Abstract
Small molecular inhibitors of hepatitis C virus (HCV) replication provide remarkable potency, but the rapid selection of resistance mutations will require that these agents be used in combination for clinical treatment. Using a model HCV replicon system, we have extended prior in vitro studies of double combinations of candidate small molecular inhibitors to studies evaluating the simultaneous use of 3 agents. This was done in an effort to anticipate conditions that might ultimately be required clinically. We formally demonstrate synergistic antiviral activity with 3-drug combinations in this model, further supporting the concept of clinical investigations of combination therapy for HCV infection.
Hepatitis C virus (HCV) infection is a major cause of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma, with >170 million individuals infected worldwide. A substantial increase in hospitalizations and medical costs related to chronic HCV is predicted over the next 1–2 decades. Therapy with pegylated interferon plus ribavirin can clear the virus in ~50% of persons infected with genotype 1 HCV infection, the most commonly encountered genotype in North America [1]. Toxicities and contraindications to interferon-based therapy prevent most patients who would otherwise be candidates for treatment from initiating and/or completing a treatment course. Thus, success rates for potential treatment candidates (as opposed to those completing a course of therapy) are ≪50% [2]. These limitations in interferon-based regimens have spurred an active search for small molecular inhibitors of HCV that could increase response rates over the short term when used with current therapy. Ultimately, it is possible that combinations of small molecular inhibitors could lead to efficacious interferon-free regimens. Clinical trials with several candidate molecules have demonstrated substantial short-term reductions in the levels of HCV RNA in plasma [3, 4]. As expected, viral isolates with reduced susceptibility have emerged rapidly [5]. The avoidance or delay of drug resistance in HIV therapeutics has been achieved by the simultaneous use of several potent agents that collectively require the virus to develop multiple resistance mutations [6]. Clinical trials that established the contemporary treatment paradigm were anticipated by in vitro studies that modeled 2- or 3-drug combination therapy in cell culture [7, 8]. These studies were critical in the planning of clinical trials of combination therapy and provided early indications that some combinations of agents were likely to be antagonistic when used together [9]. In similar studies of the HCV replicon system, we have recently reported in vitro additivity or synergy between pairs of agents directed at HCV. In particular, combinations that targeted distinct viral proteins showed greater synergy [10]. In the present report, we extend our 2-drug-combination studies, to examine whether additivity and/or synergy among anti-HCV agents could be demonstrated in cell culture when used in 3-drug combinations. Because the available protease inhibitors (PIs) all target the active site of the NS3-4A serine protease, whereas the available polymerase inhibitors target different sites on the NS5B polymerase, we analyzed the interactions of combinations of 1 PI and 2 polymerase inhibitors.
Materials and methods
To evaluate the synergistic effects of triplet combinations of small molecular inhibitors of HCV, we used the BM4-5 replicon system [11], previously used in studies in our laboratory [10]. The firefly luciferase gene was inserted into the BM4-5 replicon, in a manner described elsewhere [12], to create a luciferase/neomycin phosphotransferase fusion protein (designated “Feo”) and the replicon (BM4-5-Feo). Experiments were performed exactly as described by Wyles et al. [10]. In brief, cells were seeded into 96-well plates and incubated with compounds for 48 h. The luciferase assay (Bright-Glo; Promega) was performed according to the manufacturer’s instructions. Relative light units for each condition were determined by use of a microplate luminometer (Veritas Microplate Luminometer; Turner Biosystems) and were reported as the mean ± SE for 3 wells. The tested compounds included 2 peptidomimetic HCV PIs—BILN 2061 and a Vertex PI (kindly provided by Vicki Sato of Vertex Pharmaceuticals); 1 GlaxoSmithKline trans-lactam PI active-site mimic (kindly provided by Karen Romines of GlaxoSmithKline); 1 nucleoside analog HCV-RNA–dependent RNA polymerase inhibitor, 2′-C-methyladenosine (kindly provided by William Lee of Gilead Sciences); 1 GlaxoSmithKline benzothiadiazine RNA polymerase nonnucleoside inhibitor (NNI) (kindly provided by Karen Romines of GlaxoSmithKline); and 1 Wyeth benzofuran RNA polymerase NNI (kindly provided by Daria Hazuda of Merck) [13]. The Wyeth and GlaxoSmithKline NNIs occupy unique sites in the polymerase. The IC50 of each compound was determined independently and was used to determine the range of concentrations used for the synergy experiments. Each compound was tested both singly and in combination, at 2 2-fold serial dilutions above and below the IC50. The ratio of the 3 tested compounds remained fixed across the dosing range. Determinations of compound interactions were based on the median-effect principle and on the multiple-drug-effect equation, as described by Chou and Talalay [14]. Combination indices were determined, by use of Calcusyn (Biosoft), at the IC50, IC70, and IC90 levels. A total of 9 combinations were evaluated, with 3–5 replicates per condition. A combination index of <0.9 was considered synergistic, a combination index of ≥0.9 or ≤1.1 was considered additive, and a combination index of >1.1 was considered antagonistic. The 50%, 70%, and 90% combination indices are at the IC50, IC70, and IC90 concentrations of each drug, respectively. Cytotoxicity was assessed for each 3-compound combination, at the highest concentrations tested, by use of an MTS assay (CellTiter 96; Promega).
Results
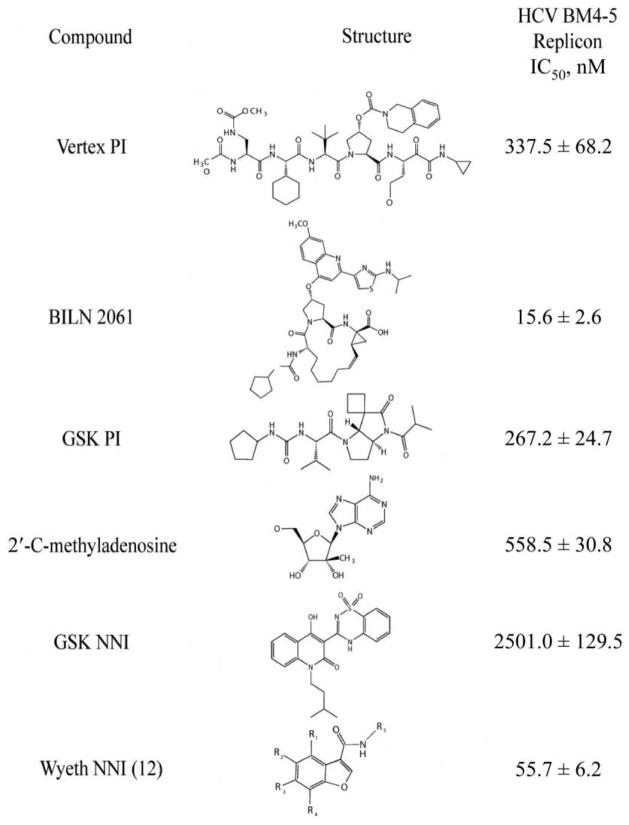
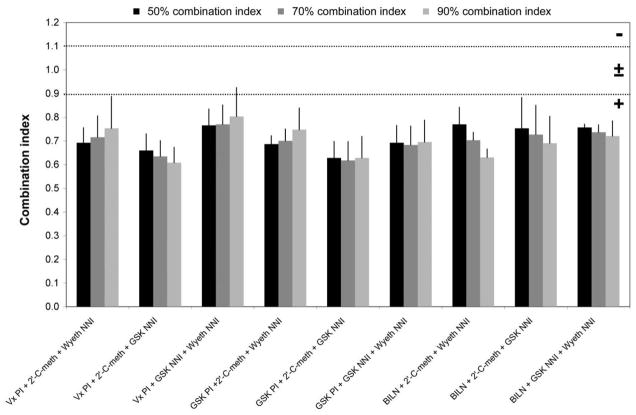
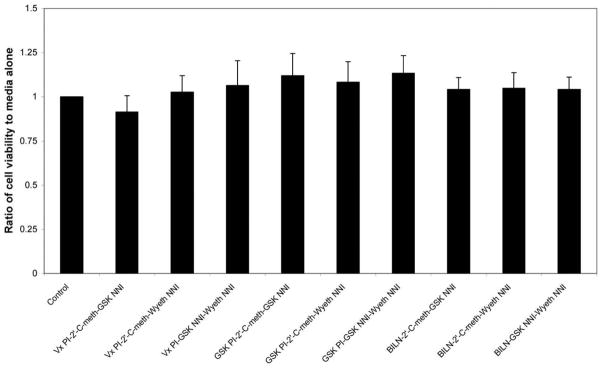
Each inhibitor showed antiviral activity in our genotype 1 replicon system (figure 1). The observed IC50 value for each of the individual compounds is listed in figure 1. Each combination that was tested demonstrated synergy at the IC50, IC70, and IC90 concentrations (figure 2). At the highest concentrations used in the studies, none of the compounds or combinations exhibited cytotoxicity (figure 3).
Figure 1.
Activity of different small molecular inhibitors in the BM4-5 replicon. The IC50 is the mean ± SE of the results from at least 3 independent experiments. GSK, GlaxoSmithKline; NNI, nonnucleoside inhibitor; PI, protease inhibitor.
Figure 2.
IC50, IC70, and IC90, for the compound combinations evaluated. The dotted lines at combination-index values 0.9 and 1.1 indicate the boundaries of an additive interaction. Combination indices are displayed as the mean ± SE of the results from at least 3 independent experiments. GSK, GlaxoSmithKline; NNI, nonnucleoside inhibitor; PI, protease inhibitor; −, antagonism; ±, additive; ±, synergy.
Figure 3.
Results of cell-viability studies, for various 3-compound combinations used in the present study. None of the combinations produced any significant loss in cell viability, compared with control wells containing media alone. Data are shown as the ratio of cell viability in wells treated with compound vs. those treated with media alone (compound A490:media A490). GSK, GlaxoSmithKline; NNI, nonnucleoside inhibitor; PI, protease inhibitor.
Conclusions
HCV shares several key biological similarities with HIV-1, thereby allowing for the rapid evolution of viral-resistance mutations under selective pressure [15]; however, unlike HIV-1, HCV replication does not involve a DNA intermediate, a feature that has redirected therapeutic strategies toward viral elimination rather than life-long suppressive therapy. The profound reductions in morbidity and mortality that accompanied the advent of highly active antiretroviral therapy in the mid-1990s depended on the development of combination antiretroviral regimens that were capable of durable viral suppression [16]. Early clinical trials with small molecular inhibitors of HCV have demonstrated substantial potency but also a propensity for rapid (i.e., within a few days) viral escape when agents are used singly [5]. On the basis of these results of antiretroviral therapy, we investigated triple combinations of small molecular inhibitors of HCV replication, focusing on combinations of drugs targeting different sites of action.
We have demonstrated that these 3-drug combinations exhibit synergy in vitro, as was the case in analogous studies that preceded the clinical investigation of highly active antiretroviral therapy [8]. Of the compounds used in these studies, several are in the same chemical class and possess a mechanism of action that is similar to that found in the compounds being developed for clinical use. The Vertex PI used in these studies is a close structural analog of VX-950, which has progressed through phase 2 human trials. The NS5B polymerase inhibitors used in these studies, both nucleoside and nonnucleosides, are members of chemical classes of compounds that are being actively developed as HCV therapeutics. They share common targets and resistance mutations with therapeutic candidates and, as such, are likely to be reasonable surrogates for use in in vitro studies.
Although there are many similarities between HCV and HIV, there are also substantial differences in biology, and approaches that were successful in the case of HIV therapy will undoubtedly require modification for treatment of HCV infection. HCV replicates to a higher level, and its protease pocket represents a target that is much less attractive than the HIV protease; it is quite conceivable that >3 small molecular inhibitors will ultimately be required for successful HCV therapy. Nonetheless, as in the case of HIV therapeutics, systematic exploitation of relevant in vitro systems are likely to provide extremely useful information that can be used for generating hypotheses for clinical trials. These studies provide further support for the concept that combinations of multiple small molecular inhibitors of HCV replication should be evaluated in rigorous clinical trials, with the view that this approach might ultimately lead to the development of interferon-free regimens that can eliminate HCV from persons infected with it.
Acknowledgments
Financial support: University of California San Diego Center for AIDS Research, funded by National Institutes of Health program 5P30 (2005 developmental grant AI-36214); National Institute of Allergy and Infectious Diseases (grant AI069989).
Footnotes
Potential conflicts of interest: R.T.S. has served as a consultant for Merck, Gilead Sciences, GlaxoSmithKline, and Vertex Pharmaceuticals.
References
- 1.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 2.Falck-Ytter Y, Kale H, Mullen KD, Sarbah SA, Sorescu L, McCullough AJ. Surprisingly small effect of antiviral treatment in patients with hepatitis C. Ann Intern Med. 2002;136:288–92. doi: 10.7326/0003-4819-136-4-200202190-00008. [DOI] [PubMed] [Google Scholar]
- 3.Reesink HW, Zeuzem S, Weegink CJ, et al. Final results of a phase 1B, multiple-dose study of VX-950, a hepatitis C virus protease inhibitor. Hepatology. 2005;42(Suppl 1):234A–5A. [Google Scholar]
- 4.Roberts S, Cooksley G, Dore G, et al. Results of a phase 1B, multiple dose study of R1626, a novel nucleoside analog targeting HCV polymerase in chronic HCV genotype 1 patients. Hepatology. 2006;44(Suppl 1):692A. [Google Scholar]
- 5.Sarrazin C, Kieffer T, Bartels D, et al. Characterization of viral variants in the HCV NS3 protease domain of genotype 1 patients that are selected during 14 days of dosing with VX-950. Hepatology. 2005;42(Suppl 1):751A. [Google Scholar]
- 6.Hammer SM, Saag MS, Schechter M, et al. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society—USA panel. Top HIV Med. 2006;14:827–43. [PubMed] [Google Scholar]
- 7.Hartshorn KL, Vogt MW, Chou TC, et al. Synergistic inhibition of human immunodeficiency virus in vitro by azidothymidine and recombinant alpha A interferon. Antimicrob Agents Chemother. 1987;31:168–72. doi: 10.1128/aac.31.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson VA, Barlow MA, Merrill DP, Chou TC, Hirsch MS. Three-drug synergistic inhibition of HIV-1 replication in vitro by zidovudine, recombinant soluble CD4, and recombinant interferon-alpha A. J Infect Dis. 1990;161:1059–67. doi: 10.1093/infdis/161.6.1059. [DOI] [PubMed] [Google Scholar]
- 9.Vogt MW, Hartshorn KL, Furman PA, et al. Ribavirin antagonizes the effect of azidothymidine on HIV replication. Science. 1987;235:1376–9. doi: 10.1126/science.2435003. [DOI] [PubMed] [Google Scholar]
- 10.Wyles DL, Kaihara KA, Vaida F, Schooley RT. Synergy of small molecular inhibitors of hepatitis C virus replication directed at multiple viral targets. J Virol. 2007;81:3005–8. doi: 10.1128/JVI.02083-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo JT, Bichko VV, Seeger C. Effect of alpha interferon on the hepatitis C virus replicon. J Virol. 2001;75:8516–23. doi: 10.1128/JVI.75.18.8516-8523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanabe Y, Sakamoto N, Enomoto N, et al. Synergistic inhibition of in-tracellular hepatitis C virus replication by combination of ribavirin and interferon-3. J Infect Dis. 2004;189:1129–39. doi: 10.1086/382595. [DOI] [PubMed] [Google Scholar]
- 13.Saha AK, Burns CJ, Del Vecchio AM, et al., inventors; Viropharma, assignee. Benzofuran compounds, compositions and methods and treatment and prophylaxis of hepatitis C viral infections and associated diseases. 60,515,944. US Patent. filed 30 October 2003 and issued 11 February 2004.
- 14.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 15.Kurosaki M, Enomoto N, Marumo F, Sato C. Evolution and selection of hepatitis C virus variants in patients with chronic hepatitis C. Virology. 1994;205:161–9. doi: 10.1006/viro.1994.1631. [DOI] [PubMed] [Google Scholar]
- 16.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]