Abstract
Central venous catheters (CVC) are widely used in the United States and are associated with 250,000 to 500,000 CVC-related infections in hospitals annually. We used a catheter made from ultraviolet-C (UVC) transmissive material to test whether delivery of UVC from the lumen would allow inactivation of microorganisms on the outer surface of CVC. When the catheter was exposed to UVC irradiation from a cold cathode fluorescent lamp (CCFL) inside the catheter lumen at a radiant exposure of 3.6 mJ/cm2, more than 6-log10 of drug-resistant Gram-positive bacteria adhered to the outer surface of the catheter were inactivated. Three to 7-log10 of drug-resistant Gram-negative bacteria and 2.8 log10 of fungi were inactivated at a radiant exposure of 11 mJ/cm2. UVC irradiation also offered a highly selective inactivation of bacteria over keratinocytes under exactly comparable conditions. After 11 mJ/cm2 UVC light had been delivered, over 6 log10 of bacteria were inactivated while the viability loss of the keratinocytes was only about 57%.
Introduction
Stable and safe vascular access has become essential to modern medical practice. The management of critically ill patients is virtually impossible without intravascular access devices. A central venous catheter (CVC), or vascular access device, is a long, thin, flexible tube used to give medicines, fluids, nutrients, or blood products over a long period of time, usually several weeks or more. A CVC is often inserted in the arm or chest through the skin into a large vein. The catheter is threaded through this vein until it reaches a large vein near the heart. CVC are widely used in the United States, and it is estimated that physicians insert more than 5 million CVC every year (1).
Although CVC have been shown to have numerous medical benefits, one of the more common risks associated with their use is the occurrence of catheter-related infections. While all catheters are known to have the potential to allow access of bacteria or other microorganisms into the bloodstream, CVC, because of their placement and longevity of use, have been known to become contaminated in a manner which can cause serious infections, including for example Staphylococcus aureus and Staphylococcus epidermidis sepsis. As a matter of fact, CVC are now the most frequent source of nosocomial bloodstream infection (2), and it has been estimated that 250,000–500,000 episodes occur in the United States annually, with an estimated 10% mortality and marginal cost to the health care system of $25,000 per episode (2).
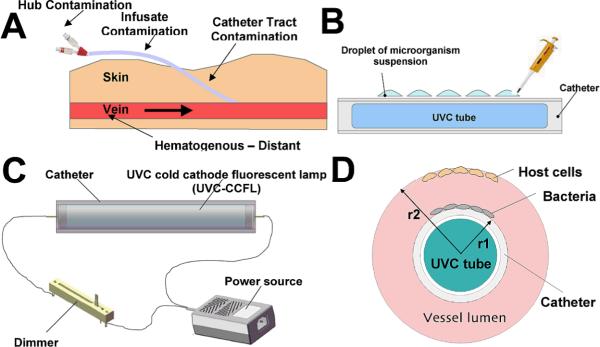
Four distinct pathways can be identified in the development of a catheter-related infection (Figure 1A) (3). First, colonization of the outer surface may start by the migration of skin resident microorganisms from the insertion site, and microbial cells may progressively move through the transcutaneous part of the dermal tunnel surrounding the catheter. Second, colonization of the internal surface may occur by colonization of the hub and intraluminal surface of the catheter during utilization, and frequent opening of the hub is now viewed as an important source of colonization. Hematogenous seeding of the catheter during bloodstream infection of any origin represents a third pathway, and lastly, contamination of the fluids or drugs intravenously administered, is sometimes responsible for outbreaks.
Figure 1.
A) Routes to develop a CVC-related infection. B) Schematic of prototype device showing a UVC cold cathode fluorescent lamp (UVC-CCFL) disposed in the lumen of a catheter. C) Schematic of UVC inactivation of microorganisms on the outer surface of catheter. D) Schematic of the geometrical selectivity of UVC inactivation of microorganisms on catheter surface over cells on vessel wall.
To prevent infection, some CVC are coated or impregnated with antibiotics or antimicrobials such as silver sulfadiazine (4). It was also suggested that catheters be flushed with antibiotic-containing solutions to disinfect the inner surface of the catheter and prevent infection (4,6). These existing methods however pose several problems and limitations. Specifically, antiseptics such as silver sulfadiazine are typically slightly acidic compositions that can cause a patient to experience an uncomfortable burning sensation at the catheter insertion point or can cause damage or irritation to the skin around the area of the catheter (6). In addition, coating a catheter with antibiotics or flushing a catheter with antibiotic-containing solutions for prophylactic purposes raises concerns about the potential emergence of antibiotic-resistant strains (8). Finally, flushing a catheter with antibiotic-containing solutions may not be effective in eliminating many microorganisms that are particularly well adhered to the inner surface of the catheter in the form of biofilms, especially for those drug-resistant strains. As a result, a pressing need exists for an improved and more reliable method to prevent CVC-related infections.
The mechanism of UVC inactivation of microorganisms is to damage the genetic material in the nucleus of the cell (9). UVC light in the range of 240 to 270 nm is strongly absorbed by the nucleic acids of an organism. The light induced damage to the DNA and RNA of an organism often results from the dimerization of pyrimidine molecules. In particular, thymine (which is only found in DNA) produces cyclobutane dimers. When these molecules are dimerized, it becomes very difficult for the nucleic acids to replicate and if replication does occur it often produces a defect which prevents the microorganisms from being viable. Within the UVC range, 254 nm is easily produced from a low-pressure mercury vapor lamp and has been shown to be close to the optimal wavelength for germicidal action (10). These lamps have been used for decades to purify environmental air and water (11,12). Use of UVC for human treatment has been limited. Since the 1970's, germicidal UVC lamps were used in operating rooms with direct exposure to patients undergoing surgery. These were abandoned by surgeons, because of the need for protective eyewear and clothing. Studies that have examined UVC inactivation of antibiotic resistant bacteria have found them to be as equally susceptible as their naïve counterparts (13).
In the present study, we investigated a technique of delivering UVC light from inside a CVC that can inactivate microorganisms on both the inner and outer surfaces of the CVC. The technique could overcome many of the problems and limitations of conventional sterilization techniques and better enable health care professions to focus on treating the underlying medical condition without having to worry about the onset of a secondary, catheter-related infection.
Materials and Methods
UVC light sources and catheter
The prototype device used in the study (Figure 1A) consisted of a UVC cold cathode fluorescent lamp (UVC-CCFL) in a quartz tube connected to a piezoelectric inverter (PhotoGlow LLC, South Yarmouth, MA) that turns 12V DC into the 600V, 5 mA current to create the electric discharge. A dimmer (PhotoGlow) was also fitted to the circuit to be able to control the amount of emission from the UVC-CCFL. Emission from the UVC-CCFL is in a cylindrical pattern that is ideal for insertion into the lumen of a catheter (Figure 1B). The diameter of the UVC-CCFL was 6 mm and the length 45 mm. The outer diameter of the catheter was 8 mm with the catheter thickness of 0.95 mm. The irradiance of the UVC-CCFL achieved 0.4 mW/cm2 at the lamp surface by tuning the dimmer in the electric circuit. The UVC irradiance was measured using a UVX radiometer (UVP Inc., Upland, CA).
In addition, a germicidal lamp (CE-12-2H; American Ultraviolet Company, Lebanon, IN), which has a larger illuminating area than the UVC-CCFL, was used as a light source to examine the susceptibility of keratinocytes and bacterial suspensions to UVC irradiation. Emission spectral measurement of both the UVC-CCFL and the germicidal lamp using a spectroradiometer (SPR-01; Luzchem Research Inc., Ottawa, ON, Canada) showed a peak emission at 254 ± 2 nm wavelength of both light sources.
Measurement of UVC transmission through catheters
Segments of three commercial catheter materials Nusil 1, Nusil extruded (Nusil Technology. LLC, Carpinteria, CA), and Polyurethane-Tecoflex 85A durometer (Lubrizol Advanced Materials, Inc, Cleveland, OH) were collected. An Evolution 300 UV-Vis Spectrophotometer (Thermo Scientific, Waltham, MA) was used to measure the UV absorbance spectrum (200–400-nm) of these catheter segments. The spectroradiometer mentioned above was used to measure the transmissivity of UVC radiation through the prototype device catheter. The UVC transmission through the catheter was defined as the ratio of the UVC irradiance measured on the outer surface of catheter to that on the surface of UVC-CCFL.
Microorganisms and culture conditions
Six species of bacteria and two species of fungi were studied. The strains used were listed in Table 1. All of the strains are biofilm-forming pathogens.
Table 1.
Microorganism strains tested in the study of UVC sterilization of catheters
| Gram (+) bacteria | S. aureus ATCC 8325-4 |
| methicillin-resistant S. aureus (MRSA) ATCC 33591 | |
| S. epidermidis ATCC35984. | |
|
| |
| Gram (−) bacteria | Escherichia coli DH5a |
| Pseudomonas aeruginosa ATCC 19660 (strain 180) | |
| Proteus mirabilis ATCC 51393 | |
|
| |
| Fungi | Candida albicans ATCC 18804 |
| Cryptococcus neoformans KN99alpha. | |
Bacteria were grown in brain-heart infusion (BHI) medium in an orbital shaking incubator (37 °C; 100 rpm) to an optical density of 0.6–0.8 at 600 nm (OD600) that corresponds to 108 cells/mL (mid-log phase). Fungi were grown in yeast peptone dextrose (YPD) medium in a shaking incubator (30 °C; 100rpm) to an optical density of 6.5 (10-fold dilutions measured) at 570 nm (OD570) that corresponds to a cell concentration of 108 cells/mL (mid-log phase). Cell growth was assessed with an Evolution 300 UV-Vis Spectrophotometer (Thermo Scientific, Waltham, MA). The suspension was then centrifuged, washed with phosphate buffered saline (PBS), and re-suspended in PBS at a density of 109 cells/mL.
UVC inactivation of microorganisms on the outer surface of catheter
Prior to UVC irradiation, droplets of 20 μl microorganism suspension containing 2×107 colony forming units (CFU) were inoculated on the outer surface of the catheter using a pipette (Figure 1C). The droplets were respectively sampled at various time points during the period of UVC irradiation for quantification of CFU.
Quantification of microorganism CFU
To measure the CFUs of microorganism suspensions, serial dilutions were performed as Figure 3A. From the suspension to be measured, 20 μl was transferred to a 1.5 mL centrifuge tube (labeled tube #1). Five other centrifuge tubes containing 90 μl of sterile PBS were labeled #2 to #6. From tube #1, 10 μl was transferred to tube #2, which was then vortexed. From tube #2, 10 μl was transferred to tube #3, and vortexed. The same procedure was used to obtain 10-fold serial dilutions for tubes #4 to #6. Once all the dilutions were obtained (1:1 to 1:105), we proceeded to plate each dilution on a square BHI (for bacteria) or YPD (for fungi) agar in an order of most (1:105) to least (1:1) diluted (Figure 3A) (14). The plates were then incubated at 37 °C (for bacteria) or 30 °C (for fungi) for 24 hours.
Figure 3.
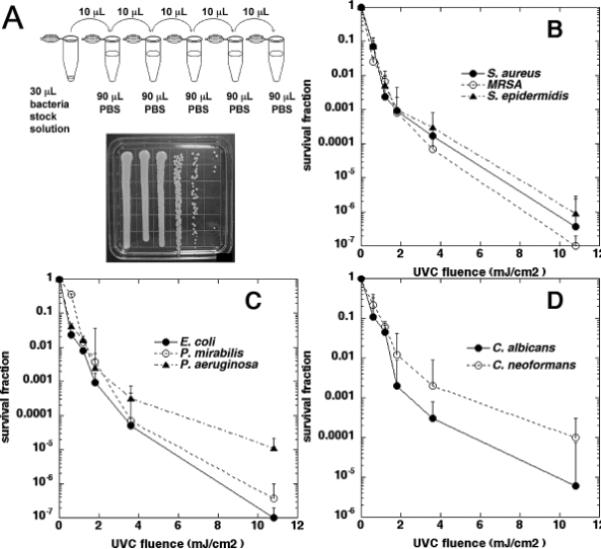
A) Quantification of colony forming units: 10-folder serial dilutions of microorganism suspensions; and streak of a set of serial dilutions of microorganism suspension on agar plate. B) Time-dependent survival fraction in response to UVC irradiation of Gram-positive bacteria on the outer surface of catheter. C) Time-dependent survival fraction in response to UVC irradiation of Gram-negative bacteria on the outer surface of catheter. D) Time-dependent survival fraction in response to UVC irradiation of fungi on the outer surface of catheter. Bars: standard deviation.
Monolayer keratinocyte cultures
PAM 212 cells, a mouse keratinocyte cell line, were used. To obtain monolayer keratinocyte cultures, keratinocytes were inoculated into 35 mm petri dishes (≈200 cells/dish) in RPMI-1640 medium (Sigma-Aldrich Co., St. Louis, MO). The dishes were then be incubated at 37°C in a humidified atmosphere of 5% CO2 for 18–24 hours prior to experiment.
UVC irradiation of monolayer keratinocyte cultures or bacterial suspensions under comparable conditions
Prior to UVC irradiation, the PAM212 culture medium was replaced with PBS and lids were removed. UVC radiation was delivered using the germicidal lamp. For each trial, five culture dishes were illuminated under UVC for 0, 3, 6, 9, and 12 seconds, respectively, at an irradiance of 1.6 mW/cm2. After UVC irradiation, PBS was removed from the 35 mm petri dishes. The cell cultures were then detached using 2 mL trypsin-0.25% EDTA solution (Sigma-Aldrich, St. Louis, MO) per dish and re-cultured to 150 mm petri dishes inoculated with 50 mL fresh culture medium. The 150 mm culture dishes were then incubated at 37 °C for 10 days for growing keratinocyte colonies to a visible size before the assay of cell viability. Culture medium in the 150 mm dishes was refreshed every three days. After 10 days incubation, the culture medium was removed from the 150 mm petri dishes, and keratinocyte viability was assessed by staining the keratinocyte colonies in the dishes with crystal violet solution. The stained keratinocyte colonies were counted manually.
Bacterial suspensions (either S. aureus or E. coli) at 10(8) cells per mL in PBS were placed into 35-mm petri dishes without lids and were treated in an identical manner to irradiation with germicidal lamp and CFU were determined by serial dilution on agar plates as described above. Experiments were performed in triplicate. Mean colony survival fractions of the 2 bacterial species combined were plotted against mean colony survival fraction of keratinocytes.
Results
Transmission of UVC through catheter
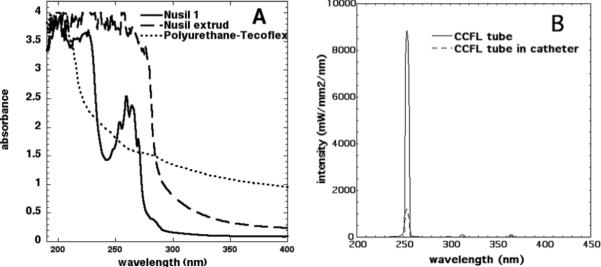
Figure 2A compares the absorbance in the UV region of the three commercial catheters used in the present study. The absorbance at 254 nm of the polyurethane material was lower than the Nusil catheters, indicating a higher transmission of the polyurethane at 254 nm compared to silicone. The NuSil extrud was completely opaque to UVC irradiation, while NuSil 1 has a significant absorption band at the emission wavelength of the UVC lamp. The polyurethane tube catheter was therefore used to construct the prototype device shown in Figure 1B. Figure 2B depicts the wavelength-dependent power intensity distribution of the UVC-CCFL detected with and without the catheter wrapping around, respectively. The transmission of the UVC through the prototype device catheter was calculated to be about 13.6%.
Figure 2.
A) Comparison of the absorbance in the UV range of two commercial catheters (NuSil 1 and NuSil extrude) and the prototype device catheter; B) Spectral power distribution of the prototype device UVC tube measured with and without catheter.
UVC inactivation of microorganisms adhered to the outer surface of catheter
Figures 3B, 3C, and 3D respectively demonstrate the effects of UVC on inactivating Gram-positive bacteria, Gram-negative bacteria, and fungi on the outer surface of catheter. When the catheter was exposed to UVC irradiation from the mercury tube inside the catheter for 60 seconds (corresponding to a radiant exposure of 3.6 mJ/cm2 on the catheter outer surface with the irradiance≈0.06 mW/cm2), 2–3 log10 of all three Gram-positive bacterial populations were inactivated, and this inactivation fraction increased to 6–7 logs after 11 mJ/cm2 of UVC had been delivered. Seven logs of inactivation represents complete sterilization. All three species (S. aureus, S. epidermidis, and MRSA) were equally susceptible to UVC inactivation. In the case of Gram-negative bacteria, 3–4 log10 were inactivated at 3.6 mJ/cm2, and this inactivation increased to 5–7 log10 after an UVC exposure of 11 mJ/cm2. It was found that P. aeruginosa was more resistant compared to P. mirabilis and E. coli (which was eradicated). For fungi, C. albicans (5-log10 inactivation after a UVC exposure of 11 mJ/cm2) was more sensitive to UVC than C. neoformans (4-log10 inactivation after a UVC exposure of 11 mJ/cm2). Overall, the fungal species were more resistant than the bacterial species.
Selectivity of UVC inactivation of microorganisms over keratinocytes
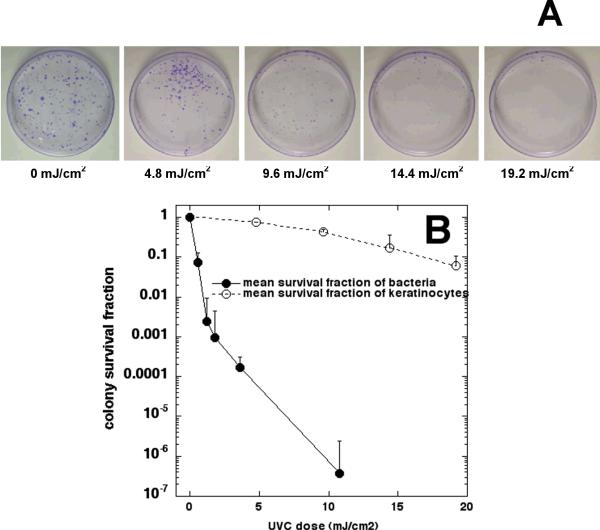
Figure 4A is a representative set of images showing the colonies of the keratinocyte cultures exposed to UVC at various radiant exposures. A clear dose response of keratinocyte viability to UVC irradiation can be observed. On average, when 11 mJ/cm2 UVC light had been delivered, as shown in Figure 4B, the viability loss of the keratinocytes was only approximately 0.24-log10 (≈57%), while over 6-log10 inactivation of bacteria was achieved under similar conditions, resulting in a more than 5-log10 selective inactivation of bacteria over keratinocytes. If we consider a 2-log10 (99%) or a 3-log10 (99.9%) inactivation of bacteria as a sufficiently therapeutic fraction, the viability loss of the keratinocytes would be only approximately 6% and 12%, respectively, at the same UVC dose.
Figure 4.
A) A representative set of images showing the colonies (crystal violet stained) of keratinocyte cultures exposed to UVC irradiation at various radiant exposures. B) Comparison of averaged fluence-dependent survival fractions in response to UVC irradiation of bacteria with that of keratinocytes using identical irradiation conditions and colony-forming assays. Bars: standard deviation.
Discussion
The idea of using UVC light for inactivating microorganisms on catheters is not new. Boudreaux (15) patented a technique of inactivating microorganisms on the inner surface of catheter using UVC light by disposing an optical fiber lengthwise into the catheter and moving the fiber up and down in the catheter. Bak et al (16) evaluated the required UVC doses to inactivate 99% bacteria in biofilms formed on the inner surface of catheters. Patient catheters (n=67) were collected and cut lengthwise into halves. The halves were then placed with the inner surface of catheters facing the direction of UVC irradiation. Microorganisms studied included S. aureus, Enterococcus faecalis, E. coli, Klebsiella pneumoniae, and Streptococcus spp. It was also suggested by Eckhardt et al that (17) the sterilization of catheter be achieved by delivering UVC light from outside the catheter. The UVC transmissivity of the catheter was increased by reducing the thickness of the catheter. However, the geometry of this technique limited its application in vivo, as the UVC light source was placed outside the catheter. The innovation of the technique presented in this study lies in the fact that it could be able to sufficiently inactivate microorganisms on both the inner and outer surfaces of a CVC in-vivo by delivering UVC light from the lumen of a catheter that is sufficiently UVC-transmissive.
The requirements for an effective inactivation of microorganisms on both the inner and outer surfaces of a CVC include: an effective UVC light source, a catheter that transmits UVC light sufficiently, evidence that UVC delivered from inside catheter inactivates microorganisms adhered to the outside of catheter, evidence that common catheter pathogens are efficiently inactivated including antibiotic resistant species, and evidence that the doses of UVC light employed will not unacceptably damage host cells or tissues surrounding the catheter. In the technique presented in this study, UVC light is produced by a cold cathode fluorescent lamp, emitting a light at 254-nm wavelength with a maximum irradiance of 0.4 mW/cm2. This wavelength is close to the optimal wavelength for bactericidal action, which is 265 nm. Compared with the idea of using an optical fiber (16), which is a point light source, UVC lamp (as a line light source) can significantly increase the efficacy of UVC sterilization of a catheter by reducing the irradiation time to achieve sufficient inactivation of microorganisms along the whole catheter.
A sufficiently UVC-transmissive catheter was used in the prototype device. Approximately 13.6% UVC can transmit to the outer surface of the catheter from the lumen of the catheter. It was shown in the present study between 7 and 2.8 -log10 of drug-resistant Gram-negative bacteria, drug-resistant Gram-positive bacteria, and fungi was inactivated when the catheter was exposed to the UVC irradiation from the mercury tube inside the catheter lumen at a dose of 3.6 to 11 mJ/cm2.
In the present study we found that UVC light gave a high level of selectivity for inactivation of microorganisms over killing of keratinocytes using directly comparable exposure conditions and colony forming assays. Over 5-log10 selective inactivation of bacteria over keratinocytes can be achieved after a UVC dose of 11 mJ/cm2. Additional geometrical selectivity may be provided by the design of a system to deliver UVC light from the lumen of a catheter so that microbial cells on either the internal or external surface of the catheter will be exposed to more UVC than host endothelial cells lining the vessel wall outside the catheter. This advantage is illustrated schematically by Figure 1D. When the outer radius of the catheter is r1, and the radius of vessel is r2, the ratio of the UVC light delivered to the vessel wall to that to the catheter outer surface will be R=(r1/r2)2. For example (assuming the cross-section of the tube is approximated by a point source) if r1=1mm and r2=3 mm, then the UVC light delivered to the vessel wall will be only 1/9 (R≈0.11) of that delivered to the outer surface of the catheter. To minimize the side effects of UVC on mammalian cells, the optimal UVC dose should be the minimal dose required to inactivate a therapeutically sufficient fraction of pathogenic microorganisms.
One limitation of the present study was that only planktonic cells of the microorganisms were investigated. It is generally accepted that biofilms form shortly after the placement of catheter. The UVC doses required to inactivate catheter biofilms can be 100-folder greater than those required to inactivate planktonic microbes due to the attenuation of UVC by extracellular polysaccharide substances of biofilm (16). It was found from the present study using the prototype device that the UVC dose required to inactivate a therapeutically sufficient fraction of planktonic bacteria (99%) on the catheter outer surface was about 1.1 mJ/cm2, corresponding to a 20-second irradiation from the prototype device. Therefore, the irradiation time of UVC required to eradicate 99% biofilms could be over 2,000 seconds (≈ 33 minutes). The irradiation time required to achieve a therapeutically effective inactivation of microorganisms (e.g. 99%) indicates the potential future use for UVC-based treatments in the clinic.
Another limitation of the present study is that only the susceptibility of keratinocytes to UVC irradiation was investigated and the effect of UVC on the endothelial cell lining of the vessel wall was not examined. The susceptibility of endothelial cells could be different from that of keratinocytes.
In conclusion, delivery of UVC light from the lumen of a sufficiently UVC-transmissive catheter may provide a promising option for the prevention of CVC-related infections. Future studies are warranted on more powerful UVC light sources and more UVC-transmissive catheters, and using animal models for in vivo studies.
Acknowledgements
This work was supported by NIH grant R01AI050875 to MRH. T.D. was supported by a Bullock-Wellman Fellowship. G.P.T was partly supported by a Massachusetts Technology Transfer Center Award.
References
- 1.McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003;348:1123–1133. doi: 10.1056/NEJMra011883. [DOI] [PubMed] [Google Scholar]
- 2.Safdar N, Kluger DM, Maki DG. A review of risk factors for catheter-related bloodstream infection caused by percutaneously inserted, noncuffed central venous catheters: implications for preventive strategies. Medicine (Baltimore) 2002;81:466–479. doi: 10.1097/00005792-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Eggimann P, Sax H, Pittet D. Catheter-related infections. Microbes Infect. 2004;6:1033–1042. doi: 10.1016/j.micinf.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Pai MP, Pendland SL, Danziger LH. Antimicrobial-coated/bonded and - impregnated intravascular catheters. Ann Pharmacother. 2001;35:1255–1263. doi: 10.1345/aph.10416. [DOI] [PubMed] [Google Scholar]
- 5.Safdar N, Maki DG. Use of vancomycin-containing lock or flush solutions for prevention of bloodstream infection associated with central venous access devices: a meta-analysis of prospective, randomized trials. Clin Infect Dis. 2006;43:474–484. doi: 10.1086/505976. [DOI] [PubMed] [Google Scholar]
- 6.Shah CB, Mittelman MW, Costerton JW, Parenteau S, Pelak M, Arsenault R, Mermel LA. Antimicrobial activity of a novel catheter lock solution. Antimicrob Agents Chemother. 2002;46:1674–1679. doi: 10.1128/AAC.46.6.1674-1679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sikora CF. Apparatus and method for sterilization of an intravenous catheter. 20080306454. USPTO Patent Application. 2008
- 8.Jones SM, Morgan M, Humphrey TJ, Lappin-Scott H. Effect of vancomycin and rifampicin on methicillin-resistant Staphylococcus aureus biofilms. Lancet. 2001;357:40–41. doi: 10.1016/S0140-6736(00)03572-8. [DOI] [PubMed] [Google Scholar]
- 9.Chang JC, Ossoff SF, Lobe DC, Dorfman MH, Dumais CM, Qualls RG, Johnson JD. UV inactivation of pathogenic and indicator microorganisms. Appl Environ Microbiol. 1985;49:1361–1365. doi: 10.1128/aem.49.6.1361-1365.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurzadyan GG, Gorner H, Schulte-Frohlinde D. Ultraviolet (193, 216 and 254 nm) photoinactivation of Escherichia coli strains with different repair deficiencies. Radiat Res. 1995;141:244–251. [PubMed] [Google Scholar]
- 11.Ko G, First MW, Burge HA. The characterization of upper-room ultraviolet germicidal irradiation in inactivating airborne microorganisms. Environ Health Perspect. 2002;110:95–101. doi: 10.1289/ehp.0211095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallbank AM, Lebtag I, Meyer W, Boehm P. Inactivation of bacteria and viruses in water: passage of germicidal ultraviolet light through Teflon. Lab Anim. 1985;19:273–274. doi: 10.1258/002367785780887374. [DOI] [PubMed] [Google Scholar]
- 13.Conner-Kerr TA, Sullivan PK, Gaillard J, Franklin ME, Jones RM. The effects of ultraviolet radiation on antibiotic resistant bacteria in vitro. Ostomy/Wound Manage. 1998;44:50–56. [PubMed] [Google Scholar]
- 14.Jett BD, Hatter KL, Huycke MM, Gilmore MS. Simplified agar plate method for quantifying viable bacteria. Biotechniques. 1997;23:648–650. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- 15.Boudreaux CP. Method and apparatus for ultraviolet radiation catheter sterilization system. 6,461,569. US patent. 2002
- 16.Bak J, Ladefoged SD, Tvede M, Begovic T, Gregersen A. Dose requirements for UVC disinfection of catheter biofilms. Biofouling. 2009;25:289–296. doi: 10.1080/08927010802716623. [DOI] [PubMed] [Google Scholar]
- 17.Eckhardt R, Jenkins GH, Kimball S. Method and apparatus for sterilizing or disinfecting catheter components. 102421. WIPO Patent Application. 2002