Abstract
A new γ-labeled marker for extracellular space is the cobaltic form of 58Co-ethylenediaminetetraacetic acid (58Co-EDTA). The cobaltic ion has a much higher affinity for EDTA than the cobaltous ion; it is prepared as a potassium salt, K+(58Co3+-EDTA4−), and is apparently biologically inert. Testing by equilibration in intact rabbits and comparing the myocardial content with that of [14C]sucrose give values of the volume of distribution in the myocardium of 0.294 ± 0.052 ml/g for 58Co-EDTA and 0.303 ± 0.051 ml/g for [14C]sucrose (SD, n = 130, for two hearts), with the ratios of 58Co-EDTA/sucrose averaging 0.973 ± 0.043 (n = 130). The average value of the extracellular fluid measured in isolated rabbit interventricular septum using Co-EDTA was 0.51 ± 0.05 ml/g (SD, n = 16) and 0.46 ± 0.04 ml/g using [14C]sucrose as an extracellular fluid space (ECF) marker. Flushing with a high concentration of nontracer Co-EDTA does not reveal any release from binding sites. The γ-energy (811 KeV), long half-life (71.4 days), stability, and lack of binding to tissue components make 58Co-EDTA a useful marker for ECF.
Keywords: volumes of distribution, interstitial fluid space, rabbit myocardium, sucrose, interventricular septum, extracellular volume, isolated hearts
The Study of Transmembranous ion flux in isolated tissue can be greatly complicated by changes in the size of the extracellular fluid space (ECF). Therefore it may be desirable to measure continuously changes in the ECF during the course of a single experiment. An extracellular marker containing a γ-emitting isotope, which can be detected in a tissue by an externally mounted NaI(T1) crystal detector, is ideally suited to such measurements. Transition metal complexes of ethylenediaminetetraacetic acid (EDTA) have been used for this purpose; the advantages of these compounds were discussed a decade ago by Brading and Jones (2). However, the commercially available compound Cr3+-EDTA4− (New England Nuclear, Boston, MA) appears not to reach a stable distribution in heart tissue (Shine et al., 11) and is contaminated with free Cr. The chelate, cobaltous EDTA (Co2+-EDTA4−) was used by Brading and Jones (2) in smooth muscle with some success, but it appeared that when the specific activity was high there was surface adsorption onto extracellular sites that gave overestimates of the volume of the ECF.
We have developed a method for preparing radiocobaltic EDTA (58Co3+-EDTA4−); we shall refer to it as 58Co-EDTA. The γ-emitting isotope of cobalt, 58Co, was chosen because it is relatively inexpensive, exhibits a strong γ emission (0.811 MeV), and has a long half-life (71.4 days). The affinity of Co3+ for the ligand is extremely high, so that dissociation of the compound is negligible.
We compared the volumes of distribution of Co-EDTA in both isolated septa and intact rabbits hearts with that of the β-emitter [14C]sucrose, which is a generally accepted standard for the estimation of extracellular space. Co-EDTA has only a slightly greater molecular size than sucrose and thus equilibrates almost as rapidly. Like sucrose it is not adsorbed onto extracellular structures or transported across cell membranes. It has the advantage that it is detectable by an external γ-detector.
Methods
Preparation of K(Co-EDTA)·2H2O
Pure unlabeled crystalline cobaltic EDTA K[Co-EDTA]·2H2O (mol wt 422.2) was obtained by the method of Dwyer, Gyarfas, and Mellor (5). Pure crystals of this compound are easily obtained by four or five recrystallizations from ethanolic solution.
The labeled compound 58Co-EDTA was made as follows. The isotope was supplied nearly carrier free as 58CoCl2 dissolved in 1 ml of dilute HCl (New England Nuclear). The 58CoCl2 solution was carefully transferred to the bottom of a liquid scintillation vial and evaporated to dryness. (It is essential to ensure that the solution is deposited at the bottom of the vial and that none of it is deposited on the sides.) When the vial was cool, 8.5 mg EDTA (acid form) and 5.6 mg potassium acetate were added to the deposit in the vial. About 1 ml of boiling water was then added to the vial, and the vial was swirled around in a beaker of water at 80°C, dissolving the chemicals and allowing the complete chelation of all radioactive cobalt. Addition of 26 mg crystalline nonradioactive CoCl2 to the solution chelated all the EDTA as cobaltous EDTA, which is pink in solution. The addition of a few drops of 30% hydrogen peroxide turned the solution from a light pink to a deep magenta color as the cobaltous EDTA oxidized to cobaltic EDTA (termed Co-EDTA). The solution was left for 1 h to ensure complete oxidation after which its volume was made up to 10 ml with distilled water. This produced a solution of Co-EDTA that was about 1 mM concentration. A small quantity of platinum black was added to the solution to decompose the hydrogen peroxide. The platinum black was subsequently removed by passing the entire solution through a 0.8 μm polycarbonate membrane (Nucleopore, Pleasanton, CA). The solution was divided into twenty 0.5-ml aliquots of about 0.24 mCi and stored at −20°C. Prior to use an aliquot was thawed and passed through a 2-cm-long column of Dowex 50 (Na form) to ensure complete removal of free 58Co2+.
Experimental Methods
In vivo experiments
These were conducted on male New Zealand White rabbits anesthetized with pentobarbital sodium (approx 50 mg/kg intravenously). The left femoral artery was cannulated for the measurement of blood pressure and withdrawal of blood samples. The left femoral vein was cannulated for the injection of tracers. The abdomen was opened, and the renal vessels were tied off to eliminate tracer loss. The animal was heparinized (400 U/kg) following the surgery.
A 1-ml control arterial blood sample and hematocrit were taken. Then a bolus of [14C]sucrose (75 μCi) and 58Co-EDTA (100 μCi) was injected into the femoral vein. Arterial blood samples (1 ml) were taken at 5-min intervals over a 25-min equilibration period to monitor the plasma isotope levels. The plasma concentrations diminished by less than 1.5% during the last 5 min of equilibration (see Fig. 1). Twenty minutes after the tracer injections the animal was put on a respirator, the chest opened, and the heart exposed. An aluminum-foil boat was slipped under the heart, and approximately 100 ml of Freon 22 at −150°C was poured over the heart at the end of the 25th minute. Within 1 s the heart stopped beating as its surface froze. Five venous blood samples were immediately taken to ensure the accuracy of the plasma isotope levels when the heart was frozen. After being frozen, the heart was immediately removed, the adipose tissue and atria were cut away, and the ventricles were sectioned into approximately 72 pieces averaging about 50 mg each with a standard deviation of 25 mg.
FIG. 1.
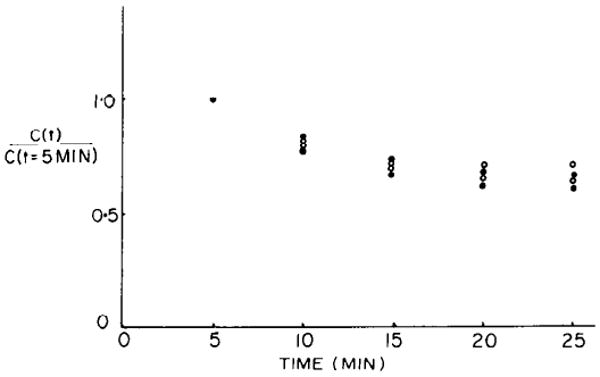
Disappearance of both 58Co-EDTA (closed circles) and [14C]sucrose (open circles) from plasma in intact rabbit. C(t), plasma cpm at time t; C(t = 5 min), plasma cpm 5 min after injecting bolus of radioactivity. Extraction of these isotopes from plasma appears to cease after 20 min.
Analysis of samples
The 58Co-EDTA activity in each sample was counted for 5 min in a well-type T1-activated NaI-crystal photomultiplier system using an automatic sample changer (Baird-Atomic model 707) and a multichannel analyzer (Tracor-Northern model MS 700). The whole energy spectrum was recorded from each sample. (Count rates for the isotope were corrected for background and for isotope decay.) The heart pieces and plasma samples were weighed and placed in individual tubes and dissolved in 1 ml of Soluene 350 (Packard).
The tubes were heated for 24 h in a 40°C water bath. The contents were then pipetted into scintillation vials (the pipette tips were saved in their respective tubes) and decolorized with 0.1 ml perchloric acid (70%) and 0.2 ml hydrogen peroxide (30%) by heating in an oven at 90°C for 45 min. The residual isotope in the empty tubes was recovered by rinsing the tubes 5 times with 2-ml aliquots of scintillation fluid, which were then pipetted into scintillation vials. The empty tubes and pipette tips showed counts of less than two times background, which were therefore less than 0.14% of the counts in the samples. The scintillation vials were shaken until the contents appeared clear, and the activities were counted using a Nuclear-Chicago Mark II liquid scintillation counter (model 6844). The quench curve samples, containing varying amounts of dissolved and decolorized rabbit heart as the quenching agent, were prepared and counted in an identical manner. Counts per minute were obtained in two spectral windows for [14C]sucrose and 58Co-EDTA, and efficiencies and spillover ratios of the tracers were calculated for the respective windows. The count rates were corrected for background and isotope decay.
The concentrations (C) of the tracers in each sample were calculated as corrected counts per minute per gram of frozen wet myocardial tissue. The ECF volume (VECF; ml water/g of myocardium) was calculated from the ECF tracer activities on the assumption of complete equilibration between plasma and interstitial fluid
where Cheart is the tracer activity in each heart piece (counts·min−1·g frozen tissue−1); Cplasma is the tracer activity of plasma (counts·min−1·g of plasma−1) averaged from the last blood sample taken just before freezing the heart and the five venous samples taken immediately after freezing; and ρp is the density of plasma (1.025 ml/g; 9). The 0.966 is the ml of water per ml plasma (6).
Perfusion of isolated interventricular septum
We also measured Co-EDTA and sucrose spaces in the isolated arterially perfused interventricular septum of the rabbit heart. Methods for dissection, mounting, and monitoring of tension were described by Langer and Brady (8). Muscles were perfused at 1.4–1.8 ml·g−1·min−1 by means of a peristaltic pump. Preparations were initially perfused for a few minutes at 35°C. Then the temperature was reduced to 25°C over a 1-h period. The septa were paced at 42 beats/min. In other experiments temperature was raised to 37°C and maintained throughout; these septa were enclosed in a temperature-regulated chamber as described by Shine et al. (11).
The composition of the modified Tyrode perfusate was as follows (in mM): Na 142, K 5 or 6, Mg 1.0, Ca 1.5 or 2.5, Cl 141 or 142, 12, 0.435, glucose 5.6, Co-EDTA either 1 mM or <8 × 10−6 M. Replacing KCl with KCo-EDTA involved negligible changes in the concentration of chloride.
Unlabeled cobaltic EDTA was assayed by means of an atomic absorption spectrophotometer equipped with a hollow cathode cobalt lamp (Varian Techtron PTY, Melbourne, Australia). Appropriate standard curves were constructed using pure crystalline unlabeled Co-EDTA· 2H2O dissolved in modified Tyrode solution. Rabbit interventricular septa were perfused with modified Tyrode solution contained 1 mM unlabeled Co-EDTA for 1 h, dried overnight at 100°C, and ashed at 600°C for 24 h in a platinum crucible. The ash was dissolved in a small quantity of 0.1 N HCl, and the volume was brought to 100 ml with Tyrode solution. Absorbance values were interpolated from the standard curve. The mean of three readings was used to calculate each concentration of unlabeled Co-EDTA.
Uptake of isotope by rabbit septa
Tissue activity of 58Co was measured with a NaI(T1) crystal probe closely apposed to the septum. At the end of an experiment the septum was removed from the perfusion apparatus, blotted, and weighed. It was then dried to constant weight at 100°C for 24 h and reweighed to measure total tissue water (by the equation: H2O content = wet wt − dry wt). Finally it was dissolved in nitric acid and assayed for isotope content by suitable γ-counting, as were the perfusate samples. In [14C]sucrose experiments, the preparation and counting techniques were the same as those used for the whole heart experiments.
Results
In vivo experiments
Experiments were performed on two rabbits. The concentrations of the markers in the plasma are plotted as a function of time after injection in Fig. 1. The stability of the concentrations from the 20th to the 25th min suggests that equilibration between plasma and interstitial fluid is complete. The distribution of the volumes for Co-EDTA and sucrose for both experiments combined are shown in Fig. 2. The mean volumes of distribution for Co-EDTA and sucrose were 0.294 ± 0.052 (SD) and 0.303 ± 0.051 ml/g, respectively (n = 130 for two hearts). The Co-EDTA and sucrose volumes were not significantly different from each other.
FIG. 2.
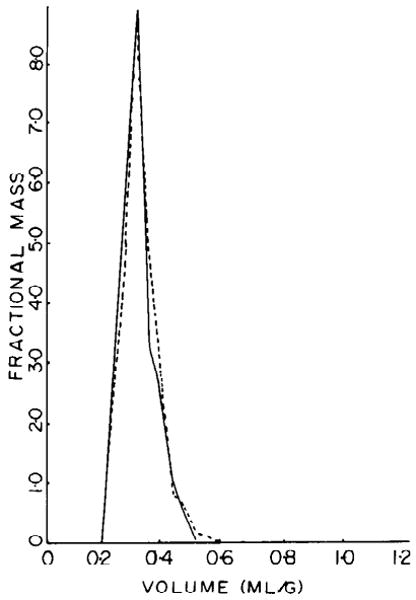
Probability density functions of myocardial volumes of distribution for 2 extracellular markers, 58Co-EDTA (solid line) and [14C]sucrose (broken line) in intact rabbit hearts. Area under each curve is unit. Mean ± SD was 0.294 ± 0.052 for 58Co-EDTA and 0.303 ± 0.051 for [14C]sucrose.
A comparison of the sucrose and the Co-EDTA volumes for the individual heart pieces is shown in Fig. 3. The data lie closely around the line of identity. The regression line is that which minimizes error equally in ordinate and abscissa. (This is actually the bisector of the y on x and x on y regression lines.) As a paired comparison, the ratios of the volumes of distribution of [14C]sucrose and 58Co-EDTA, were calculated for each piece. The ratios averaged 0.973 ± 0.043 (SD, n = 130 for two hearts). The result is as expected for compounds of similar molecular weight that exhibit no binding in the interstitial space or uptake by cells. It is worth noting that the result depicted in Fig. 3 suggests that there is considerable variation in the ECF from one small region to the next. The excellent correlation in VECF for the two markers in any given tissue sample suggests that the variability is not in the methodology but represents biological variability. Clearly, conclusions based on results of ECF measurements on large pieces of tissue must be interpreted with some caution because ECF measurements in large pieces of tissue may mask inhomogeneity.
FIG. 3.
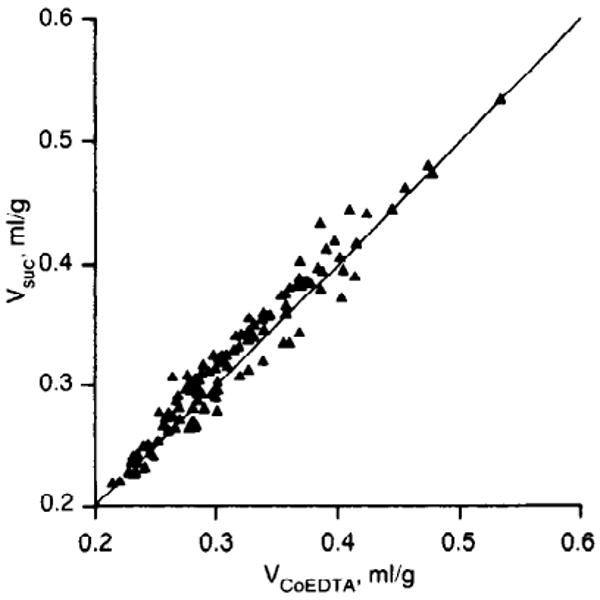
Scatter diagram of volumes of distribution of [14C]sucrose and 58Co-EDTA in 2 intact rabbit hearts. Regression is Vsuc = 1.001 ± 0.009 VCo EDTA (SE) with correlation coefficient of 0.974 and SD around mean of 0.014. Line of identity is that expected of 2 markers having identical volumes of distribution.
From this we conclude that Co-EDTA behaves almost identically to sucrose as an ECF marker and is therefore an acceptable replacement for sucrose, especially when a γ-emitter is needed.
Uptake of 58Co-EDTA by isolated interventricular septa
The uptake of 58Co-EDTA in septa paced at 42/min at 25°C is shown in Fig. 4, plotted as a percent of the plateau count rate. In each experiment, the specific activity of 58Co-EDTA in the perfusate was high enough that approximately 10,000 counts/min were detected by the probe at the plateau. The uptake pattern indicates a rapid initial phase with complete equilibration of the marker within 20–30 min. After this period no drift in the marker distribution occurred.
FIG. 4.
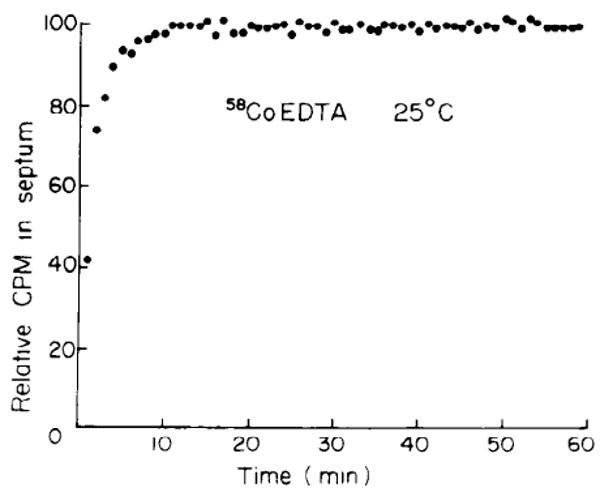
Uptake of 58Co-EDTA from modified Tyrode solution containing Co-EDTA at concn of 1 mM (25°C). Ordinate normalized for maximum uptake.
The distribution of pure unlabeled Co-EDTA was compared with that of labeled 58Co-EDTA. Septa were perfused for 1 h to ensure complete equilibration of the unlabeled compound. The quantity of unlabeled Co-EDTA in the muscle was assayed by atomic absorption spectrophotometry. For unlabeled Co-EDTA the volumes of distribution were 0.53 ± 0.05 ml/g (SD, n = 10), which were 62.2 ± 5.6% of the total water content. For labeled 58Co-EDTA, the volumes of distribution were 0.49 ± 0.01 ml/g (SD, n = 10), which were 55.7 ± 1.7% of the water content; this did not differ significantly from the value obtained with unlabeled compound (P > 0.2 by t test). The water content of the septa from the 58Co-EDTA experiments was 86.4 ± 1.7% (SD, n = 6) of tissue wet wt. The mean value for the pooled labeled and unlabeled Co-EDTA spaces was 0.51 ± 0.05 ml/g of myocardium (SD, n = 16), considerably higher than for the in vivo experiments.
We considered the possibility that surface adsorption of the marker might have occurred. Such adsorption would produce values for Co-EDTA space (VEDTA) larger than the true extracellular fluid space. Brading and Jones (2) found this to occur when the similar compound cobaltous EDTA was used as a marker in smooth muscle. If surface adsorption were occurring to any significant extent, addition of unlabeled Co-EDTA after complete equilibration of the labeled compound should produce a reduction in the apparent size of the ECF. Paced septa (42/min) were labeled with a modified Tyrode solution containing less than 8 × 10−6 M Co-EDTA. After complete equilibration of the marker, unlabeled Co-EDTA was added to the Tyrode solution to give a concentration of 1 mM. The concentration of radioactive molecules was unchanged on switching to the 1 mM concentration. The lack of any discernible change in the marker distribution (Fig. 5) indicates that there was no significant displacement of labeled molecules and therefore no surface adsorption.
FIG. 5.
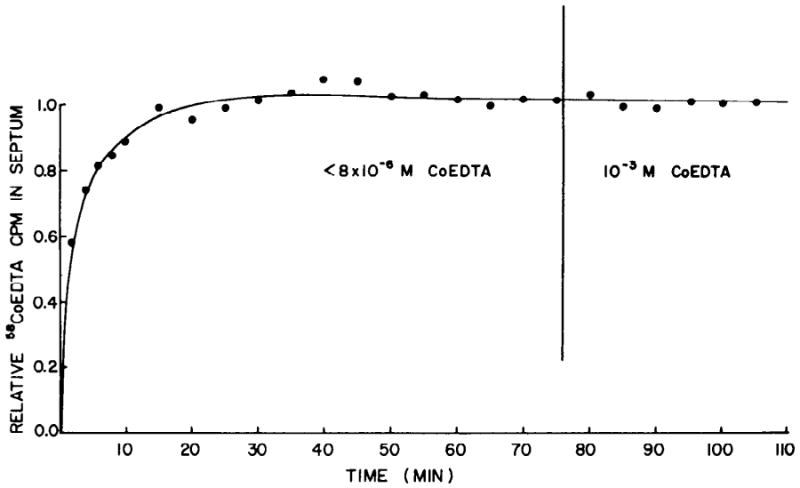
Uptake of 58Co-EDTA from modified Tyrode solution at 25°C. Initially concn of nontracer Co-EDTA was less than 8 × 10−6 M. At 75 min concn of Co-EDTA was increased to 1 mM. Concn of 58Co-EDTA remained constant throughout expt. Ordinate normalized to last point.
Comparison of Co-EDTA and sucrose distribution in isolated rabbit septa
We perfused additional septa at 25°C to measure the [14C]sucrose space. After stabilization of the septa, they were perfused with modified Tyrode solution containing 1 mM [l4C]sucrose (∼5 μCi/ml) for 1 h. Perfusion was then stopped, and the septa were blotted and prepared for liquid scintillation counting as described for the whole heart. In four septa (with a water content of 0.84 ± 0.01 ml/g), the sucrose volume of distribution was 54.9 ± 4.6% of total tissue water or 0.462 ± 0.043 (SD) ml/g wet wt. These values, obtained in a later series of experiments, are perhaps a little smaller but did not differ statistically significantly from the comparable values for Co-EDTA in the rabbit septum given above. The similarity of sucrose and Co-EDTA volumes suggests that both markers are measuring the same space in these edematous preparations.
Co-EDTA uptake at different temperatures
The experiments on isolated septa at 25°C (Figs. 4 and 5) showed that the tracer content plateaued at levels much lower than the total water space of the tissue. We perfused additional septa at 31 and 37°C (pacing 72/min at the highest temperature). At 31°C the kinetics of uptake were identical to those at 25°C. However, at 37°C the tissue counts appeared to increase slowly, never quite reaching a flat asymptote during up to 80 min of perfusion (Fig. 6). From the linear increase in count rate after the initial 30 min of uptake, the apparent rate of change VECF was 0.0076 ± 0.0058 ml/min (SD, n = 6); this is an increase of VEDTA of about 1.6%/min, or only 0.89%/min of total water. The continuing uptake of 58Co-EDTA at 37°C implies that either the ECF was continually enlarging or that the compound was entering the myocardial cells.
FIG. 6.
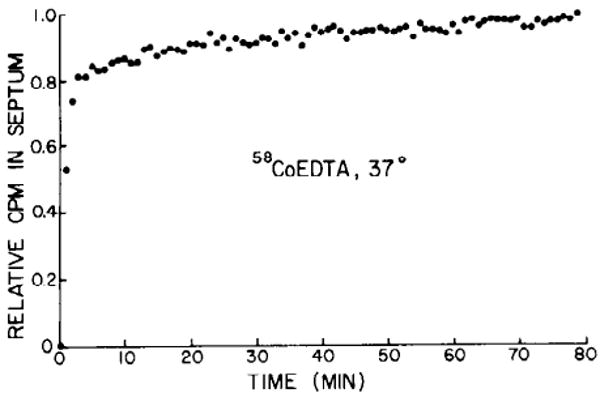
Uptake of 58Co-EDTA for 80 min at 37°C. Ordinate normalized for maximum uptake.
Discussion
The method of synthesis of radiolabeled Co-EDTA is simple and minimizes the possibility of contamination with free cobalt. The purity of the compound is not absolutely assured in the absence of the recrystallization procedure used for manufacturing the unlabeled compound. As the unlabeled compound was prepared by repeated recrystallization from ethanolic solution, it is extremely unlikely that it was contaminated to any significant extent with free cobaltous ion. It therefore provides a reasonable standard of purity. The similarity in volumes of distribution of both the labeled and unlabeled compounds suggests that they are chemically identical and that significant quantities of contaminating cobaltous ion are not present in the labeled preparation.
Although other transition metal chelates have been available for measuring the ECF (2), cobaltic EDTA has several advantages. Although the affinity of EDTA for Co3+ is extremely high (K = 1035 M−1), its affinity for divalent cations present in Tyrode solution is considerably lower, e.g., for Ca-EDTA, K = 1010.57 M−1 and for Mg-EDTA, K = 108.69 M−1 (1). Although the affinity of EDTA for Co3+ could conceivably be reduced in biological fluids, it seems reasonable to conclude that Co3+ is unlikely to be displaced from the complex in Tyrode solution to any significant extent. Therefore the possibility of binding of Co2+ to the tissue is negligible.
In this study the volumes of distribution of both sucrose and Co-EDTA appear not to differ from one another significantly. Differences might have been expected for two reasons. The molecular weight of sucrose (342) is less than that of Co-EDTA (422). Consequently sucrose would be expected to equilibrate into a slightly larger volume of the extracellular fluid than Co-EDTA. There are considerable quantities of negatively charged compounds in the extracellular space (4). Haljamae et al. (7) have attributed the exclusion of Cl− from the interstitial fluid of dogs to the presence of negatively charged carboxyl and sulfate groups associated with the interstitial ground substance. Because consideration of molecular weight and charge of the two compounds would lead one to expect a smaller distribution of Co-EDTA than sucrose, the fact that the two distributions are similar suggests that both charge and volume exclusion effects are small. The absence of effect of unlabeled Co-EDTA on the uptake of 58Co-EDTA indicates that no surface adsorption occurs. Working on smooth muscle, Brading and Jones (2) showed that cobaltous EDTA (which bears 2 negative charges) is adsorbed in the extracellular space, an effect that would not be expected in view of the likelihood of negative charges in the extracellular space. Macchia et al. (10) have found that in toad semitendinosus muscle the space exceeds the sucrose space, whereas in rat gastrocnemius the space is less than the sucrose space. They also found that these markers had apparently the same volumes of distribution in the myocardium of nephrectomized rats. These variations cannot be explained in terms of exclusion but do imply the existence of some variety in charged compounds in the extracellular space; this indicates that one must be cautious in extrapolating from one tissue to another.
It is unlikely that sucrose is bound in the extracellular space or onto the surface of host cells. The fact that Co-EDTA and sucrose share almost identical volumes of distribution suggests that the chelate is not bound in rabbit ventricle and therefore provides a reasonable estimate of extracellular space. The agreement between Co-EDTA and sucrose spaces in both the in vivo heart, with a normal ECF, and the perfused interventricular septum, with an enlarged ECF and water content, further indicates the accuracy of 58Co-EDTA as an ECF marker over a wide range of ECF sizes.
In the isolated rabbit ventricular septum the ECF measured by 58Co-EDTA was approximately 55% of the total tissue water. This value is much higher than the 30% observed in intact fresh heart muscle. A large ECF in the septum is not surprising in view of the high water content of about 85% of wet wt that is caused by exposure to aqueous perfusate. The large size of the ECF in the septal preparation does prompt concern about possible artifacts.
The lack of effect of a sudden large rise in the concentration of unlabeled cobaltic EDTA on the retention of 58Co-EDTA indicates that no surface adsorption occurs. In the apparent absence of an observable surface adsorption isotherm it is unlikely that there are many, if any, transport sites in the sarcolemma. Even so, there does remain the possibility that Co-EDTA enters the cell by passive diffusion. Under these circumstances one would expect its distribution across the cell membrane to be described by the Nernst equation [C]i/[C]0 = exp (−EF/RT) when [C]i is the internal concentration of Co-EDTA; [C]0 is the external concentration; E is membrane potential; F is Faraday's constant; R is the gas constant; and T is absolute temperature. Assuming E = −75 mV and T = 298 K then [C]i/[C]0 would be 0.05 for passive equilibration. Consequently, in the presence of free diffusion and equilibration across the membrane, the tissue activity of 58Co-EDTA would still primarily reflect its extracellular distribution. The possibility of transsarcolemmal diffusion of Co-EDTA exists in the perfused septal preparation at 37°C. However, the rate of increase of the space is very slow, amounting to 0.12%/min of the total tissue water. Therefore even if the slowest phase of the uptake at 37°C did represent transsarcolemmal diffusion, it is slow enough that the marker is still applicable to measurement of larger transient changes in the ECF. It is likely that the slow uptake observed at 37°C indicates gradual expansion of the ECF rather than transport into healthy cells. For instance, it is possible that there is continual loss of salt from the cells; the accompanying loss of water might be expected to enlarge the ECF at the expense of the cell volume (3).
In summary, we are presenting 58Co-EDTA as a γ-emitting tracer that is stable, biologically inert, appears not to enter cells, and equilibrates rapidly into extracellular space of the myocardium in vivo and in isolated preparations.
Acknowledgments
We thank Dr. Barbara Erlich and Alice Lee for their help with the absorption spectrophotometry and Carrol Harris for the isotope counting. We also thank Drs. Glenn Langer and Kenneth Shine for their encouragement and support and Edith Boettcher and Paddy O'Brien for the preparation of the manuscript.
This work was supported by National Heart, Lung, and Blood Institute Grants HL-11351, HL-23684, HL-19139, 1 T32 HL-07412, and 1 T32 HL-07403, the American Heart Association Greater Los Angeles Affiliate Fellowship No. 532-C2, and the Castera Foundation.
References
- 1.Bjerrum J, Schwartzenbach G, Sillen LG. Stability constants of metal ion complexes. London: Chem Soc. 1957:76–77. [Google Scholar]
- 2.Brading AF, Jones AW. Distribution and kinetics of CoEDTA in smooth muscle, and its uses as an extracellular marker. J Physiol London. 1969;200:387–401. doi: 10.1113/jphysiol.1969.sp008700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carslake MC, Weatherall M. Changes in the sodium, potassium and chloride of rabbit auricles treated with ouabain. J Physiol London. 1962;163:347–361. doi: 10.1113/jphysiol.1962.sp006980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Comper WD, Laurent TC. Physiological function of connective tissue polysaccharides. Physiol Rev. 1978;58:255–315. doi: 10.1152/physrev.1978.58.1.255. [DOI] [PubMed] [Google Scholar]
- 5.Dwyer FP, Gyarfas E, Mellor D. The resolution of racemization of potassium ethylenediance tetracetate cobaltate III. J Phys Chem. 1955;59:296–297. [Google Scholar]
- 6.Geigy Scientific Tables. Ardsley, NY: Geigy; 1975. [Google Scholar]
- 7.Haljamae H, Linde A, Amundson B. Comparative analyses of capsular fluid and interstitial fluid. Am J Physiol. 1974;227:1199–1205. doi: 10.1152/ajplegacy.1974.227.5.1199. [DOI] [PubMed] [Google Scholar]
- 8.Langer GA, Brady AJ. The effects of temperature upon contraction and ionic exchange in rabbit ventricular myocardium: relation to control of active state. J Gen Physiol. 1968;54:682–713. doi: 10.1085/jgp.52.3.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacLeod J. Red cell density in certain common animals. Q J Exp Physiol. 1932;22:275–280. [Google Scholar]
- 10.Macchia DD, Page E, Polimeni PI. Interstitial anion distribution in striated muscle determined with [35S]sulfate and [3H]sucrose. Am J Physiol. 1979;237(Cell Physiol. 6):C125–C130. doi: 10.1152/ajpcell.1979.237.3.C125. [DOI] [PubMed] [Google Scholar]
- 11.Shine KI, Douglas AM, Ricchiuti NV. Ischemia in isolated interventricular septum: mechanical events. Am J Physiol. 1976;231:1225–1232. doi: 10.1152/ajplegacy.1976.231.4.1225. [DOI] [PubMed] [Google Scholar]


