Abstract
Background
Genetic factors impact substantially both on alcohol consumption (AC) and on the risk for alcohol dependence (AD). However, we know little about the degree to which measures of AC index the genetic risk for AD.
Methods
We assessed a lifetime history of AD by DSM-IV criteria and four measures of AC at the time of heaviest drinking (drink frequency, regular quantity, maximum quantity, and drunk frequency) in 5,073 adult twins from same-sex pairs from the Virginia Twin Registry. Structural models were fitted using Mx.
Results
We found evidence for different genetic structure in the sexes. In women, genetic risk for AD and for the four measures of AC was entirely shared. In men, the AC measures captured 85% of the genetic risk for AD. In women, the genetic relationship with AD was strongest for drunk frequency and in men for both drunk frequency and regular quantity.
Conclusions
In a population-based sample of twins, four relatively simple measures of AC obtained for the time of lifetime heaviest drinking were able to capture all (in women) or a very large proportion (in men) of the genetic risk for the complex multi-dimensional construct of AD. If replicated, these results have practical implications for studies aiming to assess genetic risk for AD.
Keywords: Alcohol Consumption, Alcohol Dependence, Twin Studies, Heritability
Alcohol Dependence (AD) in DSM-IV is a complex construct that reflects a range of neurobiological, cognitive, and behavioral symptoms. Substantial evidence from adoption (Cadoret et al., 1985, 1987; Cloninger et al., 1981; Goodwin et al., 1973) and twin studies (Heath et al., 1997; Hrubec and Omenn, 1981; Kendler et al., 1992, 1997; McGue et al., 1992; Pickens et al., 1991; Prescott and Kendler, 1999) suggests that genetic factors impact substantially on risk for AD or AD-like alcoholic syndromes.
Twin studies have also consistently shown important genetic influences on various measures of alcohol consumption (AC) (Agrawal et al., 2009; Hansell et al., 2008; Heath et al., 1991; Hettema et al., 1999; Kaprio et al., 1991; Prescott et al., 1994; Whitfield et al., 2004). These findings raise an important question the answer to which has both theoretical and practical implications: To what extent do measures of alcohol consumption index the genetic risk for AD? Do the detailed clinical assessments of the symptoms of AD, such as tolerance, loss of control, withdrawal, and desire or inability to cut down, provide additional important information about genetic risk above and beyond that obtained from relatively simple consumption-related measures?
We are aware of two published studies that have investigated this question, both using data from the Australian Twin Registry. Whitfield and colleagues examined the interrelationship between “heavy drinking” (defined as the top quartile in AC across three questionnaire assessments) and AD defined by DSM-III-R (American Psychiatric Association, 1987) assessed at personal interview (Whitfield et al., 2004). Their best-fit model estimated the genetic correlation between heavy drinking and AD at +0.63, suggesting that their simple consumption measure captured a good deal of but far from all of the genetic risk for AD.
More recently, Grant and colleagues (2009), also using results from personal interviews, examined 5 measures of lifetime AC rated for the period of lifetime heaviest use: maximum drinks consumed in 24 hours, maximal lifetime tolerance, and weekly consumption, frequency of heavy drinking, and frequency of drinking to intoxication. They fitted a quadrivariate twin model including a common factor of these 5 consumption measures, a lifetime AD symptom count, AD symptom clustering (within a 12-month period), and a diagnosis of alcohol abuse. Their consumption measure formed a single coherent factor, which had a very high genetic correlation with both AD symptom scores (≥+0.97). Unlike the earlier results from the Australian registry, these findings suggested a very close relationship between genetic risk factors for broadly defined AC and AD symptoms and suggest that measurement of AC could, in some studies, substitute for the more laborious assessment of AD.
In this article, we re-examine this question in the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (VATSPSUD) (Kendler and Prescott, 2006). Our approach is similar to that of Grant and colleagues (2009) in some but not all ways. We also use a range of indices of AC: frequency, quantity, maximum consumption in a single day, and frequency of drinking to intoxication. (For brevity’s sake, we refer to these measures as: drink frequency, regular quantity, maximum quantity, and drunk frequency.) We examine these four indices together in a single model—rather than as a common factor—so that we can estimate the degree to which the genetic risk for AD is reflected by genetic risk factors for our 4 consumption measures. Finally, we model lifetime DSM-IV AD as a dichotomous disorder—as would be performed traditionally in most genetic and other clinical studies—rather than as a symptom count.
METHODS
Sample
Participants in this study derived from two inter-related studies of Caucasian same-sex twin pairs who participated in VATSPSUD (Kendler and Prescott, 2006). All subjects for the VATSPSUD were ascertained from the population-based Virginia Twin Registry formed from a systematic review of birth certificates in the Common-wealth of Virginia. Female–female (FF) twin pairs, from birth years 1934 to 1974, became eligible if both members previously responded to a mailed questionnaire in 1987 to 1988, the response rate to which was approximately 64%. Data on AC and AD used in this report were collected at the 4th wave of interviews (FF4), conducted in 1995 to 1997. For this wave, we succeeded in interviewing 85%of the eligible sample. Data on the male–male (MM) pairs came from a sample (birth years 1940 to 1974) initially ascertained directly from registry records, which contained all twin births. The first interview (MM1) was completed largely by phone in 1993 to 1996 and obtained a 72% response rate. This was followed by a second wave of interviews (MM2), conducted in 1994 to 1998, with a response rate of 83%. Data on AC and AD were collected at both of these waves. We used the measures of drink frequency, regular quantity, maximum quantity, and AD from MM1 because of the larger sample size, but drunk frequency was only assessed at MM2 and so those data were used.
Zygosity was determined by discriminate function analyses using standard twin questions validated against DNA genotyping in 496 pairs (Kendler and Prescott, 1999). The mean (SD) age and years of education of the twins were 36.3 (8.2) and 14.3 (2.2) at the FF4 interview and 37.0 (9.1) and 13.6 (2.6) at the MM2 interview.
The total sample size from same-sex twin pairs on which we had data for AC (that is, excluding life-time abstainers) and AD, and which were used in these analyses, was 5,073, and consisted of 2,090 complete pairs and 893 twins whose cotwins did not participate. By zygosity, the numbers of complete pairs were monozygotic (MZ) male pairs 770; dizygotic (DZ) male 598; MZ female 440; and DZ female 282.
This project was approved by the human subject committees at Virginia Commonwealth University. Written informed consent was obtained prior to face-to-face interviews and verbal consent prior to phone interviews. Interviewers had a master’s degree in a mental health-related field or a bachelor’s degree in this area plus 2 years of clinical experience. At each wave, members of a twin pair were interviewed by different interviewers who were blind to clinical information about the cotwin.
Measures
Our alcohol section began by asking about any lifetime alcohol use. In our FF4, MM1, and MM2 interviews, 8.0%, 5.0%, and 4.3% of participants, respectively, denied any lifetime alcohol use and were excluded from all subsequent analyses. We then asked about “drinking habits in the past year,” having defined an alcoholic drink as “one bottle of beer, one glass of wine, or one shot of liquor.” Drink frequency was defined as “in a typical month in the last year, how many days out of 30 did you have an alcoholic drink?” Regular quantity was defined as “On those days when you drank, how many drinks did you usually have in a day?” Maximum quantity was defined as “What is the largest number of drinks you had on any single day during the past year?” Drunk frequency was defined as “During the past year, how often did you drink enough to feel drunk?” For drink frequency, regular quantity and maximum quantity, we simply recorded the number of days per month or drinks per day. For drunk frequency, we had 11 response options: 1 = Never, 2 = Once/Year, 3 = 2 Times/Year, 4 = 3 to 6 Times/Year, 5=7 to 11 Times/Year, 6 = 1 Time/Month, 7 = 2 to 3 Times/Month, 8 = 1 to 2 Times/Week, 9 = 3 to 4 Times/Week, 10 = 5 to 6 Times/Week, and 11 = Every Day.
We then inquired “Has there ever been a period when you drank more than you have during the past 12 months?” If the answer to that is “No,” participants are skipped to the next part of the alcohol section. If the answer is “Yes,” they are asked to give us their age during their period of heaviest drinking. We then repeated the assessment of drink frequency, regular quantity, maximum quantity, and drunk frequency for a “typical month during that year when you used alcohol the most.” In our sample, 66.7% of females and 71.0% of males reported there was a period when they drank more than in the past 12 months. They reported their average age at that time was, respectively, 22.5 (5.5) and 22.2 (5.3) years. The diagnoses of lifetime AD were derived algorithmically from our interviews based on DSM-IV criteria (American Psychiatric Association, 1994).
Because of the skewed nature of the distributions of our AC measures, for our correlational and twin analyses, we polychotomized them into 5 categories with different cut-offs for males and females. For drink frequency, our 5 categories were 1 to 3, 4 to 9, 10 to 15, 16 to 27, and 28 to 30 days per month in males and 1, 2 to 3, 4 to 7, 8 to 14, and 15 to 30 days per month in females. For regular quantity, these categories were 1 to 2, 3, 4 to 5, 6 to 9, and ≥9 drinks/day for males; and 1, 2, 3, 4, and ≥5 drinks/ day for females. For maximum quantity, these categories were 1 to 5, 6 to 9, 10 to 12, 13 to 20, and ≥21 drinks/day for males and 1, 2 to 3, 4, 5 to 6, and ≥7 drinks/day for females. For drunk frequency, using the response options listed earlier, our categories were 1 to 2, 3 to 5, 6 to 7, 8, and 9 to 11 for males and 1, 2, 3 to 4, 5 to 7, and 8 to 11 for females.
Short-term test–retest reliability of our consumption measures was available from 98 male and 171 female twins who were interviewed a mean of, respectively, 4.5 and 5.7 weeks after their initial MM2 or FF4 interview. The observed polychoric correlations for males (and females) were drink frequency +0.86 (+0.90), regular quantity +0.77 (+0.80), maximum quantity +0.85 (+0.86), and drunk frequency+ 0.72 (+0.82).
Statistical Methods
Our models divide the sources of individual differences in AC and the liability to AD into three classes: additive genetic effects (A), shared family environment (C), and unique environment (E) (Kendler and Prescott, 2006). Shared environment reflects family and community experiences that increase similarity in twins who are raised together. Unique environment includes environmental experiences not shared by members of a twin pair as well as measurement error unique to each measure.
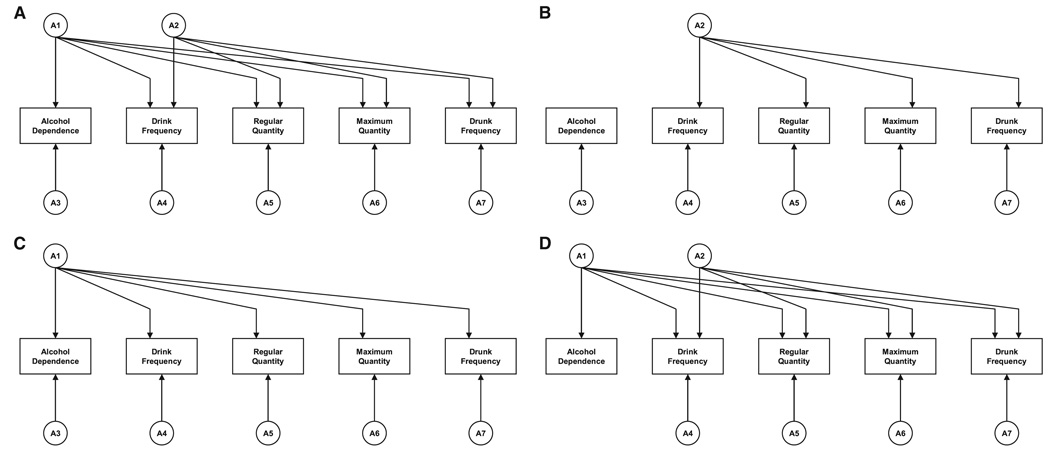
Our multivariate twin model was designed to examine the degree to which genetic influences on AD are shared with the genetic influences on our four measures of AC. This was tested in a series of nested models. The first or full model is depicted in Fig. 1A. The model contains seven separable sources of genetic risk, two of which (A1 and A2) are common factors, and five of which are specific to AD (A3) and the 4 forms of AC (A4 to A7). The first common factor—A1—influences both AD and the forms of AC. Critically, the second common factor—A2—influences only the 4 forms of AC. When the full model is fit, A2 represents common genetic risk shared by the AC measures after removing the genetic influences these measures share with AD.
Fig. 1.
(A) Our full structural model relating genetic risk for alcohol dependence (AD) and four measures of alcohol consumption (AC). The model contains seven separable sources of genetic risk, two of which (A1 and A2) are common factors and five of which (A3 through A7) are specific to AD and the 4 forms of AC. The first common factor—A1—influences both AD and the forms of AC. Critically, the second common factor—A2—influences only the 4 forms of AC. (B) The full model depicted in Fig. 1A with the deletion of factor A1. This model represents the hypothesis that genetic risk factors for AD and AC are unrelated. (C) The full model depicted in Fig. 1A with the deletion of factor A2. This model represents the hypothesis that all the genetic risk factors shared among AD and the AC measures can be explained by one common factor. However, specific genetic risk for each measure is permitted in the model through factors A3 to A7. (D) The full model depicted in Fig. 1A with the deletion of factors A2 and A3. This model evaluates the hypothesis that all the genetic risk for AD is shared with the genetic risk common to the AC measures.
We then fit submodels to test three alternative hypotheses about the overlap of genetic influences on AD and AC. First, we want to test the (unlikely) hypothesis that genetic risk for AD is unrelated to genetic risk for AC. This is performed by fitting model 1b, which just deletes factor A1.
Second, we want to examine whether any meaningful genetic differences exist between AD and AC. This is tested by fitting model 1c, which deletes factor A2, forcing all the genetic risk that is shared among the AC measures to also be shared with AD.
Third, we want to test whether all of the genetic risk for AD is shared with the measures of AC. Assuming that model 1a provided the best general fit, this would be performed by fitting model 1d, which eliminates A3—thereby forcing all the genetic risk for AD to derive from the common A1 factor. (If model 1c provided the best fit, it would be possible to test this hypothesis by eliminating A3 from that model [not pictured].)
Because our interest in these analyses was focused on the structure of genetic risk factors for AD and AC, we utilized a full Cholesky decomposition to model effects of both C and E (for details see [Neale and Cardon, 1992 p. 249–250]). This is a saturated model that we did not attempt to further simplify although we do test whether all C paths can be eliminated from the model.
The goal of our model fitting is to achieve the best possible balance of explanatory power and simplicity. This goal is operationalized by Akaike’s information criterion (AIC) (Akaike, 1987), which equals χ2 − 2df where df equals the degrees of freedom. We seek to minimize the AIC value.
Twin model fitting was performed using the Mx software package (Neale et al., 2003). Mx was unable to estimate confidence intervals for all of the parameters in our best-fit models. Therefore, these models were re-fitted in Mplus (Muthen and Muthen, 2007). These analyses provided very similar estimates to those obtained with Mx, and we report the standard errors obtained in Mplus. With categorical dependent variables (as AD), Mplus uses a weighted least squares estimation procedure rather than maximum likelihood (as in Mx). Thus, the reported standard errors are not precise, but do provide a sense of the accuracy of our parameter estimation.
RESULTS
Descriptive Statistics
During the period of lifetime heaviest drinking, males, compared to females, reported higher levels of mean [SD] drink frequency (13.9 days/month [10.2] versus 8.0 [8.4]), regular quantity (6.2 drinks/day [6.2] versus 3.0 [2.3]), maximum quantity (13.2 drinks/day [10.5] versus 5.2 [4.0]), and drunk frequency (using the categories outlined above) (5.8 [2.9] versus 4.2 [2.9]), corresponding to about once a month for males and a few times a year for females.
The phenotypic inter-correlations between our measures of AC and AD are seen in Table 1. The correlations between the four measures of AC were substantial, ranging from+0.49 to +0.73 in males and +0.56 to +0.86 in females. In both sexes, the lowest correlation was seen between drink frequency and regular quantity, and the highest between regular quantity and maximum quantity. The correlations between the AC measures and AD were also high, ranging from +0.60 to +0.67 in males, and +0.61 to +0.76 in females. In males, the liability to AD was most highly correlated with maximum quantity while in females the highest correlation was seen with drunk frequency.
Table 1.
Phenotypic Polychoric Correlations Between Alcohol Consumption Measures and Alcohol Dependence (AD) for Females (Below the Diagonal) and Males (Above the Diagonal)
| Drink frequency |
Regular quantity |
Maximum quantity |
Drunk frequency |
AD | |
|---|---|---|---|---|---|
| Drink frequency | 0.49 | 0.63 | 0.58 | 0.65 | |
| Regular quantity | 0.56 | 0.73 | 0.58 | 0.61 | |
| Maximum quantity | 0.72 | 0.86 | 0.62 | 0.67 | |
| Drunk frequency | 0.74 | 0.77 | 0.80 | 0.60 | |
| AD | 0.68 | 0.61 | 0.71 | 0.76 |
Model Fitting
We first estimated the fit of a full model (including genetic factors A1 through A7 and full Cholesky decompositions for shared and individual-specific environment—that is C and E, respectively). This model included both MM and FF pairs and constrained the parameter values (not including means) to be equal across the two sexes. We then allowed the parameters to differ in males and females and obtained a substantial improvement in fit (ΔAIC = −151.8). Therefore, for the remainder of these analyses, we estimated the models separately in the two sexes.
Results in Female–Female Pairs
Model-fitting results for the FF twin pairs are presented in Table 2. Model I was our full model (Fig. 1A) with genetic factors A1 through A7 and full Cholesky decompositions for C and E. Model II dropped (i.e., forced to zero) all C parameters with a large improvement in our fit index (AIC = −24.2), indicating that shared environment played no substantial role in twin resemblance for AD or our AC measures. Model III dropped all the A parameters which, by contrast, produced a deterioration in model fit (AIC = +3.0) suggesting that genetic factors were critical. Our further model fitting, therefore, included only A and E parameters.
Table 2.
Results of Model Fitting for Lifetime Alcohol Dependence (AD) and Four Measures of Alcohol Consumption (AC) Assessed During the Period of Heaviest Drinking Among 1,763 Twins From Female–Female Pairs
| Description | ||||||
|---|---|---|---|---|---|---|
| Model | A | C | E | ΔFit in χ2 units | ΔDF | ΔAIC |
| Ia | Full ACE model: A1 + A2 + A3 + A4 to A7 | FCD | FCD | – | – | – |
| II | Full AE model: A1 + A2 + A3 + A4 to A7 | – | FCD | +5.9 | 15 | −24.1 |
| III | Full CE model: – | FCD | FCD | +31.0 | 14 | +3.0 |
| IVb | Independent genetic influences on AD and AC: A2 + A3 + A4 to A7 | – | FCD | +122.9 | 20 | +82.9 |
| V | Single common genetic factor for AD and AC: A1 + A3 + A4 to A7 | – | FCD | +17.5 | 19 | −20.5 |
| VIc | Two common genetic factors: one for both AD and AC and one for AC: A1 + A2 + A4 to A7 |
– | FCD | +5.9 | 16 | −26.1 |
FCD, full Cholesky Decomposition; A, additive genetic effects; C, shared or common environment; E, individual specific environment; AIC, Akaike’s information criterion.
Fit of Model I: 2LL = 18,083.4, df = 8,749, AIC = 585.4. Fit changes are relative to Model I.
Multiple start values were tried to obtain more reasonable fits for this model without success.
Best-fit model.
Model IV dropped A1 from the model (Fig. 1B), thereby testing if the genetic influences on AD and AC were entirely unrelated. This model failed badly, producing a very poor fit (AIC = +82.9). Model V dropped A2 from the model (Fig. 1C), thereby testing if a single common genetic factor could explain all the sharing of genetic influences between AD and AC. This model fit moderately worse than model II (AIC = −20.5 vs. −24.2). Model VI dropped A3 from the model (Fig. 1D), thereby testing if all of the genetic influences on AD were shared with our AC measures. This model fit better than model II (AIC = −26.1) and was thereby our best-fit model.
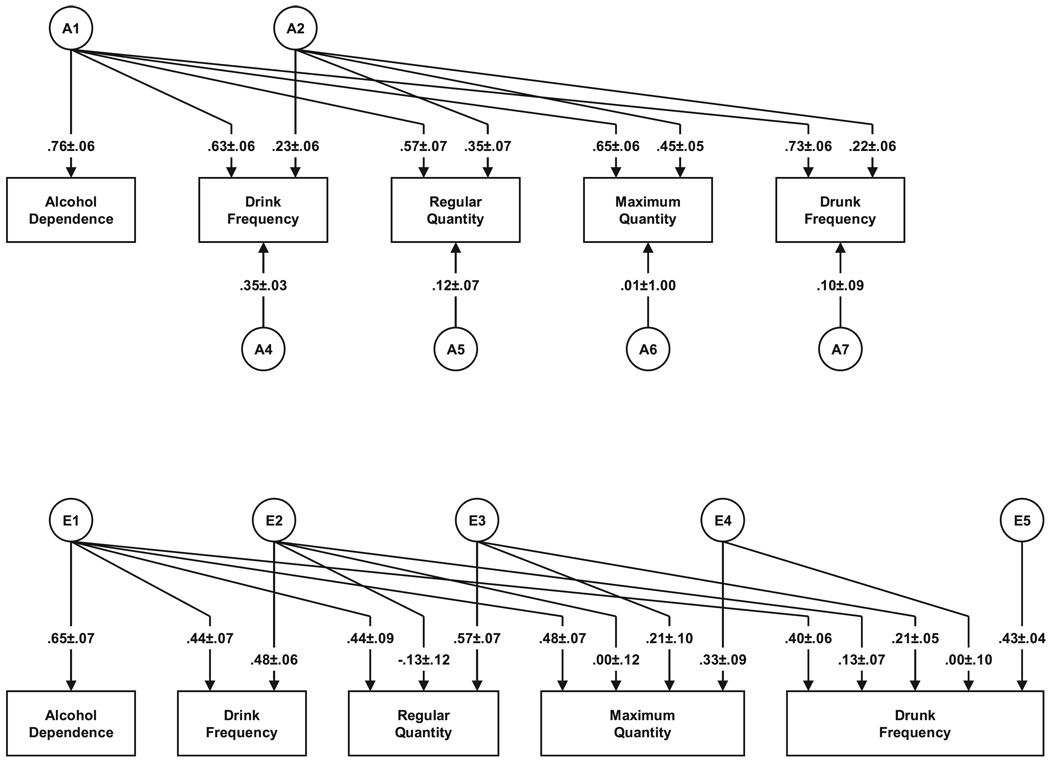
The parameter estimates and standard errors from our best-fit model in the FF twin pairs are seen in Fig. 2, and the sources of genetic influences on our 4 AC measures are seen in Table 3. We will focus here solely on results for the genetic factors. The heritability of AD in this model is estimated at 58%. Most interestingly, all of these genetic effects result from factor A1—the common factor influencing both AD and AC—and there is no evidence of specific genetic effects on AD. Thus, our model fitting suggests that in females, genetic risk for AD is entirely shared with the genetic risk factors for AC.
Fig. 2.
Parameter estimates from the best-fit model for alcohol dependence and four measures of alcohol consumption in female–female twins. The parameter estimates depicted (along with their standard errors) are factor loadings and are squared to obtain proportions of variance.
Table 3.
The Total Heritability for the Four Measures of Alcohol Consumption (AC) in Males and Females and the Proportion of Genetic Liability Shared With Alcohol Dependence (AD), Other Measures of Consumption or Unique to That Form of Consumption
| Percent shared | |||||
|---|---|---|---|---|---|
| Sex | AC measure | Heritability | With AD |
With other measures of consumption |
Unique |
| Female | Drink frequency | 57.2 | 69 | 9 | 22 |
| Regular quantity | 47.3 | 71 | 26 | 3 | |
| Maximum quantity | 62.5 | 68 | 32 | 0 | |
| Drunk frequency | 59.1 | 90 | 8 | 2 | |
| Male | Drink frequency | 49.4 | 78 | 17 | 5 |
| Regular quantity | 41.7 | 89 | 8 | 3 | |
| Maximum quantity | 54.9 | 87 | 2 | 11 | |
| Drunk frequency | 48.5 | 90 | 0 | 10 | |
In our best-fit model, the genetic relationship between our four measures of AC and AD can be assessed in two ways. The most direct measure is the magnitude of the path from the A1 genetic factor to the 4 forms of AC. By this metric, drunk frequency is by a moderate margin the form of AC that best indexes the genetic risk to AD, followed by maximum quantity. An alternative measure is the proportion of genetic risk in the AC measure that is shared with AD. As seen in Table 3, by this index, drunk frequency stands out. Over 90% of the genetic risk for drunk frequency is shared with AD compared to ~70% for the other three measures of AC.
Results in Male–Male Pairs
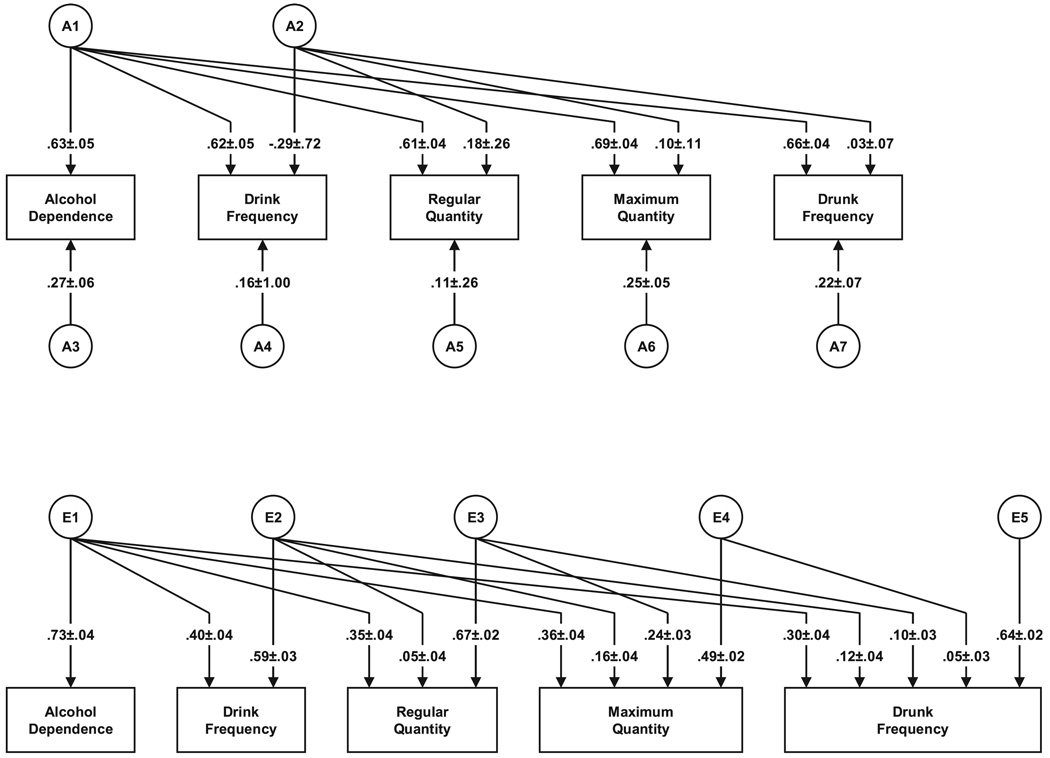
As with the FF pairs, the fit improved when we dropped all C parameters (model II) and deteriorated when we dropped all A parameters (model III) (Table 4). Compared to the full model, the model fit was not improved by dropping A1 (model IV), A2 (model V), or A3 (model VI). It is particularly noteworthy that we could not drop A3, which reflects genetic influences unique to AD. In contrast to the female twins, the best-fit model for the males was model II, parameter estimates from which are seen in Fig. 3.
Table 4.
Results of Model Fitting for Lifetime Alcohol Dependence (AD) and Four Measures of Alcohol Consumption (AC) Assessed During the Period of Heaviest Drinking Among 3,310 Twins From Male–Male Pairs
| Description | ||||||
|---|---|---|---|---|---|---|
| Model | A | C | E | ΔFit in χ2 units | ΔDF | ΔAIC |
| Ia | Full ACE model: A1 + A2 + A3 + A4 to A7 | FCD | FCD | – | – | – |
| IIb | Full AE model: A1 + A2 + A3 + A4 to A7 | – | FCD | +17.4 | 15 | −12.6 |
| III | Full CE model: – | FCD | FCD | +43.1 | 14 | +15.1 |
| IVc | Independent genetic influences on AD and AC: A2 + A3 + A4 to A7 | – | FCD | +228.3 | 20 | +188.3 |
| V | Single common genetic factor for AD and AC: A1 + A3 + A4 to A7 | – | FCD | +28.9 | 19 | −9.1 |
| VI | Two common genetic factors: one for both AD and AC and one for AC: A1 + A2 + A4 to A7 | – | FCD | +21.3 | 16 | −10.7 |
FCD, full Cholesky Decomposition; A, additive genetic effects; C, shared or common environment; E, individual-specific environment; AIC, Akaike’s information criterion.
Fit of Model I: −2LL = 37,643, df = 15,885, AIC = 5,873.1. Fit changes are relative to Model I.
Best-fit model.
Multiple start values were tried to obtain more reasonable fits for this model without success.
Fig. 3.
The best-fit model for alcohol dependence with standard errors and four measures of alcohol consumption in male–male twins.
We will again focus here solely on results for the genetic factors. The heritability for AD in this model is estimated at 47%. Of this total genetic effect, 84.5% was shared with our measures of AC. Unlike in females, there was evidence for genetic variance unique to AD (15.5%). Looking at the magnitude of the path from the A1 genetic factor to the 4 forms of AC, maximum quantity best indexes the genetic risk to AD in males, followed by drunk frequency. Looking at the proportion of genetic risk for these forms of AC shared with AD (Table 3), nearly 90% of the genetic risk for drunk frequency and regular quantity was shared with AD, compared to 87% for maximum quantity and 78% for drink frequency.
An unexpected feature of our best-fit model in males was the modest negative path from A2 to drink frequency, indicating that the residual variation in drinking frequency (after removing the genetic variation shared with AD) was negatively correlated with the quantity measures. Further model fitting indicated that the path from the A2 common factor to drunk frequency could be set to zero with no loss of fit, but that was not the case for the paths to regular and maximum quantity.
DISCUSSION
The major goal of this study was to clarify the degree to which measures of the frequency and quantity of AC reflect the genetic risk for AD as defined in DSM-IV (American Psychiatric Association, 1994). We addressed this question by examining lifetime diagnoses of AD and four measures of AC reported for the period of heaviest lifetime drinking in a large population-based sample of Virginia born twins. The four measures of AC were regular frequency, regular quantity, maximum single day consumption (maximum quantity), and frequency of drinking to intoxication (drunk frequency).
In females, the genetic risk factors for AD and for the measures of AC were entirely shared. Our four relatively simple measures of AC were able to index in women all of the genetic risk for the complex multi-dimensional construct of AD. In men, the results were slightly different. Our measures of AC captured ~85% of the genetic risk for AD, with 15% remaining that was unique to AD.
Looking at the degree to which individual AC measures reflected the genetic risk factors shared with AD (that is, factor A1), the pattern also differed somewhat across sexes in the best-fit models. In women, drunk frequency loaded most strongly by a moderate margin on the A1 factor. For men, maximum quantity had the highest loading followed closely by drunk frequency.
Our results can be usefully compared to two similar prior investigations both from the Australian Twin Registry (Grant et al., 2009; Whitfield et al., 2004). Our findings were most similar to those reported by Grant and colleagues (2009) who found, in both men and women, a very high genetic correlation between a factor derived from 5 consumption measures for the year of heaviest drinking and an AD symptom count. Although the consumption measures were not identical, and we used a dichotomous AD diagnosis and found some differences across the sexes, qualitatively both studies agreed that multiple consumption measures obtained for the period of heaviest drinking can index very closely the genetic risk for AD. Our results agree less well with the earlier report by Whitfield and colleagues (2004), which found a considerably lower genetic correlation between AC and AD. However, this study evaluated mean alcohol intake across three assessment waves rather than at the time of heaviest alcohol use, which probably explains the discrepancy in findings.
The results in our article and the Grant and colleagues (2009) study have both pragmatic and theoretical implications. Practically, our results suggest that for both genetic epidemiologic and molecular genetic investigations into alcohol use disorders, it would be feasible to closely index the genetic risk for AD by collecting relatively simple quantitative data on the frequency and quantity of AC during periods of maximum drinking. Such information can be collected quickly and potentially by self-report questionnaire (although in our study these measures were obtained at personal interview). Furthermore, these measures would yield a quantitative phenotype that may be more informative and provide greater statistical power than the dichotomous diagnosis of AD or even the semi-continuous scale of number of AD criteria. Whether measures of AC would be judged to have sufficient face validity to replace traditional measures of AD is another question that cannot be addressed by this research design. Furthermore, it is clear from our results (depicted in Figs. 2 and 3) that genetic influences on AC include factors unrelated to AD. While measures for AC may capture most of the genetic risk for AD, our results suggest that using AC measures in molecular analyses would detect some loci predictive of other aspects of AC unrelated to AD.
Theoretically, these results raise questions about the need for detailed measures of the DSM-IV criteria for AD such as tolerance, dependence, narrowing of behavioral focus and use despite persistent problems. Some of these constructs are challenging to assess especially retrospectively. Our results suggest that considerably simpler consumption-like measures for the period of heaviest drinking can index the same genetic risk factors that predispose to the subtler construct of dependence as it is imperfectly assessed by structured clinical interview.
In males, but not in females, once we had accounted for the first major genetic factor shared across AD and our four measures of AC, we found negative genetic correlations between drink frequency and the other three consumption measures. No such pattern was seen in females. Given the tentative nature of these findings, extensive speculation is unwarranted. However, these results would be expected if in males there were a different set of genetic factors, which predispose to regular low-quantity drinking (e.g., one beer a night), which is not associated with problematic use.
The heritability estimates for AD obtained in our models were slightly lower than observed in prior analyses of this sample (Kendler et al., 1992; Prescott and Kendler, 1999; Prescott et al., 1999), probably because we excluded lifetime abstainers from the current analyses. Because there is a small genetic effect on who uses alcohol, excluding the abstainers reduces the genetic variance observed in AD and AC. Nonetheless, our heritability estimates are broadly consistent with other comparable population based (Heath et al., 1997; Hrubec and Omenn, 1981; Kendler et al., 1997) and clinically ascertained twin cohorts (Pickens et al., 1991). This increases the likelihood that our results will generalize to other samples but this needs to be confirmed by future studies.
We also collected, in our twin sample, data on current AC. In parallel analyses using current consumption in the same models as reported here, the degree of genetic sharing with AD was reduced. As might be expected, to adequately index risk for AD, questions about AC must be asked about the period of lifetime heaviest drinking. Questions focused on current consumption will not be adequate.
LIMITATIONS
These results should be interpreted in the context of three potentially significant methodological limitations. First, our sample was restricted to native born white Virginia twins. Although the patterns of alcohol and drug use in this sample are broadly representative of the United States (Kendler and Prescott, 2006), these results may not extrapolate to other populations and ethnicities.
Second, we relied on participants’ retrospective recall for lifetime symptoms of AD and patterns of AC during their period of heaviest drinking. It is possible that our findings could be influenced by correlated errors of recall.
Third, we excluded lifetime nondrinkers from these analyses. Statistical methods do exist that would permit us to include these subjects and formally model the genetic and environmental contributions to alcohol initiation and then consumption patterns and risk for subsequent AD (Kendler et al., 1999) contingent upon drinking. However, the lifetime abstainers constituted such a small proportion of our sample that the gain in completeness would be more than offset by a very large increase in the complexity of our models and the number of required statistical parameters.
ACKNOWLEDGMENTS
This work was supported in part by NIH grants DA-011287, MH/AA/DA-49492, AA 011408, and AA P20017828. The authors report no competing interests. HermineMaes, PhD, kindly reviewed the Mx script.
REFERENCES
- Agrawal A, Grant JD, Littlefield A, Waldron M, Pergadia ML, Lynskey MT, Madden PA, Todorov A, Trull T, Bucholz KK, Todd RD, Sher K, Heath AC. Developing a quantitative measure of alcohol consumption for genomic studies on prospective cohorts. J Stud Alcohol Drugs. 2009;70:157–168. doi: 10.15288/jsad.2009.70.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Revised 3rd ed. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Cadoret RJ, O’Gorman TW, Troughton E, Heywood E. Alcoholism and antisocial personality. Interrelationships, genetic and environmental factors. Arch Gen Psychiatry. 1985;42:161–167. doi: 10.1001/archpsyc.1985.01790250055007. [DOI] [PubMed] [Google Scholar]
- Cadoret RJ, Troughton E, O’Gorman TW. Genetic and environmental factors in alcohol abuse and antisocial personality. J Stud Alcohol. 1987;48:1–8. doi: 10.15288/jsa.1987.48.1. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Goodwin DW, Schulsinger F, Hermansen L, Guze SB, Winokur G. Alcohol problems in adoptees raised apart from alcoholic biological parents. Arch Gen Psychiatry. 1973;28:238–243. doi: 10.1001/archpsyc.1973.01750320068011. [DOI] [PubMed] [Google Scholar]
- Grant JD, Agrawal A, Bucholz KK, Madden PA, Pergadia ML, Nelson ED, Lynskey MT, Todd RD, Todorov AA, Hansell NK, Whitfield JB, Martin NG, Heath AC. Alcohol consumption indices of genetic risk for alcohol dependence. Biol Psychiatry. 2009;2009:66. doi: 10.1016/j.biopsych.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansell NK, Agrawal A, Whitfield JB, Morley KI, Zhu G, Lind PA, Pergadia ML, Madden PA, Todd RD, Heath AC, Martin NG. Long-term stability and heritability of telephone interview measures of alcohol consumption and dependence. Twin Res Hum Genet. 2008;11:287–305. doi: 10.1375/twin.11.3.287. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Heath AC, Meyer J, Jardine R, Martin NG. The inheritance of alcohol consumption patterns in a general population twin sample: II. Determinants of consumption frequency and quantity consumed. J Stud Alcohol. 1991;52:425–433. doi: 10.15288/jsa.1991.52.425. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Corey LA, Kendler KS. A multivariate genetic analysis of the use of tobacco, alcohol, and caffeine in a population based sample of male and female twins. Drug Alcohol Depend. 1999;57:69–78. doi: 10.1016/s0376-8716(99)00053-8. [DOI] [PubMed] [Google Scholar]
- Hrubec Z, Omenn GS. Evidence of genetic predisposition to alcoholic cirrhosis and psychosis: twin concordances for alcoholism and its biological end points by zygosity among male veterans. Alcohol Clin Exp Res. 1981;5:207–215. doi: 10.1111/j.1530-0277.1981.tb04890.x. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Rose RJ, Romanov K, Koskenvuo M. Genetic and environmental determinants of use and abuse of alcohol: the Finnish Twin Cohort studies. Alcohol Alcohol Suppl. 1991;1:131–136. [PubMed] [Google Scholar]
- Kendler KS, Heath AC, Neale MC, Kessler RC, Eaves LJ. A population-based twin study of alcoholism in women. JAMA. 1992;268:1877–1882. [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychol Med. 1999;29:299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. A population-based twin study of lifetime major depression in men and women. Arch Gen Psychiatry. 1999;56:39–44. doi: 10.1001/archpsyc.56.1.39. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Genes, Environment, and Psychopathology: Understanding the Causes of Psychiatric and Substance Use Disorders. 1st ed. New York: Guilford Press; 2006. [Google Scholar]
- Kendler KS, Prescott CA, Neale MC, Pedersen NL. Temperance board registration for alcohol abuse in a national sample of Swedish male twins, born 1902 to 1949. Arch Gen Psychiatry. 1997;54:178–184. doi: 10.1001/archpsyc.1997.01830140090015. [DOI] [PubMed] [Google Scholar]
- McGue M, Pickens RW, Svikis DS. Sex and age effects on the inheritance of alcohol problems: a twin study. J Abnorm Psychol. 1992;101:3–17. doi: 10.1037//0021-843x.101.1.3. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus User’s Guide: Fifth Edition. 5th ed. Los Angeles, CA: Muthen & Muthen; 2007. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6th ed. Richmond VA: Dept. of Psychiatry, Virginia Commonwealth University Medical School; 2003. [Google Scholar]
- Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. Dordrecht, The Netherlands: Kluwer Academic Publishers B.V.; 1992. [Google Scholar]
- Pickens RW, Svikis DS, McGue M, Lykken DT, Heston LL, Clayton PJ. Heterogeneity in the inheritance of alcoholism. A study of male and female twins. Arch Gen Psychiatry. 1991;48:19–28. doi: 10.1001/archpsyc.1991.01810250021002. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Aggen SH, Kendler KS. Sex differences in the sources of genetic liability to alcohol abuse and dependence in a population-based sample of U.S. twins. Alcohol Clin Exp Res. 1999;23:1136–1144. doi: 10.1111/j.1530-0277.1999.tb04270.x. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Hewitt JK, Heath AC, Truett KR, Neale MC, Eaves LJ. Environmental and genetic influences on alcohol use in a volunteer sample of older twins. J Stud Alcohol. 1994;55:18–33. doi: 10.15288/jsa.1994.55.18. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Whitfield JB, Zhu G, Madden PA, Neale MC, Heath AC, Martin NG. The genetics of alcohol intake and of alcohol dependence. Alcohol Clin Exp Res. 2004;28:1153–1160. doi: 10.1097/01.alc.0000134221.32773.69. [DOI] [PubMed] [Google Scholar]