Abstract
Short-term caloric restriction (CR) protects the young myocardium against ischemia/reperfusion (I/R) injury through a mechanism involving AMP-activated protein kinase (AMPK). Here we ask whether a life-long CR intervention can extend this protection to the aged myocardium, and whether AMP-activated protein kinase (AMPK) plays a role in that protection. Hearts from ad libitum fed (AL) and life-long calorically restricted (LCR) mice were examined at 30 months of age by 25/90 minute global I/R, with and without AMPK inhibition (AraA). LCR hearts were protected from infarction (AL, 28±4 % vs. LCR, 10±1 %, p < 0.01) and post-ischemic functional deficit (LVDP recovery: AL, 65±8 % vs. LCR, 93±7 %, p < 0.01). Pre-ischemic AraA impaired both of these protective effects (Infarct size: LCR+AraA, 22±4 %; LVDP recovery: LCR+AraA, 82±9 %, both p vs. AL > 0.1). AMPKα phosphorylation was dramatically increased in LCR hearts prior to I/R (AL, 1.18±0.01 vs. LCR, 1.68±0.04, ratio, p < 0.0001), and accompanied by a more modest increase in total AMPKα (AL, 2.18±0.03 vs. LCR, 2.39±0.08 ratio, p < 0.05). These results indicate that life-long caloric restriction profoundly protects the aged heart against I/R injury, and suggest that AMPK may play a role in that protection.
Keywords: Diet, nutrition, preconditioning, ischemia, myocardial infarction
Recent estimates of global disease burden indicate that ischemic heart disease (IHD) accounts for 12.2 % of deaths worldwide (WHO, 2004), and among high-income nations, 89.8 % of IHD mortality occurs in individuals 60 years of age or older (WHO, 2004).
One biological contributor to this age-association of IHD mortality is a progressive impairment of myocardial resistance to ischemia/reperfusion (I/R) injury1. This impairment not only affects basal myocardial resistance to I/R, but also diminishes induction of I/R resistance by a variety of preconditioning strategies2–5. Together, these effects describe an aged myocardium that is basally susceptible to metabolic challenge, and less able to recruit cellular defenses after exposure to known protective treatments.
Caloric restriction (CR) is associated with enhanced physiological function in many species6,7, and recent evidence indicates that life-long CR can reduce cardiovascular disease (CVD) incidence by 50% in aged primates8. Shorter-term CR paradigms elicit protection against infarction5,9, post-ischemic functional deficit5,9,10, and susceptibility to arrhythmia3. However, none of these effects have been studied after life-long CR, or in an age range comparable to that at which human IHD manifests. To that end, we employ a well-described mouse model11 to test the primary hypothesis that life-long CR remains cardioprotective in animals aged to point comparable to that at which most human IHD occurs. Secondarily, we examine the role played by AMP-activated protein kinase (AMPK) in the I/R responses of the myocardium after life-long CR or ad libitum feeding. While this kinase is known to be involved in protection due to short-term CR9, some evidence suggests that its potential to protect the heart wanes over longer term interventions5.
B6D2F1 mice were purchased from the NIA at 24 months of age. At the time of delivery, all mice had undergone caloric restriction from 14 weeks of age (LCR), or been ad libitum (AL) fed according to the model developed for the Biomarkers of Aging Program (BAP)11. These treatments were replicated on-site until 30 months of age when mice were killed11. Global I/R (25/90 minute) was performed conventionally by the constant-pressure Langendorff method under normothermic conditions, as described previously12–14. The perfusate was modified slightly to better represent metabolic conditions in vivo through inclusion of 0.4 mM oleate and 10 μU.mL−1 insulin9,15. Hearts undergoing AMPK blockade were exposed to 100 μM 9-β-D-arabinofuranoside (AraA) for 20 minutes prior to ischemia, which has previously been shown to reduce myocardial AMPK phosphorylation9. Left ventricular pressure waveforms and terminal infarct size were measured by a pressure-transducing catheter and tetrazolium staining, respectively, as described previously by our laboratory12–14. Hearts from separate cohorts of AL and LCR were used for Western immunoblotting. Upon excision they were briefly rinsed, then immediately frozen in liquid nitrogen and stored at −80 °C. Further steps of tissue preparation and blotting were performed as previously described9. Sample sizes for I/R were: AL, n = 6; AL + AraA, n = 5; LCR, n = 6; LCR + AraA, n = 5. Blot data include six hearts from each group of animals. Post-ischemic cardiac function was statistically assessed by linear mixed model, where diet and AraA were included as a fixed factors and time was the sole random factor. Infarct size was treated by factorial ANOVA, and immunoblot data by Student's t-tests. An alpha level of 0.05 was assumed for all analyses.
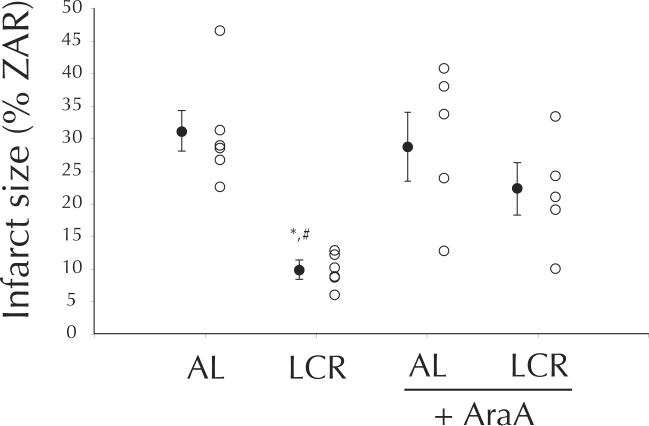
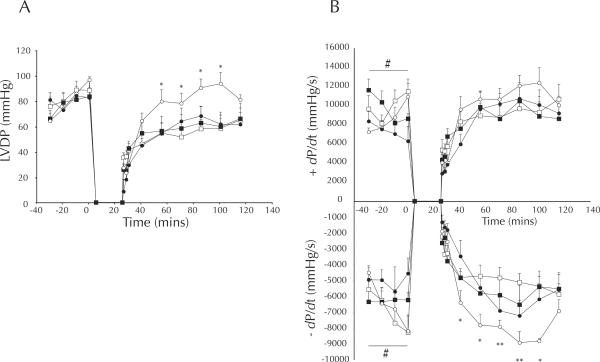
Anticipated effects of CR were observed for morphological variables at the time of death (Table 1). While LV mass was higher in AL hearts (p < 0.001), in vivo cardiac dimension (measured by short-axis M-mode echocardiography one week prior to death), was unchanged by LCR (Table 1). LCR hearts were considerably more resistant (66 %) to infarction than AL hearts (p < 0.005; LCR vs. AL, p < 0.005), and this protection was susceptible to inhibition of AMPK (LCR vs. LCR + AraA, p < 0.05, Figure 1). Similar protection was observed in terms of post-ischemic recovery of left ventricular developed pressure (LVDP), which approached pre-ischemic levels among LCR hearts during minutes 30 – 75 of reperfusion. During the same time period AL hearts recovered to only 73 % (p < 0.05 vs. LCR, Figure 2) of pre-ischemic LVDP, and AraA treated LCR hearts recovered similarly to AL at 79 %, which was significantly less than LCR alone (p < 0.05 vs. LCR, Figure 2). Western blot data suggest that, after excision, LCR hearts exhibited much higher phosphorylation of the AMPK alpha subunit (p < 0.001, Figure 3), and that this catalytic subunit was also expressed at slightly higher levels compared to AL (p < 0.05, Figure 3).
Table 1.
Tissue masses and echocardiographic dimensions for CR and AL animals at time of sacrifice.
| AL | CR | |
|---|---|---|
| Heart (mg) | 270 ± 6 | 190 ± 6 |
| Body (g) | 38 ± 2 | 25 ± 1** |
| WAT (g) | 1.2 ± 0.3 | 0.1 ± 0.0** |
| Soleus (mg) | 18 ± 6 | 13 ± 1 |
| TA (mg) | 74 ± 12 | 45 ± 4* |
| Heart: Body | 7.3 ± 0.4 | 7.4 ± 0.1 |
| TA:Body | 1.8 ± 0.5 | 1.8 ± 0.2 |
| WAT:Body | 29.2 ± 6.0 | 5.8 ± 0.5** |
|
| ||
| LVIDs | 2.0 ± 0.2 | 1.8 ± 1.7 |
| LVIDd | 3.8 ± 1.4 | 3.5 ± 1.8 |
| FS | 0.47 ± 0.04 | 0.49 ± 0.03 |
TA: Tibialis anterior, WAT: White Adipose Tissue, LVIDs: end systole Left Ventricular Internal Diameter: LVIDd: end diastole Left Ventricular Internal Diameter, FS: Fractional Shortening.
Figure 1.
Post I/R infarct size (% ZAR mass) among AL and LCR, in the absence and presence (+AraA) of pre-ischemic 9-β-d-arabinofuranoside (100 μM). Open circles are individual data, filled circles are means ± SEM. *p < 0.05 compared to AL, #p < 0.05 compared to LCR + AraA.
Figure 2.
Left ventricular function during 25/90 min global I/R, by diet (LCR, open circles; AL, open squares), and preischemic AraA (LCR + AraA, filled circles; AL + AraA, filled squares). Data are means ± SEM. Panel A) LV developed pressure (mmHg). Panel B) Rates of left ventricular pressure development (dP/dt) and decay (−dP/dt). Data are means ± SEM. # p < 0.05 diet × drug (fixed effect), * p < 0.05 CR vs. CR + AraA (pairwise contrast), ** p < 0.05 (diet, diet × drug).
Figure 3.
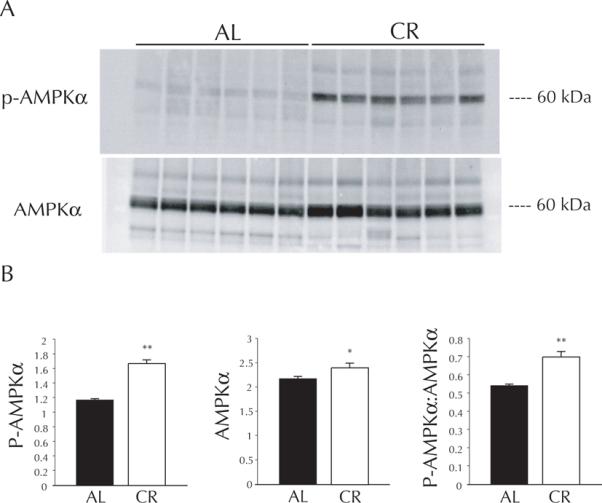
Panel A: Representative immunoblot for life-long calorically restricted (LCR) and ad-libitum (AL) hearts: P-AMPKα (top) and total AMPKα (bottom). Panel B: Densitometry for immunoblot data, presented as means ± SEM. * p < 0.05 vs. AL, ** p < 0.001 vs. AL.
The data presented here provide evidence that life-long CR elicits substantial myocardial resistance to I/R injury. This finding extends previous work by establishing CR as a strategy that remains cardioprotective after life-long intervention, and most importantly, that the conferred protection is pronounced in the otherwise I/R-susceptible aged murine heart. While the mechanisms underlying this protection are likely to involve many genes and substantial pleiotropy, our results suggest that AMPK is one active constituent of the protected myocardial phenotype accompanying life-long CR. Inhibition of AMPK by AraA markedly reduced infarct resistance and functional recovery in our LCR mice, and AraA has previously been shown to simultaneously decrease AMPKα phosphorylation and remove myocardial protection due to short-term CR9.
The means by which AMPK exerts its cardioprotective effects are unclear, in large part because the many roles that AMPK plays during I/R have not been reconciled in combination. Certainly AMPK orchestrates responses that are protective in isolation, such as translocation of GLUT4 and sarcolemmal KATP channels16,17, but it has also been proposed that AMPK contributes to I/R injury by shifting myocardial metabolism to favor less efficient fatty acid oxidation18. We cannot comment on these possibilities directly, but have attempted to permit for the latter by including physiologically representative concentrations of fatty acid and insulin in our perfusion system.
In conclusion, life-long caloric restriction elicits a myocardial phenotype that is profoundly protected against I/R injury, even at an age comparable to that at which most human ischemic events occur. AMPK activation may contribute to the mechanism by which life-long CR protects the myocardium against ischemia late in life.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the American Heart Association [AHA 0715537Z to AGE]; the National Heart, Lung, and Blood Institute [R01 HL072790 to R.M.]; and the National Institute on Aging [AG013038 to D.S., KO1-AG029337 to A.D., and KO1-AG033196-01 to L.L.].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST: None declared.
REFERENCES
- 1.Boengler K, Schulz R, Heusch G. Loss of cardioprotection with ageing. Cardiovasc Res. 2009;83:247–61. doi: 10.1093/cvr/cvp033. [DOI] [PubMed] [Google Scholar]
- 2.Abete P, Ferrara N, Cacciatore F, Madrid A, Bianco S, Calabrese C, Napoli C, Scognamiglio P, Bollella O, Cioppa A, Longobardi G, Rengo F. Angina-induced protection against myocardial infarction in adult and elderly patients: a loss of preconditioning mechanism in the aging heart? J Am Coll Cardiol. 1997;30:947–54. doi: 10.1016/s0735-1097(97)00256-8. [DOI] [PubMed] [Google Scholar]
- 3.Abete P, Testa G, Ferrara N, De Santis D, Capaccio P, Viati L, Calabrese C, Cacciatore F, Longobardi G, Condorelli M, Napoli C, Rengo F. Cardioprotective effect of ischemic preconditioning is preserved in food-restricted senescent rats. Am J Physiol Heart Circ Physiol. 2002;282:H1978–87. doi: 10.1152/ajpheart.00929.2001. [DOI] [PubMed] [Google Scholar]
- 4.Abete P, Testa G, Galizia G, Mazzella F, Della Morte D, de Santis D, Calabrese C, Cacciatore F, Gargiulo G, Ferrara N, Rengo G, Sica V, Napoli C, Rengo F. Tandem action of exercise training and food restriction completely preserves ischemic preconditioning in the aging heart. Exp Gerontol. 2005;40:43–50. doi: 10.1016/j.exger.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Shinmura K, Tamaki K, Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol Heart Circ Physiol. 2008;295:H2348–55. doi: 10.1152/ajpheart.00602.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–22. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med. 1997;337:986–94. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–4. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116:2809–17. doi: 10.1161/CIRCULATIONAHA.107.725697. [DOI] [PubMed] [Google Scholar]
- 10.Broderick TL, Belke T, Driedzic WR. Effects of chronic caloric restriction on mitochondrial respiration in the ischemic reperfused rat heart. Mol Cell Biochem. 2002;233:119–25. doi: 10.1023/a:1015506327849. [DOI] [PubMed] [Google Scholar]
- 11.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 12.Brown D, Chicco A, Jew K, Johnson M, Lynch J, Watson P, Moore R. Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not the mitochondrial, isoform of the KATP channel in the rat. Journal of Physiology. 2005;569:913–924. doi: 10.1113/jphysiol.2005.095729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown D, Lynch J, Armstrong CJ, Caruso N, Ehlers L, Johnson M, Moore R. Susceptibility of the heart to ischaemia-reperfusion injury and exercise-induced cardioprotection are sex-dependent in the rat. Journal of Physiology. 2005;564:619–630. doi: 10.1113/jphysiol.2004.081323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards AG, Rees ML, Gioscia RA, Zachman DK, Lynch JM, Browder JC, Chicco AJ, Moore RL. PKC-permitted elevation of sarcolemmal KATP concentration may explain female-specific resistance to myocardial infarction. J Physiol. 2009 doi: 10.1113/jphysiol.2009.181040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altarejos JY, Taniguchi M, Clanachan AS, Lopaschuk GD. Myocardial ischemia differentially regulates LKB1 and an alternate 5'-AMP-activated protein kinase kinase. J Biol Chem. 2005;280:183–90. doi: 10.1074/jbc.M411810200. [DOI] [PubMed] [Google Scholar]
- 16.Russell RR, 3rd, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sukhodub A, Jovanovic S, Du Q, Budas G, Clelland A, Shen M, Sakamoto K, Tian R, Jovanovic A. AMP-activated protein kinase mediates preconditioning in cardiomyocytes by regulating activity and trafficking of sarcolemmal ATP-sensitive K+ channels. Journal of Cellular Physiology. 2007;210:224–236. doi: 10.1002/jcp.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dyck JR, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology: enemy or ally? J Physiol. 2006;574:95–112. doi: 10.1113/jphysiol.2006.109389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.