Abstract
Objectives
Parents are believed having strong influence on children’s eating behaviors. However, previous findings on child-parent resemblance in dietary intakes are mixed. We systematically reviewed and meta-analyzed the association (correlations) based on published studies.
Methods
We searched related studies published since 1980 and found 24 studies meeting inclusion criteria for review and 15 for meta-regression analysis. We compared the associations between parent-child pairs, nutrients, over time, and by dietary assessment method.
Results
Most studies were based on small samples. Overall, they suggest a moderate or weak association, but findings varied remarkably. Our meta-analysis showed average Fisher’s transformed correlations were 0.20 (95% confidence interval 0.13–0.28) for fat (% energy); for energy, 0.21 (0.18, 0.24). The correlations varied by parent-child pairs, dietary assessment and countries. FFQ or mixed approaches yielded lower correlation than 24-hour recalls or food records. Child self-reported intakes showed weaker correlation, better methodology quality showed stronger correlation in fat intake (% energy), which also became weaker over time.
Conclusions
Overall, the resemblance is weak and it varied considerably across studies, nutrients, foods, parent-child pairs.
Keywords: child, parent, diet, resemblance, association, review, meta-analysis
INTRODUCTION
It has been widely believed that parents have a strong influence on children’s eating behaviors. Parents are gate keepers and can serve as role models for their children’s health-related behaviors [1–2]. Consequently, there is a widespread perception of a strong parent-child association in dietary intakes [3–10]. Surprisingly, some studies show the association is very weak [11–13]. This is likely because young people’s eating patterns are in fact influenced by many complex factors and the family environment plays only a partial role [14–16]. For example, most children, especially in industrialized countries, consume at least one meal at school. Moreover, eating snack foods is common practice among children and adults, in particular, in industrialized countries [17–18].
Further, as children grow older they become subjected to stronger peer influence and acquire greater autonomy when making food choices. Therefore, it will be of interest to systematically examine and quantify the parent-child association in dietary intakes. To our knowledge, no such effort has been made. Another related question is whether the association has become weaker over time due to many changes in the society. For instance, some of those changes may include growing independence of children, changes in home and social environments, parenting styles, growing proportion of working mothers [19], changes in food supply and distribution [15, 20–21] as well as modifications in people’s dietary intake [22–25].
The present study aimed to systematically review and assess the degree of association and similarity between children’s and their parents’ dietary intake based on studies published since 1980. Further, we compared the differences in the association between parent-child pairs, countries, and over time. We also tested the differences in the association detected across dietary assessment approaches. We hypothesized that studies using food frequency questionnaires (FFQ) were likely to report weaker associations given their better estimation of usual dietary intake but being less quantitative [26–27] compared to 24-hour recalls or multiple food records. To our knowledge, this is the first such systematic investigation to address these questions. Findings of this study will help enhance our understanding of the factors that may affect children’s dietary intake patterns and provide useful insights for developing effective intervention programs to promote healthy eating in young people.
METHODS
Literature search strategy
We first searched the PubMed database using related MESH key words, including child, adolescent, family, parent, mother, father, diet and food for studies published between January 1, 1980 and September 31st, 2009. Then using similar approaches, we conducted separate search for studies published in Spanish and Chinese using other database that we have access to (see below). We could not examine studies published in other languages.
The titles and abstracts of the related studies were examined on screen first for exclusion and inclusion. Papers that could not be excluded on the basis of the abstracts were obtained in full and reviewed for suitability for inclusion. Only studies that have provided results (e.g., correlation coefficients, intraclass correlation coefficients) about the association both children’s and their parents’ dietary intakes (including energy, nutrients and food groups) were included. Studies that aimed at correlating dietary intake of children with their parents’ knowledge and attitudes about dietary intake were excluded. There was no restriction regarding children’s age. Then, the full papers that met our selection criteria were carefully reviewed.
Our literature search resulted in a total of 24 papers [3–13, 28–40] finally included in the review and 15 in our meta-analysis (which reported correlations for intakes of total energy and fat). Most these papers were obtained through initial screening, while some were identified by using the “Related Article” feature on Pubmed and reference lists in the selected articles. In addition, some studies identified in the course of reading or brought to our attention by colleagues and experts consulted were included.
Note that our search of PubMed using different combinations of the related MESH key words yielded 2590 related abstracts, but only 23 studies met our inclusion criteria. Our search of Embase using the related key words identified 455 related abstracts, but only one met our inclusion criteria for systematic review; our search of Índice Médico Español identified 15 related abstracts, none met our inclusion criteria. These are two comprehensive database of biomedical research published in Spanish. We searched the Chinese National Knowledge Infrastructure, the most comprehensive database of biomedical research published in China, for papers published in Chinese, identified 344 related abstracts, but none met our inclusion criteria.
Data extraction
Using a standardized data extraction form, we extracted and tabulated the related data. Information extracted included first author’s name, study publication year, country of data collection, sample characteristics (e.g., age, gender, ethnicity/race of the participants, sample size, socioeconomic status (SES)), type of parent-child pairs, methods of dietary assessment, main research findings, whether and what confounders and covariates were controlled for, and main strengths and limitations. Some studies did not provide all the related details.
Most studies reported correlation coefficients computed Pearson or Spearman rank correlation coefficients. Our meta-regression analysis (was simply termed ‘meta-analysis’ in our study) was conducted based on the reported correlation coefficients (used as ‘data points’) and those that could be approximately interpreted as correlation coefficients such as intra-class correlation coefficients. Each of these correlation coefficients were reported for a specific parent-child pair and data points were classified according to gender of parent, child or both for each of these coefficients. The 15 studies provided [3–12, 28, 31–33, 36] 117 data points energy and fat intakes and for different types of parent-child pairs (i.e., parent-child (PC), parent-son (PS), parent-daughter (PD), father-son (FS), father-daughter (FD), mother-son (MS), mother-daughter (MD)). Note that we selected energy because it helps reflect overall diet and affects people’s risk of obesity, which has become a global epidemic. We selected fat intake, both as absolute (in grams) and relative intake (i.e., % of energy derived from fat), to reflex dietary composition and quality, and fat intake affects the risk of a number of chronic diseases such as cardiovascular disease. The other nutrients were not included also due to their small number of data points.
Assessment of methodological quality of each study
This was assessed systematically (see Appendix A). Each study was assigned 7 subscores (from 1-worst to 3-best for each category) for different components (sample size, age range of children, dietary assessment, nutrients and foods reported, types of parent-child dyads, adjustment for potential confounders, and representativeness of sample) and a total score. The total score ranged between 0 and 21.
Appendix A.
Criteria for assessing the methodological quality of included studies ^
| Score | Sample size* | Age range of children | Dietary assessment | Nutrients and foods reported | Types of parent- child dyads # | Adjustment for potential confounders | Representativeness of sample |
|---|---|---|---|---|---|---|---|
| 1 | <300 dyads | Not specified, or <5 year range (e.g. 2–6 years) | Short FFQ<=20 questions or single- day recall or record | Only food groups or 1–2 of energy, fat, fat% energy | Reported results for 1–2 or only combined (parent- child) | None | Not representative, convenience sample |
| 2 | 300–500 dyads | 5 to <10 years range | middle | All of energy, fat, fat % energy with/without type of fat | for 3–4 types | Age and sex of parents and/or children adjusted | Representative of small area |
| 3 (best) | >=500 dyads | both children and adolescents, >=10 years range | at least >=3 day recall or records, long FFQ or combination of methods | Multiple dietary variables (eg>8): all of energy, fat, % energy from fat + food groups | for 5 types (=2*2+1) | Above + other characteristics, eg, SES | Nationally representative |
The total score ranged between 0 and 21 for each study.
Based on the largest sample size available for any of the parent-child dyads studied.
In total five types: parent-child, father-son, father-daughter, mother-son, mother-daughter.
The scores for each study were assessed by two coauthors (raters) in a blinded manner, and the agreement between their scores was tested thereafter. When discrepancies were found, a third rater scored the study considering the other two raters’ assessment. Next, they discussed to reach a consensus. The correlation between the initial scores assigned by the first two raters was 0.88 and their average difference was 0.6. The correlations between the total scores assigned by the third rater and one of the first two raters were 0.94 and 0.95 and their average differences were 0.1 and 0.4, respectively. Based on median of the final total scores the studies were considered as good- vs. less good quality studies. This binary variable was added in our meta-analysis to assess its impact on the correlations.
Statistical analysis
We conducted a set of analyses. The mutually exclusive specific categories of parent-child pair (e.g., mother-daughter) were considered in some, while in some another more general classification, termed “non-exclusive,” was used (e.g., mother-child includes mother-child, mother-son and mother-daughter data points). We first estimated the arithmetic mean, standard deviation, and range of the correlation coefficients by the non-exclusive categories of parent-child pairs. Correlations of 0.10–<0.30 are considered to be weak; r=0.30–<0.50 as moderate; while r>=0.50 indicate a strong association.
Next, we tested the differences in correlation coefficients between nutrients and within each parent-child pair using ANOVA and pairwise t-tests. Statistical significance of the correlation coefficients was considered as well, the proportion significant across nutrients and within parent-child pairs was compared using χ2 test.
Moreover, using the Fisher’s z transformation, we converted Pearson’s or Spearman’s correlation coefficients to z’s to obtain approximate normality, and then calculated a mean transformed correlation weighted by the sample sizes in the studies[41], with its 95% confidence interval (95%CI), taking into account heterogeneity between data points, and thus using random effect estimates. Subsequent in-depth analyses however focused mainly on Fisher’s z transformed correlation coefficients.
Considering the small sample size and the expected heterogeneity nature of these studies (e.g., large variations in people’s dietary intakes, study design and dietary assessment approaches), we conducted meta-regression analyses with random-effects and added a limited number of predictors, using Fisher’s z transformed correlation with their corresponding standard errors as the outcome variable. Three models were conducted: model 1 included study-level characteristics (such as publication year, study country, and methods of dietary assessment, overall study quality) as the predictors; models 2 and 3 forced parent-child dyads to be additional predictors. Models 2–3 were reduced, given the limited sample size, by backward elimination of study-level predictors with p<0.10.
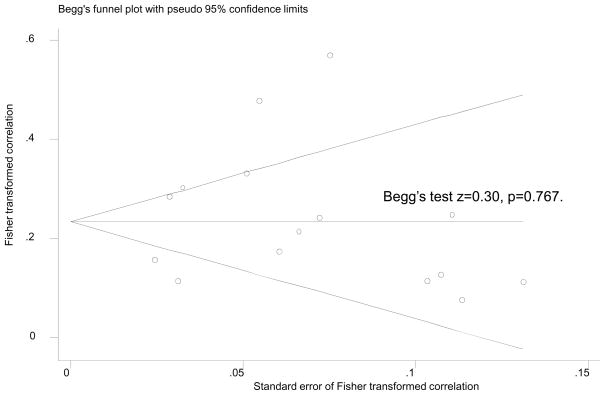
Taking the average of Fisher’s z transformed correlations and their corresponding average standard errors for each study, we tested homogeneity of the effect size between studies using Q-test, which indicated heterogeneity across studies included in our meta-analysis (Q= 81.93, d.f.=14; P<0.001). Finally, we assessed publication bias through Begg’s funnel plot, by plotting Fisher’s transformed correlation values against their standard errors and by the Begg’s adjusted rank correlation test[42–43]. Our analysis indicated no publication bias (Appendix B). All analysis was performed using STATA release 11 (College Station, TX)[44], particularly using the meta, metabias and metareg commands for the meta-analyses among others. Statistical significance was set at P<0.05.
Appendix B.
Publication bias assessment: Begg’s funnel plot and Begg’s test1
1Using Fisher’s transformed correlation coefficient with its SE per study datapoint, aggregating datapoints means per study to assess publication bias. N=15 studies. Continuity corrected Begg’s test is presented.
RESULTS
Findings of systematic review
Table 1 presents the detailed characteristics and main findings of the 24 studies we identified that reported parent-child associations in dietary intakes. The studies differed considerably in their design, study samples, settings and findings, and used various methods such as 24 hour recalls, dietary records, FFQ, or a mixed approach for dietary assessment. Very few of them are based on large, national samples, and over half were conducted in the U.S.
Table 1.
Main characteristics and findings of relevant studies that examined the association between children’s and their parents’ dietary intakes1
Summary of the main characteristics and findings of 24 related studies
| Ref. | Author, year; Study methodolog y strength score2 |
Country | Sample size | Child age | Other sample characteristics | Dietary assessment | Whether child intake was self- reported | Main findings | Main strengths and limitations, and additional important findings and notes |
|---|---|---|---|---|---|---|---|---|---|
| [3] | Laskarzewski et al., 1980 Score=12 |
U.S. | 294 children 294 parents |
6–19 y | 4/5 (n=234) of the sample were white families, and 1/5 (n=60) were black families | One 24-hr dietary recall. Data were obtained on the same day for 1/3 of the families (n=99), on different days within a week for 2/3 families (n=195). | Yes |
Spearman correlation: All parent-child pairs (n=294) CHO: 0.28* saturated fat: 0.15* PUFA: 0.19* calories: 0.24* cholesterol: 0.004 One parent-one child (n=198) CHO: 0.30* saturated fat: 0.15* PUFA: 0.22* calories: 0.26* cholesterol: −0.004 Note: Nutrient intake was expressed as per kg body weight. Pearson correlations (not presented) were similar to the Spearman correlations. |
Strengths: Sampled both white and black US children. Limitations: Only used one 24- hr recall. Additional findings: ANOVA adjusted for covariates shows that 23–97% of the variation in children’s intakes was accounted for by those of parents’ (23% for cholesterol among all parent-child pairs, and 97% for CHO of black fathers over age 40 and their children). The proportion for black parent-child pairs were higher than that for white for CHO, saturated fat, and calories. |
| [5] | Perusse et al., 1988 Score=17 |
Canada | 885 children 712 parents From 375 families |
mean age of 14.5 for males and 14.8 for females | French descent Quebec city area | 3-day dietary record for children and parents | Yes |
Intraclass correlation: Parent-child: Energy (kcal): 0.25* Energy (kcal/kg/d): 0.26* CHO (g): r=0.26* Fat (g): r=0.27* Protein (g): r=0.26* CHO (% energy): r=0.29* |
Strengths: Used the BETA model to separate phenotypic variance of intake measurements into biological and cultural. |
| [4] | Patterson, et al., 1988 Score=14 |
U.S. | 132 Anglo (A) children and 154 parents 169 Mexican- American (MA) children and 143 parents |
9–15 y | Minority sample | one 24-hr recall, 3-day food record, FFQ | Yes |
Correlation for food frequency: Father-younger child: A: 0.56* MA: 0.17 Father-older child: A: 0.29 MA: 0.34* Mother-younger child: A: 0.36* MA: 0.29* Mother-older child: A: 0.42* MA: 0.42* |
Strengths: a) BMI was adjusted; b) multiple dietary assessment approaches. Limitations: Correlations based on 24-hour recall and 3-day food record were not reported. |
| [6] | Oliveria et al., 1992 Score=14 |
U.S. | 91 children; 87 mothers and 83 fathers | 3–5 y | white middle class families | 4 sets of 3-day food record at 3- month intervals | No (complete d by mother with help from child or adult personnel in day care) |
Pearson correlation: parent-child (n=83): Energy: 0.20 Protein 0.37* CHO: 0.31* Total fat: 0.32* Saturated fat: 0.47* MUFA: 0.29* PUFA: 0.27* Cholesterol: 0.41* Calcium: 0.30* Father-child (n=83): Energy: 0.16 Protein 0.34* CHO: 0.18 Total fat: 0.15 Saturated fat: 0.34* MUFA: 0.13 PUFA: 0.10 Cholesterol: 0.34* Calcium: 0.21 Father-son (n=50): Energy: 0.19 Protein 0.25 CHO: 0.25 Total fat: 0.15 Saturated fat: 0.40* MUFA: 0.15 PUFA: 0.06 Cholesterol: 0.45* Calcium: 0.15 Father-daughter (n=33): Energy: −0.03 Protein 0.25 CHO: 0.03 Total fat: 0.12 Saturated fat: 0.22 MUFA: 0.09 PUFA: 0.18 Cholesterol: 0.10 Calcium: 0.33 Mother-child (n=87): Energy: 0.17 Protein 0.29* CHO: 0.37* Total fat: 0.46* Saturated fat: 0.48* MUFA: 0.43* PUFA: 0.33* Cholesterol: 0.37* Calcium: 0.29* Mother-son (n=54): Energy: 0.03 Protein 0.14 CHO: 0.28* Total fat: 0.40* Saturated fat: 0.43* MUFA: 0.43* PUFA: 0.31* Cholesterol: 0.19 Calcium: 0.22* Mother-daughter (n=33): Energy: 0.22 Protein 0.42* CHO: 0.47* Total fat: 0.49* Saturated fat: 0.51* MUFA: 0.42* PUFA: 0.35* Cholesterol: 0.56* Calcium: 0.30 |
Strengths: a) 4 sets of 3-day food records were used to catch seasonal variations in intakes; b) the correlations (except for total energy intake) were adjusted for total energy intake and parents’ age; c) compared correlations stratified by number of meals eaten at home by parents Additional findings: Correlations were much higher when parents had more meals (fathers>=15 vs less, mothers >=18 vs less) at home, eg, for energy: Father-child: 0.29 vs 0.07 Mother-child: 0.35 vs 0.11 |
| [7] | Rossow and Rise, 1994 Score=12 |
Norway | 337 children, 324 mothers, 323 fathers | 16–20 y | both urban and rural areas | short questions on fat intake (milk and butter/margarine consumption) | Yes |
Spearman correlation: Mother-child: 0.47 Father-child: 0.42 Children were more likely to have a low-fat diet if their parents did so: mother’s low: OR: 5.00 (2.97, 8.41) father’s low: OR: 4.44 (2.56, 7.85) |
Strengths: Nationally representative sample. Limitations: Weak dietary assessment. |
| [8] | Stafleu et al., 1994 Score=14 |
the Netherlands | 97 young adult women and 200 mothers and grandmother s | 20–30 y | Sampled from low-income inhabitants in Helmond where death rates from ischaemic heart disease were higher than the national average | validated 104- item FFQ (Feunekes et al, 1993) | Yes |
Pearson correlation: Younger-middle generation: Energy (kJ): 0.22* Total fat (% energy): 0.19 Saturated fat (% energy): 0.26* MUFA: 0.22* PUFA: 0.20 Cholesterol (mg): 0.21* Middle-older generation: Energy (kJ): 0.08 Total fat (% energy): −0.02 Saturated fat (% energy): 0.09 MUFA: 0.04 PUFA: 0.10 Cholesterol (mg): 0.29* Younger-older generation: Energy (kJ): 0.14 Total fat (% energy): 0.12 Saturated fat (% energy): 0.05 MUFA: −0..00 PUFA: 0.33* Cholesterol (mg): 0.05 Spearman correlation: Younger-middle generation: Oils and fats: 0.19 VF: 0.12 Middle-older generation: Oils and fats: 0.20* VF: 0.15 Younger-older generation: Oils and fats: 0.09 VF: −0.05 |
Strengths: a) studied 3 generations who were adults and lived apart; b) Validated FFQ was administered by interviewer. Limitations: Small sample size. |
| [9] | Vauthier et al., 1996 Score=16 |
France | 774 children and 774 parents from 387 families | 7–21 y | Caucasian middle-class families from a longitudinal survey related to cardiovascular disease, only families with two parents (<65 yrs) and two children (>7 yrs) were included. | 3-day food consumption diary | Mixed: most self reported; for young children, by mothers with help from the children) |
Family correlation: Parent-child (n=1548): Energy (kcal): 0.30 Protein (% energy): 0.33 Fat (% energy): 0.34 CHO (% energy): 0.31 Father-son (n=365): Energy (kcal): 0.35* Protein (% energy): 0.36 Fat (% energy): 0.39 CHO (% energy): 0.37 Father-daughter (n=409): Energy (kcal): 0.33* Protein (%):0.36 Fat (%): 0.28 CHO (%): 0.26 Mother-son (n=365): Energy (kcal): 0.24* Protein (% energy): 0.26 Fat (% energy): 0.31 CHO (% energy): 0.28 Mother-daughter (n=409): Energy (kcal): 0.26* Protein (% energy): 0.31 Fat (% energy): 0.40 CHO (% energy): 0.35 |
Strengths: a) Took into account the number of meals shared. b) Large sample size and reported results by parent- child pairs. Additional findings: Cultural inheritance represented 30–40% of dietary intake variance for children. With increasing number of meals eaten together (>45/week vs <=45/week), between-generation components increased by 10% for fat and CHO, but unchanged for protein intake. |
| [10] | Adelekan and Adeodu, 1997 Score=11 |
Nigeria | 108 mother- child pairs | 3–5 y | Sampled from rural areas, mothers were largely illiterate and farmers | 3 consecutive days of 24-hour recalls | No, by mothers |
Pearson correlation: energy (kJ): 0.39* protein (g): 0.07 total fat (g): 0.64* iron (mg): 0.55* |
Strengths: Mothers’ age was controlled for. |
| [11] | Feunekes et al., 1997 Score=17 |
the Netherlands | 1077 households | 1–30 y | Derived from the national food surveys of 1987 and 1992. | 2-day diet records | Mixed: most self reported, byt for those aged<=13 y, by parents |
Pearson correlation: Father-son (n=914): Energy (MJ): 0.19* Total fat (% energy): 0.40* Saturated fat (% energy): 0.43* MUFA (% energy): 0.38* PUFA (% energy): 0.50* Cholesterol (mg/MJ): 0.41* Father-daughter (n=900): Energy (MJ): 0.24* Total fat (% energy): 0.39* Saturated fat (% energy): 0.37* MUFA (% energy): 0.42* PUFA (% energy): 0.48* Cholesterol (mg/MJ): 0.46* Mother-son (n=1003): Energy (MJ): 0.09* Total fat (% energy): 0.37* Saturated fat (% energy): 0.43* MUFA (% energy): 0.38* PUFA (% energy): 0.50* Cholesterol (mg/MJ): 0.47* Mother-daughter (n=998): Energy (MJ): 0.24* Total fat (% energy): 0.44* Saturated fat (% energy): 0.45* MUFA (% energy): 0.45* PUFA (% energy): 0.50* Cholesterol (mg/MJ): 0.55* |
Strengths: a) large sample size; b) wide child age range; c) child age was controlled for. Additional findings: Associations were higher for foods eaten at home than foods eaten outside of the home. Moderate within- family intake correlations were still found for families with a therapeutic dieting parent (not presented). |
| [12] | Feunekes et al., 1998 Score=10 |
The Netherlands | 347 adolescents 309 mothers 270 fathers |
15 y | Urban and rural adolescents were selected from five schools | Self- administered FFQ from the Dutch National Food Consumption Survey (1987- 1988) | Yes |
Pearson correlation: Mother-child: Energy (MJ): 0.19* Energy (MJ/kg): 0.22* fat (% energy): 0.19* Saturated fat (% energy): 0.23* MUFA (% energy): 0.20* PUFA (% energy): 0.38* Cholesterol (mg/MJ):0.00 Father-child: Energy (MJ): 0.13* Energy (MJ/kg): 0.10 Total fat (% energy): 0.18* Saturated fat (% energy): 0.24* MUFA (% energy): 0.26* PUFA (% energy): 0.16* Cholesterol (mg/MJ):0.22* |
|
| [34] | Billon S et al, 2002 Score=16 |
France | 398 families (398 fathers, 398 mothers, 383 sons, and 416 daughters) | (mean±sd) girl:14.3± 3.8 y; boy: 14.0±3.5 y | middle-class French families | 3 day food consumption diary | Yes | Correlation for absolute breakfast energy intake (BEI): FS: 0.25; FD: 0.19; MS:0.26; MD:0.27; Correlations for relative breakfast energy intake (RBEI): FS:0.23; FD: 0.19; MS:0.27; MD:0.21 [Note: RBEI =BEI/total energy intake over the 3 days.] |
Strengths: a)Used absolute and relative breakfast energy intake; b) adjusted for physical activity, smoking, BMI, alcohol consumption Limitations: limited sample in mid-class families in one city |
| [13] | Cullen et al., 2002 Score=12 |
US | 132 children 132 parents |
mean age=10y; 4th–6th grade | Included multiple ethnic groups: white (42%), African Am (20%), Mexican Am (30%), Asian Am (8%) | Children: up to 7-day food records; parents, 21-item FFQ regarding fat practice over the previous week. | Yes |
Spearman correlation: Low fat practices: 0.28* |
Strengths: a) included multiple ethnic groups; b) Relative strong dietary assessment. |
| [29] | Fisher et al., 2002 Score=12 |
US | 191 girls and their parents | 5 y | 1) Non-Hispanic White; 2) Parents were in their mid-30s, well-educated, and majority were employed |
Parents: FFQ; Children: 3 24-hour recalls | No, by mothers in the presence of their daughters |
Correlation coefficient (based on path coefficient from structural model): VF: 0.23* |
Strengths: Used structural equations modeling enable to find the net correlation accounting for other factors. Limitations: FFQ and 24-hr recalls gave different scales of intake, and the data from children and their parents might not be comparable. |
| [35] | Longbottom et al, 2002 Score=9 |
Scotland | 36 children (12 boys) and their mothers | 5.5–8.5 y (mean/SD: 6.5±1.0) | 4-day weighed food records for the mother and their children, respectively | Yes |
Spearman’s correlation for density (weight/per 1000 kcal) for mother-child pairs for various foods: crude mean r for 26 food groups: 0.34 fat and oil: 0.24 fish: 0.15 meat & product: 0.21 fruit:0.74** Note: We calculated the crude mean r. |
Strengths: Used data on weighed food intakes. Limitations: small sample size. |
|
| [36] | Mitchell et al., 2003 Score=15 |
US | 1364 members of 42 large Mexican American families | >16 y | The San Antonio Family Heart Study (SAFHS), a prospective study of cardiovascular risk in Mexican American families | FFQ modified for Mexican American population | Yes |
Age and sex-adjusted familial correlations: Parent-offspring: Energy: 0.14 Fat (% energy): 0.09 Parent-parent: Energy: 0.27 Fat (% energy): 0.15 Sibling-sibling: energy: 0.10 fat (% energy): 0.04 Note: P values were not reported, but the discussion stated, "For nearly all of these dietary variables, the correlations were statistically significant." |
Strengths: a) concurrent assessment of dietary intake of parent–sibling pairs; b) large sample size; c) reported sibling- sibling and parent- parent correlations. Limitations: a) a highly selective sample; b) small sample size. |
| [37] | Runyan et al, 2003 Score=9 |
USA | 72 pairs of daughters and premenopausal mothers | 11–14 y (12.8±0.8 y) | mother aged 33- 51 years old with mean age as 42.4±4.2 | 3-day food records and a calcium intake survey | Yes |
Pearson correlation Calcium: 0.33* |
Limitations: small sample size |
| [32] | Stanton et al., 2003 Score=11 |
US | 404 children and their mothers | 12–15 y | 1) 72% white, 28% black; 2) selected from rural areas 3) 97% were female parents; 4) Mothers’ age: 27–71 y; education: 39% <HS, 24% college graduates, 28.5% HS |
Same 35-item FFQ for children and parents | Yes |
Pearson correlation: Fat: Mother-daughter: 0.30** Mother-son: 0.11 Mother-child: 0.22** White dyads: 0.23** Black dyads: 0.18 Fiber: Mother-daughter: 0.13* Mother-son: −0.00 Mother-child: 0.06 White dyads: 0.10 Black dyads: 0.04 |
Strengths: Ethnic diversity |
| [31] | Park et al., 2004 Score=15 |
South Korea | 231 children and 260 parents from 134 families | 11–19 y | 2) Excluded: obesity secondary to hypothyroidism or Cushing’s disease severe debilitating diseases or cancer Excluded: women lactating or pregnant, treated with anti- obesity agents, lost more than 10% of normal weight over the past 6 months. | FFQ from Korean National Health and Nutrition Survey Usual intake over the past 6 months | Yes |
Intraclass correlation analysis: Father-son: Energy: −0.03 CHO (% energy): 0.12 Protein (% energy): 0.02 Fat (% energy): 0.20 Saturated fat: 0.04 Cholesterol: −0.06 Father-daughter: Energy: 0.20 CHO (% energy): −0.10 Protein (% energy): 0.07 Fat (% energy): −0.01 Saturated fat: 0.02 Cholesterol: 0.03 Mother-son: Energy: 0.10 CHO (% energy): 0.23* Protein (% energy): 0.23* Saturated fat: 0.28* Cholesterol: 0.27* Mother-daughter: Energy: 0.27* CHO (% energy): 0.17 Protein (% energy): 0.31* Fat (% energy): 0.09 Saturated fat: 0.25* Cholesterol: 0.14 |
Strengths: Use of adjusted models (ICC adjusted for age, age-square and age-cubed). |
| [30] | Galloway et al., 2005 Score=12 |
US | 173 girl- mother pairs | 7 y at baseline 9 y at follow-up | Non-Hispanic white girls from central Pennsylvania | Three 24-hr recalls when girls were 7, and again when girls were 9 | No, by mothers |
Correlation coefficient (based on path coefficient from structural model): Mothers’ VF intake when girls were 7 vs. girls’ VF intake at age 9: 0.36** |
Strengths: a) used structural equations modeling; b) longitudinal design. |
| [40] | da Veiga and Sichieri, 2006 Score=12 |
Brazil | 391 fathers, 486 mothers, 287 boys, 256 girls | 12–18 y | A cross-sectional population-based survey in Rio de Janeiro | Parents: validated FFQ; Children: adult FFQ plus five new food items obtained from a pretest among adolescents | Yes |
Spearman correlation: Rice (1 soupspoon): Father-daughter: 0.45* Father-son: 0.52* Mother-daughter: 0.49* Mother-son: 0.50* Vegetables (1 portion): Father-daughter: 0.36* Father-son: 0.49* Mother-daughter: 0.46* Mother-son: 0.36* Milk products (1 glass or slice or unit): Father-daughter: 0.51* Father-son: 0.63* Mother-daughter: 0.60* Mother-son: 0.58* Meat (4–6 oz): Father-daughter: 0.53* Father-son: 0.63* Mother-daughter: 0.56* Mother-son: 0.58* Soda (1 glass): Father-daughter: 0.25* Father-son: 0.42* Mother-daughter: 0.39* Mother-son: 0.39* Mean correlation of foods for mother-child pairs: By level of maternal education: ≤4 y: 0.49 5–7 y: 0.40 >8 y: 0.41 By monthly per capita income: US$<100: 0.48 US$100–199: 0.45 US$≥200: 0.36 |
Strengths: a) concurrent assessment of dietary intake of parent–child pairs; b) used a validated comprehensive FFQs; c) tested effect of family socioeconomic level on food intake resemblance. |
| [33] | Wang et al., 2009 Score=15 |
US | 121 child- mother pairs | 10–14 y | African American children from urban areas of Chic ago | the Youth and Adolescent Questionnaire(Y AQ) developed by Harvard Univ and the adult version of the FFQ | Yes |
Spearman correlation: Energy (kcal): Mother-daughter: 0.26* Mother-son: −0.24 Mother-child: 0.04 Fat (g): Mother-daughter: 0.30* Mother-son: −0.21 Mother-child: 0.07 Fat (% energy): Mother-daughter: 0.11 Mother-son: 0.19 Mother-child: 0.16 Fiber (g): Mother-daughter: 0.12 Mother-son: −0.08 Mother-child: 0.02 Calcium (mg): Mother-daughter: 0.19 Mother-son: −0.19 Mother-child: 0.02 |
Strengths: a) used comparable comprehensive FFQs for children and their mothers; b) studied the factors might be associated with the resemblance. Limitations: A selective US population group. |
| [28] | Beydoun and Wang., 2009 Score=20 |
US | 1061 fathers, 1230 mothers, 1370 sons and 1322 daughters | 2–18 y | A nationally representative multi-stage stratified data from the USDA CSFII 1994–96 | Two 24 hour recalls | Mixed: most self reported, but for children aged 2–9 y, 7% and 11% self- reported dietary intake on days 1 and 2, respectively) | Multivariate-adjusted standardized regression coefficient (can be considered as adjusted correlations): Total dietary quality score: Parent-child: 0.26* Mother-daughter: 0.18 Mother-son: 0.28* Father-daughter: 0.28* Father-son: 0.29* Energy (kcal): Parent-child: 0.22* Mother-daughter: 0.26* Mother-son: 0.23* Father-daughter: 0.14* Father-son: 0.29* Fat (g): Parent-child: 0.24* Mother-daughter: 0.24* Mother-son: 0.28* Father-daughter: 0.18* Father-son: 0.27* Fat (% energy): Parent-child: 0.01 Mother-daughter: 0.02 Mother-son: −0.04 Father-daughter: 0.02 Father-son: 0.01 VF: Parent-child: 0.29 Mother-daughter: 0.37 Mother-son: 0.31 Father-daughter: 0.21 Father-son: 0.29 |
Strengths: a) Used nationally representative data and sampling design complexity was accounted for. b) adjusted for some covariates Limitations: a) Only two 24-h recalls used. b) Clustering at the household level. Additional findings: The study provided many more results about other dietary intake measures including other nutrients and food groups and the correlations by parent-child characteristics such as race, age and family income. |
| [38] | Papas et al, 2009 Score=9 |
USA | 109 adolescent mothers and their toddler children | 13 months old | primiparous, low-income, African American mothers and their toddlers | Toddler dietary variety was measured with a 73-item feeding checklist; maternal dietary variety was measured with the Youth Adolescent Food Frequency Questionnaire (YAQ). | No, by mothers |
Spearman correlation: fruit: 0.26** vegetables: 0.40** snacks/disserts: 0.50** meats: 0.29** diary: 0.18* soda: 0.25* |
Strengths: longitudinal study Limitations: 1) highly selective sample; b) dietary assessment: maternal dietary variety was measured as a frequency of food items consumed over the past year, but toddlers’, by food items consumed over the previous week. |
| [39] | López- Alvarenga et al., 2007 Score=11 |
Mexico | 552 children (406 subjects filled the questionnaires for both parents and the child and 146 only filled the questionnaires for one parent (father or mother) and the child). | 8–12 y | From the initial sample (n=668), 300 participants were attending a low SES school and 368 participants came from a medium- high SES school. | The families filled in one questionnaire made up of 43 questions about parents’ habits, 34 regarding the children’s habits and 31 questions related with food intake frequency and quantity. | No, by parents |
First canonical root for child- parent food preference correlations: Yogurt, Oaxaca cheese and yellow cheese: 0.75** Skimmed milk, skimmed yogurt and diet soda: 0.67** Omelet, bread and beans: 0.80** Eggs, pork, butter, fried potatoes, tacos, soups, sandwiches and pizza: 0.77** Fish: 0.79** Sausages, beef, poultry and oat: 0.71** French fries, sweet bread, cakes and sweetened cereals: 0.81** VF: 0.74** Sweets and soft drinks: 0.74** Whole milk: 0.50** |
Limitations: a) only limited food groups were included in the questionnaires; b) Assessed food preferences, but not actual food intakes; c) The canonical root method may be an overestimate compared to regular correlation. |
These studies were listed by publication year. CHO, carbohydrate; MUFA, monounsaturated fat; PUFA, polyunsaturated fat; VF, vegetable and fruit; % of energy, % of total energy derived from the nutrient.
The methodological quality of each study is assessed based on the study design, study sample, data collection including how dietary assessment, data analysis and results reported (see Appendix for related details). Seven separate scores (range: 1 to 3 best) was assigned for each of these features, and then a total score (the highest score was 3*7=21) was calculated for each study. The final total score for each study was used in our meta-analysis for those studies included in the meta-analysis.
P<0.05;
P<0.01.
Overall, they suggest moderate to weak correlation coefficients, though findings varied remarkably across studies and nutrients. For example, in a recent study, based on nationally representative U.S. data with diet being assessed using two 24-hour recalls in the mid-1990s, we examined a set of dietary intake measures including an overall dietary quality index score (the Healthy Eating Index), which measured the overall dietary pattern and quality. This is the largest available study (included 2,692 child-parent pairs) that assessed the familiar association in dietary intakes. The parent-child correlations were weak or moderate (correlations were ranged 0.20–0.33 for key dietary measures such as the dietary quality score and total energy intake) for various parent-child pairs, but the resemblance was stronger in some groups for some intake measures (e.g., 0.56 for total fat intake as grams in the ‘other ethnic group’)[28]. In addition, the resemblance was stronger for younger children’s (<10 years of age) than their older counterparts (≥10 years of age) in terms of overall dietary quality. In another Chicago-based study we assessed the resemblance in 121 pairs of urban low-income African-American adolescents and their mothers [33]. Their dietary intakes were assessed using two similar FFQ developed by Harvard University, one for adults and one for children. None of the mother-son correlations for nutrients and food groups were greater than 0.20. Mother-daughter pairs had stronger correlations (0.26 for energy and 0.30 for fat).
The second largest study was conducted among 1,077 households in the Netherlands, based on 2-day dietary records collected in the 1987 and 1992 national surveys [11]. The study also showed a weak to moderate parent-child correlations, although there were considerable variation in the correlations, ranged from 0.55 for mother-daughter pairs’ cholesterol intake to 0.09 for mother-son pairs’ energy intake.
Table 2 summarizes the characteristics of the studies included in our review and meta-analysis. Only the 15 studies that reported parent-child correlations in energy and fat intakes were include in our meta-analysis. They provided a total of 117 data points (i.e., correlation values; 45 for energy, 45 for total fat and 27 for fat as % energy).
Table 2.
Summary of the characteristics of included studies and data points (i.e., correlation coefficients)
| All 24 studies included in review | The 15 studies included in meta- analysis1 | Data points from the 15 studies included in meta-analysis2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | Energy | Fat | Fat (% energy) | |||||
| n | % | n | % | n | % | ||||||
| Total | 24 | 100 | 15 | 100 | 45 | 100 | 45 | 100 | 27 | 100 | |
| Populations | USA | 13 | 54.2 | 8 | 53.3 | 27 | 60.0 | 36 | 80.0 | 10 | 37.0 |
| European countries | 8 | 33.3 | 5 | 33.3 | 13 | 28.9 | 8 | 17.8 | 13 | 48.1 | |
| Other countries | 3 | 12.5 | 2 | 13.3 | 5 | 11.1 | 1 | 2.2 | 4 | 14.8 | |
| Publication Year | 1980s | 3 | 12.5 | 3 | 20.0 | 12 | 26.7 | 19 | 42.2 | 1 | 3.7 |
| 1990s | 7 | 29.2 | 7 | 46.7 | 20 | 44.4 | 15 | 33.4 | 13 | 48.1 | |
| 2000s | 14 | 45.8 | 5 | 33.3 | 13 | 28.9 | 11 | 24.4 | 13 | 48.2 | |
| Dietary assessment method | 24 hr recalls or records | 12 | 50.0 | 7 | 46.7 | 25 | 55.6 | 17 | 37.8 | 15 | 55.6 |
| FFQ | 9 | 37.5 | 6 | 40.0 | 12 | 26.7 | 8 | 17.8 | 12 | 44.4 | |
| Mixed | 2 | 8.3 | 1 | 6.7 | 8 | 17.8 | 18 | 40.0 | 0 | 0.0 | |
| Other | 1 | 4.2 | 1 | 6.7 | 0 | 0.00 | 2 | 4.4 | 0 | 0.0 | |
| Children’s intake self-reported | Yes | 15 | 65.2 | 10 | 66.7 | 24 | 53.3 | 29 | 64.4 | 13 | 48.1 |
| No | 8 | 34.8 | 5 | 33.3 | 21 | 46.7 | 16 | 35.6 | 14 | 51.8 | |
| Parent-child pair (exclusive)3 | parent-child | __ | __ | __ | __ | 7 | 15.6 | 2 | 4.4 | 4 | 14.8 |
| parent-son | __ | __ | __ | __ | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| parent-daughter | __ | __ | __ | __ | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| father-son | __ | __ | __ | __ | 4 | 9.0 | 2 | 4.4 | 4 | 14.8 | |
| father-daughter | __ | __ | __ | __ | 4 | 9.0 | 2 | 4.4 | 4 | 14.8 | |
| father-child | __ | __ | __ | __ | 8 | 17.8 | 12 | 26.7 | 1 | 3.7 | |
| mother-son | __ | __ | __ | __ | 5 | 11.1 | 4 | 8.9 | 5 | 18.5 | |
| mother-daughter | __ | __ | __ | __ | 7 | 15.6 | 6 | 13.3 | 7 | 26.0 | |
| mother-child | __ | __ | __ | __ | 10 | 22.2 | 17 | 37.8 | 2 | 7.4 | |
| Parent-child pair (non-exclusive)4 | parent-child | __ | __ | __ | __ | 45 | 100.0 | 45 | 100.00 | 27 | 100.0 |
| parent-son | __ | __ | __ | __ | 9 | 20.0 | 6 | 13.3 | 9 | 33.3 | |
| parent-daughter | __ | __ | __ | __ | 11 | 24.4 | 8 | 17.8 | 11 | 40.7 | |
| father-son | __ | __ | __ | __ | 4 | 8.9 | 2 | 4.4 | 4 | 14.8 | |
| father-daughter | __ | __ | __ | __ | 4 | 8.9 | 2 | 4.4 | 4 | 14.8 | |
| father-child | __ | __ | __ | __ | 16 | 35.6 | 16 | 35.6 | 9 | 33.3 | |
| mother-son | __ | __ | __ | __ | 5 | 11.1 | 4 | 8.9 | 5 | 18.5 | |
| mother-daughter | __ | __ | __ | __ | 7 | 15.6 | 6 | 13.3 | 7 | 25.9 | |
| mother-child | __ | __ | __ | __ | 22 | 48.9 | 27 | 60.0 | 14 | 51.8 | |
Studies which were included in the meta-regression analysis for parent-child correlations in energy, fat and fat (%energy).
The characteristics of data points differed significantly by nutrient type based on the χ2 test of independence (p<0.05) for most characteristics except self-report by children. Based on one-way ANOVA, quality score did not differ significantly between nutrient measures.
Exclusive categories (e.g., father-child pairs did not include father-son or father-daughter) of parent-child pairs based on actual data points as presented in studies.
Non-exclusive categories: pooled categories based on the exclusive ones grouping data points according to broader categories, e.g. parent-child includes all exclusive parent-child pairs.
Most characteristics of these data points were significantly different across nutrients considered. For instance, while approximately 60% of the energy data points were from US studies, it was 80% and 37% for fat and fat (% of energy), respectively. In addition, fat (% energy) was more likely than the other two to be assessed using FFQ (44% of data points) compared to energy (27%) and fat (18%). However, study overall quality score and self-report of diet by child (yes vs. no) did not differ significantly by nutrient data points.
We grouped parent-child pairs using a mutually exclusive and a non-exclusive method, respectively. For the mutually exclusive method, it is important to note that none of the data points considered parent-son or parent-daughter correlations. In the case of total energy intake, the majority of data points consisted of correlations between mothers and their children or fathers and their children, irrespective of children’s gender. The same was true for fat intake. However, in the case of fat (% of energy), the distribution of data points by mutually exclusive parent-child pair was more even, with the highest proportion being for mother-daughter correlations (26%).
Meta-analyses and meta-regression analyses for energy and fat intakes
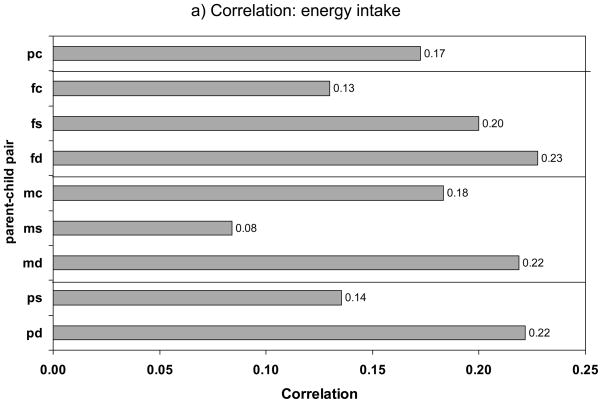
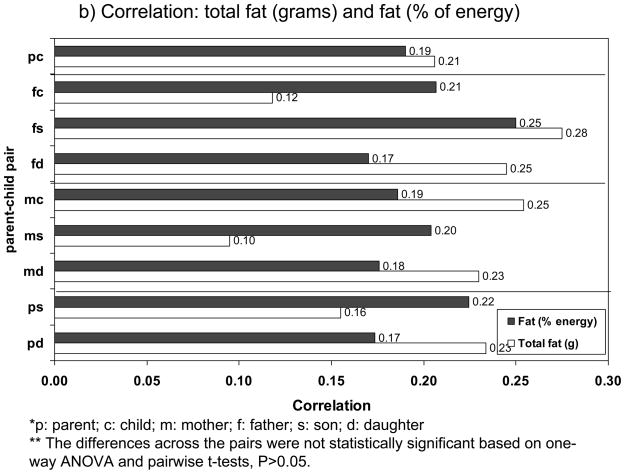
Our meta-analyses showed that, overall, parent-child correlations (not Fisher transformed) for fat intake in grams and % energy were similar, 0.20±0.19 vs. 0.19±0.15; for energy intake, it was 0.17±0.14. This pattern was not replicated, however, across non-exclusive parent-child pairs. In fact, it was only consistent in the case of parent-son, father-son and mother-son correlations. Analysis of variance and pairwise t-tests indicated that mean correlations did not differ significantly between the three intake measures considered within each parent-child pair (p>0.05), which may be due to the small sample size (Table 3). Mean correlations for the three intake measures across parent-child pairs are shown in Figure 1a and 1b. These mean correlations ranged between 0.08 and 0.28, which is considered weak to moderate correlation.
Table 3.
Meta-analysis: Comparison of parent-child correlations of energy and fat intakes, by type of parent-child pairs1
| All, pc | ps | pd | fc | mc | fs | fd | ms | md | |
|---|---|---|---|---|---|---|---|---|---|
| Energy (n) | (45) | (9) | (11) | (16) | (22) | (4) | (4) | (5) | (7) |
| Mean r | 0.17 | 0.13 | 0.22 | 0.13 | 0.18 | 0.20 | 0.23 | 0.08 | 0.22 |
| SD | 0.14 | 0.18 | 0.08 | 0.16 | 0.14 | 0.17 | 0.08 | 0.19 | 0.07 |
| Range | −0.24; 0.39 | −0.24; 0.35 | 0.02; 0.33 | −0.19; 0.35 | −0.24; 0.39 | −0.03; 0.35 | 0.14; 0.33 | −0.24; 0.24 | 0.08; 0.27 |
| Fisher’s transformed r (95% CI) | 0.21 (0.18; 0.24) | 0.18 (0.10; 0.26) | 0.18 (0.10; 0.26) | 0.18 (0.12; 0.24) | 0.21 (0.16; 0.25) | 0.23 (0.12; 0.34) | 0.23 (0.14; 0.33) | 0.13 (0.02; 0.24) | 0.25 (0.21; 0.29) |
| Total fat (n) | (45) | (6) | (8) | (16) | (27) | (2) | (2) | (4) | (6) |
| Mean r | 0.20 | 0.16 | 0.23 | 0.11 | 0.26 | 0.27 | 0.24 | 0.10 | 0.23 |
| SD | 0.19 | 0.19 | 0.11 | 0.22 | 0.17 | 0.01 | 0.15 | 0.21 | 0.11 |
| Range | −0.23; 0.64 | −0.21; 0.28 | 0.02; 0.35 | −0.23; 0.42 | −0.21; 0.64 | 0.27; 0.28 | 0.14; 0.35 | −0.21; 0.28 | 0.02; 0.33 |
| Fisher’s transformed r (95% CI) | 0.25 (0.21; 0.29) | 0.23 (0.16; 0.30) | 0.25 (0.17; 0.33) | 0.18 (0.10; 0.26) | 0.28 (0.22; 0.34) | 0.28 (0.24; 0.33) | 0.25 (0.03; 0.47) | 0.16 (0.04; 0.28) | 0.26 (0.18; 0.34) |
| Fat % energy (n) | (27) | (9) | (11) | (9) | (14) | (4) | (4) | (5) | (7) |
| Mean | 0.19 | 0.22 | 0.17 | 0.21 | 0.19 | 0.25 | 0.17 | 0.20 | 0.17 |
| SD | 0.15 | 0.16 | 0.18 | 0.17 | 0.15 | 0.18 | 0.20 | 0.16 | 0.18 |
| Range | −0.04; 0.44 | −0.04; 0.40 | −0.02; 0.44 | −0.01; 0.40 | −0.04; 0.44 | 0.01; 0.40 | −0.01; 0.39 | −0.04; 0.37 | −0.02; 0.44 |
| Fisher’s transformed r (95% CI) | 0.20 (0.13; 0.28) | 0.23 (0.08; 0.38) | 0.19 (0.06; 0.32) | 0.22 (0.08; 0.36) | 0.20 (0.08; 0.32) | 0.26 (0.02; 0.51) | 0.19 (−0.04; 0.41) | 0.21 (−0.01; 0.44) | 0.19 (−0.00; 0.39) |
p: parent; c: child; m: mother; f: father; s: son; d: daughter
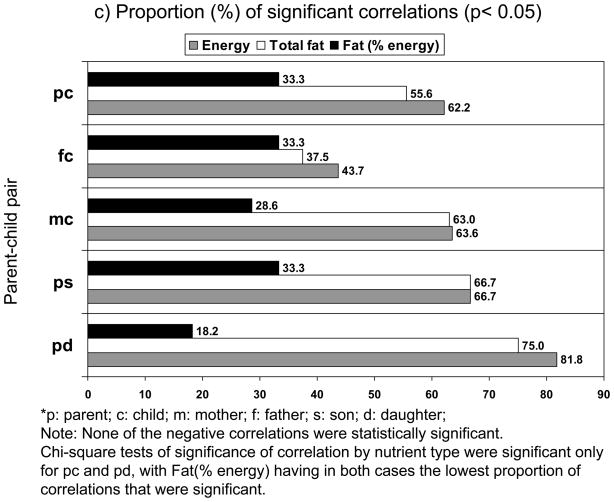
Figure 1.
Mean correlation coefficients of dietary intakes and proportion (%) of significant correlations (p< 0.05), by parent-child relationship type*
*p: parent; c: child; m: mother; f: father; s: son; d: daughter
** The differences across the pairs were not statistically significant based on one-way ANOVA and pairwise t-tests, P>0.05.
Note: None of the negative correlations were statistically significant.
Chi-square tests of significance of correlation by nutrient type were not significant across pairs.
Further, we tested whether the proportion of significant correlations (i.e. p<0.05 for null hypothesis that correlation=0) differed between nutrients across parent-child pairs (Figure 1c). We only considered pairs that had enough power to conduct a χ2 test. While in most parent-child pairs, energy intake had the highest proportion, the differences in proportions between nutrients were only statistically significant in parent-daughter dyad (for energy=82%, for fat=75%, for fat as % of energy=18%; p=0.005).
Means of Fisher’s transformed r with their 95% CI were estimated using random effects models, taking into account the associated standard error. In general, estimated Fisher’s transformed correlations became stronger, and most were significantly greater than 0 (see 95% CI, Table 3).
Table 4 presents the results examining study and data point characteristics in relation to the magnitude of correlations (Fisher’s z transformed). In models 1A through 1C, only these predictors were considered and the full model is presented. In the case of energy intake (Model 1A), dietary assessment was the only significant predictor with FFQ revealing weaker correlation by −0.307 compared to multiple 24-hour recalls or records. In contrast, in model 1B (fat intake), the only significant predictor was study country, non-European countries showed a significantly higher correlation compared to the U.S. (p<0.001). In model 1C, correlation in fat (% energy) was significantly lower over time (β=−0.032 per decade; p<0.001), higher based on FFQ compared to 24 hr recalls or records (β=0.473; p=0.013), lower for child self-reported intake (β=−0.365; p=0.046), and higher with better study quality (β =0.278; <0.001).
Table 4.
Meta-analysis: Tests of the differences in the Fisher’s transformed correlation coefficients, by country, publication year, dietary assessment approach, whether child dietary intake was self-reported, study quality, and type of parent-child pairs 1
| A. Energy |
B. Fat |
C. Fat (% of energy) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | P value | β | SE | P value | β | SE) | P value | |
| Model 1 | (n=45) | (n=45) | (n=27 | ||||||
| Study country (ref=US) | |||||||||
| Europe | 0.093 | 0.057 | 0.105 | 0.040 | 0.091 | 0.660 | −0.020 | 0.102 | 0.840 |
| Other | 0.123 | 0.065 | 0.057 | 0.428 | 0.102 | <0.0012 | −0.157 | 0.093 | 0.095 |
| Publication year | 0.006 | 0.005 | 0.206 | 0.000 | 0.008 | 0.960 | −0.032 | 0.009 | <0.0012 |
| Dietary assessment (ref=24 hr recall or record) | |||||||||
| FFQ | −0.307 | 0.133 | 0.0212 | −0.189 | 0.225 | 0.401 | 0.473 | 0.190 | 0.0132 |
| Mixed | −0.117 | 0.066 | 0.078 | −0.098 | 0.067 | 0.147 | __ | __ | __ |
| Other | __ | __ | __ | 0.073 | 0.257 | 0.775 | __ | __ | __ |
| Children’s intake self- reported (ref=no) | |||||||||
| Yes | 0.13 | 0.10 | 0.200 | 0.037 | 0.172 | 0.830 | −0.365 | 0.183 | 0.0462 |
| Overall study quality (ref=low) | |||||||||
| High (study quality score>the median of studies included; M=13.5) | −0.09 | 0.06 | 0.124 | −0.086 | 0.114 | 0.449 | 0.278 | 0.077 | <0.001 |
| Model 2 Parent-child pairs (ignore child gender; ref=parent-child) | (n=45) | (n=45) | (n=27) | ||||||
| Mother-child | −0.026 | 0.035 | 0.462 | −0.025 | 0.076 | 0.747 | −0.012 | 0.032 | 0.711 |
| Father-child | −0.046 | 0.037 | 0.221 | −0.102 | 0.080 | 0.203 | −0.010 | 0.032 | 0.751 |
| Model 3 Parent-child pairs (consider child gender; ref=parent-child) | (n=45) | (n=45) | (n=27) | ||||||
| Parent-son | −0.039 | 0.036 | 0.263 | −0.095 | 0.084 | 0.255 | −0.029 | 0.042 | 0.483 |
| Parent-daughter | 0.006 | 0.035 | 0.852 | −0.068 | 0.082 | 0.411 | −0.034 | 0.041 | 0.402 |
| Other pairs | −0.004 | 0.042 | 0.929 | −0.033 | 0.074 | 0.649 | −0.093 | 0.081 | 0.250 |
In all models, the full models included publication year, study country, method of dietary assessment, overall study quality, and whether child dietary data were self-reported as the independent variables. Models 2 and 3 forced types of parent-child pair as additional predictors. Backward elimination was used in those two models and only predictors with p-value<0.10 were retained..
p<0.05 for null hypothesis that β=0.
Further, we tested whether the correlations differed by the types of parent-child pairs, in particular, the influence of parental and child gender, but the small sample sizes limited our statistical power. None of the differences were significant (p>0.05). In model 2, type of parent-child pair was entered as an additional predictor and forced to be retained. The other predictors from Model 1 were only retained if their associated p-values in the full model was <0.10. In model 3, a different variant of the parent-child pair variable was introduced focusing on the gender of the child.
DISCUSSION
In the present study, we systematically reviewed and analyzed the relevant studies published since 1980. In order to quantify the associations, we calculated the average correlation coefficients and the variations across intake variables and child-parent pairs. We also compared the differences in the associations across study population groups, over time, as well as dietary intake assessments. Our research showed that only a relatively small number of (n=24) previous studies have examined the child-parent association in dietary intakes. Most of them are based on small samples and about half are conducted in the U.S. Only two of them, a recent study we conducted in the US [28] and another one from the Netherland [11], are based on national data. Very limited studies compared the differences between different parent-child pairs or tested the resemblance in overall dietary intake pattern [28].
Nevertheless, these studies revealed that although the reported degree of association and similarity varied considerably across studies, nutrients and foods. Overall, the association is weak. For example, our meta-analysis shows that on average, the mean correlation coefficient was only 0.17 (SD=0.14; range, −0.24–0.39) for energy intake and 0.19 (SD=0.15; range, −0.04–0.44) for fat intake (% energy). We suspect these weak correlation coefficients probably reflect the fact that the parental and family influence on young people’s dietary intake is not as strong as many people have speculated. In addition, it is also possible that the difficulty to assess children’s and their parents’ intakes accurately using comparable assessment methods (i.e., measurement) have weakened the observed association.
Our review and meta-analysis also reveals several other interesting differences in the correlations. First, the differences in the association are noticeable across nutrient intake variables. For instance, the correlation seemed to be slightly stronger for fat intake than for energy intake. This may be due to the potentially greater similarity in dietary composition, and the possibility that many parents in some societies (e.g., Western societies) may want to control for their total energy intake due to concerns of weight gain and obesity.
Second, our meta-analysis provided some evidence supporting our hypothesis that the association has become weaker over time as indicated by the effect of publication year for fat intake (as % of energy, lowered by 0.032 per year). In the case of energy intake and fat intake (as grams), we cannot rule out the possibility that the lack of significant finding may be due to small sample sizes. In addition, as a marker for parental influence on children’s eating behaviors, fat intake as % of energy may be better that energy intake. Family members could share many food items, but parents might restrict total caloric intake due to concerns of weight gain.
Third, our meta-analysis findings suggest parent-child pairs in the U.S. seem to have weaker association in intakes of energy and total fat compared to other non-European countries. We suspect these differences are contributed by differences in food environment (e.g., food supply and availabilities) and parenting styles between the U.S. and other countries. For example, US children may have more opportunities to eat away from home including school meals and to eat snack foods without the presence of their parents (thus, weaker association). We suspect the parent-child similarity in dietary intakes would be stronger in at least some developing countries as children and their parents are more likely to eat more meals at home (or children were more likely to eat food brought from home in schools), less likely to eat snack foods, and eat the same kinds of foods compared to industrialized countries. For example, a study in Brazil showed that the average correlation between consumption of food groups by mother-child pairs by maternal education levels was 0.49 for <=4 years and 0.41 for >4 years, respectively[40]. Another study in Mexico also showed a good correlation between parental and child food preferences[39]. However, the limited available studies in other countries, especially developing countries, do not allow us to fully explore the between-population differences in our meta-analysis.
In addition, the use of different dietary assessment methods affected findings regarding parent-child correlation in dietary intake. Studies using FFQ yielded lower correlation in energy intake than those using 24-hour recalls or food records. This may be due to the fact that the latter may be conducted for the same days for both parents and children, and thus yielded stronger correlations, while FFQ assesses frequency of a wider range of foods over a longer period of time (e.g., usually over the past year)[26–27]. However, when examining fat as % of energy (indicating dietary composition), FFQ revealed a stronger parent-child correlation.
The present study has several strengths including its inclusion of related studies published over the past three decades since 1980 that we could find and application of several statistical analysis approaches including regression analysis to compare differences between several factors that we hypothesized might affect the child-parent association in dietary intakes, and test of publication bias. In addition, we assessed the methodology quality of studies being reviewed and in our meta-analysis tested its influence on the observed parent-child correlation in intakes.
On the other hand, as a meta-analysis, our study was limited by the small number of data points provided by available studies. Only 15 studies were included, which provided a relatively small number of data points. This limited the statistical power and our models to test the differences across sample characteristics. Second, over half of the studies were from the U.S. and only three were conducted in developing countries. Third, another possible good way to characterize the resemblance would be based on food groups instead of nutrients. However, the limited available results did not allow us to conduct such meta-analysis. In addition, we could only search related studies published in limited languages and data sources.
Our findings have a number of implications. First, they provide useful insight to guide future research. More future studies are needed to study the parent-child resemblance in diet, the differences in the association between population groups, the determinants, and related secular trends. It is desirable to carry out such studies based on nationally representative data with sound dietary assessment. Application of more sophisticated statistical analysis approaches such as multilevel models than simple correlations in order to account for the influence of potential confounders on the results is desirable.
Second, the weak associations we found challenge the widely held belief that children’s and their parents’ dietary intakes are alike. Although we also believe that parents have an important influence on their children’s dietary intake, especially at young ages (e.g. [28]) we suggest that this influence should not be over-stated or interpreted. More attention should be given to the influence of the other players in children’ eating patterns such as that of schools, local food environment and peer influence, government’s guidelines and policies that regulate school meals, the broader food environment that is influenced by food production, distribution and advertisement. A growing number of studies show that people’s dietary intakes are affected by the complex interactions of large number of variables at different levels [14, 16, 45].
Third, more research is needed in developing countries and societies that are under more remarked social and nutrition transitions. Finally, with the many changes that many societies have been experiencing, we suspect parental influence on children’s dietary intake is likely to continue to diminish. To help young people to develop lifelong healthy eating habits, parents and families should not be the sole primary focus, more vigorous and population-based approaches are needed. In addition, parents need be better empowered to assist their children to eat a healthy diet.
Summary Boxes.
What this paper adds:
What is already known on this subject? Parents are believed having a strong influence on children’s eating behaviors as role model and gatekeeper. However, previous findings on child-parent resemblance in dietary intakes are mixed. Some provided evidence to support the association, while others did not.
What does this study add? It provides a systematic review of 24 related studies published since 1980 and an analysis of the association (correlations) based on 15 published studies. Overall, this systematic analysis showed a moderate or weak association (average correlations were approximately 0.2), and the related published findings varied remarkably across studies, nutrients, parent-child pairs, and by some other study characteristics including dietary assessment methods.
Acknowledgments
The study was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, 1R03HD058077-01A1, R03HD058077-01A1S1), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK, R01DK81335-01A1), and the National Institute on Aging Intramural Research Program. We would also like to thank Drs. Xinxue Liu and Ting Wang from Peking University Health Science Center in China and Ms Silvia Bel Serrat for their assistance in searching related studies published in Chinese and Spanish.
Footnotes
The authors declare no conflicts of interest.
Licence statement:
“The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in JECH and any other BMJPGL products and sublicences such use and exploit all subsidiary rights, as set out in our licence (http://jech.bmj.com/ifora/licence.pdf).”
Authors’ contributions
Youfa Wang contributed to study design, data collection and analysis, results interpretation, write up and revision of paper, obtaining funding support and administration; May Beydoun, study design, data collection and analysis, results interpretation, write up and revision of the paper; Ji Li and Yinghui Liu, data collection and revision of the paper; and Luis Moreno, data collection and revision of the paper.
References
- 1.Golan M, Crow S. Parents are key players in the prevention and treatment of weight-related problems. Nutr Rev. 2004;62:39–50. doi: 10.1111/j.1753-4887.2004.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 2.Savage JS, Fisher JO, Birch LL. Parental influence on eating behavior: conception to adolescence. J Law Med Ethics. 2007;35:22–34. doi: 10.1111/j.1748-720X.2007.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laskarzewski P, Morrison JA, Khoury P, et al. Parent-child nutrient intake interrelationships in school children ages 6 to 19: the Princeton School District Study. Am J Clin Nutr. 1980;33:2350–5. doi: 10.1093/ajcn/33.11.2350. [DOI] [PubMed] [Google Scholar]
- 4.Patterson TL, Rupp JW, Sallis JF, et al. Aggregation of dietary calories, fats, and sodium in Mexican-American and Anglo families. Am J Prev Med. 1988;4:75–82. [PubMed] [Google Scholar]
- 5.Perusse L, Tremblay A, Leblanc C, et al. Familial resemblance in energy intake: contribution of genetic and environmental factors. Am J Clin Nutr. 1988;47:629–35. doi: 10.1093/ajcn/47.4.629. [DOI] [PubMed] [Google Scholar]
- 6.Oliveria SA, Ellison RC, Moore LL, et al. Parent-child relationships in nutrient intake: the Framingham Children’s Study. Am J Clin Nutr. 1992;56:593–8. doi: 10.1093/ajcn/56.3.593. [DOI] [PubMed] [Google Scholar]
- 7.Rossow I, Rise J. Concordance of parental and adolescent health behaviors. Soc Sci Med. 1994;38:1299–305. doi: 10.1016/0277-9536(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 8.Stafleu A, Van Staveren WA, de Graaf C, et al. Family resemblance in energy, fat, and cholesterol intake: a study among three generations of women. Prev Med. 1994;23:474–80. doi: 10.1006/pmed.1994.1065. [DOI] [PubMed] [Google Scholar]
- 9.Vauthier JM, Lluch A, Lecomte E, et al. Family resemblance in energy and macronutrient intakes: the Stanislas Family Study. Int J Epidemiol. 1996;25:1030–7. doi: 10.1093/ije/25.5.1030. [DOI] [PubMed] [Google Scholar]
- 10.Adelekan DA, Adeodu OO. Interrelationship in nutrient intake of Nigerian mothers and their children: nutritional and health implications. Afr J Med Med Sci. 1997;26:63–5. [PubMed] [Google Scholar]
- 11.Feunekes GI, Stafleu A, de Graaf C, et al. Family resemblance in fat intake in The Netherlands. Eur J Clin Nutr. 1997;51:793–9. doi: 10.1038/sj.ejcn.1600494. [DOI] [PubMed] [Google Scholar]
- 12.Feunekes GI, de Graaf C, Meyboom S, et al. Food choice and fat intake of adolescents and adults: associations of intakes within social networks. Prev Med. 1998;27:645–56. doi: 10.1006/pmed.1998.0341. [DOI] [PubMed] [Google Scholar]
- 13.Cullen KW, Lara KM, de Moor C. Familial concordance of dietary fat practices and intake. Fam Community Health. 2002;25:65–75. doi: 10.1097/00003727-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 14.French SA, Story M, Jeffery RW. Environmental influences on eating and physical activity. Annu Rev Public Health. 2001;22:309–35. doi: 10.1146/annurev.publhealth.22.1.309. [DOI] [PubMed] [Google Scholar]
- 15.Popkin BM. Global nutrition dynamics: the world is shifting rapidly toward a diet linked with noncommunicable diseases. Am J Clin Nutr. 2006;84:289–98. doi: 10.1093/ajcn/84.1.289. [DOI] [PubMed] [Google Scholar]
- 16.Vereecken CA, Inchley J, Subramanian SV, et al. The relative influence of individual and contextual socio-economic status on consumption of fruit and soft drinks among adolescents in Europe. Eur J Public Health. 2005;15:224–32. doi: 10.1093/eurpub/cki005. [DOI] [PubMed] [Google Scholar]
- 17.Jahns L, Siega-Riz AM, Popkin BM. The increasing prevalence of snacking among US children from 1977 to 1996. J Pediatr. 2001;138:493–8. doi: 10.1067/mpd.2001.112162. [DOI] [PubMed] [Google Scholar]
- 18.Zizza C, Siega-Riz AM, Popkin BM. Significant increase in young adults’ snacking between 1977–1978 and 1994–1996 represents a cause for concern! Prev Med. 2001;32:303–10. doi: 10.1006/pmed.2000.0817. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Census Bureau. 2006 http://www.census.gov/
- 20.Du S, Lu B, Zhai F, et al. A new stage of the nutrition transition in China. Public Health Nutr. 2002;5:169–74. doi: 10.1079/PHN2001290. [DOI] [PubMed] [Google Scholar]
- 21.Young LR, Nestle M. The contribution of expanding portion sizes to the US obesity epidemic. Am J Public Health. 2002;92:246–9. doi: 10.2105/ajph.92.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adair LS, Popkin BM. Are child eating patterns being transformed globally? Obes Res. 2005;13:1281–99. doi: 10.1038/oby.2005.153. [DOI] [PubMed] [Google Scholar]
- 23.Nicklas TA, Demory-Luce D, Yang SJ, et al. Children’s food consumption patterns have changed over two decades (1973–1994): The Bogalusa heart study. J Am Diet Assoc. 2004;104:1127–40. doi: 10.1016/j.jada.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 24.Nicklas TA, Morales M, Linares A, et al. Children’s meal patterns have changed over a 21-year period: the Bogalusa Heart Study. J Am Diet Assoc. 2004;104:753–61. doi: 10.1016/j.jada.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen SJ, Siega-Riz AM, Popkin BM. Trends in food locations and sources among adolescents and young adults. Prev Med. 2002;35:107–13. doi: 10.1006/pmed.2002.1037. [DOI] [PubMed] [Google Scholar]
- 26.Patterson RE, Kristal AR, Tinker LF, et al. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 27.Rockett HR, Wolf AM, Colditz GA. Development and reproducibility of a food frequency questionnaire to assess diets of older children and adolescents. J Am Diet Assoc. 1995;95:336–40. doi: 10.1016/S0002-8223(95)00086-0. [DOI] [PubMed] [Google Scholar]
- 28.Beydoun MA, Wang Y. Parent-child dietary intake resemblance in the United States: Evidence from a large representative survey. Soc Sci Med. 2009;68:2137–44. doi: 10.1016/j.socscimed.2009.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher JO, Mitchell DC, Smiciklas-Wright H, et al. Parental influences on young girls’ fruit and vegetable, micronutrient, and fat intakes. J Am Diet Assoc. 2002;102:58–64. doi: 10.1016/s0002-8223(02)90017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galloway AT, Fiorito L, Lee Y, et al. Parental pressure, dietary patterns, and weight status among girls who are “picky eaters”. J Am Diet Assoc. 2005;105:541–8. doi: 10.1016/j.jada.2005.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park HS, Yim KS, Cho SI. Gender differences in familial aggregation of obesity-related phenotypes and dietary intake patterns in Korean families. Ann Epidemiol. 2004;14:486–91. doi: 10.1016/j.annepidem.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Stanton CA, Fries EA, Danish SJ. Racial and gender differences in the diets of rural youth and their mothers. Am J Health Behav. 2003;27:336–47. doi: 10.5993/ajhb.27.4.5. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Li J, Caballero B. Resemblance in dietary intakes between urban low-income African-American adolescents and their mothers: the healthy eating and active lifestyles from school to home for kids study. J Am Diet Assoc. 2009;109:52–63. doi: 10.1016/j.jada.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Billon S, Lluch A, Gueguen R, et al. Family resemblance in breakfast energy intake: the Stanislas Family Study. Eur J Clin Nutr. 2002;56:1011–9. doi: 10.1038/sj.ejcn.1601440. [DOI] [PubMed] [Google Scholar]
- 35.Longbottom PJ, Wrieden WL, Pine CM. Is there a relationship between the food intakes of Scottish 5(1/2)–8(1/2)-year-olds and those of their mothers? J Hum Nutr Diet. 2002;15:271–9. doi: 10.1046/j.1365-277x.2002.00374.x. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell BD, Rainwater DL, Hsueh WC, et al. Familial aggregation of nutrient intake and physical activity: results from the San Antonio Family Heart Study. Ann Epidemiol. 2003;13:128–35. doi: 10.1016/s1047-2797(02)00255-7. [DOI] [PubMed] [Google Scholar]
- 37.Runyan SM, Stadler DD, Bainbridge CN, et al. Familial resemblance of bone mineralization, calcium intake, and physical activity in early-adolescent daughters, their mothers, and maternal grandmothers. J Am Diet Assoc. 2003;103:1320–5. doi: 10.1016/s0002-8223(03)01075-7. [DOI] [PubMed] [Google Scholar]
- 38.Papas MA, Hurley KM, Quigg AM, et al. Low-income, African American adolescent mothers and their toddlers exhibit similar dietary variety patterns. J Nutr Educ Behav. 2009;41:87–94. doi: 10.1016/j.jneb.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 39.López-Alvarenga JC, Vázquez-Velázquez V, Bolado-García VE, González-Barranco J, Castañeda-López J, Robles L, Velásquez-Alva C, Aguirre-Hernándezh R, Comuzziea A. Influencia de los padres sobre las preferencias alimentarias en niños de dos escuelas primarias con diferente estrato económico. Estudio ESFUERSO. Gac Méd Méx. 2007;143:463–9. [PubMed] [Google Scholar]
- 40.daVeiga GV, Sichierib R. Correlation in food intake between parents and adolescents depends on socioeconomic level. Nutrition Research. 2006:517–23. [Google Scholar]
- 41.Donner A, Rosner B. On inferences concerning a common correlation coefficient. Applied Statistics. 1980;29:69–72. [Google Scholar]
- 42.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 43.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 44.STATA. Statistics/Data Analysis: Release 11.0. Texas: Stata Corporation; 2009. [Google Scholar]
- 45.Popkin BM, Duffey K, Gordon-Larsen P. Environmental influences on food choice, physical activity and energy balance. Physiol Behav. 2005;86:603–13. doi: 10.1016/j.physbeh.2005.08.051. [DOI] [PubMed] [Google Scholar]