Abstract
Context
Depressive symptoms commonly follow coronary artery bypass (CABG) surgery and are associated with worse clinical outcomes.
Objective
To test the effectiveness of telephone-delivered collaborative care for post-CABG depression versus doctors’ usual care.
Design
Single-blind effectiveness trial.
Setting
Seven Pittsburgh-area university-based and community hospitals.
Participants
302 depressed post-CABG patients and a non-depressed comparison group of 151 randomly sampled post-CABG patients recruited between 3/2004 and 9/2007 and followed as outpatients.
Intervention
8-Months of telephone-delivered collaborative care provided by nurses working with patients’ primary care physicians and supervised by a study psychiatrist and study primary care physician.
Main Outcome Measures
Mental health-related quality of life (HRQoL) as measured by the SF-36 MCS at 8-months follow-up; secondary outcome measures included mood symptoms (Hamilton Rating Scale for Depression (HRS-D)), physical HRQoL (SF-36 PCS) and functioning (Duke Activity Status Index (DASI)); and hospital readmissions.
Results
Depressed intervention patients (N=150) reported greater improvements (all P ≤ 0.02) in mental HRQoL (SF-36 MCS: Δ 3.2 points; 95% CI: 0.5–6.0), physical functioning (DASI: Δ 4.6 points; 1.9–7.3), and mood symptoms (HRS-D: Δ3.1 points (1.3–4.9); and were more likely to report a ≥ 50% decline in HRS-D score from baseline (50.0% vs. 29.6%; NNT 4.9 (3.2–10.4)) than depressed patients randomized to their physicians’ usual care (N=152) (P<0.001). Depressed men were particularly likely to benefit from the intervention (SF-36 MCS: Δ 5.7 points (2.2–9.2); P=0.001) and tended to have a lower incidence of rehospitalization for cardiovascular causes than depressed men receiving usual care (13% vs. 23%; P=0.07) or depressed women (19% vs. 11%; P=0.22). However, the mean HRQoL and physical functioning of depressed intervention patients did not reach that of our non-depressed comparison group.
Conclusions
Compared to usual care, telephone-delivered collaborative care for post-CABG depression resulted in improved HRQoL, physical functioning, and mood symptoms at 8-months follow-up.
Keywords: Depression, coronary artery bypass surgery, randomized clinical trial, collaborative care, coronary artery disease
Coronary artery bypass graft (CABG) surgery is one of the most common and costly medical procedures performed in the U.S.1 Its main indications are the relief of angina and improvement in quality of life.2 Yet up to half of post-CABG patients report depressive symptoms in the peri-operative period,3 and they are more likely to experience poorer health-related quality of life (HRQoL),4 worse functional status, continued chest pains5, 6 and higher rates of re-hospitalization and death following CABG independent of cardiac status, medical co-morbidity, or the extent of surgery.7–11
Although the mechanism whereby depression affects post-CABG outcomes remains unknown,12 widely generalizable strategies to detect and effectively treat post-CABG depression are of great interest. Several treatment trials for depression have been conducted in cardiac populations but most achieved less than anticipated benefits with regard to reducing mood symptoms13–19 or cardiovascular morbidity.13–16, 19, 20 Moreover, none utilized the proven effective “collaborative care” approach21 recently endorsed by a National Institutes of Health expert consensus panel.22
Unlike earlier interventions for treating depressed cardiac patients that utilized either a single antidepressant,13, 15, 17, 18 counseling modality,20 or antidepressant in combination with counseling,14, 19 collaborative care emphasizes a flexible real-world treatment “package” that involves active follow-up by a non-physician “care manager” who: adheres to evidence-based treatment protocols; supports patients with timely education about their illness; considers patients’ prior treatment experiences and current preferences; teaches self-management techniques; actively involves primary care physicians (PCPs) in their patients’ care through regular exchanges of real-time information; proactively monitors treatment responses and suggests adjustments when indicated; and facilitates co-management or transfer of care to local specialists when patients either do not respond to treatment, are clinically complicated, or upon patient or PCP request.23, 24
This report presents the main outcomes findings from “Bypassing the Blues” (BtB), the first randomized trial of a collaborative care strategy for treating depression following an acute cardiac event. The primary objective of BtB was to compare the impact on HRQoL of our treatment strategy for post-CABG depression with doctors’ usual care at 8-months follow-up. Our secondary objectives were to evaluate the effectiveness of our intervention on mood symptoms, physical health, and cardiovascular morbidity. BtB also included a non-depressed comparison group to facilitate contrasts with depressed subjects on our baseline and follow-up measures.25
METHODS
Patients
We screened post-CABG patients for depression prior to hospital discharge at 2 university-affiliated and 5 community Pittsburgh-area hospitals, implementing a protocol approved by the Institutional Review Board of the University of Pittsburgh, each participating hospital, and an independent data and safety monitoring board appointed by our funding agency.25 From 3/2004 to 9/2007, trained nurse-recruiters identified medically-stable patients who had just undergone CABG and obtained their signed informed consent to undergo our screening procedure.
We administered the two-item Patient Health Questionnaire (PHQ-2)26 and considered an affirmative answer to either item as a positive depression screen.27 We required patients to have a Folstein Mini-Mental Status Exam28 score ≥ 24 as evidence of mental competence to provide consent; have no current alcohol dependence or other substance abuse disorder; not be in treatment with a mental health specialist (MHS), express active suicidality, or a history of psychotic illness or bipolar disorder; be discharged home or to short-term rehabilitation; and speak English, have no communication barrier, and own a telephone. Upon confirmation, the study nurse obtained the patient’s signed informed consent permitting us to recontact him or her following hospital discharge to confirm protocol-eligibility prior to our randomization procedure. We orally and via mail encouraged all PHQ-2 screen-positive patients to contact their PCP to discuss this clinical finding.
Mood symptoms commonly follow CABG and may represent the normal sequelae of surgery (e.g., fatigue, sleeplessness).29 Therefore, we administered the PHQ-930 two-weeks following hospitalization via telephone to confirm the PHQ-2 screen, and required patients score ≥ 10 indicating at least a moderate level of depressive symptoms to remain protocol-eligible.27
Assessments and Outcome Measures
Nurse-recruiters collected information on patients’ self-reported race31 and sociodemographic characteristics, and conducted a detailed chart review of co-morbid medical conditions, extent of surgery, and medication use. Following confirmation of protocol-eligibility at two-weeks and at 2-, 4-, and 8-month follow-up, blinded telephone assessors administered the: SF-3632, 33 to determine generic mental (MCS) and physical (PCS) HRQoL; 12-item Duke Activity Status Index (DASI)34 to determine disease-specific physical functioning; and the17-item Hamilton Rating Scale for Depression (HRS-D) to track mood symptoms.35 Minimally clinically important changes have been defined as ≥ 3 point or 0.25 effect size (ES) improvements36, 37 on these measures,38, 34 and a meaningful recovery from depression as ≥ 50% reduction from baseline symptoms.39 We selected the SF-36 MCS as our primary outcome measure as it assesses a wider domain of functioning and it is more extensively used as an outcome measure among cardiac patients than the HRS-D or any other mood questionnaire. We also administered the PRIME-MD Mood and Anxiety Modules to determine the presence of major depression and an anxiety disorder, respectively.40 Following the 2-week baseline assessment and after each follow-up contact, we mailed the patient a $20 check for his/her time ($80 total).
Assessors inquired about any hospitalizations patients experienced since their last assessment. When these events or a death was detected, relevant medical records and/or death certificates were sought and forwarded to two physicians for independent review and classification of the event (Endpoint Classification Committee). When not in complete agreement, the event was discussed at a meeting and adjudicated by consensus. We also abstracted and quantified process measures of depression care from the electronic registry used by our care managers to document treatment.25
Randomization Procedure
Following confirmation of protocol-eligibility and completion of the 2-week assessment, we randomized depressed patients to either the intervention or “usual care” group in a 1:1 ratio in blocks of 4 according to a computer-generated random assignment sequence stratified by hospital site, prepared in advance by our statistician (S.M.), entered into the computer-assisted telephone interview program used by our assessors, and concealed until after the 2-week call. A study nurse or project coordinator then informed patients of their treatment assignment and notified their PCP.
Non-Depressed Comparison Group
We randomly sampled approximately one PHQ-2 screen-negative patient who was not using an antidepressant and met all other protocol-eligibility criteria for every two randomized depressed post-CABG study subjects, stratified by participating hospital and gender, and oversampled by race. Later, the patient was required to score <5 on the 2-week PHQ-9 to continue participation.
Intervention
Initial Telephone Contact
As described elsewhere,25 a nurse care manager telephoned intervention patients to: review their psychiatric history; provide basic psychoeducation about depression and its impact on cardiac disease; and describe treatment options. The latter included: (1) a workbook to enhance patients’ understanding and ability to self-care for depression41; (2) initiation or adjustment of antidepressant pharmacotherapy prescribed under their PCPs’ direction; (3) “watchful-waiting” for mildly elevated mood symptoms; or (4) referral to a local MHS.
Case-Review
After the initial patient contact, the nurse care manager presented his/her clinical information to the study psychiatrist (C.F.R.) and internist (B.L.R.) at a weekly case-review session which focused on newly randomized patients and those with severe mood symptoms. The presented information included an overview of each patient’s clinical course including serial PHQ-9 scores, pharmacotherapy usage, workbook chapters covered, MHS referral status, and additional details to inform decision making (e.g., prior antidepressant usage, and individual PHQ-9 item scores).
Following a case discussion, the clinical management team formulated treatment recommendations consistent with each patient’s prior experiences, current treatment preferences, and insurance coverage. The nurse conveyed these recommendations to the patient via telephone, and to his/her PCP for consideration via fax, telephone, or mail depending upon the urgency, and updated the study team about the patient’s progress at the next case-review session.
Antidepressant Pharmacotherapy
Selective serotonin reuptake inhibitor (SSRI) antidepressants are considered safe for use in cardiac patients,42 with no evidence indicating superior efficacy for any one in treatment-naïve patients.43 Therefore, for those lacking a history of prior SSRI use or brand preference, we typically recommended citalopram as it: has limited drug-drug interactions; requires few dosage adjustments; and is available in generic form. For depressed patients already using an SSRI, we advised a dosage increase or a switch to another SSRI if they were at the maximum amount. We generally recommended two SSRI trials before switching to a serotonin norepinephrine reuptake inhibitor (SNRI) or bupropion, other antidepressants with low cardiovascular toxicity.25
PCPs prescribed and approved all adjustments to their patients’ pharmacotherapy and we never dispensed any medications. However, study nurses offered to telephone antidepressant prescriptions to patients’ pharmacies under PCPs’ verbal order so as to promote adherence with our treatment recommendations.
Mental Health Referral
We advised referral to a local MHS in the event of poor treatment response, severe psychopathology, complex psychosocial problems, and/or patient preference. The care manager offered to assist by identifying a provider within the patient’s insurance network, and/or facilitating the initial appointment. Following the date of the scheduled visit, the nurse contacted the patient to confirm the appointment was kept, and telephoned him/her monthly to monitor mood symptoms and promote adherence with follow-up MHS appointments.
Follow-Up
During the “acute phase” of treatment,44 the care manager telephoned patients bi-weekly to review lesson plans; monitor antidepressant pharmacotherapy; administer the PHQ-9 to assess treatment response; encourage PCP and MHS follow-up; and inform the patient of new treatment recommendations generated at our weekly case-review sessions. Depending upon the patient’s treatment choice(s), symptoms, and motivation, these telephone contacts lasted 15–45 minutes and continued for two to four months. The patient subsequently transitioned to the “continuation phase” of care during which the care manager contacted him/her every 1–2 months until completion of our 8-month intervention.
Usual Care
For ethical reasons,45 we informed “usual care” patients of their depression status as well as their PCPs. However, we provided no treatment advice unless we detected suicidality on a follow-up assessment.
Blinding
Telephone assessors were blinded as to patients’ randomization and baseline depression status and they cautioned subjects at the beginning of each call not to divulge their treatment assignment. Given the nature of our intervention, neither patients nor their PCPs were blinded to the treatment assignment.
Data Safety Monitoring
We programmed our data management system to identify: depressed subjects whose blinded HRS-D score increased ≥ 25% above their 2-week baseline score; and non-depressed comparison subjects who scored ≥ 10 the HRS-D. If indicated following a review, we wrote to the treating PCP and offered to identify local MHSs and provide additional depression treatment advice. Whenever staff uncovered suicidality, they were instructed to immediately contact a study psychiatrist to determine the level of threat and convey treatment advice to the patient and his/her PCP.
Statistical Analyses
Women may derive less benefit from CABG surgery than men,46–50 and depressed women exposed to a psychosocial intervention following myocardial infarction may experience worse cardiac outcomes than women exposed to a control condition51 or men.14, 48 Therefore, we powered our trial to conduct an intent-to-treat (ITT) analysis within each gender on the SF-36 MCS, our primary outcome measure. We hypothesized our intervention would produce a ≥ 0.5 greater ES improvement on the SF-36 MCS versus “usual care” at 8-months follow-up. We selected this time-point to allow a therapeutic alliance to develop between patients and their care managers and sufficient time for several therapeutic trials, if necessary, of antidepressant pharmacotherapy and counseling to take effect. Randomizing 150 depressed men (or women) would provide 83% power to detect a medium ES difference ≥ 0.5 using a 2-tailed α=0.05 and assuming 10% attrition, and 80% power to detect an ES ≥ 0.3 using our full sample (N=300).
We compared baseline sociodemographic, clinical, and functional status measures by both randomization and baseline depression status using t-tests for continuous data, chi-square analyses for categorical data, and controlling for multiple comparisons using the Hochberg method.52 To calculate changes in score and effect sizes on all depressed-randomized patients with 95% confidence intervals (CI), we used a repeated measures mixed effect model with treatment, time (4 time points), gender, and all 2- and 3-factor interaction terms with subject intercepts treated as a random effect to account for individual differences at randomization, and time treated as a fixed effect categorical variable. We used the restricted maximum likelihood inferential procedure to fit our mixed models under missing-at-random and unstructured covariance matrix assumptions (SAS Proc Mixed); multiple imputation techniques to calculate missing 8-month HRS-D scores; chi-square tests to compare differences in the proportions patients who achieved a remission; and the number needed to treat (NNT) using the reciprocal of the difference in response rates. Cumulative event rates were calculated using Kaplan-Meier survival analyses with log-rank chi-square tests for determining statistical significance. All P-values are 2-tailed with significance levels at P≤ 0.05, and all statistical tests of outcomes measures are 2-group comparisons involving depressed-randomized groups only.
RESULTS
Of the 2,485 post-CABG patients consenting to our PHQ-2 depression screening procedure, 56% (1,387) screened positive prior to hospital discharge (Figure 1). At two-week follow-up, 1,100 remained protocol-eligible and completed the PHQ-9. Of these, 337 (31%) scored ≥ 10 and 302 (90%) accepted randomization. Additionally, we randomly selected 151 non-depressed post-CABG patients for a total of 453 study subjects. By June 1, 2008, following the last 8-month follow-up contact, we ascertained vital status of all 453 (100%) and identified 4 deaths, all from non-suicide causes, and no serious or unexpected adverse events.
Figure 1. Flowchart of participants in the Trial.
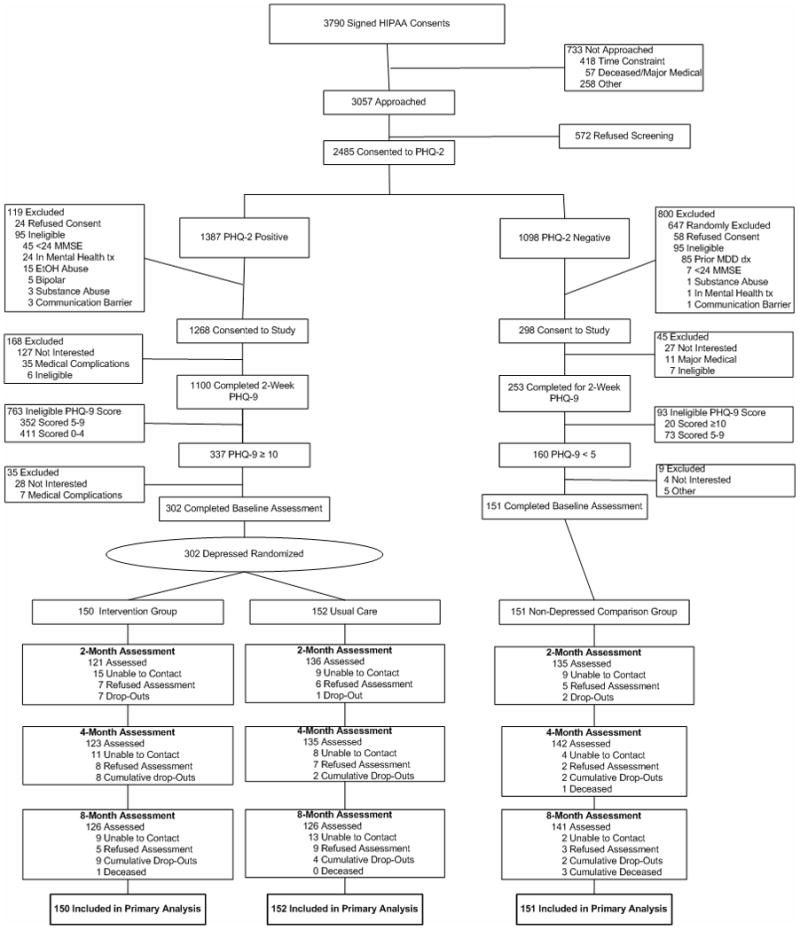
HIPAA, Health Insurance Portability and Accountability Act; PHQ-2, 2-item Patient Health Questionnaire; PHQ-9, 9-item Patient Health Questionnaire.
Overall, 13% (60/453) did not complete their 8-month telephone assessment, and missed assessments for any reason did not differ by randomization or baseline depression status. Further inspection of the reasons for withdrawal among those depressed and randomized revealed they were mostly at these patients’ request or due to loss of follow-up contact.
Depressed intervention and usual care patients were similar on all baseline clinical, sociodemographic, and surgical criteria (Table 1). However, compared to our non-depressed comparison group, depressed patients tended to be slightly younger and were more likely to be non-Caucasian, have chronic obstructive pulmonary disease, use tobacco, and to report prior treatment for depression, and lower levels of HRQoL and physical functioning (all P≤ 0.03). Compared to depressed males, depressed females also reported higher rates of co-morbid anxiety and prior depression treatment.
Table 1.
Baseline sociodemographic, clinical, and mental health characteristics by randomization status and baseline depression status.
| Depressed Intervention (n=150) | Depressed Usual Care (n=152) | Non-Depressed Comparison Group (n=151) | Depressed Intervention vs. Usual Care P* | Depressed vs. Non-Depressed Comparison P† | |
|---|---|---|---|---|---|
| Age, mean (SD) | 64 (10.8) | 64 (11.2) | 66 (9.6) | 0.44 | 0.03 |
| Male, % (N) | 54 (81) | 63 (96) | 63 (95) | 0.11 | 0.38 |
| Caucasian, % (N) | 88 (132) | 93 (142) | 81 (122) | 0.13 | <0.01 |
| > High School education, % (N) | 57 (86) | 54 (82) | 52 (78) | 0.55 | 0.42 |
| Married, % (N) | 64 (96) | 69 (105) | 69 (104) | 0.41 | 0.84 |
| Working, part-time or full-time, % (N) | 41 (61) | 32 (49) | 38 (57) | 0.07 | 0.37 |
| SF-36 MCS, mean (SD)‡ | 43.2 (11.2) | 42.8 (11.8) | 61.5 (5.8) | 0.72 | <0.001¶ |
| SF-36 PCS, mean (SD)‡ | 31.3 (7.0) | 30.2 (7.1) | 37.4 (7.4) | 0.19 | <0.001¶ |
| Duke Activity Status Index, mean (SD)‡ | 7.1 (5.8) | 7.7 (7.6) | 13.2 (6.4) | 0.41 | <0.001¶ |
| Perceived Social Support Scale, mean (SD) | 70.0 (10.6) | 68.8 (11.2) | 73.2 (12.2) | 0.36 | <0.001¶ |
| Hypertension, % (N) | 87 (131) | 80 (122) | 81 (122) | 0.10 | 0.43 |
| Diabetes, % (N) | 40 (60) | 45 (68) | 39 (59) | 0.40 | 0.50 |
| Hyperlipidemia, % (N) | 85 (128) | 77 (117) | 74 (112) | 0.06 | 0.09 |
| Stroke, % (N) | 8 (12) | 8 (12) | 5 (8) | 0.97 | 0.30 |
| Chronic obstructive pulmonary disease, % (N) | 21 (32) | 23 (35) | 9 (14) | 0.72 | <0.01 |
| Chronic renal insufficiency, % (N) | 13 (19) | 7 (10) | 11 (16) | 0.07 | 0.74 |
| Myocardial infarction, % (N) | 49 (73) | 44 (67) | 45 (68) | 0.42 | 0.79 |
| Congestive heart failure, % (N) | 28 (42) | 20 (31) | 21 (31) | 0.12 | 0.38 |
| Percent ejection fraction, mean (SD) N | 51 (12.3) 145 | 50 (12.6) 147 | 51 (13.0) 144 | 0.28 | 0.92 |
| Tobacco use, past year, % (N) | 25 (37) | 30 (45) | 14 (21) | 0.59 | 0.005 |
| Angiotensin-converting enzyme inhibitors, % (N) | 39 (59) | 34 (52) | 28 (42) | 0.36 | 0.06 |
| Aspirin, % (N) | 84 (126) | 80 (121) | 81 (123) | 0.32 | 0.93 |
| Beta-blockers, % (N) | 81 (122) | 77 (117) | 82 (124) | 0.35 | 0.37 |
| Calcium channel blocker, % (N) | 14 (21) | 17 (26) | 12 (18) | 0.46 | 0.30 |
| Lipid-lowering medication, % (N) | 73 (109) | 67 (102) | 78 (118) | 0.29 | 0.05 |
| CABG Type, % (N) | |||||
| Off-pump | 15 (23) | 19 (29) | 21 (31) | 0.39 | 0.39 |
| On-pump | 85 (127) | 81 (123) | 79 (120) | ||
| Total CABG surgery time, hours, mean (SD) | 4.2 (1.7) | 4.1 (1.5) | 3.9 (1.5) | 0.75 | 0.22 |
| Cross clamp time, minutes, mean (SD) | 86 (47) 111 | 75 (38) 115 | 77 (39) 103 | 0.06 | 0.48 |
| Total number if bypass grafts, median (range) | 3 (1–7) | 3 (1–6) | 4 (1–7) | 0.64 | 0.11 |
| PHQ-9 score, mean (SD)§ | 13.5 (3.2) | 13.6 (3.6) | 1.9 (1.4) | 0.81 | <0.001¶ |
| Hamilton Rating Scale for Depression, mean (SD)§ | 16.5 (7.1) | 15.9 (6.9) | 3.0 (2.6) | 0.44 | 0.001¶ |
| Major depression, % (N) | 37 (55) | 40 (61) | 0 (0) | 0.54 | <0.001¶ |
| Anxiety disorder, % (N)|| | 31 (46) | 28 (43) | 0 (0) | 0.65 | <0.001¶ |
| Visits with a mental health professional, % (N) | |||||
| Lifetime | 31 (46) | 24 (37) | 5 (8) | 0.22 | <0.001¶ |
| Within last 2 years | 3 (4) | 3 (5) | 0 (0) | 0.69 | <0.001¶ |
| Received treatment from a PCP for depression, % (N) | |||||
| Lifetime | 23 (35) | 25 (38) | 2 (3) | 0.74 | <0.001¶ |
| Within last 2 years | 17 (25) | 16 (25) | 1 (1) | 0.69 | <0.001¶ |
| Antidepressant pharmacotherapy, % (N) | |||||
| Lifetime | 37 (55) | 36 (54) | 1 (2) | 0.84 | <0.001¶ |
| Within last 2 years | 22 (33) | 26 (40) | 1 (1) | 0.22 | <0.001¶ |
| Baseline | 26 (39) | 28 (43) | 0 (0) | 0.55 | 0.001¶ |
Abbreviations: SF-36 MCS, Medical Outcomes Study Short Form Mental Component Scale; SF-36 PCS, Medical Outcomes Study Short form Physical Component Scale; CABG, coronary artery bypass graft surgery;
Depressed Intervention (N=150) vs. Depressed Usual Care (N=152)
Depressed (N=302) vs. Non-Depressed Comparison Group (N=151)
Higher scores indicate better health-related quality of life
Higher scores indicate more severe symptoms
Panic, generalized anxiety, or anxiety NOS
Differences remain significant after using Hochberg method for multiple comparisons
Clinical Outcomes
From baseline to 8-months follow-up, intervention patients achieved significant clinical improvements on the SF-36 MCS (3.2 points (95% CI: 0.5–6.0), P=0.02; ES: 0.30 (0.17–0.52), P=0.01) and our key secondary outcome measures, compared to patients receiving usual post-CABG care (Table 2 and Figure 2), however we detected no differences in outcomes by recruitment site. While these improvements became evident at 2-months follow-up (Figure 3), the mean level of HRQoL and physical functioning for intervention patients never attained that of our non-depressed comparison group. Overall, 50% (75/150) of intervention subjects’ reported ≥ 50% reduction in mood symptoms from baseline versus 29.6% (45/152) of those in usual care (ES 0.42 (0.19–0.65); P<0.001), and the number needed to treat (NNT) to produce one additional treatment response was 4.9 (3.2–10.4) (Table 3).
Table 2.
Baseline to 8-Month mixed model estimates of mean change scores by randomization status.
| All Patients (n=302) | Intervention (n=150) | Usual Care (n=152) | Between Group Difference (95% CI) | P |
|---|---|---|---|---|
| SF-36 MCS, mean (SE) | ||||
| Baseline | 43.1 (1.0) | 42.5 (1.0) | ||
| 8-Month follow-up | 50.0 (1.0) | 46.2 (1.1) | ||
| Δ Baseline to 8-Month | 6.8 (1.0) | 3.6 (1.0) | 3.2 (0.5–6.0) | 0.02 |
| HRS-D, mean (SE) | ||||
| Baseline | 16.6 (0.6) | 16.0 (0.6) | ||
| 8-Month follow-up | 9.0 (0.7) | 11.4 (0.7) | ||
| Δ Baseline to 8-Month | 7.6 (0.6) | 4.5 (0.6) | 3.1 (1.3–4.9) | 0.001 |
| SF-36 PCS, mean (SE) | ||||
| Baseline | 31.2 (0.8) | 30.3 (0.8) | ||
| 8-Month follow-up | 44.0 (0.8) | 41.4 (0.8) | ||
| Δ Baseline to 8-Month | 12.8 (0.8) | 11.1 (0.8) | 1.6 (−0.5–3.8) | 0.14 |
| DASI, mean (SE) | ||||
| Baseline | 7.1 (0.9) | 7.9 (0.9) | ||
| 8-Month Follow-up | 25.2 (1.0) | 21.4 (1.0) | ||
| Δ Baseline to 8-Month | 18.1 (1.0) | 13.5 (1.0) | 4.6 (1.9–7.3) | 0.001 |
| Men (n=177) | Intervention (n=81) | Usual Care (n=96) | ||
| SF-36 MCS, mean (SE) | ||||
| Baseline | 44.3 (1.3) | 43.3 (1.2) | ||
| 8-Month follow-up | 52.1 (1.4) | 45.4 (1.3) | ||
| Δ Baseline to 8-Month | 7.8 (1.3) | 2.1 (1.2) | 5.7 (2.2–9.2) | 0.001 |
| HRS-D, mean (SE) | ||||
| Baseline | 15.7 (0.8) | 15.8 (0.8) | ||
| 8-Month follow-up | 7.8 (0.9) | 10.9 (0.8) | ||
| Δ Baseline to 8-Month | 7.9 (0.8) | 4.9 (0.8) | 3.0 (0.8–5.3) | 0.009 |
| SF-36 PCS, mean (SE) | ||||
| Baseline | 31.9 (1.0) | 30.0 (1.0) | ||
| 8-Month follow-up | 46.6 (1.1) | 41.0 (1.0) | ||
| Δ Baseline to 8-Month | 14.6 (1.0) | 11.1 (1.0) | 3.6 (0.8–6.3) | 0.01 |
| DASI, mean (SE) | ||||
| Baseline | 7.5 (1.2) | 7.3 (1.1) | ||
| 8-Month Follow-up | 29.3 (1.3) | 22.9 (1.2) | ||
| Δ Baseline to 8-Month | 21.8 (1.3) | 15.6 (1.2) | 6.1 (2.7–9.6) | 0.001 |
| Women (n=125) | Intervention (n=69) | Usual Care (n=56) | ||
| SF-36 MCS, mean (SE) | ||||
| Baseline | 42.0 (1.4) | 41.7 (1.6) | ||
| 8-Month follow-up | 47.8 (1.6) | 46.9 (1.7) | ||
| Δ Baseline to 8-Month | 5.9 (1.5) | 5.1 (1.6) | 0.7 (−3.3–4.9) | 0.74 |
| HRS-D, mean (SE) | ||||
| Baseline | 17.6 (0.9) | 16.2 (1.0) | ||
| 8-Month follow-up | 10.2 (1.0) | 12.0 (1.1) | ||
| Δ Baseline to 8-Month | 7.4 (0.9) | 4.2 (1.0) | 3.2 (0.5–5.9) | 0.02 |
| SF-36 PCS, mean (SE) | ||||
| Baseline | 30.5 (1.1) | 30.6 (1.2) | ||
| 8-Month follow-up | 41.4 (1.2) | 41.8 (1.3) | ||
| Δ Baseline to 8-Month | 10.9 (1.2) | 11.2 (1.3) | −0.3 (−3.6–3.0) | 0.86 |
| DASI, mean (SE) | ||||
| Baseline | 6.6 (1.3) | 8.5 (1.5) | ||
| 8-Month follow-up | 21.1 (1.4) | 19.9 (1.6) | ||
| Δ Baseline to 8-Month | 14.5 (1.4) | 11.4 (1.6) | 3.1 (−1.1–7.3) | 0.14 |
SE indicates standard error; CI confidence interval
Table 3.
Proportion achieving 50% decline from baseline HRS-D score at 8-month follow-up.*
| Intervention (n=150) | Usual Care (n=152) | Effect Size (95% CI) | NNT (95% CI) | P | |
|---|---|---|---|---|---|
| All (n=302) | 50.0% (75/150) | 29.6% (45/152) | 0.42 (0.19–0.65) | 4.9 (3.2–10.4) | <0.001 |
| Men (n=177) | 60.5% (49/81) | 33.3% (32/96) | 0.55 (0.26–0.85) | 3.4 (2.4–7.7) | <0.001 |
| Women (n=125) | 37.7% (26/69) | 23.2% (13/56) | 0.32 (−0.04–0.67) | 6.9 (3.3-∞) | 0.08 |
Multiple imputation used to address missing 8-month follow-up assessments (17%; 50/302).
CI indicates confidence interval
Clinical Outcomes by Gender
At 8-months follow-up, depressed post-CABG men randomized to our intervention reported a 5.7 point (P=0.001) improvement on the SF-36 MCS and improvements on our other key secondary measures (Table 2 and Figure 2). Overall, 60.5% (49/81) of intervention men versus 33.3% (32/96) of usual care men reported a ≥ 50% reduction in HRS-D score from baseline (0.55 ES (0.26–0.85); P<0.001), while 37.7% (26/69) vs. 23.2% (13/56) of women did so improve (0.32 ES (−0.04–0.67); P=0.08) (Table 3). Although we found a significant gender by treatment interaction on the SF-36 PCS (F=5.25, df=1,302, P=0.02), we did not identify any other gender by treatment or 3-way (gender × treatment × time) interactions on our other outcome measures.
Rehospitalizations
By 8-months follow-up we identified 207 rehospitalizations including 85 (41%) for cardiovascular causes (Table 4). Overall, 33% of depressed intervention patients, 32% of usual care patients, and 25% of non-depressed comparison subjects were rehospitalized (Figure 4). Although depressed patients reported an overall similar incidence of rehospitalization for cardiovascular causes, men randomized to our intervention tended to have a lower incidence of rehospitalization than those in usual care (13% vs. 23%; P=0.07).
Table 4.
Primary diagnosis for incidence of rehospitalization by study group and gender.
| Depressed Intervention | Depressed Usual Care | Non-Depressed Comparison Group | All | ||||
|---|---|---|---|---|---|---|---|
| Males (n=25)* | Females (n=22)* | Males (n=32)* | Females (n=14)* | Males (n=25)* | Females (n=15)* | ||
| Total Rehospitalizations, N | 34 | 51 | 46 | 22 | 33 | 21 | 207 |
| Cardiac/Cardiovascular Rehospitalizations, N | 12 | 19 | 25 | 10 | 12 | 7 | 85 |
| Arrhythmia | 0 | 1 | 5 | 0 | 4 | 1 | 11 |
| Congestive heart failure | 2 | 4 | 8 | 3 | 2 | 3 | 22 |
| Myocardial infarction, non-fatal | 0 | 2 | 1 | 0 | 0 | 0 | 3 |
| Perscutaneous coronary revascularization† | 3 | 0 | 3 | 2 | 4 | 0 | 12 |
| Revascularization, non-cardiac | 3 | 2 | 2 | 0 | 1 | 0 | 8 |
| Stroke | 0 | 0 | 1 | 1 | 0 | 1 | 3 |
| Surgery, cardiac‡ | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| Unstable angina | 3 | 3 | 4 | 4 | 0 | 1 | 15 |
| Other | 0 | 7 | 1 | 0 | 0 | 1 | 9 |
| Non-Cardiac/Cardiovascular Rehospitalizations, N | 21 | 32 | 21 | 12 | 21 | 14 | 121 |
| Bleeding event, all | 2 | 0 | 4 | 0 | 1 | 1 | 8 |
| GI bleed | 2 | 0 | 3 | 0 | 1 | 1 | 7 |
| Chest pain, non-cardiac | 1 | 3 | 0 | 1 | 0 | 2 | 7 |
| Infection, all | 5 | 15 | 4 | 4 | 7 | 3 | 38 |
| Infection related to CABG | 2 | 5 | 2 | 1 | 1 | 2 | 13 |
| Malignancy | 2 | 0 | 0 | 0 | 1 | 3 | 6 |
| Neurological event # | 1 | 5 | 1 | 0 | 1 | 0 | 8 |
| Pulmonary disease | 0 | 0 | 1 | 2 | 0 | 0 | 3 |
| Surgery, non-cardiac | 9 | 3 | 3 | 2 | 7 | 1 | 25 |
| Trauma | 0 | 1 | 1 | 2 | 3 | 1 | 8 |
| Other | 1 | 5 | 7 | 1 | 1 | 3 | 18 |
| Psychiatric Rehospitalizations, N | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Suicidal ideation | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
Number of patients with at least one rehospitalization event
e.g., Stent and/or angioplasty.
e.g., Valve repair; no patient had redo-CABG by 8-months follow-up.
Non-stroke events.
Processes of Care Management
Of the 150 patients in our 8-month intervention, 83% had three or more telephone care manager contacts by 4-month follow-up, and the median number of contacts was 10 (range: 0–28) (Table 5). While the number of contacts did not differ by gender, men were more likely to use the workbook (91% (74/81) vs. 78% (54/69); P=0.02), and women were more likely to use pharmacotherapy (59% (41/69) vs. 43% (35/81); P=0.05). Rates of self-reported pharmacotherapy usage increased from baseline levels at all follow-up points; however, these rates were higher in intervention than in usual care patients. Furthermore, rates of MHS care were low and did not differ by randomization status (e.g., 4% intervention vs. 6% usual care at 8-months).
Table 5.
Intervention process measures of care.
| Intervention | Usual Care | Difference (95% CI) | P | |
|---|---|---|---|---|
| Care manager contacts, median (range) | ||||
| 2-Months | 3 (0–8) | N/A | -- | -- |
| 4-Months | 5.5 (0–14) | N/A | -- | -- |
| 8-Months | 10 (0–28) | N/A | -- | -- |
| ≥ 3 Registry mentions of workbook use | ||||
| 2-Months | 65% (97/150) | N/A | -- | -- |
| 4-Months | 75% (113/150) | N/A | -- | -- |
| 8-Months | 85% (128/150) | N/A | -- | -- |
| Antidepressant Pharmacotherapy, self-reported* | ||||
| Baseline | 15% (22/150) | 9% (13/152) | 6% (−1–13) | 0.10 |
| 2-Months | 44% (54/122) | 26% (35/135) | 18% (7–30) | 0.002 |
| 4-Months | 42% (51/122) | 26% (35/134) | 16% (4–27) | 0.008 |
| 8-Months | 44% (55/126) | 31% (40/127) | 13% (1–24) | 0.05 |
| Mental health specialist visit, self-reported | ||||
| 2-Months | 3% (4/122) | 2% (3/135) | 1% (−3–5) | 0.60 |
| 4-Months | 4% (5/122) | 1% (2/134) | 3% (−1–7) | 0.20 |
| 8-Months | 4% (5/126) | 6% (7/127) | −2% (−7–4) | 0.56 |
Antidepressant pharmacotherapy includes selective serotonin reuptake inhibitors (SSRIs), selective serotonin norepinephrine reuptake inhibitors (SNRIs), bupropion, and mirtazapine
COMMENT
Bypassing the Blues is the first clinical trial of a collaborative care strategy for treating depression following an acute cardiac event. We found collaborative care for treating post CABG depression to improve mental HRQoL and physical functioning and reduce mood symptoms at 8-month follow-up. The internal and external validity of our findings are strengthened by multiple design elements including: a randomized study design with blinded assessments of outcomes; patient recruitment from both academically-affiliated and community hospitals; use of a time-efficient depression case-identification strategy27 recommended by the American Heart Association42; telephone delivery of our intervention; consideration of patients’ treatment preferences; and stipulation that patients obtain antidepressant pharmacotherapy from their PCP and MHS counseling at their prevailing cost.
The observed ES improvement on self-report measures such as the HRS-D is at the upper-end reported by a meta-analysis of 37 collaborative care trials for depressed primary care patients (0.25 ES; 0.18–0.32),21 and resembles the ES obtained from more intensive forms of psychotherapy53 and pharmacotherapy.54, 55 The effectiveness of our treatment strategy also compares favorably to the ES improvements in HRS-D reported by the Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) (ES: 0.14; −0.06–0.35),13 the citalopram arm of the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy Trial (CREATE) (0.29; 0.05–0.52),19 the psychotherapy-based Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) trial (0.22; 0.11–0.33)14 and the counseling arm of CREATE (−0.22; −0.46–0.01).19 Moreover, neither those trials nor any other investigating the impact of treating depression in patients with cardiovascular disease15, 20, 56 were linked to primary care, delivered primarily via telephone, and/or required patients to obtain their own pharmacotherapy and MHS care at cost. Our findings and mode of intervention delivery, thus have major public health implications for medically frail individuals, those living in rural settings, and others with physical challenges impeding face-to-face depression treatment.
The generalizability of our study findings possibly is limited as recruitment occurred in just one U.S. region. Nevertheless, the sociodemographic and clinical characteristics of participants resembled those enrolled in other CABG studies.5, 8, 9, 11, 20 Additionally, nurse-recruiters were required to obtain patients’ prior consent through a hospital staff member before they could even approach to obtain consent to administer the PHQ-2. This potentially introduced a selection bias if severely depressed patients were less likely to participate in our screening procedures. Since a substantial minority of patients failed to clinically respond to our depression intervention, it is vital to identify post-CABG patients most likely to become “treatment resistant” so as to develop more effective treatments for them.57 Identifying the intervention components which maximally contribute to our outcomes is also of great interest. However, collaborative care is a complex intervention involving a number of separate mechanisms that have proved difficult to disentangle from the non-specific effects of increased attention by the care manager.58, 59
A critical barrier to wider dissemination of collaborative care is the persisting lack of financial support for it in typical U.S. practice settings.60 However, it is an integral component of the “medical home” model presently advocated by leading professional organizations to reimburse PCPs for providing high-quality chronic illness care.24, 61 Given the $32,201 mean cost of a CABG-associated rehospitalization,62 $14,471 annual expenses per Medicare beneficiary,63 and relationship of co-morbid depression with a doubling of health care costs independent of physical illness burden,64, 65 post-CABG patients are an attractive target for a depression treatment program likely to prove cost-effective66 and possibly cost-saving. For example, a trial of collaborative care for treating depression in diabetics reduced total medical costs compared with usual care at a median 12-month intervention expenditure of $546.67 We are analyzing the cost-effectiveness of our intervention and plan to report those findings in a separate report.
CONCLUSIONS
Compared to usual care, telephone-delivered collaborative care for post-CABG depression can improve HRQoL, physical functioning, and mood symptoms at 8-months follow-up. Additional research is necessary to develop improved treatments for women and patients with resistant depression, and to examine the economic impact of this intervention.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01 HL70000 (Rollman) and P30 MH71944 (Reynolds), and by the UPMC Endowed Chair in Geriatric Psychiatry (Reynolds).
Bypassing the Blues Study Team
We wish to thank the members of our dedicated study team for their efforts: Erin August, RN*, Amy Boyles, MSW*, Lori Freund*, Lillian Giles, RN*, Kyle Holleran, BS*, Elizabeth Hunt, BS*, Cynthia Kravetz, BS*, Sabrina Leshko, BS*, Jessica Minydzak, BS*, Carol Mitchell, RN, BSN*, Eleanor Shirley, MA*, Susan Spillane, RN*, and Sharon Stover, RN* (University of Pittsburgh, Pittsburgh, Pennsylvania).
Consultants
We thank our study consultants Nancy Frasure-Smith, PhD (McGill University, Montreal, Canada), Kenneth E. Freedland, PhD* (Washington University, St Louis, Missouri), and Mark Hlatky, MD* (Stanford University, Palo Alto, California) for their critical input particularly during the planning and implementation stages of our study.
Data Center, Center for Research on Health Care
Timothy Bragg*, Deljo Ann Gannon*, Donald W. Grimm*, Doris Rubio, PhD*, and Terry Sefcik, MS* (University of Pittsburgh, Pittsburgh, Pennsylvania).
Data and Safety Monitoring Board
Christopher M. O’Connor, MD* (chair) (Duke University, Durham, North Carolina); Robert M. Carney, PhD*, Washington University, St Louis, Missouri); J. Michael DiMaio, MD* (University of Texas Southwestern Medical Center, Dallas, Texas); Mark T. Hughes, MD* (Johns Hopkins, Baltimore, Maryland); and Steven Roose, MD* (Columbia University, New York, New York).
Endpoint Classification Committee
Wishwa Kapoor, MD, MPH* (chair), Linda Cadaret, MD, Peter Counihan, MD*, Robert Cook, MD*, Rebecca Drayer, MD, Matt Freiberg, MD*, Jordan Karp, MD, Oscar Marroquin, MD*, Natalia Morone, MD, Mamoo Nakamura, MD*, Jamal Rana, MD*, Steven Reis, MD*, Mark Roberts, MD*, Hilary Tindle, MD, Steven Weisbord, MD, and Ellen Whyte, MD (University of Pittsburgh, Pittsburgh, Pennsylvania); Vinayak Hegde, MD*, and Nosheen Javed, MD* (The Western Pennsylvania Hospital, Pittsburgh, Pennsylvania).
Study Site Principal Investigators
Peter Counihan, MD* (Presbyterian and Passavant Hospitals, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania); Michael H. Culig, MD* (The Western Pennsylvania Hospital, Pittsburgh, Pennsylvania); Venkataraman Krishnaswami, MD* (Mercy Hospital of Pittsburgh, Pittsburgh, Pennsylvania); Venkat R. Machiraju, MD* (Shadyside Hospital, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania); Sang B. Park, MD* (Jefferson Regional Medical Center, Jefferson Hills, Pennsylvania); and Mark M. Suzuki, MD* (Westmoreland Regional Hospital, Greensburg, PA).
Support and Role of the Sponsor
This work was supported by NIH grants R01 HL70000 (Rollman) and P30 MH71944 (Reynolds) and by the UPMC Endowed Chair in Geriatric Psychiatry (Reynolds). The National Heart Lung and Blood Institute (NHLBI) and National Institute of Mental Health (NIMH) had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. We gratefully thank our NHLBI project officer, Susan Czajkowski, PhD for her long-term support of our efforts.
Financial Disclosures
Charles F. Reynolds III, M.D. reports receiving pharmaceutical supplies for his NIH-sponsored work from Forest Laboratories, Pfizer, Bristol-Myers Squibb, Wyeth, and Eli Lilly, and Sati Mazumdar, Ph.D. reports stock ownership in Forest Laboratories. None of the other authors report any other potential financial conflicts (e.g., speakers’ bureaus, stock, industry grant support, or consulting relationships). We designate persons listed in our Acknowledgement section who were compensated for their role on the project with an asterix (*)
Previous Presentations
Parts of this manuscript were presented at the annual national meetings of the American Psychosomatic Society (Chicago, Illinois; March 5, 2009), Society for Behavioral Medicine (Montreal, Canada; April 25, 2009), and Society for General Internal Medicine (Miami, Florida; May 14, 2009).
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart Lung and Blood Institute.
Footnotes
Trial Registration: Clinicaltrials.gov Identifier: NCT00091962 (http://clinicaltrials.gov/ct2/show/NCT00091962?term=rollman+cabg&rank=1)
Author Contributions:
Dr Rollman had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Rollman, Herbeck Belnap, Kapoor, Mazumdar, Reynolds, Schulberg.
Acquisition of data: Rollman, Counihan, Herbeck Belnap, Kapoor, LeMenager, Reynolds.
Analysis and interpretation of data: Rollman, Herbeck Belnap, Houck, Mazumdar, Reynolds, Schulberg.
Drafting of the manuscript: Rollman, Houck, Mazumdar.
Critical revision of the manuscript for important intellectual content: Rollman, Counihan, Herbeck Belnap, Houck, Kapoor, LeMenager, Mazumdar, Reynolds, Schulberg.
Statistical analysis: Houck, Mazumdar.
Obtained funding: Rollman, Reynolds.
Administrative, technical, or material support: Rollman, Counihan, Herbeck Belnap, Houck, Kapoor, LeMenager, Reynolds, Schulberg.
Study supervision: Rollman, Herbeck Belnap, Houck, Kapoor, LeMenager, Reynolds.
References
- 1.Lloyd-Jones D, Adams R, et al. Heart Disease and Stroke Statistics--2009 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2004;110(14):e340–437. [PubMed] [Google Scholar]
- 3.Pignay-Demaria V, Lesperance F, Demaria RG, Frasure-Smith N, Perrault LP. Depression and Anxiety and Outcomes of Coronary Artery Bypass Surgery. Ann Thorac Surg. 2003;75:314–321. doi: 10.1016/s0003-4975(02)04391-6. [DOI] [PubMed] [Google Scholar]
- 4.Goyal TM, Idler EL, Krause TJ, Contrada RJ. Quality of life following cardiac surgery: impact of the severity and course of depressive symptoms. Psychosomatic Med. 2005;67(5):759–765. doi: 10.1097/01.psy.0000174046.40566.80. [DOI] [PubMed] [Google Scholar]
- 5.Mallik S, Krumholz HM, Lin ZQ, et al. Patients with depressive symptoms have lower health status benefits after coronary artery bypass surgery. Circulation. 2005;111(3):271–277. doi: 10.1161/01.CIR.0000152102.29293.D7. [DOI] [PubMed] [Google Scholar]
- 6.Doering LV, Moser DK, Lemankiewicz W, Luper C, Khan S. Depression, healing, and recovery from coronary artery bypass surgery. Am J Crit Care. 2005;14(4):316–324. [PubMed] [Google Scholar]
- 7.Rumsfeld JS, Ho PM, Magid DJ, et al. Predictors of health-related quality of life after coronary artery bypass surgery. Ann of Thoracic Surgery. 2004;77(5):1508–1513. doi: 10.1016/j.athoracsur.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 8.Blumenthal JA, Lett HS, Babyak MA, et al. Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet. 2003;362(9384):604–609. doi: 10.1016/S0140-6736(03)14190-6. [DOI] [PubMed] [Google Scholar]
- 9.Connerney I, Shapiro PA, McLaughlin JS, Bagiella E, Sloan RP. Relation between depression after coronary artery bypass surgery and 12-month outcome: a prospective study. Lancet. 2001;358(9295):1766–1771. doi: 10.1016/S0140-6736(01)06803-9. [DOI] [PubMed] [Google Scholar]
- 10.Oxlad M, Stubberfield J, Stuklis R, Edwards J, Wade TD. Psychological risk factors for cardiac- related hospital readmission within 6 months of coronary artery bypass graft surgery. J Psychosom Res. 2006;61(6):775–781. doi: 10.1016/j.jpsychores.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Burg MM, Benedetto CM, Soufer R. Pre-surgical depression impacts 6-month outcome in patients undergoing coronary artery bypass graft (CABG) surgery. Psychosom Med. 2003;65:508–510. doi: 10.1097/01.psy.0000077509.39465.79. [DOI] [PubMed] [Google Scholar]
- 12.Carney RM, Freedland KE. Depression in patients with coronary heart disease. Am J Med. 2008;121(11 Suppl 2):S20–27. doi: 10.1016/j.amjmed.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Glassman AH, O’Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288(6):701–709. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 14.Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: The Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA. 2003;289(23):3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 15.O’Connor CM, Jiang W, Silva SG, et al. Safety and Efficacy of Sertraline plus Nurse Facilitated Supportive Intervention (NFSI) versus Placebo plus Nurse Facilitated Supportive Intervention for Depression in Patients with CHF (SADHART-CHF) J Cardiac Failure. 2008;14(9):797. [Google Scholar]
- 16.van Melle JP, de Jonge P, Honig A, et al. Effects of antidepressant treatment following myocardial infarction. Br J Psychiatry. 2007;190:460–466. doi: 10.1192/bjp.bp.106.028647. [DOI] [PubMed] [Google Scholar]
- 17.Honig A, Kuyper AMG, Schene AH, et al. Treatment of post-myocardial infarction depressive disorder: a randomized, placebo-controlled trial with mirtazapine. Psychosomatic Med. 2007;69(7):606–613. doi: 10.1097/PSY.0b013e31814b260d. [DOI] [PubMed] [Google Scholar]
- 18.Strik JJ, Honig A, Lousberg R, et al. Efficacy and safety of fluoxetine in the treatment of patients with major depression after first myocardial infarction: findings from a double-blind, placebo-controlled trial. Psychosomatic Med. 2000;62(6):783–789. doi: 10.1097/00006842-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Lesperance F, Frasure-Smith N, Koszycki D, et al. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA. 2007;297(4):367–379. doi: 10.1001/jama.297.4.367. [DOI] [PubMed] [Google Scholar]
- 20.Freedland KE, Skala JA, Carney RM, et al. Treatment of Depression After Coronary Artery Bypass Surgery: A Randomized Controlled Trial. Arch Gen Psychiatry. 2009;66(4):387–396. doi: 10.1001/archgenpsychiatry.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006;166:2314–2321. doi: 10.1001/archinte.166.21.2314. [DOI] [PubMed] [Google Scholar]
- 22.Davidson KW, Kupfer DJ, Bigger JT, et al. Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute Working Group Report. Psychosom Med. 2006;68(5):645–650. doi: 10.1097/01.psy.0000233233.48738.22. [DOI] [PubMed] [Google Scholar]
- 23.Von Korff M, Gruman J, Schaefer J, Curry SJ, Wagner EH. Collaborative management of chronic illness: Essential elements. Ann Intern Med. 1997;127:1097–1102. doi: 10.7326/0003-4819-127-12-199712150-00008. [DOI] [PubMed] [Google Scholar]
- 24.Coleman K, Austin BT, Brach C, Wagner EH. Evidence On The Chronic Care Model In The New Millennium. Health Aff. 2009;28(1):75–85. doi: 10.1377/hlthaff.28.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rollman BL, Belnap Herbeck B, Lemenager M, Mazumdar S, Schulberg HC, Reynolds CF., III The Bypassing the Blues Treatment Protocol: Stepped Collaborative Care for Treating Post-CABG Depression. Psychosom Med. 2009;71:217–230. doi: 10.1097/PSY.0b013e3181970c1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 27.McManus D, Pipkin SS, Whooley MA. Screening for depression in patients with coronary heart disease. Am J Cardio. 2005;96(8):1076–1081. doi: 10.1016/j.amjcard.2005.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Pirraglia PA, Peterson JC, Williams-Russo P, Gorkin L, Charlson ME. Depressive symptomatology in coronary artery bypass graft surgery patients. Int’l J Geriatr Psych. 1999;14(8):668–680. doi: 10.1002/(sici)1099-1166(199908)14:8<668::aid-gps988>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NOTICE: NOT- OD-01-053. Bethesda, MD: 2001. NIH Policy on Reporting Race and Ethnicity Data: Subjects in Clinical Research. [Google Scholar]
- 32.Ware J, Kosinski M, Keller S. SF-36 physical and mental health summary scales: A user’s manual. 2. Boston: New England Medical Center; 1994. [Google Scholar]
- 33.Watson EK, Firman DW, Baade PD, Ring I. Telephone administration of the SF-36 health survey: Validation studies and population norms for adults in Queensland. Australian & New Zealand J Public Health. 1996;20(4):359–363. doi: 10.1111/j.1467-842x.1996.tb01046.x. [DOI] [PubMed] [Google Scholar]
- 34.Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (The Duke Activity Status Index) Am J Cardiology. 1989;64(10):651–654. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 35.Freedland KE, Skala JA, Carney RM, et al. The Depression Interview and Structured Hamilton (DISH): Rationale, Development, Characteristics, and Clinical Validity. Psychosomatic Med. 2002;64(6):897–905. doi: 10.1097/01.psy.0000028826.64279.29. [DOI] [PubMed] [Google Scholar]
- 36.Hays RD, Woolley JM. The concept of clinically meaningful difference in health-related quality-of- life research. How meaningful is it? Pharmacoeconomics. 2000;18(5):419–423. doi: 10.2165/00019053-200018050-00001. [DOI] [PubMed] [Google Scholar]
- 37.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. London: Academic Press; 1988. [Google Scholar]
- 38.Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Quality of Life Rsearch. 2001;10(5):405–413. doi: 10.1023/a:1012588218728. discussion 415–420. [DOI] [PubMed] [Google Scholar]
- 39.Prien RF, Carpenter LL, Kupfer DJ. The definition and operational criteria for treatment outcome of major depressive disorder. A review of the current research literature. Arch Gen Psychiatry. 1991;48(9):796–800. doi: 10.1001/archpsyc.1991.01810330020003. [DOI] [PubMed] [Google Scholar]
- 40.Spitzer RL, Williams JBW, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care: the PRIME-MD 1000 study. JAMA. 1994;272:1749–1756. [PubMed] [Google Scholar]
- 41.Katon W, Ludman E, Simon G. The Depression Helpbook. Boulder, CO: Bull Publishing; 2002. [Google Scholar]
- 42.Lichtman JH, Bigger JT, Jr, Blumenthal JA, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118(17):1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 43.Qaseem A, Snow V, Denberg TD, Forciea MA, Owens DK Physicians. Using Second-Generation Antidepressants to Treat Depressive Disorders: A Clinical Practice Guideline from the American College of Physicians. Ann Intern Med. 2008;149(10):725–733. doi: 10.7326/0003-4819-149-10-200811180-00007. [DOI] [PubMed] [Google Scholar]
- 44.Depression in Primary Care. Treatment of Major Depression. Vol. 1. Vol. 2. Rockville, MD: U.S. Dept of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; 1993. Detection and Diagnosis. AHCPR Publication Nos. 93–0550 & 93–0551. [Google Scholar]
- 45.Reynolds CF, 3rd, Degenholtz H, Parker LS, et al. Treatment as usual (TAU) control practices in the PROSPECT Study: managing the interaction and tension between research design and ethics. Int’ J Geriatric Psychiatry. 2001;16(6):602–608. doi: 10.1002/gps.466. [DOI] [PubMed] [Google Scholar]
- 46.Con AH, Linden W, Thompson JM, Ignaszewski A. The psychology of men and women recovering from coronary artery bypass surgery. J Cardiopulmonary Rehabil. 1999;19(3):152–161. doi: 10.1097/00008483-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Ayanian JZ, Guadagnoli E, Cleary PD. Physical and psychosocial functioning of women and men after coronary artery bypass surgery. JAMA. 1995;274(22):1767–1770. [PubMed] [Google Scholar]
- 48.Schneiderman N, Saab PG, Catellier DJ, et al. Psychosocial treatment within sex by ethnicity subgroups in the Enhancing Recovery in Coronary Heart Disease clinical trial. Psychosomatic Med. 2004;66(4):475–483. doi: 10.1097/01.psy.0000133217.96180.e8. [DOI] [PubMed] [Google Scholar]
- 49.Phillips Bute B, Mathew J, Blumenthal JA, et al. Female gender is associated with impaired quality of life 1 year after coronary artery bypass surgery. Psychosom Med. 2003;65(6):944–951. doi: 10.1097/01.psy.0000097342.24933.a2. [DOI] [PubMed] [Google Scholar]
- 50.Vaccarino V, Lin ZQ, Kasl SV, et al. Sex differences in health status after coronary artery bypass surgery. Circulation. 2003;108(21):2642–2647. doi: 10.1161/01.CIR.0000097117.28614.D8. [DOI] [PubMed] [Google Scholar]
- 51.Frasure-Smith N, Lesperance F, Prince RH, et al. Randomised trial of home-based psychosocial nursing intervention for patients recovering from myocardial infarction. Lancet. 1997;350(9076):473–479. doi: 10.1016/S0140-6736(97)02142-9. [DOI] [PubMed] [Google Scholar]
- 52.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9(7):811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 53.Churchill R, Hunot V, Corney R, et al. A systematic review of controlled trials of the effectiveness and cost-effectiveness of brief psychological treatments for depression. Health Technol Assess. 2001;5(35):1–173. doi: 10.3310/hta5350. [DOI] [PubMed] [Google Scholar]
- 54.Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287(14):1840–1847. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- 55.Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry. 2006;59(11):990–996. doi: 10.1016/j.biopsych.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 56.Thombs BD, de Jonge P, Coyne JC, et al. Depression screening and patient outcomes in cardiovascular care: a systematic review. JAMA. 2008;300(18):2161–2171. doi: 10.1001/jama.2008.667. [DOI] [PubMed] [Google Scholar]
- 57.Carney RM, Freedland KE. Treatment-resistant depression and mortality after acute coronary syndrome. Am J Psychiatry. 2009;166(4):410–417. doi: 10.1176/appi.ajp.2008.08081239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bower P, Gilbody S, Richards D, Fletcher J, Sutton A. Collaborative care for depression in primary care. Making sense of a complex intervention: systematic review and meta-regression. Br J Psychiatry. 2006;189:484–493. doi: 10.1192/bjp.bp.106.023655. [DOI] [PubMed] [Google Scholar]
- 59.Williams JW, Jr, Gerrity M, Holsinger T, Dobscha S, Gaynes B, Dietrich A. Systematic review of multifaceted interventions to improve depression care. Gen Hosp Psychiatry. 2007;29(2):91–116. doi: 10.1016/j.genhosppsych.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 60.Pincus HA, Houtsinger JK, Bachman J, Keyser D. Depression in primary care: bringing behavioral health care into the mainstream. Health Aff. 2005;24(1):271–276. doi: 10.1377/hlthaff.24.1.271. [DOI] [PubMed] [Google Scholar]
- 61.Iglehart JK. No place like home-testing a new model of care delivery. N Engl J Med. 2008;359(12):1200–1202. doi: 10.1056/NEJMp0805225. [DOI] [PubMed] [Google Scholar]
- 62.Brown PP, Kugelmass AD, Cohen DJ, et al. The frequency and cost of complications associated with coronary artery bypass grafting surgery: results from the United States Medicare program. Ann Thorac Surg. 2008;85(6):1980–1986. doi: 10.1016/j.athoracsur.2008.01.053. [DOI] [PubMed] [Google Scholar]
- 63.Medicare: Medicare Spending and Financing Fact Sheet. Menlo Park, CA: The Henry J. Kaiser Family Foundation; May, 2009. [Google Scholar]
- 64.Unutzer J, Schoenbaum M, Katon WJ, et al. Healthcare costs associated with depression in medically ill fee-for-service Medicare participants. J Am Geriatr Soc. 2009;57(3):506–510. doi: 10.1111/j.1532-5415.2008.02134.x. [DOI] [PubMed] [Google Scholar]
- 65.Arnow BA, Blasey CM, Lee J, et al. Relationships among depression, chronic pain, chronic disabling pain, and medical costs. Psychiatr Serv. 2009;60(3):344–350. doi: 10.1176/ps.2009.60.3.344. [DOI] [PubMed] [Google Scholar]
- 66.Gilbody S, Bower P, Whitty P. Costs and consequences of enhanced primary care for depression: systematic review of randomised economic evaluations. Br J Psychiatry. 2006;189:297–308. doi: 10.1192/bjp.bp.105.016006. [DOI] [PubMed] [Google Scholar]
- 67.Katon WJ, Russo JE, Von Korff M, et al. Long-term effects on medical costs of improving depression outcomes in patients with depression and diabetes. Diabetes Care. 2008;31(6):1155–1159. doi: 10.2337/dc08-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


