Abstract
A transient, ischemia-resistant phenotype known as “ischemic tolerance” can be established in brain in a rapid or delayed fashion by a preceding noninjurious “preconditioning” stimulus. Initial preclinical studies of this phenomenon relied primarily on brief periods of ischemia or hypoxia as preconditioning stimuli, but it was later realized that many other stressors, including pharmacologic ones, are also effective. This review highlights the surprisingly wide variety of drugs now known to promote ischemic tolerance, documented and to some extent mechanistically characterized in preclinical animal models of stroke. Although considerably more experimentation is needed to thoroughly validate the ability of any currently identified preconditioning agent to protect ischemic brain, the fact that some of these drugs are already clinically approved for other indications implies that the growing enthusiasm for translational success in the field of pharmacologic preconditioning may be well justified.
One goal common to all preclinical stroke research is to identify molecular mediators of neurovascular injury or protection and to devise therapies to either block or enhance these mechanisms to improve outcome. Investigations of preconditioning and ischemic tolerance (IT) [1], [2] are no different: The receptors, signal transduction pathways, transcriptional regulatory elements, micro and messenger RNA and protein profiles, and subcellular organelle function that are modified by the preconditioning stimulus are all suitable targets for therapeutics. At first pass, the patient population that suffers from cerebral ischemic injury due to unpredictable focal stroke, cardiac arrest, or subarachnoid hemorrhage represents, by definition, one that is unlikely to derive benefit from preconditioning research. However, the novel endogenous survival pathways identified in preclinical IT studies may ultimately become targets for drugs that protect brain even when acutely administered after the precipitating event. Importantly, a significant number of other patients – those in which we can anticipate a period of cerebral ischemia following transient ischemic attack, aneurysm clipping, subarachnoid hemorrhage, carotid endarterectomy or stenting, asymptomatic carotid stenosis, coronary bypass, and cardiac valve replacement – represent defined at-risk populations ideally suited for translational therapeutic preconditioning. The candidate drugs that might underpin clinical trials for this latter group of patients actually comprise a relatively long – and therefore promising – list, particularly if the current foundation of preclinical studies is expanded with intention. This review will highlight many of these.
Overview
In the initial years of cerebral IT research, the majority of studies utilized brief ischemia in vivo, or oxygen-glucose deprivation (OGD) in vitro, as the preconditioning stimulus. However, as time passed, the effectiveness of other IT-promoting preconditioning stimuli was slowly realized. The ability of a nonischemic, nonhypoxic stimulus to protect against subsequent cerebral ischemia was initially referred to as “cross-tolerance”, but with the increasing number of preclinical pharmacologic preconditioning studies appearing in the literature, this nomenclature is no longer in frequent use. More important is the implication that the ability of disparate stimuli to trigger both the metabolic changes and the up- or down-regulation of expression of the hundreds of genes responsible for establishing IT suggests that many of these stimuli share a common, but limited, set of overlapping molecular signaling pathways that may be amenable to activation by pharmacologic preconditioning mimetics. Some of these preconditioning-inducing agents are particularly attractive from a translational standpoint, given their demonstrated low toxicity and minimal side effects in humans. Even though several nonischemic or nonhypoxic stimuli that are not pharmacologic (hyperthermia, spreading depression, hyperbaric oxygen, exercise, etc.) have shown efficacy as preconditioning triggers in some stroke models, including various ‘immunological preconditioning’ strategies [2], for the purposes of this review, only pharmacology-based preconditioning regimens will garner the spotlight. Not included are prophylactic approaches to neuroprotection that, in essence, represent acute or chronic pretreatments in which the drug is present when ischemia strikes. Rather, the focus here will be on classical or delayed pharmacologic preconditioning wherein the singular or final drug treatment of a series precedes the ischemic event by many hours or days, and the obligatory genomic reprogramming that largely defines the ischemia-tolerant phenotype is promoted.
After almost two decades of preclinical research, the number of pharmacologic stimuli that induce a state of IT is noteworthy (Table 1). However, the relative depth and breadth of research on any specific pharmacologic paradigm for establishing preconditioning-induced IT remains extremely uneven. For example, some agents that are clinically approved for other indications and that are safe and well tolerated in human patients have received scant attention as preconditioning stimuli, even though a precedent exists for demonstrated protection from ischemic brain injury in at least one laboratory preconditioning study. Conversely, some compounds continue to receive considerable experimental attention in animal studies (albeit sometimes disproportionately from only a handful of laboratories), and we have uncovered a number of their respective induction and expression mechanisms, despite the fact that, even though efficacious in rodents, these agents are unlikely to be approved for clinical use. The volatile anesthetics and the KATP channel openers (KCOs) are the two classes of drugs that break this pattern, given that a relatively large number of laboratory studies have characterized the effectiveness of these already clinically approved drugs; these and other examples from this latter category will be discussed further below. While not dismissing the value of in vitro models (including organotypic slices and cell culture), the majority of studies cited in this review will be those conducted in animals subjected to transient or permanent focal ischemia, or global ischemia, since the latter models are necessary stepping stones on the road to demonstrating the neuro-, glial-, and vasculo-protective efficacy of a particular preconditioning treatment, which, in turn, lay the groundwork for clinical trials [3].
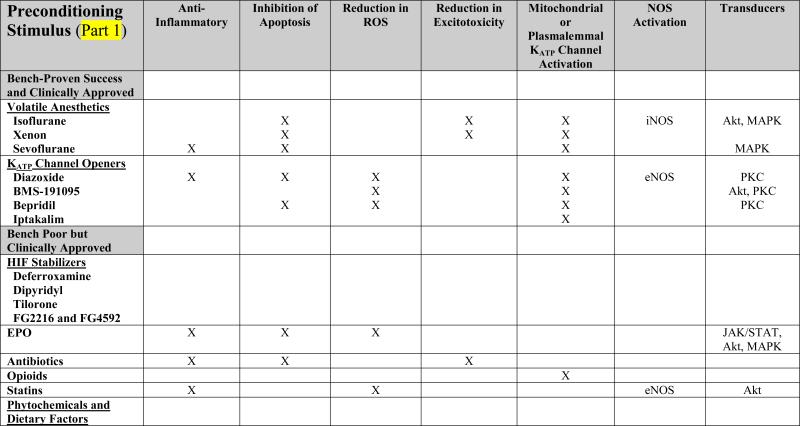
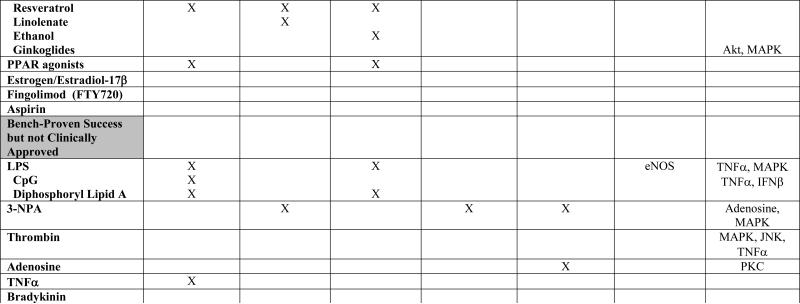
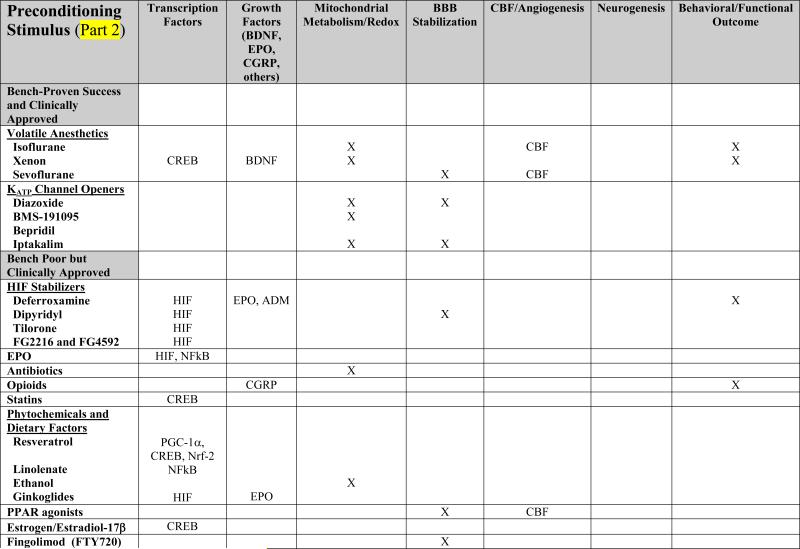
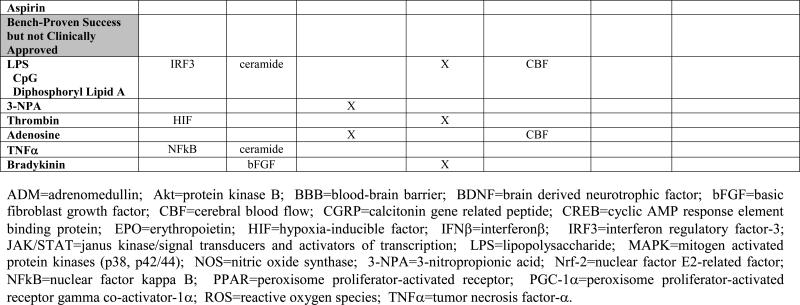
TABLE 1. Mechanistic Basis of Pharmacologic Preconditioning.
Shown below is evidence to date, obtained primiarly from in vivo preclinical studies of both focal and global ischemia, and in both adult and neonate models of preconditioning-induced delayed ischemic tolerance, regarding the mechanisms whereby various pharmacologic preconditioning stimuli exert their beneficial effects. Drugs are categorized in parallel with their presentation in the text. An “X” indicates that data are available supporting that particular mechanism; in some instances, the specific molecule(s) or “effect” are listed. A lack of an entry in any given row/column space does not necessarily mean that the indicated mediator is not involved in promoting the ischemia-tolerant state in response to that particular stimulus, but only that no studies yet exist to support such involvement.
|
|
|
|
Tested at the bench, and clinically approved
The volatile anesthetics and the KCOs probably rank as the most well studied and best understood pharmacologic preconditioning agents already in widespread clinical use (Table 1). One family of drugs that have received a significant amount of preclinical attention in adult [4], [5], [6] and neonatal [7], [8], [9] rodent IT models are the volatile anesthetics; together with their proven safety profiles, these agents are ripe for translational application. To date, isoflurane is the most thoroughly investigated preconditioning anesthetic, but, more recently, xenon [10], [11] and sevoflurane [12], [11], [13], [14] have garnered attention as well. Further mechanism-based animal studies of both rapid and delayed ischemic preconditioning with sevoflurane, the current inhalational anesthetic of choice for human surgery, are warranted. Mechanistically, studies implicate inducible nitric oxide synthase (iNOS), the MAP kinases, Akt, and KATP channels, as critical to establishing the IT phenotype following anesthetic preconditioning, and also reveal gender and sex hormone dependencies [15], [16] (Table 1).
Different subtypes of KATP channels exist in different subcellular locations and in different tissues; at least nine structure-dependent families of KCOs have now been identified, some of which, like diazoxide, are selective for the mitochondrial KATP channel. Studies of the ability of chromakalim and selective mitochondrial KCOs like the blood-brain barrier permeable diazoxide to precondition in a classical fashion against global [17], [18], [19] and focal [19], [20] ischemia in rodents are many, as are investigations conducted in in vitro models. Moreover, rapid diazoxide preconditioning provided both morphological and functional protection in a canine model of brain injury by hypothermic cardiac arrest [21]. In vitro studies, and some animal investigations, suggest that KCO-induced IT is associated with post-ischemic reductions in proinflammatory and apoptotic mediators, reactive oxygen species, and blood-brain barrier breakdown, along with increases in Akt, endothelial nitric oxide synthase (eNOS), heat shock proteins, and antioxidant enzymes (Table 1). But the many molecular pathways leading from channel opening to the manifestation of cytoprotective neuronal and vascular phenotypes remain to be clarified. Notably, accumulating evidence indicates that activation of mitochondrial KCOs and modulation of mitochondrial function are key means by which many other pharmacologic (and nonpharmacologic) preconditioning stimuli ultimately manifest their protective effects [22].
Approved for clinic applications, but relatively unexplored at the bench
A surprisingly long list of FDA-approved drugs (e.g., deferroxamine [DFX], erythropoietin [EPO], antibiotics, opioids, statins, phytochemicals, peroxisome proliferator-activated receptor [PPAR] agonists, estrogen, and certain immunosuppressants) have shown preconditioning efficacy in a small number of in vitro and/or in vivo models of ischemic brain injury (Table 1). Expanding efforts to assess these pharmacologic stimuli for the robustness of their protective effects in other models of IT should be prioritized given their high translational potential. Some of the primary features that render these compounds particularly attractive in this regard are detailed below.
Drugs that stabilize the transcription factor hypoxia-inducible factor (HIF) isoforms HIF-1α and/or HIF-2α look promising as preconditioning stimuli for anticipated cerebral ischemia. Deferroxamine, an iron chelator used for over 30 years in the treatment of assorted chronic anemias and iron poisoning – and now in clinical trials for intracerebral hemorrhage [23] – is an effective preconditioning compound in models of neonatal [24], [25] and adult [26],[27] stroke. As a result of its ability to inhibit members of the HIF-stabilizing, iron-dependent prolyl hydroxylase family secondary to binding iron, deferroxamine is just one of several preconditioning treatments (e.g., LPS, inflammatory cytokines, thrombin, nitric oxide) that may induce IT in this mechanistic fashion. The transcription of hundreds of survival- and angiogenesis-promoting genes (e.g., vascular endothelial growth factor [VEGF], EPO), as well as the modulation in cellular energy metabolism, that HIF induces [28], [29], are thought to contribute significantly to the ischemia tolerant state. A number of small molecule inhibitors of prolyl hydroxlase enzymes are under active investigation to leverage this phenotype, including the blood-brain barrier permeable tilorone [27] and Fibrogen's FG-2216 and FG-4592 – now in phase II clinical trials for kidney disease patients with anemia – and may eventually prove useful as preconditioning therapeutics for HIF stabilization. However, studies of the effects of neuron-specific HIF-1α deletion on stroke outcome (in the absence of preconditioning) are controversial [30], [31], suggesting that helpful preischemic, but harmful postischemic, effects of at least neuronal HIF are involved. Therefore, understanding, and controlling, the pharmacokinetics of prolyl hydroxylase inhibition and other HIF regulatory factors will be critical to the success of such approaches.
Evidence from preclinical studies indicates that EPO, like nitric oxide, can serve as both inducer [32], [33] and effector [26], [34], [35], [36] of the ischemia-tolerant phenotype. The ability of exogenous EPO to trigger IT might mimic, in part, a paracrine-based signaling system for hypoxic/ischemic preconditioning wherein HIF-driven gene expression changes occurring in astrocytes lead to the synthesis and release of EPO and other downstream target proteins (e.g., adrenomedullin, VEGF), which then mediate the IT response in neurons [33], [37]. The mechanistic basis of EPO's beneficial effects with respect to postischemic treatment protocols in animals is multi-fold [38], [39]; [40] (Table 1). Clinically, recombinant human EPO (rhEPO) is FDA-approved for hematopoiesis and has been used by millions of people. However, while a phase II stroke trial for rhEPO showed significant improvements across several outcome measures [41], the outcome of the phase III stroke trial was less encouraging (potentially confounded by co-treatment with recombinant tissue plasminogen activator) [42].
Interestingly, some antibiotics also exhibit preconditioning effects. In particular, administration of the macrolide erythromycin to rats protected pyramidal cell function when hippocampal slices from these animals were rendered severely hypoxic [43], and improved survival of both hippocampal and neocortical neurons following transient global ischemia [44]. Follow up microarray studies suggest that this protection may be afforded secondary to poststroke transcriptional suppression of proinflammatory genes [45]. The morphologic and functional protection against both transient and permanent focal stroke injury afforded by preconditioning with the third generation cephalosporin antibiotic ceftriaxone was also associated with a reduction in postischemic inflammatory mediators (e.g., TNFα and MMP9), as well as increases in the expression of the astrocyte glutamate transporter protein [46]. Assuming that these improvements in outcome are not the result of reductions in poststroke infection [47], [48], the fact that specific members of the commonly prescribed antibiotic drug family are efficacious as preconditioning stimuli represent provocative findings with high translational potential.
In a similar fashion, opioid preconditioning may be another attractive way to induce rapid or delayed IT in the clinic, particularly if single doses prove effective, and the addiction/withdrawal concerns associated with chronic opioid use can be avoided. However, experimental support for this possibility is limited at present to a handful of studies. In particular, morphine effectively preconditioned Purkinje cells in cerebellar slices against simulated ischemic injury [49], and a delta-opioid receptor antagonist blocked the ability of both hypoxia to precondition cultured rat cortical neurons against glutamate toxicity [50] and electroacupuncture to precondition rats against transient focal stroke [51]. Elsewhere in the CNS, systemic morphine administration was demonstrated to promote retinal ischemic tolerance, while the nonselective opioid antagonist naloxone blocked ischemic preconditioning-induced retinal IT [52].
Statins represent another class of widely used drugs that might find utility for preconditioning at risk patients. One finding in the ongoing Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial was that high-dose atorvastatin reduced the incidence of subsequent stroke in patients without known coronary artery disease, but who had already suffered a recent stroke or transient ischemic attack [53]; whether this is representative of a statin-based preconditioning effect is open to debate, as is the notion that regular statin therapy might chronically precondition the brain and improve stroke outcome in patients taking this drug. The few relevant laboratory studies published to date hint rather strongly that statins might actually be useful for acute preconditioning, given that administration of a single dose of simvistatin is neuroprotective in the postnatal-day-7 rat model of hypoxia-ischemia [54], and that rosuvastatin protects cultured neurons from OGD-induced cell death [55]. Although reductions in the postischemic elaboration of inflammatory cytokines and increases in Akt and CREB activation was observed in the former model [56] (Table 1), more preclinical studies are clearly needed to support this promising avenue of inducing both short- and long-lasting cerebroprotection.
The ability of several agents commonly found in specific foodstuffs to serve as preconditioning triggers is consistent with the notion that, like exercise, phytochemicals and other dietary factors may engender an acute – or with regular consumption perhaps even a chronic – ischemia-tolerant state [57]. Despite the attractiveness of this concept, the study of the CNS effects of phytochemicals with respect to preconditioning and the possible mechanistic basis for such an effect is really in its infancy. The best developed example to date would be resveratrol, a polyphenolic derivative from grape skins, that is an effective preconditioning compound for focal and global ischemia [58], [59], [60]. Whether its mechanism of action involves alterations in histone deacetylation secondary to sirtuin activation, increases in PPAR gamma co-activator 1α (PGC-1α) expression, modulations in mitochondrial redox, protease release and/or other effects, remains unclear. Similarly, in a gerbil forebrain ischemia model, short-term oral administration of grape polyphenol extract is protective [61]. Even the polyunsaturated fatty acid linolenate, a common ingredient of vegetable oil, can precondition the gerbil brain after short-term administration [62]. While obviously more of a causative agent in disease induction and progression than a potential treatment, ethanol is a molecule that can precondition a number of tissues against ischemic and other injuries, including the stroked brain [63]. It should not be unexpected that “Eastern” medicinal herbs and therapeutics – well tolerated by patients for centuries – might also exhibit preconditioning effects suitable for cerebral ischemic protection. To my knowledge, a single study presents evidence for such an effect: Ginkoglides (constituents of the nonflavone fraction of a Ginkgo biloba extract) preconditioned against simulated ischemic injury in the C6 rat glioma cell line by a HIF1α-, MAPK-, and Akt-dependent mechanism [64].
Many other clinically approved drugs that promote IT in brain are worthy of mention. In particular, three ligand-activated, nuclear transcription factor isoforms in the PPAR family that regulate gene expression in a number of unique ways exert neuroprotective effects when given after stroke [65]. However, studies also demonstrate that, following a two-week treatment with the PPARα agonist fenofibrate, reductions in lesion size are realized following focal ischemic injury in the rat, secondary to vascular-based protective effects (reductions in postischemic inflammation and improved vascular reactivity) [66], [67]. Given the rather widespread clinical use of fibrates for hyperlipidemia, the ability of this and other PPAR agonists – perhaps those acting at the gamma (the thiazolidinediones [68]) and delta receptors – to not provide stroke prophylaxis per se but rather to precondition the brain following single or repeated application deserves more research scrutiny. Treating rats with estrogen [69] or the primary naturally-occurring estrogen hormone estradiol-17β [70] is also an effective preconditioning strategy, and may leverage the natural neuroprotective advantage pre-menopausal women exhibit regarding their incidence of stroke. The immunosuppresive drug fingolimod (FTY-720), which is in Phase II and Phase III trials for the treatment of multiple sclerosis, preconditions the mouse brain against focal ischemic injury [71]; its phosphorylation and subsequent activity at different sphingosine-1-phosphate receptors may underlie this effect, although cytosolic phospholipase A2 inhibition and other signaling actions may contribute as well. Thus, studies to determine whether these and/or other immunosuppression-based features of FTY-720 contribute to its preconditioning effect, and whether other FDA-approved immunomodulatory drugs might also promote IT, are worthy pursuits. Finally, one of Western medicine's ‘miracle’ drugs, acetylsalicylic acid (aspirin), shows both rapid and delayed preconditioning-like effects [72], [73], but, surprisingly, no follow up studies were forthcoming from this early provocative work.
Finally, there are a number of nonischemic/nonhypoxic, nonpharmacologic interventions reported to date that trigger robust ischemic tolerance in brain, including exercise [74], [75], [76], [77], acupuncture [78], [79], [51], hyperbaric oxygen (see Zhang et al., this issue), transcranial magnetic stimulation [80], and caloric restriction [81], to name a few. With the exception of exercise and hyperbaric oxygen, studies of the ability of antecedent treatment with these agents to protect brain are relatively rare. Ultimately though, the signaling pathways and molecules that lead to the requisite metabolomic and genomic changes induced by these interventions, when identified, may become viable targets of a pharmacologic preconditioning strategy.
Tested at the bench, but unsuitable for clinic applications
As alluded to earlier, there are some pharmacologic agents (e.g., lipopolysaccharide [LPS], NMDA, and the metabolic inhibitor 3-nitropropionic acid [3-NPA]) that have received significant experimental focus. However, these agents are unlikely to be approved for clinical use because of toxicity concerns related to the difficulties of dose titration and the magnitude of noncerebral, off-target side effects. Other drugs in this category include exogenously administered thrombin [82], [83], adenosine [17], [84], TNFα [85], bradykinin [86], and oxidative stress-inducing agents [87] (Table 1). However, as elaborated on below, analogues of these compounds may eventually be developed that provide the same therapeutic, IT-promoting benefit with considerably fewer safety, tolerability, and dosing concerns, so careful reviews of the collected mechanistic data underpinning these studies are merited.
The ability of low doses of LPS, a common endotoxin derived from the cell membrane of specific gram-negative bacteria, to precondition ischemic brain is a case-in-point. Following a very early study showing LPS-mediated protection against permanent focal stroke in rats [88], preconditioning with low doses of this endotoxin in rat [89], [90] and mouse [91] transient focal stroke models and neonatal hypoxia-ischemia models [92] is now well established. Notably, LPS is one of the few preconditioning stimuli to be efficacious in a large animal model of ischemic brain injury (hypothermic circulatory arrest in pigs) [93]. Mechanistically, the ischemia-tolerant state is one exhibiting a transient anti-inflammatory phenotype that, in turn, appears to result from LPS stimulating a proinflammatory activation of the innate immune system. Toll-like receptor activation likely plays a role in mediating this somewhat paradoxical response to LPS, and perhaps other preconditioning stimuli as well [94].
3-NPA depresses oxidative phosphorylation secondary to the irreversible inhibition of succinate dehydrogenase. Initially, neuroprotection from OGD was documented by the rapid preconditioning of brain slices with this metabolic inhibitor [95]. But even when administered systemically, 3-NPA can promote a state of delayed cerebral ischemic tolerance that protects against both transient [96], [97] and permanent [98], [99] focal stroke in a number of rodent species; its effects in models of global ischemia are more controversial [100], [101], [102]. While this particular compound will not be considered for clinical trials, its ability to transiently reduce cellular metabolism, as observed with the prolyl hydroxylase inhibitors and to some extent by preconditioning with aspirin [72] and antibiotics [43], suggests that a finely controlled, tissue- and/or cell-specific suppression of mitochondrial metabolism [22] could serve as a general therapeutic approach to inducing ischemic tolerance, akin to that found in hibernating and anoxia-tolerant vertebrates. If generally true, then how to achieve and temporally regulate such an effect in an organ-specific manner presents one of many significant translational research challenges in this field.
Additional considerations
With respect to treating patients by oral or intravenous routes, the efficacy of some systemically administered, pharmacologic preconditioning strategies suggests many provocative implications for therapeutic consideration. For example, “whole animal” preconditioning with hypoxia or hyperbaric oxygen, or with systemic (intravenous or intraperitoneal) administration of agents such as LPS, 3-NPA, various cytokines, resveratrol, etc., must require the sequential participation of all cells of the neurovascular unit to account for the pan-cerebral IT they promote. In other words, protection by centrally delivered preconditioning drugs that do not readily cross the blood-brain barrier (which excludes morphine, ethanol, and a few others) likely entails the following intercellular signaling sequence: Cerebrovascular endothelial cells sense and respond in an integrated fashion to a circulating molecular signal (or alterations in oxygenation) and transducer the stress/survival signal to surrounding astrocytes and neurons by yet another series of intercellular molecular signals. The extent to which disparate systemic preconditioning stimuli activate distinct or overlapping signaling pathways in and between these different cells, and the degree to which the resulting gene expression patterns are shared among them, is unclear. As one example, there is evidence that TNFα serves as a downstream mediator in response to hypoxia [103], ischemia [104], hyperoxia, LPS [91], exercise [76], and other preconditioning stimuli. From a treatment standpoint, these findings suggest IT may be achieved in humans by a drug that does not actually have to cross the blood-brain barrier, but rather one that is capable of activating the appropriate cerebral endothelial receptors. And, even though isolated neurons or other resident brain cells can be preconditioned in culture with a variety of stimuli, it may be possible that a non-cerebral tissue (e.g., the liver), or its specialized vascular endothelium, is the indirect “mediator” of systemically delivered preconditioning treatments by virtue of its “response” to such treatments (e.g., releasing cytokines into the blood). Thus, these tissues, and not the brain per se, may be suitable therapeutic targets for stroke preconditioning. The ability of remote preconditioning (brief mesenteric or limb skeletal muscle ischemia) to protect ischemic brain [105], [106] underscores such a possibility.
Next steps
To date, the endpoints used in many preclinical IT studies, even for the more ‘popular’ pharmacologic preconditioning agents, tend to be morphologic; more functional outcome measures, and more long-term follow up studies at clinically relevant time points, are needed in both focal and global ischemia models. Ultimately, drumming up solid preclinical efficacy for any lead preconditioning drug will require detailed pharmacokinetic studies to identify the time dependency of its effect, and the dose that is neither impotent nor toxic. Ideally, documentation that such a treatment is efficacious in large animal models [21], [93], in females [73], [107], in aged animals where IT may be blunted [108], and those with co-morbidities and other known stroke risk factors is also needed.
Of course, no pharmacologic treatment is without unintended side effects and related concerns. Analogues of one or more of the aforementioned pharmacologic agents that exhibit less than ideal clinical profiles might ultimately promote cerebral IT just as effectively, but with a wider safety and tolerability profile. Current examples of this include dipyridyl, a lipid soluble iron chelator effective in preconditioning in a photothrombosis model [109] that may prove less problematic in certain preconditioning paradigms than deferroxamine. The nonmethylated cytosine-guanine bacterial oligonucleotide CpG, which acts as a toll-like receptor 9 ligand [110], [111], and the nonharmful endotoxin analogue diphosphoryl lipid A [112], seem to protect the mouse brain at magnitudes similar to those achieved with LPS. One or more of the nonhematopoietic, cerebroprotective EPO analogues that appear to activate unique EPO receptor populations in brain that are linked to neuroprotective signaling pathways [40] may also exhibit robust qualities as a preconditioning agent. And the hypotensive, hyperglycemic, and other side effects of mitochondrial-selective KCO drugs like diazoxide might be avoided by using the analogues BMS-191095 [113] and bepridil [114], the latter a clinically approved anti-anginal medication. Iptakalim, a relatively new KCO that crosses the blood-brain barrier to act selectively at SUR2-type of KATP channels, without adversely affecting the pancreatic SUR1-type of channels, shows preconditioning-like neurovascular protective effects in a rat model of high altitude hypoxic brain injury, even when administered by gavage [115]. Ultimately, phase I and II clinical trials will be necessary for our most promising preconditioning drugs to define dosing, tolerability, and efficacy in humans, but these translational steps may fail like many others if such preclinical studies are not designed carefully and do not adhere to the updated STAIR criteria [116].
Given the number and diversity of pharmacologic treatments that currently promote IT in animals, some of which are already approved for clinical use, and considering that the very concept of finding utility in what was viewed by many not so long ago as a field of endeavor without clinical ramifications, the potential for translational success looks bright. In all likelihood, the preconditioning treatment regimen of choice will have to be modified significantly depending on the nature of the anticipated ischemic event (as is true in our animal models), be it stroke, subarachnoid hemorrhage, or any number of planned or emergency neurosurgical or cardiac surgeries, or neuroradiological interventions. Preconditioning ‘cocktails’ may ultimately be utilized to induce mechanism- and cell-specific IT across all cells of the neurovascular unit. Also, we may find pharmacologic preconditioning employed in conjunction with post-stroke treatments – particularly with the thrombolytics. As the field of pharmacogenomics evolves [117], it will be exciting to define and implement individualized preconditioning treatments based on personal genetic profiles. And despite the many issues regarding chronic drug administration of any kind, some can envision a future in which a form of preconditioning-like prophylaxis is pharmacologically established in a vitamin-mimicking fashion for patients with defined combinations of the more ‘standard’ risk factors (e.g., age, race, genetic history, smoking, hypertension, diabetes) that we already know are associated with adverse cerebrovascular events.
Acknowledgments
A note of appreciation to Drs. Brad K. Wacker and Ann M. Stowe for critical review of this manuscript and helpful discussion. The author regrets space limitations that prevented the citation of many noteworthy and relevant studies. Support for the author's laboratory from NIH grants RO1 HL79278, PO1 NS32636, and the Spastic Paralysis Research Foundation of the Illinois-Eastern Iowa District of Kiwanis International.
References
- 1.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7(6):437–48. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- 2.Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009 Apr;8(4):398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endres M, Engelhardt B, Koistinaho J, Lindvall O, Meairs S, Mohr JP, et al. Improving outcome after stroke: overcoming the translational roadblock. Cerebrovasc Dis (Basel, Switzerland) 2008;25(3):268–78. doi: 10.1159/000118039. [DOI] [PubMed] [Google Scholar]
- 4.Kapinya KJ, Prass K, Dirnagl U. Isoflurane induced prolonged protection against cerebral ischemia in mice: a redox sensitive mechanism? NeuroReport. 2002;13(11):1431–5. doi: 10.1097/00001756-200208070-00017. [DOI] [PubMed] [Google Scholar]
- 5.Zheng S, Zuo Z. Isoflurane preconditioning induces neuroprotection against ischemia via activation of P38 mitogen-activated protein kinases. Mol Pharmacol. 2004 May;65(5):1172–80. doi: 10.1124/mol.65.5.1172. [DOI] [PubMed] [Google Scholar]
- 6.Kitano H, Kirsch JR, Hurn PD, Murphy SJ. Inhalational anesthetics as neuroprotectants or chemical preconditioning agents in ischemic brain. J Cereb Blood Flow Metab. 2007 Jun;27(6):1108–28. doi: 10.1038/sj.jcbfm.9600410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao P, Zuo Z. Isoflurane preconditioning induces neuroprotection that is inducible nitric oxide synthase-dependent in neonatal rats. Anesthesiology. Sep. 2004;101(3):695–703. doi: 10.1097/00000542-200409000-00018. [DOI] [PubMed] [Google Scholar]
- 8.McAuliffe JJ, Joseph B, Vorhees CV. Isoflurane-delayed preconditioning reduces immediate mortality and improves striatal function in adult mice after neonatal hypoxia-ischemia. Anesthes Analges. 2007 May;104(5):1066–77. doi: 10.1213/01.ane.0000260321.62377.74. tables of contents. [DOI] [PubMed] [Google Scholar]
- 9.Zhao P, Peng L, Li L, Xu X, Zuo Z. Isoflurane preconditioning improves long-term neurologic outcome after hypoxic-ischemic brain injury in neonatal rats. Anesthesiology. 2007 Dec;107(6):963–70. doi: 10.1097/01.anes.0000291447.21046.4d. [DOI] [PubMed] [Google Scholar]
- 10.Ma D, Hossain M, Pettet GK, Luo Y, Lim T, Akimov S, et al. Xenon preconditioning reduces brain damage from neonatal asphyxia in rats. J Cereb Blood Flow Metab. 2006;26(2):199–208. doi: 10.1038/sj.jcbfm.9600184. [DOI] [PubMed] [Google Scholar]
- 11.Luo Y, Ma D, Ieong E, Sanders RD, Yu B, Hossain M, et al. Xenon and sevoflurane protect against brain injury in a neonatal asphyxia model. Anesthesiology. 2008 Nov;109(5):782–9. doi: 10.1097/ALN.0b013e3181895f88. [DOI] [PubMed] [Google Scholar]
- 12.Payne RS, Akca O, Roewer N, Schurr A, Kehl F. Sevoflurane-induced preconditioning protects against cerebral ischemic neuronal damage in rats. Brain Res. 2005 Feb 9;1034(1-2):147–52. doi: 10.1016/j.brainres.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Codaccioni JL, Velly LJ, Moubarik C, Bruder NJ, Pisano PS, Guillet BA. Sevoflurane preconditioning against focal cerebral ischemia: inhibition of apoptosis in the face of transient improvement of neurological outcome. Anesthesiology. 2009 Jun;110(6):1271–8. doi: 10.1097/ALN.0b013e3181a1fe68. [DOI] [PubMed] [Google Scholar]
- 14.Ding Q, Wang Q, Deng J, Gu Q, Hu S, Li Y, et al. Sevoflurane preconditioning induces rapid ischemic tolerance against spinal cord ischemia/reperfusion through activation of extracellular signal-regulated kinase in rabbits. Anesthes Analges. 2009 Oct;109(4):1263–72. doi: 10.1213/ane.0b013e3181b2214c. [DOI] [PubMed] [Google Scholar]
- 15.Clarkson AN. Anesthetic-mediated protection/preconditioning during cerebral ischemia. Life Sci. 2007 Mar 6;80(13):1157–75. doi: 10.1016/j.lfs.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Traystman RJ, Murphy SJ. Inhalational anesthetics as preconditioning agents in ischemic brain. Curr Opinion Pharmacol. 2008 Feb;8(1):104–10. doi: 10.1016/j.coph.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heurteaux C, Lauritzen I, Widmann C, Lazdunski M. Essential role of adenosine, adenosine A1 receptors, and ATP-sensitive K+ channels in cerebral ischemic preconditioning. Proc Natl Acad Sci USA. 1995;92(10):4666–70. doi: 10.1073/pnas.92.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenzser G, Kis B, Bari F, Busija DW. Diazoxide preconditioning attenuates global cerebral ischemia-induced blood-brain barrier permeability. Brain Res. 2005;1051(1-2):72–80. doi: 10.1016/j.brainres.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe M, Katsura K, Ohsawa I, Mizukoshi G, Takahashi K, Asoh S, et al. Involvement of mitoKATP channel in protective mechanisms of cerebral ischemic tolerance. Brain Res. 2008 Oct 31;1238:199–207. doi: 10.1016/j.brainres.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 20.Mayanagi K, Gaspar T, Katakam PV, Busija DW. Systemic administration of diazoxide induces delayed preconditioning against transient focal cerebral ischemia in rats. Brain Res. 2007 Sep 7;1168:106–11. doi: 10.1016/j.brainres.2007.06.071. [DOI] [PubMed] [Google Scholar]
- 21.Shake JG, Peck EA, Marban E, Gott VL, Johnston MV, Troncoso JC, et al. Pharmacologically induced preconditioning with diazoxide: a novel approach to brain protection. Annals Thorac Surg. 2001 Dec;72(6):1849–54. doi: 10.1016/s0003-4975(01)03192-7. [DOI] [PubMed] [Google Scholar]
- 22.Dirnagl U, Meisel A. Endogenous neuroprotection: mitochondria as gateways to cerebral preconditioning? Neuropharmacology. 2008 Sep;55(3):334–44. doi: 10.1016/j.neuropharm.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Selim M. Deferoxamine mesylate: a new hope for intracerebral hemorrhage: from bench to clinical trials. Stroke. 2009 Mar;40(3 Suppl):S90–1. doi: 10.1161/STROKEAHA.108.533125. [DOI] [PubMed] [Google Scholar]
- 24.Bergeron M, Gidday JM, Yu AY, Semenza GL, Ferriero DM, Sharp FR. Role of hypoxia-inducible factor-1 in hypoxia-induced ischemic tolerance in neonatal rat brain. Ann Neurol. 2000;48(3):285–96. [PubMed] [Google Scholar]
- 25.Jones NM, Kardashyan L, Callaway JK, Lee EM, Beart PM. Long-term functional and protective actions of preconditioning with hypoxia, cobalt chloride, and desferrioxamine against hypoxic-ischemic injury in neonatal rats. Pediatr Res. 2008 Jun;63(6):620–4. doi: 10.1203/PDR.0b013e31816d9117. [DOI] [PubMed] [Google Scholar]
- 26.Prass K, Ruscher K, Karsch M, Isaev N, Megow D, Priller J, et al. Desferrioxamine induces delayed tolerance against cerebral ischemia in vivo and in vitro. J Cereb Blood Flow Metab. 2002;22(5):520–5. doi: 10.1097/00004647-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Ratan RR, Siddiq A, Aminova L, Langley B, McConoughey S, Karpisheva K, et al. Small molecule activation of adaptive gene expression: tilorone or its analogs are novel potent activators of hypoxia inducible factor-1 that provide prophylaxis against stroke and spinal cord injury. Ann NY Acad Sci. 2008 Dec;1147:383–94. doi: 10.1196/annals.1427.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006 Mar;3(3):187–97. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Aragones J, Fraisl P, Baes M, Carmeliet P. Oxygen sensors at the crossroad of metabolism. Cell Metab. 2009 Jan 7;9(1):11–22. doi: 10.1016/j.cmet.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Helton R, Cui J, Scheel JR, Ellison JA, Ames C, Gibson C, et al. Brain-specific knockout of hypoxia-inducible factor-1alpha reduces rather than increases hypoxic-ischemic damage. J Neurosci. 2005 Apr 20;25(16):4099–107. doi: 10.1523/JNEUROSCI.4555-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC. Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci. 2007 Jun 6;27(23):6320–32. doi: 10.1523/JNEUROSCI.0449-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernaudin M, Marti HH, Roussel S, Divoux D, Nouvelot A, MacKenzie ET, et al. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19(6):643–51. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Ruscher K, Freyer D, Karsch M, Isaev N, Megow D, Sawitzki B, et al. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci. 2002;22(23):10291–301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prass K, Scharff A, Ruscher K, Lowl D, Muselmann C, Victorov I, et al. Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietin. Stroke. 2003;34(8):1981–6. doi: 10.1161/01.STR.0000080381.76409.B2. [DOI] [PubMed] [Google Scholar]
- 35.Malhotra S, Savitz SI, Ocava L, Rosenbaum DM. Ischemic preconditioning is mediated by erythropoietin through PI-3 kinase signaling in an animal model of transient ischemic attack. J Neurosci Res. 2006 Jan;83(1):19–27. doi: 10.1002/jnr.20705. [DOI] [PubMed] [Google Scholar]
- 36.Gu GJ, Li YP, Peng ZY, Xu JJ, Kang ZM, Xu WG, et al. Mechanism of ischemic tolerance induced by hyperbaric oxygen preconditioning involves upregulation of hypoxia-inducible factor-1alpha and erythropoietin in rats. J Appl Physiol. 2008 Apr;104(4):1185–91. doi: 10.1152/japplphysiol.00323.2007. [DOI] [PubMed] [Google Scholar]
- 37.Chavez JC, Baranova O, Lin J, Pichiule P. The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J Neurosci. 2006 Sep 13;26(37):9471–81. doi: 10.1523/JNEUROSCI.2838-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rabie T, Marti HH. Brain protection by erythropoietin: a manifold task. Physiology. 2008 Oct;23:263–74. doi: 10.1152/physiol.00016.2008. [DOI] [PubMed] [Google Scholar]
- 39.Minnerup J, Heidrich J, Rogalewski A, Schabitz WR, Wellmann J. The efficacy of erythropoietin and its analogues in animal stroke models: a meta-analysis. Stroke. 2009 Sep;40(9):3113–20. doi: 10.1161/STROKEAHA.109.555789. [DOI] [PubMed] [Google Scholar]
- 40.Siren AL, Fasshauer T, Bartels C, Ehrenreich H. Therapeutic potential of erythropoietin and its structural or functional variants in the nervous system. Neurotherapeutics. 2009 Jan;6(1):108–27. doi: 10.1016/j.nurt.2008.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8(8):495–505. [PMC free article] [PubMed] [Google Scholar]
- 42.Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009 Dec;40(12):e647–56. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- 43.Huber R, Kasischke K, Ludolph AC, Riepe MW. Increase of cellular hypoxic tolerance by erythromycin and other antibiotics. NeuroReport. 1999;10(7):1543–6. doi: 10.1097/00001756-199905140-00027. [DOI] [PubMed] [Google Scholar]
- 44.Brambrink AM, Koerner IP, Diehl K, Strobel G, Noppens R, Kempski O. The antibiotic erythromycin induces tolerance against transient global cerebral ischemia in rats (pharmacologic preconditioning). Anesthesiology. 2006 Jun;104(6):1208–15. doi: 10.1097/00000542-200606000-00016. [DOI] [PubMed] [Google Scholar]
- 45.Koerner IP, Gatting M, Noppens R, Kempski O, Brambrink AM. Induction of cerebral ischemic tolerance by erythromycin preconditioning reprograms the transcriptional response to ischemia and suppresses inflammation. Anesthesiology. 2007 Mar;106(3):538–47. doi: 10.1097/00000542-200703000-00019. [DOI] [PubMed] [Google Scholar]
- 46.Chu K, Lee ST, Sinn DI, Ko SY, Kim EH, Kim JM, et al. Pharmacological induction of ischemic tolerance by glutamate transporter-1 (EAAT2) upregulation. Stroke. 2007 Jan;38(1):177–82. doi: 10.1161/01.STR.0000252091.36912.65. [DOI] [PubMed] [Google Scholar]
- 47.Meisel C, Prass K, Braun J, Victorov I, Wolf T, Megow D, et al. Preventive antibacterial treatment improves the general medical and neurological outcome in a mouse model of stroke. Stroke. 2004 Jan;35(1):2–6. doi: 10.1161/01.STR.0000109041.89959.4C. [DOI] [PubMed] [Google Scholar]
- 48.Klehmet J, Harms H, Richter M, Prass K, Volk HD, Dirnagl U, et al. Stroke-induced immunodepression and post-stroke infections: lessons from the preventive antibacterial therapy in stroke trial. Neuroscience. 2009 Feb 6;158(3):1184–93. doi: 10.1016/j.neuroscience.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 49.Lim YJ, Zheng S, Zuo Z. Morphine preconditions Purkinje cells against cell death under in vitro simulated ischemia-reperfusion conditions. Anesthesiology. 2004 Mar;100(3):562–8. doi: 10.1097/00000542-200403000-00015. [DOI] [PubMed] [Google Scholar]
- 50.Zhang J, Qian H, Zhao P, Hong SS, Xia Y. Rapid hypoxia preconditioning protects cortical neurons from glutamate toxicity through delta-opioid receptor. Stroke. 2006 Apr;37(4):1094–9. doi: 10.1161/01.STR.0000206444.29930.18. [DOI] [PubMed] [Google Scholar]
- 51.Xiong LZ, Yang J, Wang Q, Lu ZH. Involvement of delta-and mu-opioid receptors in the delayed cerebral ischemic tolerance induced by repeated electroacupuncture preconditioning in rats. Chin Med J. 2007 Mar 5;120(5):394–9. [PubMed] [Google Scholar]
- 52.Husain S, Potter DE, Crosson CE. Opioid receptor-activation: retina protected from ischemic injury. Invest Ophthalmol Vis Sci. 2009 Aug;50(8):3853–9. doi: 10.1167/iovs.08-2907. [DOI] [PubMed] [Google Scholar]
- 53.Amarenco P, Bogousslavsky J, Callahan A, 3rd, Goldstein LB, Hennerici M, Rudolph AE, et al. High-dose atorvastatin after stroke or transient ischemic attack. New Engl J Med. 2006 Aug 10;355(6):549–59. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 54.Balduini W, Mazzoni E, Carloni S, De Simoni MG, Perego C, Sironi L, et al. Prophylactic but not delayed administration of simvastatin protects against long-lasting cognitive and morphological consequences of neonatal hypoxic-ischemic brain injury, reduces interleukin-1beta and tumor necrosis factor-alpha mRNA induction, and does not affect endothelial nitric oxide synthase expression. Stroke. 2003 Aug;34(8):2007–12. doi: 10.1161/01.STR.0000080677.24419.88. [DOI] [PubMed] [Google Scholar]
- 55.Domoki F, Kis B, Gaspar T, Snipes JA, Parks JS, Bari F, et al. Rosuvastatin induces delayed preconditioning against oxygen-glucose deprivation in cultured cortical neurons. Am J Physiol. 2009 Jan;296(1):C97–105. doi: 10.1152/ajpcell.00366.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carloni S, Girelli S, Buonocore G, Longini M, Balduini W. Simvastatin acutely reduces ischemic brain damage in the immature rat via Akt and CREB activation. Exp Neurol. 2009 Aug 5;220(1):82–9. doi: 10.1016/j.expneurol.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 57.Mattson MP, Cheng A. Neurohormetic phytochemicals: Low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006 Nov;29(11):632–9. doi: 10.1016/j.tins.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 58.Gao D, Zhang X, Jiang X, Peng Y, Huang W, Cheng G, et al. Resveratrol reduces the elevated level of MMP-9 induced by cerebral ischemia-reperfusion in mice. Life Sci. 2006 Apr 25;78(22):2564–70. doi: 10.1016/j.lfs.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 59.Liu YG, Wang XD, Zhang XB. Effects of resveratrol on inflammatory process induced by focal cerebral ischemia-reperfusion in rats. China Journal Chinese Materia Medica. 2007 Sep;32(17):1792–5. [PubMed] [Google Scholar]
- 60.Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, Perez-Pinzon MA. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009 Mar 31;159(3):993–1002. doi: 10.1016/j.neuroscience.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Q, Sun AY, Simonyi A, Miller DK, Smith RE, Luchtefeld RG, et al. Oral administration of grape polyphenol extract ameliorates cerebral ischemia/reperfusion-induced neuronal damage and behavioral deficits in gerbils: comparison of pre- and post-ischemic administration. J Nut Biochem. 2009 May;20(5):369–77. doi: 10.1016/j.jnutbio.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blondeau N, Widmann C, Lazdunski M, Heurteaux C. Polyunsaturated fatty acids induce ischemic and epileptic tolerance. Neuroscience. 2002;109(2):231–41. doi: 10.1016/s0306-4522(01)00473-0. [DOI] [PubMed] [Google Scholar]
- 63.Wang Q, Sun AY, Simonyi A, Kalogeris TJ, Miller DK, Sun GY, et al. Ethanol preconditioning protects against ischemia/reperfusion-induced brain damage: role of NADPH oxidase-derived ROS. Free Radic Biol Med. 2007 Oct 1;43(7):1048–60. doi: 10.1016/j.freeradbiomed.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He W, Qian Zhong M, Zhu L, Christopher Q, Du F, Yung WH, et al. Ginkgolides mimic the effects of hypoxic preconditioning to protect C6 cells against ischemic injury by upregulation of hypoxia-inducible factor-1 alpha and erythropoietin. Intl J Biochem Cell Biol. 2008;40(4):651–62. doi: 10.1016/j.biocel.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 65.Collino M, Patel NS, Thiemermann C. PPARs as new therapeutic targets for the treatment of cerebral ischemia/reperfusion injury. Therap Adv Cardiovasc Dis. 2008 Jun;2(3):179–97. doi: 10.1177/1753944708090924. [DOI] [PubMed] [Google Scholar]
- 66.Deplanque D, Gele P, Petrault O, Six I, Furman C, Bouly M, et al. Peroxisome proliferator-activated receptor-alpha activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. J Neurosci. 2003 Jul 16;23(15):6264–71. doi: 10.1523/JNEUROSCI.23-15-06264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ouk T, Laprais M, Bastide M, Mostafa K, Gautier S, Bordet R. Withdrawal of fenofibrate treatment partially abrogates preventive neuroprotection in stroke via loss of vascular protection. Vasc Pharmacol. 2009 Sep 2; doi: 10.1016/j.vph.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 68.Villacorta L, Schopfer FJ, Zhang J, Freeman BA, Chen YE. PPARgamma and its ligands: therapeutic implications in cardiovascular disease. Clin Sci (Lond) 2009 Feb;116(3):205–18. doi: 10.1042/CS20080195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raval AP, Bramlett H, Perez-Pinzon MA. Estrogen preconditioning protects the hippocampal CA1 against ischemia. Neuroscience. 2006 Sep 15;141(4):1721–30. doi: 10.1016/j.neuroscience.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 70.Raval AP, Saul I, Dave KR, DeFazio RA, Perez-Pinzon MA, Bramlett H. Pretreatment with a single estradiol-17beta bolus activates cyclic-AMP response element binding protein and protects CA1 neurons against global cerebral ischemia. Neuroscience. 2009 May 5;160(2):307–18. doi: 10.1016/j.neuroscience.2009.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wacker BK, Park TS, Gidday JM. Hypoxic preconditioning-induced cerebral ischemic tolerance: role of microvascular sphingosine kinase 2. Stroke. 2009 Oct;40(10):3342–8. doi: 10.1161/STROKEAHA.109.560714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Riepe MW, Kasischke K, Raupach A. Acetylsalicylic acid increases tolerance against hypoxic and chemical hypoxia. Stroke. 1997 Oct;28(10):2006–11. doi: 10.1161/01.str.28.10.2006. [DOI] [PubMed] [Google Scholar]
- 73.Kasischke K, Huber R, Li H, Timmler M, Riepe MW. Primary hypoxic tolerance and chemical preconditioning during estrus cycle in mice. Stroke. 1999 Jun;30(6):1256–62. doi: 10.1161/01.str.30.6.1256. [DOI] [PubMed] [Google Scholar]
- 74.Endres M, Gertz K, Lindauer U, Katchanov J, Schultze J, Schrock H, et al. Mechanisms of stroke protection by physical activity. Ann Neurol. 2003;54(5):582–90. doi: 10.1002/ana.10722. [DOI] [PubMed] [Google Scholar]
- 75.Ding YH, Young CN, Luan X, Li J, Rafols JA, Clark JC, et al. Exercise preconditioning ameliorates inflammatory injury in ischemic rats during reperfusion. Acta Neuropathol (Berl) 2005;109(3):237–46. doi: 10.1007/s00401-004-0943-y. [DOI] [PubMed] [Google Scholar]
- 76.Guo M, Lin V, Davis W, Huang T, Carranza A, Sprague S, et al. Preischemic induction of TNF-alpha by physical exercise reduces blood-brain barrier dysfunction in stroke. J Cereb Blood Flow Metab. 2008 Aug;28(8):1422–30. doi: 10.1038/jcbfm.2008.29. [DOI] [PubMed] [Google Scholar]
- 77.Krarup LH, Truelsen T, Gluud C, Andersen G, Zeng X, Korv J, et al. Prestroke physical activity is associated with severity and long-term outcome from first-ever stroke. Neurology. 2008 Oct 21;71(17):1313–8. doi: 10.1212/01.wnl.0000327667.48013.9f. [DOI] [PubMed] [Google Scholar]
- 78.Xiong L, Lu Z, Hou L, Zheng H, Zhu Z, Wang Q, et al. Pretreatment with repeated electroacupuncture attenuates transient focal cerebral ischemic injury in rats. Chin Med J. 2003 Jan;116(1):108–11. [PubMed] [Google Scholar]
- 79.Wang Q, Xiong L, Chen S, Liu Y, Zhu X. Rapid tolerance to focal cerebral ischemia in rats is induced by preconditioning with electroacupuncture: window of protection and the role of adenosine. Neurosci Lett. 2005 Jun 10-17;381(1-2):158–62. doi: 10.1016/j.neulet.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 80.Ogiue-Ikeda M, Kawato S, Ueno S. Acquisition of ischemic tolerance by repetitive transcranial magnetic stimulation in the rat hippocampus. Brain Res. 2005 Mar 10;1037(1-2):7–11. doi: 10.1016/j.brainres.2004.10.063. [DOI] [PubMed] [Google Scholar]
- 81.Yu ZF, Mattson MP. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J Neurosci Res. 1999 Sep 15;57(6):830–9. [PubMed] [Google Scholar]
- 82.Jiang Y, Wu J, Hua Y, Keep RF, Xiang J, Hoff JT, et al. Thrombin-receptor activation and thrombin-induced brain tolerance. J Cereb Blood Flow Metab. 2002 Apr;22(4):404–10. doi: 10.1097/00004647-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 83.Granziera C, Thevenet J, Price M, Wiegler K, Magistretti PJ, Badaut J, et al. Thrombin-induced ischemic tolerance is prevented by inhibiting c-jun N-terminal kinase. Brain Res. 2007 May 7;1148:217–25. doi: 10.1016/j.brainres.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 84.Blondeau N, Plamondon H, Richelme C, Heurteaux C, Lazdunski M. K(ATP) channel openers, adenosine agonists and epileptic preconditioning are stress signals inducing hippocampal neuroprotection. Neuroscience. 2000;100(3):465–74. doi: 10.1016/s0306-4522(00)00304-3. [DOI] [PubMed] [Google Scholar]
- 85.Nawashiro H, Tasaki K, Ruetzler CA, Hallenbeck JM. TNF-alpha pretreatment induces protective effects against focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 1997;17(5):483–90. doi: 10.1097/00004647-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 86.Ping A, Chun ZX, Xue XY. Bradykinin preconditioning induces protective effects against focal cerebral ischemia in rats. Brain Res. 2005 Oct 19;1059(2):105–12. doi: 10.1016/j.brainres.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 87.Ohtsuki T, Matsumoto M, Kuwabara K, Kitagawa K, Suzuki K, Taniguchi N, et al. Influence of oxidative stress on induced tolerance to ischemia in gerbil hippocampal neurons. Brain Res. 1992;599(2):246–52. doi: 10.1016/0006-8993(92)90398-s. [DOI] [PubMed] [Google Scholar]
- 88.Tasaki K, Ruetzler CA, Ohtsuki T, Martin D, Nawashiro H, Hallenbeck JM. Lipopolysaccharide pre-treatment induces resistance against subsequent focal cerebral ischemic damage in spontaneously hypertensive rats. Brain Res. 1997 Feb 14;748(1-2):267–70. doi: 10.1016/s0006-8993(96)01383-2. [DOI] [PubMed] [Google Scholar]
- 89.Bordet R, Deplanque D, Maboudou P, Puisieux F, Pu Q, Robin E, et al. Increase in endogenous brain superoxide dismutase as a potential mechanism of lipopolysaccharide-induced brain ischemic tolerance. J Cereb Blood Flow Metab. 2000;20(8):1190–6. doi: 10.1097/00004647-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 90.Bastide M, Gele P, Petrault O, Pu Q, Caliez A, Robin E, et al. Delayed cerebrovascular protective effect of lipopolysaccharide in parallel to brain ischemic tolerance. J Cereb Blood Flow Metab. 2003;23(4):399–405. doi: 10.1097/01.WCB.0000050064.57184.F2. [DOI] [PubMed] [Google Scholar]
- 91.Rosenzweig HL, Minami M, Lessov NS, Coste SC, Stevens SL, Henshall DC, et al. Endotoxin preconditioning protects against the cytotoxic effects of TNFalpha after stroke: a novel role for TNFalpha in LPS-ischemic tolerance. J Cereb Blood Flow Metab. 2007 Oct;27(10):1663–74. doi: 10.1038/sj.jcbfm.9600464. [DOI] [PubMed] [Google Scholar]
- 92.Eklind S, Mallard C, Arvidsson P, Hagberg H. Lipopolysaccharide induces both a primary and a secondary phase of sensitization in the developing rat brain. Pediatr. Res. 2005 Jul;58(1):112–6. doi: 10.1203/01.PDR.0000163513.03619.8D. [DOI] [PubMed] [Google Scholar]
- 93.Hickey EJ, You X, Kaimaktchiev V, Stenzel-Poore M, Ungerleider RM. Lipopolysaccharide preconditioning induces robust protection against brain injury resulting from deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2007 Jun;133(6):1588–96. doi: 10.1016/j.jtcvs.2006.12.056. [DOI] [PubMed] [Google Scholar]
- 94.Marsh BJ, Williams-Karnesky RL, Stenzel-Poore MP. Toll-like receptor signaling in endogenous neuroprotection and stroke. Neuroscience. 2009 Feb 6;158(3):1007–20. doi: 10.1016/j.neuroscience.2008.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Riepe MW, Esclaire F, Kasischke K, Schreiber S, Nakase H, Kempski O, et al. Increased hypoxic tolerance by chemical inhibition of oxidative phosphorylation: “chemical preconditioning”. J Cereb Blood Flow Metab. 1997;17(3):257–64. doi: 10.1097/00004647-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 96.Horiguchi T, Kis B, Rajapakse N, Shimizu K, Busija DW. Opening of mitochondrial ATP-sensitive potassium channels is a trigger of 3-nitropropionic acid-induced tolerance to transient focal cerebral ischemia in rats. Stroke. 2003 Apr;34(4):1015–20. doi: 10.1161/01.STR.0000063404.27912.5B. [DOI] [PubMed] [Google Scholar]
- 97.Pera J, Zawadzka M, Kaminska B, Szczudlik A. Influence of chemical and ischemic preconditioning on cytokine expression after focal brain ischemia. J Neurosci Res. 2004;78(1):132–40. doi: 10.1002/jnr.20232. [DOI] [PubMed] [Google Scholar]
- 98.Wiegand F, Liao W, Busch C, Castell S, Knapp F, Lindauer U, et al. Respiratory chain inhibition induces tolerance to focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19(11):1229–37. doi: 10.1097/00004647-199911000-00007. [DOI] [PubMed] [Google Scholar]
- 99.Hoshi A, Nakahara T, Ogata M, Yamamoto T. The critical threshold of 3-nitropropionic acid-induced ischemic tolerance in the rat. Brain Res. 2005 Jul 19;1050(1-2):33–9. doi: 10.1016/j.brainres.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 100.Sugino T, Nozaki K, Hashimoto N. Activation of mitogen-activated protein kinases in gerbil hippocampus with ischemic tolerance induced by 3-nitropropionic acid. Neurosci Lett. 2000;278(1-2):101–4. doi: 10.1016/s0304-3940(99)00906-4. [DOI] [PubMed] [Google Scholar]
- 101.Kato K, Shimazaki K, Kamiya T, Amemiya S, Inaba T, Oguro K, et al. Differential effects of sublethal ischemia and chemical preconditioning with 3-nitropropionic acid on protein expression in gerbil hippocampus. Life Sci. 2005 Oct 21;77(23):2867–78. doi: 10.1016/j.lfs.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 102.Garnier P, Bertrand N, Demougeot C, Prigent-Tessier A, Marie C, Beley A. Chemical preconditioning with 3-nitropropionic acid: lack of induction of neuronal tolerance in gerbil hippocampus subjected to transient forebrain ischemia. Brain Res Bull. 2002 May;58(1):33–9. doi: 10.1016/s0361-9230(02)00753-0. [DOI] [PubMed] [Google Scholar]
- 103.Liu J, Ginis I, Spatz M, Hallenbeck JM. Hypoxic preconditioning protects cultured neurons against hypoxic stress via TNF-alpha and ceramide. Am J Physiol. 2000;278(1):C144–C53. doi: 10.1152/ajpcell.2000.278.1.C144. [DOI] [PubMed] [Google Scholar]
- 104.Pradillo JM, Romera C, Hurtado O, Cardenas A, Moro MA, Leza JC, et al. TNFR1 upregulation mediates tolerance after brain ischemic preconditioning. J Cereb Blood Flow Metab. 2005 Feb;25(2):193–203. doi: 10.1038/sj.jcbfm.9600019. [DOI] [PubMed] [Google Scholar]
- 105.Dave KR, Saul I, Prado R, Busto R, Perez-Pinzon MA. Remote organ ischemic preconditioning protect brain from ischemic damage following asphyxial cardiac arrest. Neurosci Lett. 2006 Aug 14;404(1-2):170–5. doi: 10.1016/j.neulet.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 106.Ren C, Gao X, Steinberg GK, Zhao H. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience. 2008 Feb 19;151(4):1099–103. doi: 10.1016/j.neuroscience.2007.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kitano H, Young JM, Cheng J, Wang L, Hurn PD, Murphy SJ. Gender-specific response to isoflurane preconditioning in focal cerebral ischemia. J Cereb Blood Flow Metab. 2007 Jul;27(7):1377–86. doi: 10.1038/sj.jcbfm.9600444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.He Z, Crook JE, Meschia JF, Brott TG, Dickson DW, McKinney M. Aging blunts ischemic-preconditioning-induced neuroprotection following transient global ischemia in rats. Curr Neurovasc Res. 2005 Dec;2(5):365–74. doi: 10.2174/156720205774962674. [DOI] [PubMed] [Google Scholar]
- 109.Methy D, Bertrand N, Prigent-Tessier A, Mossiat C, Stanimirovic D, Beley A, et al. Beneficial effect of dipyridyl, a liposoluble iron chelator against focal cerebral ischemia: in vivo and in vitro evidence of protection of cerebral endothelial cells. Brain Res. 2008 Feb 8;1193:136–42. doi: 10.1016/j.brainres.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 110.Marsh BJ, Stevens SL, Hunter B, Stenzel-Poore MP. Inflammation and the emerging role of the toll-like receptor system in acute brain ischemia. Stroke. 2009 Mar;40(3 Suppl):S34–7. doi: 10.1161/STROKEAHA.108.534917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stevens SL, Ciesielski TM, Marsh BJ, Yang T, Homen DS, Boule JL, et al. Toll-like receptor 9: a new target of ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2008 May;28(5):1040–7. doi: 10.1038/sj.jcbfm.9600606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Toyoda T, Kassell NF, Lee KS. Induction of tolerance against ischemia/reperfusion injury in the rat brain by preconditioning with the endotoxin analog diphosphoryl lipid A. J Neurosurg. 2000 Mar;92(3):435–41. doi: 10.3171/jns.2000.92.3.0435. [DOI] [PubMed] [Google Scholar]
- 113.Gaspar T, Snipes JA, Busija AR, Kis B, Domoki F, Bari F, et al. ROS-independent preconditioning in neurons via activation of mitoK(ATP) channels by BMS-191095. J Cereb Blood Flow Metab. 2008 Jun;28(6):1090–103. doi: 10.1038/sj.jcbfm.9600611. [DOI] [PubMed] [Google Scholar]
- 114.Gaspar T, Kis B, Snipes JA, Lenzser G, Mayanagi K, Bari F, et al. Neuronal preconditioning with the antianginal drug, bepridil. J Neurochem. 2007 Aug;102(3):595–608. doi: 10.1111/j.1471-4159.2007.04501.x. [DOI] [PubMed] [Google Scholar]
- 115.Zhu HL, Luo WQ, Wang H. Iptakalim protects against hypoxic brain injury through multiple pathways associated with ATP-sensitive potassium channels. Neuroscience. 2008 Dec 10;157(4):884–94. doi: 10.1016/j.neuroscience.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 116.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009 Jun;40(6):2244–50. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Meschia JF. Pharmacogenetics and Stroke. Stroke. 2009 Sep 17;40(11):3641–5. doi: 10.1161/STROKEAHA.109.562231. [DOI] [PMC free article] [PubMed] [Google Scholar]


