The visual neurosciences. Cambridge, MA: MIT Press; 2004. pp. 159–173. [Google Scholar]
- Kovács I, Kozma P, Fehér A, Benedek G. Late maturation of visual spatial integration in humans. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:12204–12209. doi: 10.1073/pnas.96.21.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landy MS, Graham N. Visual perception of texture. In: Chalupa LM, Werner JS, editors. The visual neurosciences. Cambridge, MA: MIT Press; 2004. pp. 1106–1118. [Google Scholar]
- Landy MS, Oruç I. Properties of second-order spatial frequency channels. Vision Research. 2002;42:2311–2329. doi: 10.1016/s0042-6989(02)00193-1. [DOI] [PubMed] [Google Scholar]
- Larsson J, Landy MS, Heeger DJ. Orientation-selective adaptation to first- and second-order patterns in human visual cortex. Journal of Neurophysiology. 2006;95:862–881. doi: 10.1152/jn.00668.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TL, Kingdon A, Ellemberg D, Maurer D. Orientation discrimination in 5-year-olds and adults tested with luminance-modulated and contrast-modulated gratings. Journal of Vision. 2007;7(4):9, 1–11. doi: 10.1167/7.4.9. http://www.journalofvision.org/content/7/4/9. [PubMed] [Article] [DOI] [PubMed]
- Mareschal I, Baker CL., Jr A cortical locus for the processing of contrast-defined contours. Nature Neuroscience. 1998a;1:150–154. doi: 10.1038/401. [DOI] [PubMed] [Google Scholar]
- Mareschal I, Baker CL., Jr Temporal and spatial response to second-order stimuli in cat area 18. Journal of Neurophysiology. 1998b;80:2811–2823. doi: 10.1152/jn.1998.80.6.2811. [DOI] [PubMed] [Google Scholar]
- Merigan WH. Cortical area V4 is critical for certain texture discriminations, but this effect is not dependent on attention. Visual Neuroscience. 2000;17:949–958. doi: 10.1017/s095252380017614x. [DOI] [PubMed] [Google Scholar]
- Norcia AM, Pei F, Bonneh Y, Hou C, Sampath V, Pettet MW. Development of sensitivity to texture and contour information in the human infant. Journal of Cognitive Neuroscience. 2005;17:569–579. doi: 10.1162/0898929053467596. [DOI] [PubMed] [Google Scholar]
- Parker A, Hawken M. Capabilities of monkey cortical cells in spatial-resolution tasks. Journal of the Optical Society of America A. 1985;2:1101–1114. doi: 10.1364/josaa.2.001101. [DOI] [PubMed] [Google Scholar]
- Price TA, Kiorpes L, Movshon JA. Differential development of dorsal and ventral streams. Society for Neuroscience Abstracts; Neuroscience Meeting Planner; San Diego, CA: Society for Neuroscience; 2004. [Google Scholar]
- Rieth C, Sireteanu R. Texture segmentation and ‘pop-out’ in infants and children: The effect of test field size. Spatial Vision. 1994;8:173–191. doi: 10.1163/156856894x00323. [DOI] [PubMed] [Google Scholar]
- Rodman HR, Scalaidhe SP, Gross CG. Response properties of neurons in temporal cortical visual areas of infant monkeys. Journal of Neurophysiology. 1993;70:1115–1136. doi: 10.1152/jn.1993.70.3.1115. [DOI] [PubMed] [Google Scholar]
- Sireteanu R, Encke I, Bachert I. Saliency and context play a role in infants’ texture segmentation. Vision Research. 2005;45:2161–2176. doi: 10.1016/j.visres.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Sireteanu R, Rieth C. Texture segregation in infants and children. Behavioural Brain Research. 1992;49:133–139. doi: 10.1016/s0166-4328(05)80203-7. [DOI] [PubMed] [Google Scholar]
- Solomon JA, Sperling G. 1st- and 2nd-order motion and texture resolution in central and peripheral vision. Vision Research. 1995;35:59–64. doi: 10.1016/0042-6989(94)e0077-x. [DOI] [PubMed] [Google Scholar]
- Song Y, Baker CL., Jr Neuronal response to texture- and contrast-defined boundaries in early visual cortex. Visual Neuroscience. 2007;24:65–77. doi: 10.1017/S0952523807070113. [DOI] [PubMed] [Google Scholar]
- Stavros KA, Kiorpes L. Behavioral measurement of temporal contrast sensitivity development in macaque monkeys (Macaca nemestrina) Vision Research. 2008;48:1335–1344. doi: 10.1016/j.visres.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter A, Hwang D. A comparison of the dynamics of simple (Fourier) and complex (non-Fourier) mechanisms in texture segregation. Vision Research. 1999;39:1943–1962. doi: 10.1016/s0042-6989(98)00241-7. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Ohzawa I. Surround suppression of V1 neurons mediates orientation-based representation of high-order visual features. Journal of Neurophysiology. 2009;101:1444–1462. doi: 10.1152/jn.90749.2008. [DOI] [PubMed] [Google Scholar]
- Wilson HR, Bergen JR. A four mechanism model for threshold spatial vision. Vision Research. 1979;19:19–32. doi: 10.1016/0042-6989(79)90117-2. [DOI] [PubMed] [Google Scholar]
- Wolfson SS, Landy MS. Discrimination of orientation-defined texture edges. Vision Research. 1995;35:2863–2877. doi: 10.1016/0042-6989(94)00302-3. [DOI] [PubMed] [Google Scholar]
- Zheng J, Zhang B, Bi H, Maruko I, Watanabe I, Nakatsuka C, et al. Development of temporal response properties and contrast sensitivity of V1 and V2 neurons in macaque monkeys. Journal of Neurophysiology. 2007;97:3905–3916. doi: 10.1152/jn.01320.2006. [DOI] [PubMed] [Google Scholar]
- Zhou YX, Baker CL., Jr Envelope-responsive neurons in areas 17 and 18 of cat. Journal of Neurophysiology. 1994;72:2134–2150. doi: 10.1152/jn.1994.72.5.2134. [DOI] [PubMed] [Google Scholar]