Abstract
Objective
To determine the prevalence of obesity and evaluate how accurately standard anthropometric measures identify obesity among women with SLE.
Methods
Dual-energy x-ray absorptiometry (DEXA), height, weight, and waist and hip circumference were collected from 145 women with SLE. Three anthropometric proxies of obesity (body mass index (BMI) ≥30 kg/m2, waist circumference (WC) ≥88 cm, and waist-hip ratio (WHR) ≥0.85) were compared to a DEXA-based obesity criterion. Correspondence between measures was assessed with Cohen’s kappa. Receiver operating characteristic (ROC) curves determined optimal cut-points for each anthropometric measure, relative to DEXA. Framingham cardiovascular risk scores were compared among women who were classified as not obese by both traditional and revised anthropometric definitions, obese by both definitions, and obese only by the revised definition.
Results
28%, 29%, 41%, and 50% were classified as obese by WC, BMI, WHR, and DEXA, respectively. Correspondence between anthropometric and DEXA-based measures was moderate. Women misclassified by anthropometric measures had less truncal fat and more appendicular lean and fat mass. Cut-points were identified for anthropometric measures to better approximate DEXA estimates of percent body fat: BMI ≥26.8 kg/m2, waist circumference ≥84.75 cm., and waist-hip ratio ≥0.80. Framingham risk scores were significantly higher in women classified as obese by either traditional or revised criteria.
Conclusions
A large percentage of this group of women with SLE was obese. Substantial portions of women were misclassified by anthropometric measures. Utility of revised cut-points compared to traditional cut-points in identifying risk of cardiovascular disease or disability remains to be examined in prospective studies, but results from the Framingham risk score analysis suggest that traditional cut-points exclude a significant number of at-risk women with SLE.
Obesity is a growing public health problem, and is associated with a variety of health problems such as increased risk of cardiovascular disease, osteoarthritis, and disability. Among the few studies that have examined obesity or body composition in systemic lupus erythematosus (SLE), rates of obesity appear to be higher than in the general population (1–4). Individuals with SLE have an elevated risk of both cardiovascular disease and disability, but the increase in risk for these conditions that may be conferred by obesity is not known.
In most large-scale studies, obesity has been estimated from body mass index (BMI), calculated as body weight adjusted for height (weightkg/heightm2). For the general population, BMI is assumed to be an adequate proxy measure for body fatness, but for some groups, its inaccuracy has been demonstrated. For example, in rheumatoid arthritis, there is evidence that body weight or BMI may not accurately reflect the amount of body fat(5). The chronic inflammation seen in RA can affect body composition and metabolism, resulting in rheumatoid cachexia, or the loss of lean body mass, particularly in skeletal muscle(6, 7). Rheumatoid cachexia may occur with little or no weight loss, indicating that muscle mass is lost in conjunction with increased fat mass(7). In other words, an individual may have a BMI within a normal range, but have greater fat mass than suggested by the BMI. This is important because an overabundance of fat tissue may create unhealthy levels of hormones, proteins, and cytokines, producing inflammation that may elevate the risk of cardiovascular disease or other disease processes(8). Additionally, relatively low muscle mass may cause muscle weakness, leading to disability.
An additional limitation of BMI is that it does not provide an indication of fat distribution. Abdominal fat appears to be particularly relevant for cardiovascular and metabolic disease. Other anthropometric proxy measures of body composition or obesity, such as waist circumference and the ratio of waist circumference to hip circumference, may provide better estimates of fat distribution and the risk of cardiovascular disease and diabetes, although limitations also exist with these measures(9).
Studies seeking a more accurate view of body composition often use whole dual-energy x-ray absorptiometry (DEXA)(1, 10–19), which provides good estimates of body fat and muscle. DEXA is not feasible to use on a wide-scale basis, primarily because of the cost and lack of access to equipment(9), but results from a DEXA study can provide an indication of the accuracy of proxy measures, such as BMI. Studies examining the accuracy of BMI, or other proxy measures of obesity, have not been conducted in SLE.
The goals of these analyses were to (1) determine the proportion of a cohort of women with SLE who are obese using DEXA and three commonly used anthropometric proxy measures: BMI, waist circumference, and waist-hip ratio; (2) assess the correspondence of obesity determinations among the four methods; (3) evaluate the accuracy of the standard obesity cut-points of the anthropometric measures for women with SLE, compared to a DEXA determination of obesity; and (4) examine cardiovascular risk relative to obesity classifications.
Methods
Subjects
The sample for the present study was drawn from participants in the UCSF Lupus Outcomes Study (LOS). Participants in the LOS had formerly participated in a study of genetic risk factors for SLE outcomes (20, 21) and were recruited from both clinical and community-based sources, including UCSF-affiliated clinics (22%), non-UCSF rheumatology offices (11%), lupus support groups and conferences (26%), and newsletters, websites and other forms of publicity (41%). SLE diagnoses have been verified by medical record review. Additional details regarding the LOS are reported by Yelin et al (22). LOS participants who lived in the greater San Francisco Bay Area were recruited for an in-person assessment in the UCSF Clinical and Translational Science Institute’s Clinical Research Center (CRC) that included measurement of body composition. Exclusion criteria were non-English-speaking, younger than age 18, current oral prednisone dose of 50 mg or greater, current pregnancy, uncorrected vision problems that would interfere with reading ability, and joint replacement within 1 year.
325 individuals were asked to participate; 74 (22.8%) were ineligible (35 lived too far away, 25 were too ill, 9 had had recent surgery, 2 were pregnant, 2 had poor English skills, and 1 had cognitive problems). Of the 251 eligible individuals, 84 (33.5%) declined participation. Reasons for declining were primarily related to transportation (n=12) and scheduling difficulties (n = 39). 163 individuals completed study visits; DEXAs were completed for 157, 145 women and 12 men. Only the 145 women are included in these analyses. Sociodemographic and health-related characteristics of the study sample are shown in Table 1.
Table 1.
Subject characteristics (n = 145)
| Mean (SD) | % (n) | |
|---|---|---|
| Sociodemographic | ||
| Age, years | 47.9 (12.2) | |
| Race/ethnicity | ||
| White, non Hispanic | 54.5 (79) | |
| Hispanic | 10.3 (15) | |
| African American | 14.5 (21) | |
| Asian | 12.4 (18) | |
| Other, unknown | 8.3 (12) | |
| Education, ≤ high school | 13.1 (19) | |
| Income, < poverty | 13.0 (18) | |
| Smoking | ||
| Current | 4.2 (6) | |
| Former | 33.3 (48) | |
| Never | 62.5 (90) | |
| Health-related | ||
| Disease duration, years | 15.8 (9.2) | |
| SLAQ score* | 12.4 (7.0) | |
| Daily dose of glucocorticoids | ||
| 0 | 54.8 (74) | |
| 1–4 mg/day | 5.2 (7) | |
| 5–9 mg/day | 20.7 (28) | |
| 10–14 mg/day | 17.0 (23) | |
| 15–19 mg/day | 3.7 (5) | |
| ≥ 20 mg/day | 2.2 (3) | |
SLAQ = Systemic Lupus Activity Questionnaire
The study was approved by the UCSF Committee on Human Research.
Measures
Body composition
Anthropometric measures
Height was measured with a wall-mounted stadiometer. Weight was measured with subjects wearing light indoor clothing and no shoes. Body mass index (BMI) was calculated as weight (Kg) divided by height (meters2). Obesity by BMI was defined as BMI ≥ 30 kg/m2 (23). Waist and hip circumferences were measured with a non-stretch measuring tape that applies a consistent amount of tension to the tape (Gullick II Tape Measure). Waist circumference (WC) was measured at the mid-point between the lower border of the ribs and the iliac crest. Hip circumference was measured at the widest point over the buttocks. Two measurements were taken at each point, and the average measure used. Women with a waist circumference ≥ 88 cm were classified as obese(23). Waist-hip ratio (WHR) was calculated by dividing the average waist circumference by the average hip circumference. Women with a WHR ≥ 0.85 were classified as obese(23).
Dual Energy X-ray Absorptiometry (DEXA)
Body composition and regional body fat distribution were assessed in the CRC using a Lunar Prodigy™ Dual Energy X-ray Absorptiometry (DEXA) system. The DEXA is able to differentiate bone, muscle and fat and calculates total body mass (kilograms), fat mass (grams), percent fat, and lean body mass (grams), as well as the regional distribution of these components (left arm, leg, and trunk; right arm, leg, and trunk; and total arm, leg, and trunk). The technique has been used extensively in determination of bone density (dual energy x-ray absorptiometry), has been expanded for use in determination of soft tissue mass (24–26), and has been validated as a method of assessing body composition in both younger and older persons. It has good reported reproducibility, and is sensitive to small changes in body composition(10). The precision errors (1SD) for percent fat in soft tissue are 1.4%, for fat mass 1.0 Kg, and for lean tissue mass 0.8 Kg(25). DEXA has previously been successfully used to assess body composition among individuals with RA(11, 16–18) and SLE(1, 19), and in studies of aging(10),(12, 14).
There is no agreed-upon standard definition of obesity based on percent body fat(27). Although other definitions of obesity based on percent fat have been suggested(28), we used the definitions suggested by Gallagher, which linked percent fat to the National Institutes of Health BMI guidelines. (These guidelines define obesity as BMI ≥30 kg/m2.) In that study, the average percent body fat for individuals with BMI between 30–35 kg/m2 (obese, but not morbidly obese) from three samples from the US, UK, and Japan was ascertained by DEXA. Average body fat percentages for these obese individuals were calculated separately for sex, age, and race groups. We used those percentages as the criteria for defining obesity, based on individuals’ age and race. Body fat percentage criteria ranged from 38% for African American women aged 20–39, to 43% for white women aged 60–79.
Other variables
Socio-demographic (e.g., age, race/ethnicity, education, income, smoking status) characteristics were obtained from the baseline LOS telephone interview. Disease activity was assessed using the Systemic Lupus Activity Questionnaire (SLAQ) a validated, self-report measure of disease activity in SLE (29, 30). The SLAQ was taken from the LOS interview that most closely preceded the CRC visit. Glucocorticoid use was assessed at the time of the visit.
Additional data were collected at the clinic visit that permitted calculation of the Framingham cardiovascular risk score(31), including total cholesterol and high-density lipoprotein levels, blood pressure, treatment of hypertension, and presence of diabetes. Serum lipids were obtained through non-fasting blood draws. Although fasting measurements may be ideal, non-fasting measures of total cholesterol and HDL have been found to very closely approximate fasting levels(32). Blood pressure was measured by registered nurses while subjects were in a seated position. Treatment of hypertension and presence of diabetes were ascertained by subject self-report.
Analysis
Correspondence among obesity classifications was assessed using Cohen’s kappa. Among women who were classified as obese by one of the DEXA criteria, we compared regional body composition characteristics of those who were and were not correctly classified by anthropometric methods, including the relative distribution of fat tissue (trunk versus appendicular [arms and legs combined]).
Receiver operating characteristic (ROC) curves were calculated to determine the optimal cut-points for each anthropometric measure, relative to the DEXA-based classification. Two threshold selection methods were used: the Youden and a second technique that determines the proximity to perfect correspondence (referred to in this paper as a “Distance to Perfect” index)(33). Briefly, the Youden Index determines the maximum vertical distance from the ROC curve to the diagonal reference, or ‘chance’ line; i.e., the “optimal” cut-point corresponds to the point on the ROC curve farthest from the reference line, which has also been used as a measure of the accuracy of a diagnostic test in clinical epidemiology(34). Similarly, the Distance to Perfect Index selects the point on the ROC curve that is closest to the upper left-hand corner of the graph (0,1), which represents perfect classification(35), thereby minimizing misclassification. We calculated the sensitivity, specificity, and positive and negative predictive value of each anthropometric measure using both the established and new cut-points, compared to the DEXA-based obesity classification.
To examine the potential usefulness of the revised cut-points, we calculated Framingham cardiovascular disease risk scores (based on age, HDL cholesterol, total cholesterol, systolic blood pressure, smoking, and diabetes(31)) for three groups: women who would not be considered obese by traditional or revised definition (e.g., BMI ≤ 26.7 kg/m2), women who would be considered obese by the revised definition but not the traditional definition (e.g., BMI 26.8 – 29.9 kg/m2), and women who would be considered obese by both definitions (e.g., BMI ≥ 30 kg/m2). Differences in the risk score among the three groups were tested with analysis of variance, followed by post-hoc means comparisons with the Tukey method. This analysis was performed for each of the three anthropometric measures.
Results
Subject characteristics
Mean age of the 145 women in the analysis was 48 (± 12.2) years (Table 1). Approximately half (54.3%) were white non-Hispanic, 13.6% were African American, 12.9% were Asian, 10.7% were Hispanic, and 8.6% fell into another category. Twelve percent had incomes below the poverty level. Four percent were current smokers and one-third were former smokers. The average duration of SLE was 15.8 (±9.2) years, and the average SLAQ score was 12.4. Roughly half of the sample (47.8%) was taking oral corticosteroids at the time of assessment, with half of those taking less than 10 mg per day.
Prevalence of obesity
There were substantial variations in the estimates of obesity prevalence (Table 2). Anthropometric measures yielded estimates of 28.3% (waist-hip ratio), 29.2% (BMI), and 41.4% (waist circumference). By contrast, the DEXA estimate was higher, estimating that 49.7% were obese.
Table 2.
Body Composition Characteristics
| Body composition measure | Mean (SD) | Range | % (n) | |
|---|---|---|---|---|
| Body mass index (BMI) | 27.2 (6.8) | 17.6 – 45.4 | ||
| BMI category | Underweight (<18.5) | 2.1 (3) | ||
| Normal (18.5 ≤ BMI <25) | 45.8 (66) | |||
| Overweight (25 ≤ BMI < 30) | 22.9 (33) | |||
| Obese (≥30) | 29.2 (42) | |||
| Waist circumference (WC) | 86.8 (17.1) | 62 – 143 | ||
| WC category | Normal (< 80 cm) | 44.8 (65) | ||
| Overweight (80 – 87) | 13.8 (20) | |||
| Obese (≥88 cm) | 41.4 (60) | |||
| Waist-hip ratio (WHR) | 0.81 (0.08) | 0.65 – 1.15 | ||
| WHR category | Not obese (< 0.85) | 71.7 (104) | ||
| Obese (≥0.85) | 28.3 (41) | |||
| % fat from dual energy x-ray absorptiometry (DEXA) | 40.8 (9.3) | 19.5 – 58.9 | ||
| Not obese | 50.3 (73) | |||
| Obese(44) | 49.7 (72) |
Correspondence among classifications
The DEXA classification corresponded fairly well with BMI and waist circumference classifications (kappa = 0.59 and 0.64, respectively). In contrast, the waist-hip ratio estimate of obesity did not correspond well with the DEXA-based measure (kappa = 0.27).
Among women who were classified as obese by DEXA, examination of more detailed results from the DEXA revealed total and regional differences in body composition between those classified as obese versus not obese by an anthropometric method (Table 4). Women who were obese by DEXA and not obese by BMI had significantly lower overall body fat, their trunk fat comprised a significantly lower proportion of their total body mass, and their appendicular lean mass comprised a significantly greater proportion of their total body mass. For example, women who were obese by DEXA but not obese by BMI had a lower total percent body fat (45.7% vs. 50.8%, p = <.0001), a lower proportion of total body mass as trunk fat (23.5% vs. 27.2%, p < .0001), and a higher proportion of total body mass as appendicular lean mass (23.4% vs. 21.2, p = .0002). A similar pattern was noted for women who were misclassified by waist circumference; in addition, however, both appendicular lean and fat mass were greater than among women correctly classified as obese. For women who were misclassified by waist-hip ratio, trunk fat composed a significantly lower proportion of total mass, and appendicular fat a significantly higher proportion, but appendicular lean mass was not significantly different than for women correctly classified as obese.
Table 4.
Body composition characteristics of individuals with and without discrepancies in obesity classifications between DEXA-based and anthropometric measures (includes only individuals classified as obese by DEXA measure (n = 72)
| Body composition characteristic from DEXA | Classification from anthropometric measure | Anthropometric measure |
||
|---|---|---|---|---|
| BMI | Waist circumference | Waist hip ratio | ||
| % fat | Not obese | 45.7 | 44.7 | 48.4 |
| Obese | 50.8 | 50.0 | 48.9 | |
| p* | <.0001 | <.0001 | .65 | |
| Trunk fat/total fat1 | Not obese | 51.5 | 49.7 | 51.1 |
| Obese | 53.7 | 54.0 | 55.4 | |
| p* | .11 | .004 | .001 | |
| Trunk fat/total mass2 | Not obese | 23.5 | 22.2 | 24.7 |
| Obese | 27.2 | 26.9 | 27.0 | |
| p* | <.0001 | <.0001 | .006 | |
| Appendicular fat/total fat3 | Not obese | 45.6 | 47.3 | 46.4 |
| Obese | 44.0 | 43.7 | 42.2 | |
| p* | .23 | .01 | .002 | |
| Appendicular lean/total mass4 | Not obese | 23.4 | 24.4 | 22.4 |
| Obese | 21.2 | 21.4 | 21.8 | |
| p* | .0001 | <.0001 | .34 | |
% of total fat from trunk fat
% of total mass from trunk fat
% of total fat from appendicular fat
% of total mass from appendicular lean mass
Similar results were noted for women misclassified compared to the other two DEXA criteria.
Cut-point analysis
ROC analyses identified new cut-points for each of the anthropometric measures, using the DEXA-defined obesity classification as the criterion. In each case, the cut-point was lower than that commonly used, a higher proportion of the sample was classified as obese, and the correspondence between DEXA-defined obesity and the anthropometric measure improved.
The revised BMI definitions of obesity were ≥26.4 kg/m2 and ≥26.8 kg/m2, derived from the Distance to perfect and Youden methods, respectively (Table 5). For further consideration, we chose to use the higher of these two cutpoints, 26.8 kg/m2, as it was the more conservative (i.e., closer to the traditional cut-point of 30 kg/m2). The revised BMI cut-point produced a sensitivity of 0.80, specificity of 0.95, and correct classification of 87.5% of the sample. For waist circumference, the revised obesity cutpoint was ≥ 84.75 centimeters, with sensitivity of 0.85, specificity of 0.89, and correct classification of 86.8%. The revised criterion for WHR was ≥ 0.80, which provided a sensitivity of 0.69, specificity of 0.67, and correct classification of 68.1%.
Table 5.
Results of receiver operating curve analyses to estimate revised obesity criteria for women with SLE
| BMI |
Waist |
Waist hip ratio |
|||||
|---|---|---|---|---|---|---|---|
| Original | Revised1 | Revised2 | Original | Revised | Original | Revised | |
| Cutpoint | ≥30 | ≥26.4 | ≥26.8 | ≥88 cm | ≥84.75 cm | ≥0.85 | ≥0.80 |
| % classified obese | 29.2 | 44.4 | 42.2 | 41.4 | 47.9 | 28.3 | 51.4 |
| Sensitivity | 0.59 | 0.83 | 0.80 | 0.74 | 0.85 | 0.42 | 0.69 |
| Specificity | 1.00 | 0.93 | 0.95 | 0.90 | 0.89 | 0.85 | 0.67 |
| Positive predictive value | 1.00 | 0.92 | 0.93 | 0.88 | 0.88 | 0.73 | 0.68 |
| Negative predictive value | 0.72 | 0.85 | 0.83 | 0.78 | 0.85 | 0.60 | 0.69 |
| Kappa coefficient | 0.59 | 0.76 | 0.75 | 0.64 | 0.74 | 0.27 | 0.36 |
| % correctly classified | 79.9 | 88.2 | 87.5 | 82.1 | 86.8 | 63.4 | 68.1 |
From “Distance to perfect” method
From Youden method
Association with Framingham cardiovascular disease risk scores
For each anthropometric measure, women were categorized as either not obese by either traditional or revised criterion, obese by both criteria, or obese only by the revised criterion. In each case, the risk scores of the two obese groups were not significantly different from each other, and the risk score of the non-obese group was significantly lower than the scores of either obese group. For example, 56.7% (80 women) were classified as not obese by either criterion (BMI <26.8); their mean risk score was 5.7 (± 6.6) (Figure 1). Forty-two women (29.8%) were classified as obese by both criteria (BMI ≥ 30); their mean risk score was 9.1 (± 5.6). The remaining 19 women (13.5%) were classified as obese only by the revised criterion (BMI 26.8 – 29.9); their mean risk score was 9.4 (±8.1). The analysis of variance revealed a significant overall difference among the mean risk scores (p = 0.007). Post-hoc means comparisons found significant difference between the non-obese group and both of the obese groups.
Figure 1.
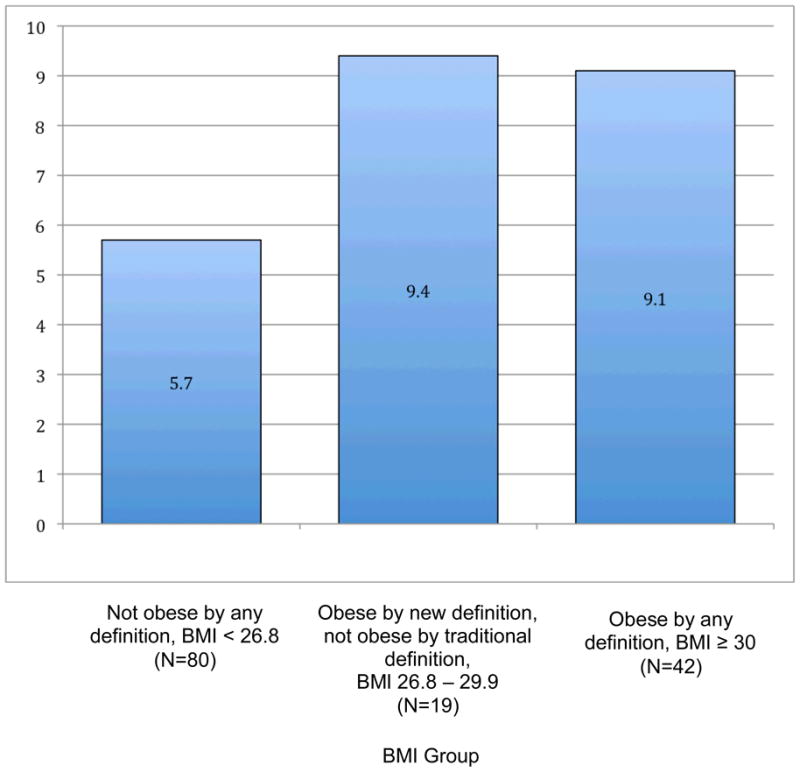
Framingham Risk Scores by BMI Group (see definitions below)
Discussion
A large proportion of this group of women with SLE was obese. Using the most common body composition measure, BMI, almost 30% were obese; using a more sensitive measure, DEXA, half met the criterion for obesity. Substantial portions of women were misclassified by the anthropometric measures. The majority of misclassifications were due to women who were obese by the DEXA standard but did not meet the anthropometric criterion for obesity; relatively few women were found to be obese by anthropometric methods and not obese by DEXA. Women who were misclassified as not obese by anthropometric measures exhibited different patterns of fat distribution than those who were correctly classified, and tended to have a lower proportion of their fat accumulation in the trunk and a greater proportion in their arms and/or legs.
Our analyses suggest new cut-points for defining obesity among women with SLE: BMI ≥ 26.8 kg/m2, waist circumference ≥ 84.75 cm, and a waist-hip ratio ≥ 0.80. In each case, the revised cut-points are substantially lower than those traditionally used. For example, the traditional BMI cut-point for obesity is ≥ 30 kg/m2; the revised cut-point is closer to the traditional cut-point used to define overweight (25.0 kg/m2). The inaccuracy of BMI in identifying high adiposity in mid-range BMI values has been noted previously. For example, a meta-analysis yielded a pooled sensitivity of BMI to identify excess body fat of 0.50, and a pooled specificity of 0.90, with considerable heterogeneity among studies(36). The BMI cut-point we identified is similar to cut-points derived in other studies(37, 38). For example, in a study using NHANES data, a BMI of 25.5 was identified as the best cut-point to identify high body fat (>35% for women). In our study and those cited above, results suggest that lean mass was relatively lower, and fat mass relatively higher than might be expected by BMI. Whether this is a general population trend or relevant specifically to SLE is not known. Likewise, whether disease factors (e.g., inflammation), treatment (e.g., glucocorticoid use), or behavior (e.g., low physical activity), or the combination of these, have a differential impact on the body composition, including infiltration of muscle with fat, of women with SLE is not known.
Sensitivity and specificity of BMI and WC to detect obesity using the revised cut-points was high. The waist-hip ratio, however, did not perform well, even when using an adjusted cut-point, so this measure should be used with caution as a proxy for estimating obesity in women with SLE. Others have also found that WHR did not correspond with body fatness as well as BMI and WC(39).
There is a strong relationship between obesity, defined by BMI and waist circumference, and cardiovascular morbidity(9, 23). However, the actual risk conferred by high BMI or waist circumference is that of high adiposity. An examination of NHANES data found that among more than 6,000 women who had “normal” BMI’s, almost one-third had body fat greater than 35%(40). Among this group of “normal weight obese” women, metabolic syndrome, dyslipidemia, and cardiovascular disease were each elevated. Because of the elevated rate of cardiovascular disease in SLE(41), this phenomenon might be expected to be even more prevalent. We found elevated cardiovascular risk scores among women meeting both the traditional and revised anthropometric criteria of obesity. Although the absolute 10-year risk of a cardiovascular event was relatively low for all groups, the risk of both obese groups (~5.5% for the two BMI obese groups) was about 80% higher than that of the non-obese group (~3%). Annual monitoring of BMI is recommended as a quality indicator to screen for cardiovascular risk(42), but a lower BMI cut-point to define risk conferred by high adiposity may be appropriate for women with SLE to permit earlier and/or better identification of individuals at risk. In addition, based on findings from rheumatoid arthritis, in which women who had higher levels of appendicular fat had greater risk of disability, these new cut points may also be more useful in predicting development or progression of disability than the traditional ones.
This study included a relatively small number of women with SLE (n = 145), so larger studies may yield different results, as may studies that include subjects who are different from this cohort in racial/ethnic composition or disease severity. In addition, prospective studies are clearly needed to identify the value of the suggested revised cutpoints in terms of identifying both cardiovascular or disability risk. It is also possible that other analyses of body composition, such as studies of fat infiltration into muscle, may yield information regarding alterations of body composition among women with SLE that confer additional risk for poor health or functional outcomes(12, 43).
In conclusion, we suggest consideration of revised criteria to define obesity in women with SLE when using anthropometric methods. These revised criteria provide greater sensitivity to body fat and greater correspondence with DEXA-defined obesity. Using these cut-points, both BMI and waist circumference provided robust proxies of DEXA-defined obesity. Waist-hip ratio was less useful, and based on these data, would not be recommended as a proxy measure for obesity in women with SLE. Our results suggest that cardiovascular risk of women who meet the revised obesity criterion is equivalent to that of women who meet traditional anthropometric obesity criteria, suggesting that the traditional criteria may under-estimate obesity-related cardiovascular risk. The utility of the revised cut-points compared to the traditional cut-points in identifying risk of cardiovascular disease or disability remains to be examined in prospective studies.
Table 3.
Correspondence among obesity estimates
| BMI* |
Waist circumference |
Waist hip ratio |
|||||
|---|---|---|---|---|---|---|---|
| Not obese | Obese | Not obese | Obese | Not obese | Obese | ||
| DEXA | Not obese | 50.7% | 0 | 45.5% | 4.8% | 42.8% | 7.6% |
| Obese | 20.1% | 29.2% | 13.1% | 36.6% | 29.0% | 20.7% | |
| Kappa | .59 | .64 | .27 | ||||
BMI = body mass index
References
- 1.Kipen Y, Strauss B, Morand E. Body composition in systemic lupus erythematosus. Br J Rheumatol. 1998;37:514–9. doi: 10.1093/rheumatology/37.5.514. [DOI] [PubMed] [Google Scholar]
- 2.Petri M. Detection of coronary artery disease and the role of traditional risk factors in the Hopkins Lupus Cohort. Lupus. 2000;9:170–5. doi: 10.1191/096120300678828226. [DOI] [PubMed] [Google Scholar]
- 3.Petri M, Perez-Gutthann S, Spence D, Hochberg M. Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Am J Med. 1992;93:513–9. doi: 10.1016/0002-9343(92)90578-y. [DOI] [PubMed] [Google Scholar]
- 4.Bernatsky S, Boivin J, Joseph L, St Pierre Y, Moore A, Rajan R, Clarke A. Prevalence of factors influencing cancer risk in women with lupus: social habits, reproductive issues, and obesity. J Rheumatol. 2002;29:2251–4. [PubMed] [Google Scholar]
- 5.Elkan A, Engvall I, Cederholm G, Hafström I. Rheumatoid cachexia, central obesity, and malnutrition in patients with low-active rheumatoid arthritis: feasibility of anthropometry, Mini Nutritional Assessment and body composition techniques. Eur J Nutr. 2009;48:315–22. doi: 10.1007/s00394-009-0017-y. [DOI] [PubMed] [Google Scholar]
- 6.Roubenoff R. Exercise and inflammatory disease. Arthritis Rheum (Arthritis Care Res) 2003;49:263–6. doi: 10.1002/art.11008. [DOI] [PubMed] [Google Scholar]
- 7.Walsmith J, Roubenoff R. Cachexia in rheumatoid arthritis. Int J Cardiol. 2002;85:89–99. doi: 10.1016/s0167-5273(02)00237-1. [DOI] [PubMed] [Google Scholar]
- 8.Perry C, Alekel D, Ritland L, Bhupathiraju S, Stewart J, Hanson L, Matvienko O, Kohut M, Reddy M, Van Loan M, Genschel U. Centrally located body fat is related to inflammatory markers in healthy postmenopausal women. Menopause. 2008;15:619–27. doi: 10.1097/gme.0b013e318159f1a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snijder M, van Dam R, Visser M, Seidell J. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol. 2006;35:83–92. doi: 10.1093/ije/dyi253. [DOI] [PubMed] [Google Scholar]
- 10.Visser M, Pahor M, Tylavsky F, Kritchevsky S, Cauley J, Newman A, Blunt B, Harris T. One- and two-year change in body composition as measured by DXA in a population-based cohort of older men and women. J Appl Physiol. 2003;94:2368–74. doi: 10.1152/japplphysiol.00124.2002. [DOI] [PubMed] [Google Scholar]
- 11.Rall L, Walsmith J, Snydman L, Reichlin S, Veldhuis J, Kehayias J, Abad L, Lundgren N, Roubenoff R. Cachexia in rheumatoid arthritis is not explained by decreased growth hormone secretion. Arthritis Rheum. 2002;46:2574–7. doi: 10.1002/art.10714. [DOI] [PubMed] [Google Scholar]
- 12.Visser M, Kritchevsky S, Goodpaster B, Newman A, Nevitt M, Stamm E, Harris T. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the Health, Aging, and Body Composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Visser M, Ma R, Baumgartner R, Kotler D, Gallagher D, Heymsfield Skeletal muscle mass: evluation of neutron activation and dual-energy x-ray absorptiometry methods. J Appl Physiol. 1996;80:824–31. doi: 10.1152/jappl.1996.80.3.824. [DOI] [PubMed] [Google Scholar]
- 14.Visser M, Fuerst T, Lang T, Salamone L, Harris T. Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. Health, Aging, and Body Composition Study--Dual-Energy X-ray Absorptiometry and Body Composition Working Group. J Appl Physiol. 1999;87:1513–20. doi: 10.1152/jappl.1999.87.4.1513. [DOI] [PubMed] [Google Scholar]
- 15.Houtkooper L, Going S, Sproul J, Blew R, Lohman T. Comparison of methods for assessing body-composition changes over 1 y in postmenopausal women. Am J Clin Nutr. 2000;72:401–96. doi: 10.1093/ajcn/72.2.401. [DOI] [PubMed] [Google Scholar]
- 16.Roubenoff R, Walsmith J, Lundgren N, Snydman L, Dolnikowski G, Roberts S. Low physical activity reduces total energy expenditure in women with rheumatoid arthritis: implications for dietary intake recommendations. Am J Clin Nutr. 2002;76:774–9. doi: 10.1093/ajcn/76.4.774. [DOI] [PubMed] [Google Scholar]
- 17.Walsmith J, Abad L, Kehayias J, Roubenoff R. Tumor necrosis factor-α production is associated with less body cell mass in women with rheumatoid arthritis. J Rheumatol. 2004;31:23–9. [PubMed] [Google Scholar]
- 18.Westhovens R, Nijs J, Taelman V, Dequeker J. Body composition in rheumatoid arthritis. Br J Rheumatol. 1997;36:444–8. doi: 10.1093/rheumatology/36.4.444. [DOI] [PubMed] [Google Scholar]
- 19.Kipen Y, Briganti E, Strauss B, Littlejohn G, Morand E. Three year follow-up of body composition changes in pre-menopausal women with systemic lupus erythematosus. Rheumatology. 1999;38:59–65. doi: 10.1093/rheumatology/38.1.59. [DOI] [PubMed] [Google Scholar]
- 20.Freemer M, King TJ, Criswell L. Association of smoking with dsDNA autoantibody production in systemic lupus erythematosus. Ann Rheum Dis. 2006;65:581–4. doi: 10.1136/ard.2005.039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thorburn C, Prokunina-Olsson L, Sterba K, Lum R, Seldin M, Alarcon-Riquelme M, Criswell L. Association of PCDC1 genetic variation with risk and clinical manifestations of systemic lupus erythematosus in a multiethnic cohort. Genes Immun. 2007 Mar 8; doi: 10.1038/sj.gene.6364383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yelin E, Trupin L, Katz P, Criswell L, Yazdany J, Gillis J, Panopolis P. Work dynamics among persons with systemic lupus erythematosus. Arthritis Rheum (Arthritis Care Res) 2007;57:56–63. doi: 10.1002/art.22481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Obesity: preventing and managing the global epidemic. Geneva: World Health Organization; 2000. [PubMed] [Google Scholar]
- 24.Heymsfield S. Dual photon absorptiometry: comparison of bone mineral and soft tissue mass measurements in vivo with established methods. Am J Clin Nutr. 1989;49:1283–9. doi: 10.1093/ajcn/49.6.1283. [DOI] [PubMed] [Google Scholar]
- 25.Mazess R. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51:1106–12. doi: 10.1093/ajcn/51.6.1106. [DOI] [PubMed] [Google Scholar]
- 26.Wang J. body fat from body density: underwater weighing vs dual-phon absorptiometry. Am J Physiol. 1989;256:E829–E34. doi: 10.1152/ajpendo.1989.256.6.E829. [DOI] [PubMed] [Google Scholar]
- 27.Expert WHO Committee on Physical Status. Physical status: the use and interpretation of anthropometry: report of a WHO expert committee. 1995. [PubMed] [Google Scholar]
- 28.Schutz Y, Kyle U, Pichard C. Fat-free mass index and fat max index percentiles in Caucasians aged 18–98y. Int J Obesity. 2002;26:953–60. doi: 10.1038/sj.ijo.0802037. [DOI] [PubMed] [Google Scholar]
- 29.Karlson E, Daltroy L, Rivest C, Ramsey-Goldman R, Wright E, Patrtridge A, Liang M, Fortin P. Validation of a systemic lupus activity questionnaire (SLAQ) for population studies. Lupus. 2003;12:280–6. doi: 10.1191/0961203303lu332oa. [DOI] [PubMed] [Google Scholar]
- 30.Yazdany J, Yelin E, Panopalis P, Trupin L, Julian L, Katz P. Validation of the systemic lupus erythematosus activity questionnaire in a large obsevational cohort. Arthritis Rheum (Arthritis Care Res) 2008;59:136–43. doi: 10.1002/art.23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Agostino R, Vasan R, Pencina M, Wolf P, Cobain M, Massaro J, Kannel W. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 32.The Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youden W. Index for rating diagnostic tests. Cancer. 1950;3:32–5. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 34.Kraemer H. Evaluating medical tests: objective and quantitative guidelines. Newbury Park, CA: Sage Publications; 1992. [Google Scholar]
- 35.Coffin M, Sukhatme S. Receiver operating characteristic studies and measurement errors. Biometrics. 1997;53:823–37. [PubMed] [Google Scholar]
- 36.Okorodudu D, Jumean M, Montori V, Romero-Corral A, Somers V-JJ, Erwin P, Lopez-Jimenez F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obesity. 2010;34:791–9. doi: 10.1038/ijo.2010.5. [DOI] [PubMed] [Google Scholar]
- 37.Romero-Corral A, Somers V, Sierra-Johnson J, Thomas R, Collazo-Clavell M, Korinek J, Allison T, Batsis J, Sert-Kuniyoshi F, Lopez-Jimenez F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obesity. 2008;2008:959–66. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahman M, Berenson A. Accuracy of current body mass index obesity classification for white, black, and hispanic reproductive-age women. Obstet Gynecol. 2010;115:982–8. doi: 10.1097/AOG.0b013e3181da9423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor R, Keil D, Gold E, Williams S, Goulding A. Body mass index, waist girth, and waist-to-hip ratio as indexes of total and regional adiposity in women: evaluation using receiver operating characteristic curves. Am J Clin Nutr. 1998;67:44–9. doi: 10.1093/ajcn/67.1.44. [DOI] [PubMed] [Google Scholar]
- 40.Romero-Corral A, Somers V, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, Jensen M, Parati G, Lopez-Jimenez F. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J. 2010;31:737–46. doi: 10.1093/eurheartj/ehp487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hak A, Karlson E, Feskanich D, Stampfer M, Costenbader K. Systemic lupus erythematosus and the risk of cardiovascular disease: results from teh nurses’ health study. Arthritis Rheum (Arthritis Care Res) 2009;61:1396–402. doi: 10.1002/art.24537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yazdany J, Panopalis P, Gillis J, Schmajuk G, MacLean C, Wofsy D, Yelin E. A quality indicator set for systemic lupus erythematosus. Arthritis Rheum (Arthritis Care Res) 2009;61:370–7. doi: 10.1002/art.24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delmonico M, Harris T, Visser M, Park S, Conroy M, Valsquez-Mieyer P, Boudreau R, Manini T, Nevitt M, Newman A, Goodpaster B, Health A Body Composition Study. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–985. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gallagher D, Heymsfield S, Heo M, Jebb S, Murgatroyd P, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;2000:72. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]


