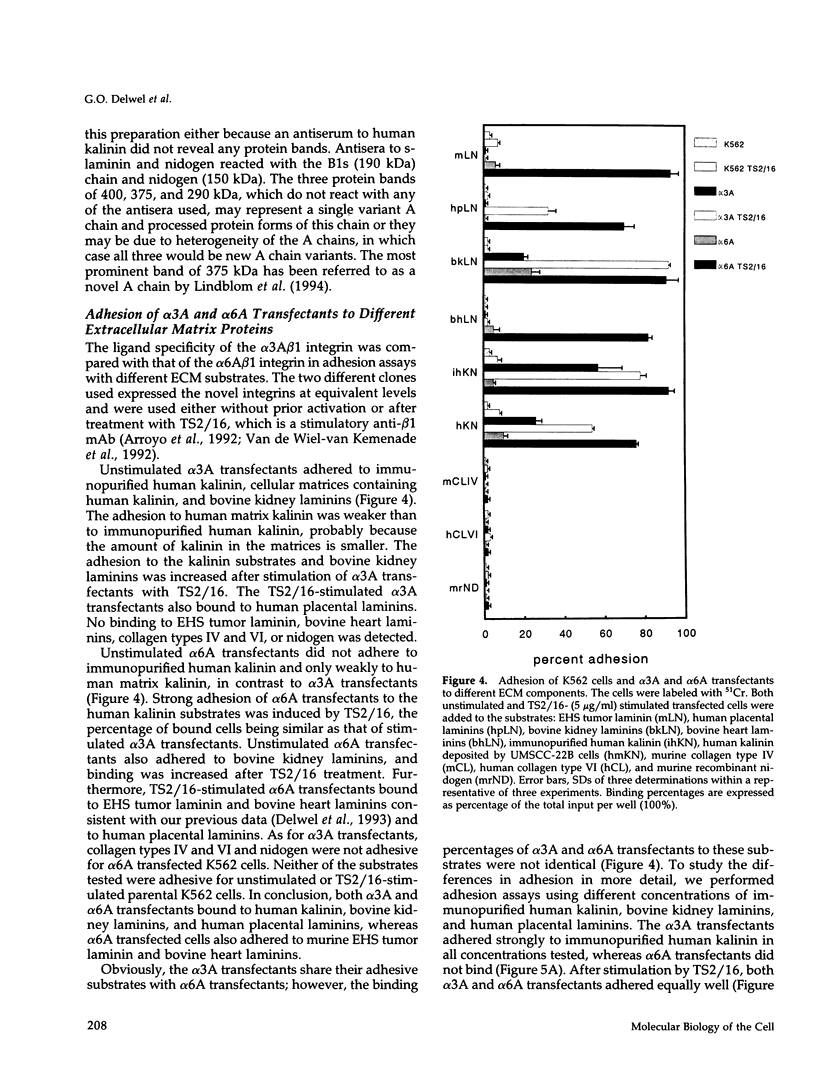
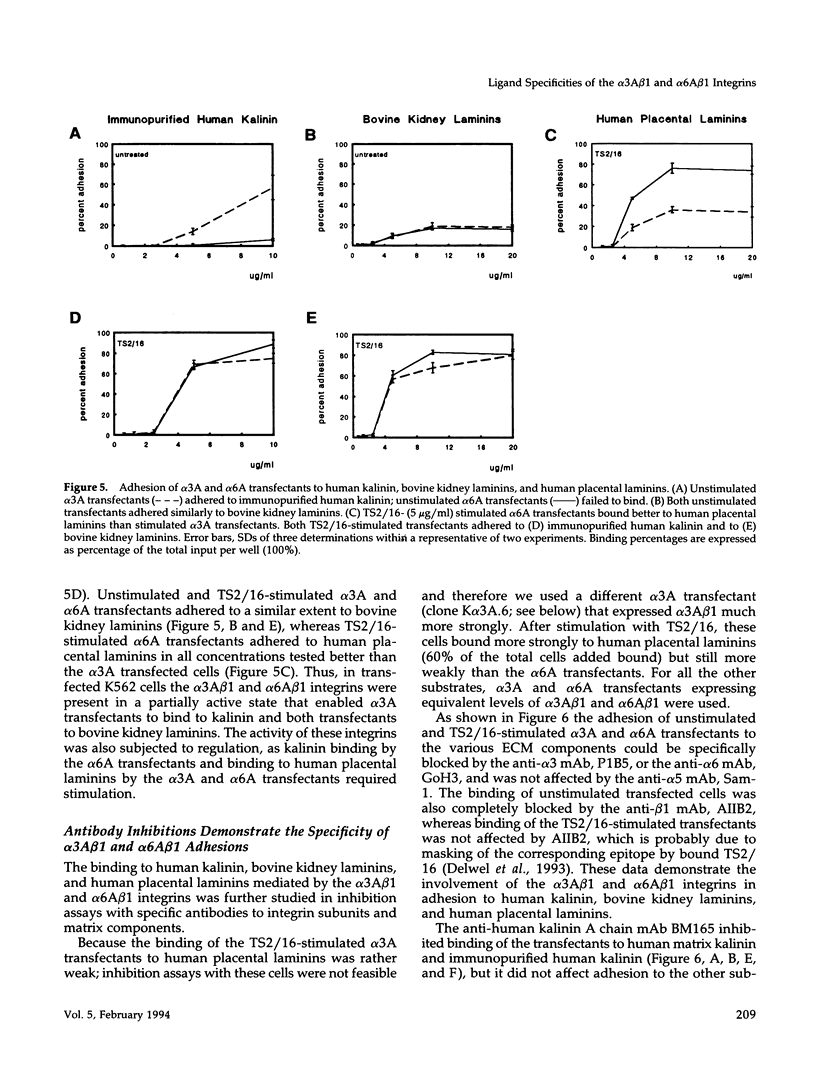
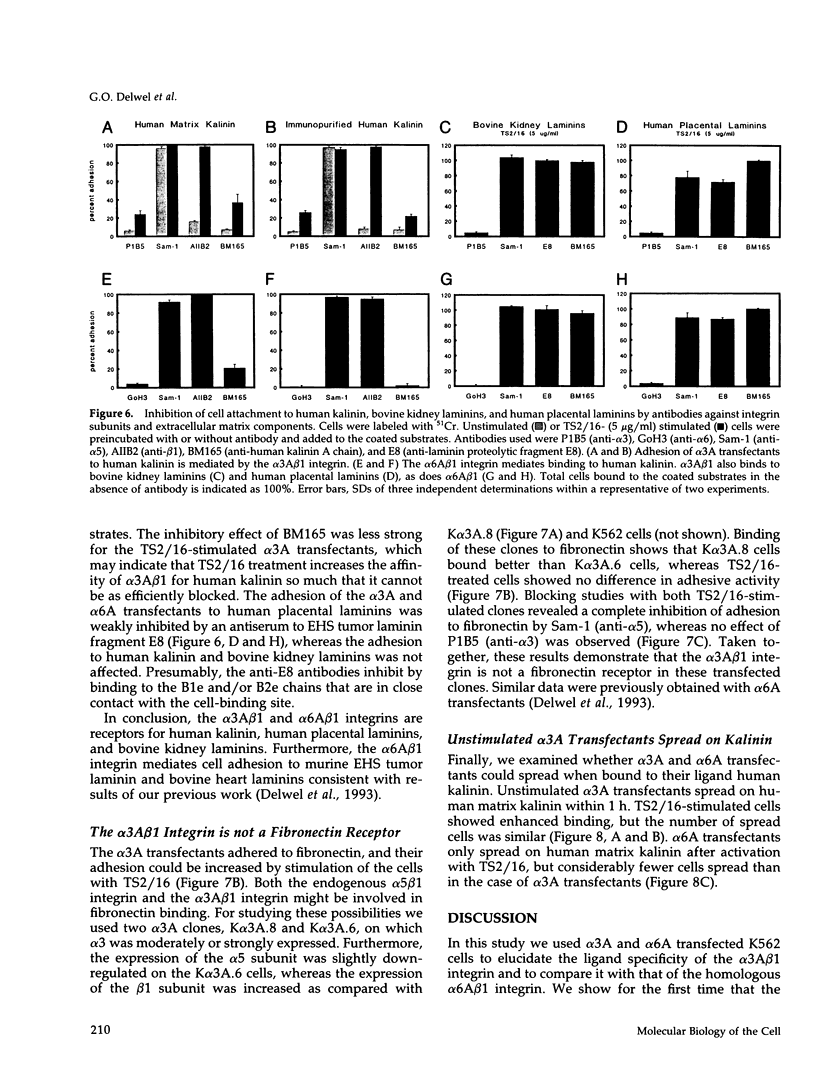
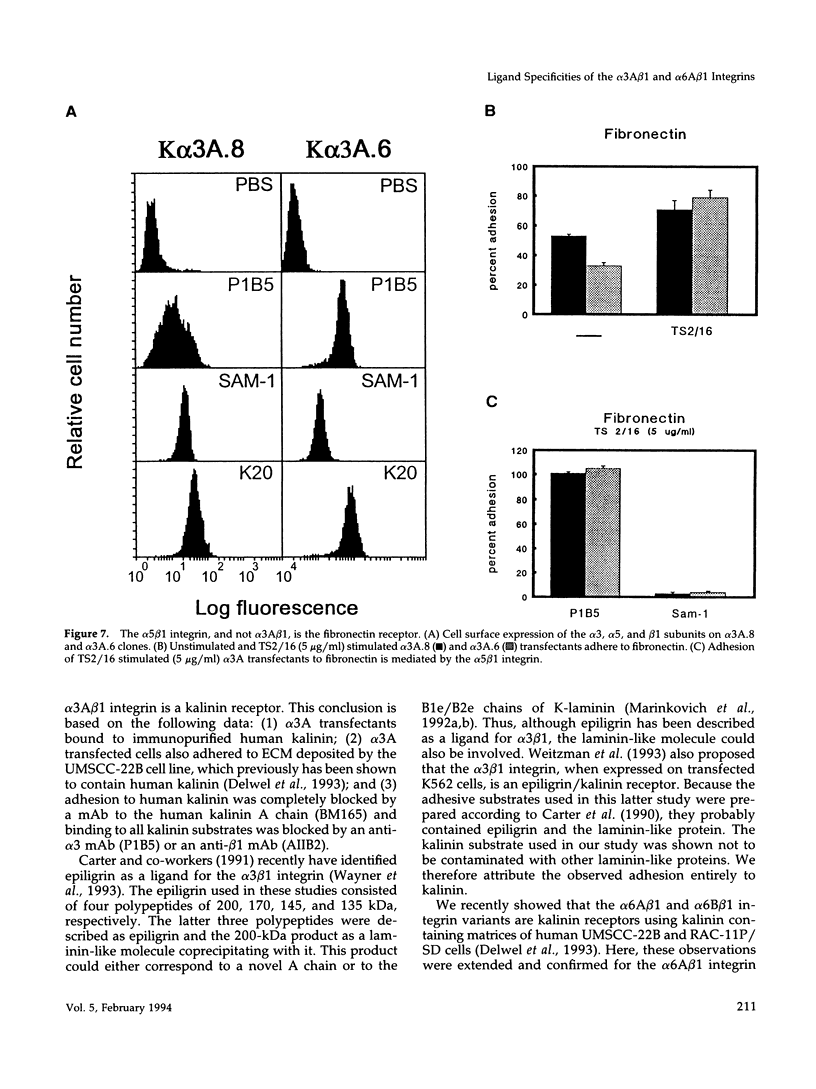
Abstract
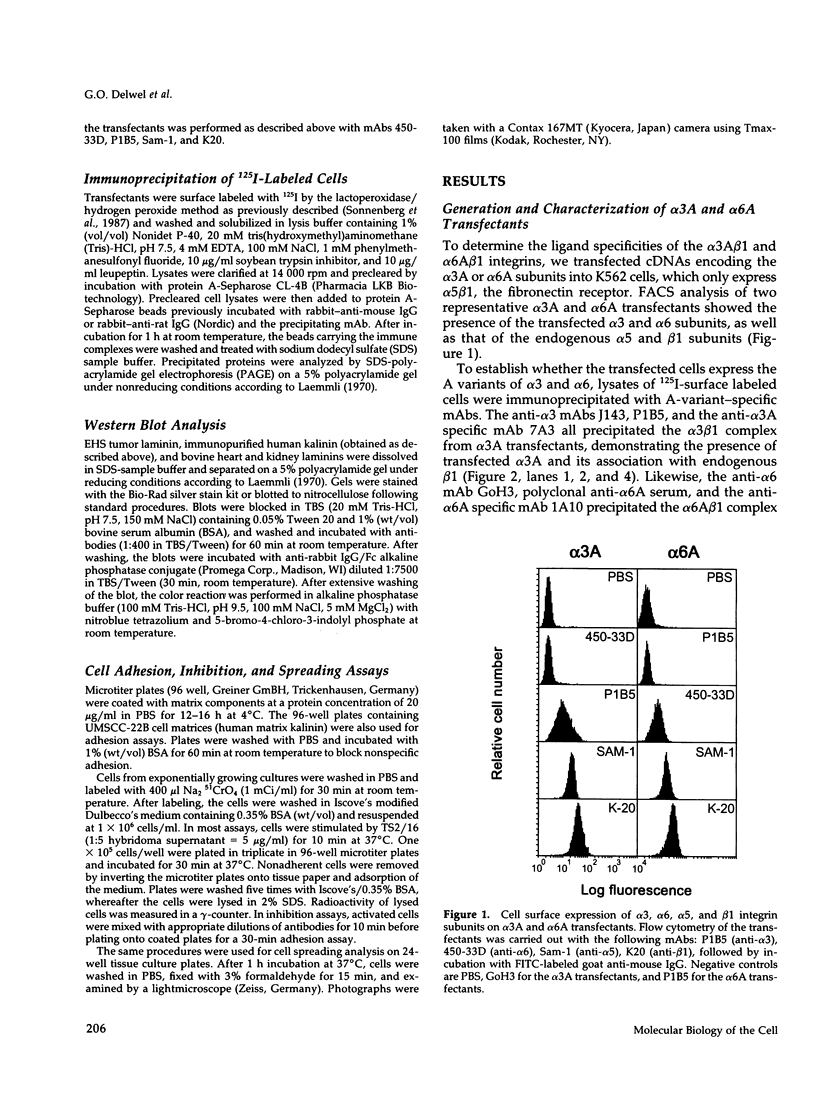
The ligand specificity of the alpha 3A beta 1 integrin was analyzed using K562 cells transfected with full-length alpha 3A cDNA and was compared with that of alpha 6A beta 1 in similarly transfected K562 cells. Clones were obtained that showed comparable surface expression of either alpha 3A beta 1 or alpha 6A beta 1 integrins. Those expressing alpha 3A beta 1 attached to and spread on immunopurified human kalinin and cellular matrices containing human kalinin, which is a particular isoform of laminin. In addition, alpha 3A transfectants adhered to bovine kidney laminins possessing a novel A chain variant. Binding to kalinin was blocked by a monoclonal antibody against the A chain constituent of kalinin and adhesion to both kalinin and kidney laminins by anti-alpha 3 and beta 1 monoclonal antibodies. The alpha 3A transfected cells bound more strongly to kalinin and bovine kidney laminins after treatment with the beta 1 stimulatory antibody TS2/16. A distinctly weaker and activation-dependent adhesion of alpha 3A transfectants was observed on human placental laminins possessing the Am chain variant (merosin), and no adhesion occurred on bovine heart laminins and murine EHS tumor laminin. Further inactive substrates were fibronectin, nidogen, and collagen types IV and VI, indicating that the alpha 3A beta 1 integrin is a much less promiscuous receptor than thought before. By contrast, alpha 6A transfected cells adhered to all laminin isoforms when stimulated with TS2/16. Adhesion also occurred only on bovine kidney laminins in the absence of TS2/16. These results demonstrate that both alpha 3A beta 1 and alpha 6A beta 1 integrins are typical laminin receptors but that their affinity and activation dependence for binding to various laminin isoforms differ considerably.
Full text
PDF



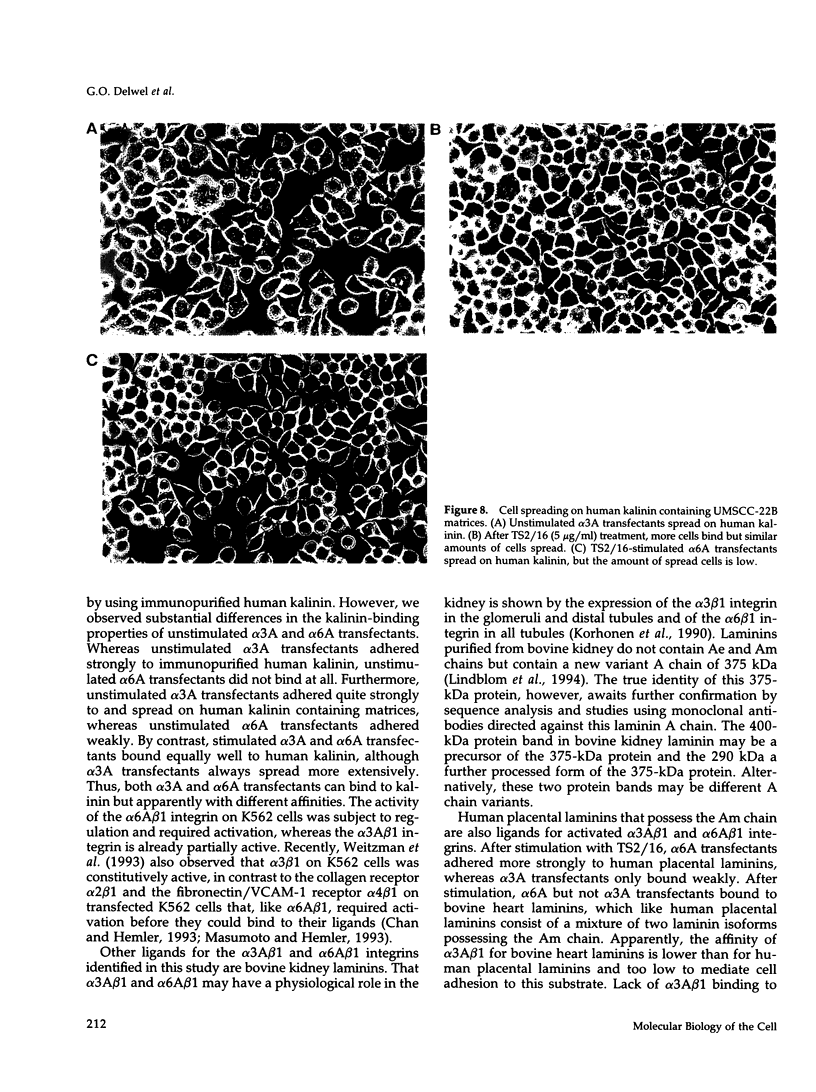



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amiot M., Bernard A., Tran H. C., Leca G., Kanellopoulos J. M., Boumsell L. The human cell surface glycoprotein complex (gp 120,200) recognized by monoclonal antibody K20 is a component binding to phytohaemagglutinin on T cells. Scand J Immunol. 1986 Jan;23(1):109–118. doi: 10.1111/j.1365-3083.1986.tb01948.x. [DOI] [PubMed] [Google Scholar]
- Arroyo A. G., Sánchez-Mateos P., Campanero M. R., Martín-Padura I., Dejana E., Sánchez-Madrid F. Regulation of the VLA integrin-ligand interactions through the beta 1 subunit. J Cell Biol. 1992 May;117(3):659–670. doi: 10.1083/jcb.117.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck K., Hunter I., Engel J. Structure and function of laminin: anatomy of a multidomain glycoprotein. FASEB J. 1990 Feb 1;4(2):148–160. doi: 10.1096/fasebj.4.2.2404817. [DOI] [PubMed] [Google Scholar]
- Brown J. C., Goodman S. L. Different cellular receptors for human placental laminin and murine EHS laminin. FEBS Lett. 1991 Apr 22;282(1):5–8. doi: 10.1016/0014-5793(91)80432-3. [DOI] [PubMed] [Google Scholar]
- Carter W. G., Ryan M. C., Gahr P. J. Epiligrin, a new cell adhesion ligand for integrin alpha 3 beta 1 in epithelial basement membranes. Cell. 1991 May 17;65(4):599–610. doi: 10.1016/0092-8674(91)90092-d. [DOI] [PubMed] [Google Scholar]
- Carter W. G., Wayner E. A., Bouchard T. S., Kaur P. The role of integrins alpha 2 beta 1 and alpha 3 beta 1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990 Apr;110(4):1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan B. M., Hemler M. E. Multiple functional forms of the integrin VLA-2 can be derived from a single alpha 2 cDNA clone: interconversion of forms induced by an anti-beta 1 antibody. J Cell Biol. 1993 Jan;120(2):537–543. doi: 10.1083/jcb.120.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedhar S., Jewell K., Rojiani M., Gray V. The receptor for the basement membrane glycoprotein entactin is the integrin alpha 3/beta 1. J Biol Chem. 1992 Sep 15;267(26):18908–18914. [PubMed] [Google Scholar]
- Delwel G. O., Hogervorst F., Kuikman I., Paulsson M., Timpl R., Sonnenberg A. Expression and function of the cytoplasmic variants of the integrin alpha 6 subunit in transfected K562 cells. Activation-dependent adhesion and interaction with isoforms of laminin. J Biol Chem. 1993 Dec 5;268(34):25865–25875. [PubMed] [Google Scholar]
- Domloge-Hultsch N., Gammon W. R., Briggaman R. A., Gil S. G., Carter W. G., Yancey K. B. Epiligrin, the major human keratinocyte integrin ligand, is a target in both an acquired autoimmune and an inherited subepidermal blistering skin disease. J Clin Invest. 1992 Oct;90(4):1628–1633. doi: 10.1172/JCI116033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrig K., Leivo I., Argraves W. S., Ruoslahti E., Engvall E. Merosin, a tissue-specific basement membrane protein, is a laminin-like protein. Proc Natl Acad Sci U S A. 1990 May;87(9):3264–3268. doi: 10.1073/pnas.87.9.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elices M. J., Hemler M. E. The human integrin VLA-2 is a collagen receptor on some cells and a collagen/laminin receptor on others. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9906–9910. doi: 10.1073/pnas.86.24.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elices M. J., Urry L. A., Hemler M. E. Receptor functions for the integrin VLA-3: fibronectin, collagen, and laminin binding are differentially influenced by Arg-Gly-Asp peptide and by divalent cations. J Cell Biol. 1991 Jan;112(1):169–181. doi: 10.1083/jcb.112.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. W., Mayer U., Nischt R., Aumailley M., Reinhardt D., Wiedemann H., Mann K., Timpl R., Krieg T., Engel J. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. EMBO J. 1991 Nov;10(11):3137–3146. doi: 10.1002/j.1460-2075.1991.tb04875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlsen K. R., Dickerson K., Argraves W. S., Engvall E., Ruoslahti E. Subunit structure of a laminin-binding integrin and localization of its binding site on laminin. J Biol Chem. 1989 Nov 15;264(32):19034–19038. [PubMed] [Google Scholar]
- Gehlsen K. R., Dillner L., Engvall E., Ruoslahti E. The human laminin receptor is a member of the integrin family of cell adhesion receptors. Science. 1988 Sep 2;241(4870):1228–1229. doi: 10.1126/science.2970671. [DOI] [PubMed] [Google Scholar]
- Green T. L., Hunter D. D., Chan W., Merlie J. P., Sanes J. R. Synthesis and assembly of the synaptic cleft protein S-laminin by cultured cells. J Biol Chem. 1992 Jan 25;267(3):2014–2022. [PubMed] [Google Scholar]
- Hemler M. E., Sanchez-Madrid F., Flotte T. J., Krensky A. M., Burakoff S. J., Bhan A. K., Springer T. A., Strominger J. L. Glycoproteins of 210,000 and 130,000 m.w. on activated T cells: cell distribution and antigenic relation to components on resting cells and T cell lines. J Immunol. 1984 Jun;132(6):3011–3018. [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988 Aug 11;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst F., Admiraal L. G., Niessen C., Kuikman I., Janssen H., Daams H., Sonnenberg A. Biochemical characterization and tissue distribution of the A and B variants of the integrin alpha 6 subunit. J Cell Biol. 1993 Apr;121(1):179–191. doi: 10.1083/jcb.121.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst F., Kuikman I., van Kessel A. G., Sonnenberg A. Molecular cloning of the human alpha 6 integrin subunit. Alternative splicing of alpha 6 mRNA and chromosomal localization of the alpha 6 and beta 4 genes. Eur J Biochem. 1991 Jul 15;199(2):425–433. doi: 10.1111/j.1432-1033.1991.tb16140.x. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Leong J. M. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990 Mar 9;60(5):861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- Jaspars L. H., van der Linden H. C., Scheffer G. L., Scheper R. J., Meijer C. J. Monoclonal antibody 4C7 recognizes an endothelial basement membrane component that is selectively expressed in capillaries of lymphoid follicles. J Pathol. 1993 Jun;170(2):121–128. doi: 10.1002/path.1711700205. [DOI] [PubMed] [Google Scholar]
- Kallunki P., Sainio K., Eddy R., Byers M., Kallunki T., Sariola H., Beck K., Hirvonen H., Shows T. B., Tryggvason K. A truncated laminin chain homologous to the B2 chain: structure, spatial expression, and chromosomal assignment. J Cell Biol. 1992 Nov;119(3):679–693. doi: 10.1083/jcb.119.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor R. R., Mattes M. J., Lloyd K. O., Old L. J., Albino A. P. Biochemical analysis of two cell surface glycoprotein complexes, very common antigen 1 and very common antigen 2. Relationship to very late activation T cell antigens. J Biol Chem. 1987 Nov 5;262(31):15158–15165. [PubMed] [Google Scholar]
- Keizer G. D., Te Velde A. A., Schwarting R., Figdor C. G., De Vries J. E. Role of p150,95 in adhesion, migration, chemotaxis and phagocytosis of human monocytes. Eur J Immunol. 1987 Sep;17(9):1317–1322. doi: 10.1002/eji.1830170915. [DOI] [PubMed] [Google Scholar]
- Kennel S. J., Epler R. G., Lankford T. K., Foote L. J., Dickas V., Canamucio M., Cavalierie R., Cosimelli M., Venturo I., Falcioni R. Second generation monoclonal antibodies to the human integrin alpha 6 beta 4. Hybridoma. 1990 Jun;9(3):243–255. doi: 10.1089/hyb.1990.9.243. [DOI] [PubMed] [Google Scholar]
- Korhonen M., Ylänne J., Laitinen L., Virtanen I. The alpha 1-alpha 6 subunits of integrins are characteristically expressed in distinct segments of developing and adult human nephron. J Cell Biol. 1990 Sep;111(3):1245–1254. doi: 10.1083/jcb.111.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. H., Cheng Y. F., Clyman R. Human microvascular endothelial cells use beta 1 and beta 3 integrin receptor complexes to attach to laminin. J Cell Biol. 1990 Sep;111(3):1233–1243. doi: 10.1083/jcb.111.3.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Languino L. R., Gehlsen K. R., Wayner E., Carter W. G., Engvall E., Ruoslahti E. Endothelial cells use alpha 2 beta 1 integrin as a laminin receptor. J Cell Biol. 1989 Nov;109(5):2455–2462. doi: 10.1083/jcb.109.5.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivo I., Engvall E. Merosin, a protein specific for basement membranes of Schwann cells, striated muscle, and trophoblast, is expressed late in nerve and muscle development. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1544–1548. doi: 10.1073/pnas.85.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblom A., Marsh T., Fauser C., Engel J., Paulsson M. Characterization of native laminin from bovine kidney and comparison with other laminin variants. Eur J Biochem. 1994 Jan 15;219(1-2):383–392. doi: 10.1111/j.1432-1033.1994.tb19950.x. [DOI] [PubMed] [Google Scholar]
- Lotz M. M., Korzelius C. A., Mercurio A. M. Human colon carcinoma cells use multiple receptors to adhere to laminin: involvement of alpha 6 beta 4 and alpha 2 beta 1 integrins. Cell Regul. 1990 Feb;1(3):249–257. doi: 10.1091/mbc.1.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovich M. P., Lunstrum G. P., Burgeson R. E. The anchoring filament protein kalinin is synthesized and secreted as a high molecular weight precursor. J Biol Chem. 1992 Sep 5;267(25):17900–17906. [PubMed] [Google Scholar]
- Marinkovich M. P., Lunstrum G. P., Keene D. R., Burgeson R. E. The dermal-epidermal junction of human skin contains a novel laminin variant. J Cell Biol. 1992 Nov;119(3):695–703. doi: 10.1083/jcb.119.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumoto A., Hemler M. E. Multiple activation states of VLA-4. Mechanistic differences between adhesion to CS1/fibronectin and to vascular cell adhesion molecule-1. J Biol Chem. 1993 Jan 5;268(1):228–234. [PubMed] [Google Scholar]
- Nurcombe V., Aumailley M., Timpl R., Edgar D. The high-affinity binding of laminin to cells. Assignation of a major cell-binding site to the long arm of laminin and of a latent cell-binding site to its short arms. Eur J Biochem. 1989 Mar 1;180(1):9–14. doi: 10.1111/j.1432-1033.1989.tb14608.x. [DOI] [PubMed] [Google Scholar]
- Odermatt E., Risteli J., van Delden V., Timpl R. Structural diversity and domain composition of a unique collagenous fragment (intima collagen) obtained from human placenta. Biochem J. 1983 May 1;211(2):295–302. doi: 10.1042/bj2110295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott U., Odermatt E., Engel J., Furthmayr H., Timpl R. Protease resistance and conformation of laminin. Eur J Biochem. 1982 Mar;123(1):63–72. doi: 10.1111/j.1432-1033.1982.tb06499.x. [DOI] [PubMed] [Google Scholar]
- Paulsson M., Deutzmann R., Timpl R., Dalzoppo D., Odermatt E., Engel J. Evidence for coiled-coil alpha-helical regions in the long arm of laminin. EMBO J. 1985 Feb;4(2):309–316. doi: 10.1002/j.1460-2075.1985.tb03630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson M., Saladin K., Engvall E. Structure of laminin variants. The 300-kDa chains of murine and bovine heart laminin are related to the human placenta merosin heavy chain and replace the a chain in some laminin variants. J Biol Chem. 1991 Sep 15;266(26):17545–17551. [PubMed] [Google Scholar]
- Paulsson M., Saladin K. Mouse heart laminin. Purification of the native protein and structural comparison with Engelbreth-Holm-Swarm tumor laminin. J Biol Chem. 1989 Nov 5;264(31):18726–18732. [PubMed] [Google Scholar]
- Pfaff M., Aumailley M., Specks U., Knolle J., Zerwes H. G., Timpl R. Integrin and Arg-Gly-Asp dependence of cell adhesion to the native and unfolded triple helix of collagen type VI. Exp Cell Res. 1993 May;206(1):167–176. doi: 10.1006/excr.1993.1134. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Rousselle P., Lunstrum G. P., Keene D. R., Burgeson R. E. Kalinin: an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol. 1991 Aug;114(3):567–576. doi: 10.1083/jcb.114.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R., Engvall E., Butkowski R., Hunter D. D. Molecular heterogeneity of basal laminae: isoforms of laminin and collagen IV at the neuromuscular junction and elsewhere. J Cell Biol. 1990 Oct;111(4):1685–1699. doi: 10.1083/jcb.111.4.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A., Gehlsen K. R., Aumailley M., Timpl R. Isolation of alpha 6 beta 1 integrins from platelets and adherent cells by affinity chromatography on mouse laminin fragment E8 and human laminin pepsin fragment. Exp Cell Res. 1991 Dec;197(2):234–244. doi: 10.1016/0014-4827(91)90428-w. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A., Janssen H., Hogervorst F., Calafat J., Hilgers J. A complex of platelet glycoproteins Ic and IIa identified by a rat monoclonal antibody. J Biol Chem. 1987 Jul 25;262(21):10376–10383. [PubMed] [Google Scholar]
- Sonnenberg A., Linders C. J., Modderman P. W., Damsky C. H., Aumailley M., Timpl R. Integrin recognition of different cell-binding fragments of laminin (P1, E3, E8) and evidence that alpha 6 beta 1 but not alpha 6 beta 4 functions as a major receptor for fragment E8. J Cell Biol. 1990 Jun;110(6):2145–2155. doi: 10.1083/jcb.110.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A., Modderman P. W., Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988 Dec 1;336(6198):487–489. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- Takada Y., Murphy E., Pil P., Chen C., Ginsberg M. H., Hemler M. E. Molecular cloning and expression of the cDNA for alpha 3 subunit of human alpha 3 beta 1 (VLA-3), an integrin receptor for fibronectin, laminin, and collagen. J Cell Biol. 1991 Oct;115(1):257–266. doi: 10.1083/jcb.115.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y., Wayner E. A., Carter W. G., Hemler M. E. Extracellular matrix receptors, ECMRII and ECMRI, for collagen and fibronectin correspond to VLA-2 and VLA-3 in the VLA family of heterodimers. J Cell Biochem. 1988 Aug;37(4):385–393. doi: 10.1002/jcb.240370406. [DOI] [PubMed] [Google Scholar]
- Tamura R. N., Rozzo C., Starr L., Chambers J., Reichardt L. F., Cooper H. M., Quaranta V. Epithelial integrin alpha 6 beta 4: complete primary structure of alpha 6 and variant forms of beta 4. J Cell Biol. 1990 Oct;111(4):1593–1604. doi: 10.1083/jcb.111.4.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R., Paulsson M., Dziadek M., Fujiwara S. Basement membranes. Methods Enzymol. 1987;145:363–391. doi: 10.1016/0076-6879(87)45021-0. [DOI] [PubMed] [Google Scholar]
- Timpl R. Structure and biological activity of basement membrane proteins. Eur J Biochem. 1989 Apr 1;180(3):487–502. doi: 10.1111/j.1432-1033.1989.tb14673.x. [DOI] [PubMed] [Google Scholar]
- Tomaselli K. J., Hall D. E., Flier L. A., Gehlsen K. R., Turner D. C., Carbonetto S., Reichardt L. F. A neuronal cell line (PC12) expresses two beta 1-class integrins-alpha 1 beta 1 and alpha 3 beta 1-that recognize different neurite outgrowth-promoting domains in laminin. Neuron. 1990 Nov;5(5):651–662. doi: 10.1016/0896-6273(90)90219-6. [DOI] [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common beta subunits. J Cell Biol. 1987 Oct;105(4):1873–1884. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G., Piotrowicz R. S., Kunicki T. J. The function of multiple extracellular matrix receptors in mediating cell adhesion to extracellular matrix: preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion to fibronectin and react with platelet glycoproteins Ic-IIa. J Cell Biol. 1988 Nov;107(5):1881–1891. doi: 10.1083/jcb.107.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Gil S. G., Murphy G. F., Wilke M. S., Carter W. G. Epiligrin, a component of epithelial basement membranes, is an adhesive ligand for alpha 3 beta 1 positive T lymphocytes. J Cell Biol. 1993 Jun;121(5):1141–1152. doi: 10.1083/jcb.121.5.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman J. B., Pasqualini R., Takada Y., Hemler M. E. The function and distinctive regulation of the integrin VLA-3 in cell adhesion, spreading, and homotypic cell aggregation. J Biol Chem. 1993 Apr 25;268(12):8651–8657. [PubMed] [Google Scholar]
- Werb Z., Tremble P. M., Behrendtsen O., Crowley E., Damsky C. H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989 Aug;109(2):877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziober B. L., Vu M. P., Waleh N., Crawford J., Lin C. S., Kramer R. H. Alternative extracellular and cytoplasmic domains of the integrin alpha 7 subunit are differentially expressed during development. J Biol Chem. 1993 Dec 15;268(35):26773–26783. [PubMed] [Google Scholar]
- van de Wiel-van Kemenade E., van Kooyk Y., de Boer A. J., Huijbens R. J., Weder P., van de Kasteele W., Melief C. J., Figdor C. G. Adhesion of T and B lymphocytes to extracellular matrix and endothelial cells can be regulated through the beta subunit of VLA. J Cell Biol. 1992 Apr;117(2):461–470. doi: 10.1083/jcb.117.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]