Abstract
Summary
The interferon-producing plasmacytoid dendritic cells (pDCs) share common progenitors with antigen-presenting classical dendritic cells (cDCs), yet they possess distinct morphology and molecular features resembling those of lymphocytes. It is unclear whether the unique cell fate of pDCs is actively maintained in the steady state. We report that the deletion of transcription factor E2-2 from mature peripheral pDCs caused their spontaneous differentiation into cells with cDC properties. This included the loss of pDC markers, increase in MHC class II expression and T cell priming capacity, acquisition of dendritic morphology and induction of cDC signature genes. Genome-wide chromatin immunoprecipitation revealed direct binding of E2-2 to key pDC-specific and lymphoid genes, as well as to certain genes enriched in cDCs. Thus, E2-2 actively maintains the cell fate of mature pDCs and opposes the “default” cDC fate, in part through direct regulation of lineage-specific gene expression programs.
Highlights
* Deletion of E2-2 from mature pDCs causes phenotypic conversion to cDC-like cells;
* E2-2-deficient pDCs acquire the morphology and gene expression signature of cDCs;
* E2-2 directly binds to multiple pDC-enriched target genes;
* E2-2 maintains Bcl11a and represses Id2 expression in pDCs
Introduction
Dendritic cells (DCs) of the immune system efficiently recognize pathogens through pattern recognition receptors such as Toll-like receptors (TLRs), secrete multiple cytokines and activate naïve T cells during primary responses. The latter property distinguishes them among other innate immune cell types, and establishes a key link between innate and adaptive immunity (Steinman and Idoyaga, 2010). In humans and in experimental animals, DCs are represented by two major lineages, classical or conventional DCs (cDCs) and plasmacytoid DCs (pDCs) (Merad and Manz, 2009). The cDCs show high surface expression of MHC class II (MHC II) and of the integrin CD11c, and even in the steady state have outstanding capacity for naïve T cell priming. Murine cDCs comprise two phenotypically and functionally distinct subsets, distinguished by surface expression of CD8α in the lymphoid organs and CD103 in tissues; similar subsets have been proposed for the human cDCs (Shortman and Heath, 2010). In contrast, pDCs are specialized in TLR-mediated recognition of viral nucleic acids and high-level secretion of type I interferon (IFN) in response to viruses (Liu, 2005; Swiecki and Colonna, 2010). The unique IFN secretion capacity of pDCs is facilitated by baseline expression of IRF7, the transcriptional “master regulator” of IFN response; conversely, it is tightly controlled by pDC-specific inhibitory receptors such as human ILT7 and BDCA-2 and murine Siglec-H (Gilliet et al., 2008). The pDCs express low MHC II and CD11c and display distinct markers such as CD45RA (also known as B220), Ly-6c and Bst2, which is highly specific for murine pDCs in the steady state (Blasius et al., 2006) and also serves as ILT7 ligand in humans (Cao et al., 2009). The pDCs in the steady state have poor T cell stimulatory capacity, whereas upon activation by viruses and TLR ligands they can efficiently present and cross-present Ag to T cells (Villadangos and Young, 2008).
Multiple lines of evidence suggest that cDCs and pDCs are closely related. First, both lineages are postulated to develop in the same pathway (Liu and Nussenzweig, 2010) from a bone marrow (BM) progenitor termed common DC progenitor (CDP), or pro-DC (Naik et al., 2007; Onai et al., 2007). This common developmental pathway critically depends on cytokine Flt3 ligand (Flt3L), its receptor Flt3 and downstream transcription factor Stat3 (Schmid et al., 2010). Second, genome-wide expression profiles of human and murine pDCs are most similar to those of the respective cDCs, but distinct from lymphocytes or myeloid cells (Robbins et al., 2008). Third, in vitro activation by viruses or cytokines induces human pDC differentiation into cells with morphological and functional properties of cDCs (Soumelis and Liu, 2006). In contrast, pDCs differ from cDCs in several key aspects. Most obviously, cDCs exhibit typical DC morphology with prominent cytoplasmic veils and protrusions (dendrites), whereas pDCs have round morphology of a secretory lymphocyte. Indeed, pDCs display many salient features of lymphocytes such as relatively long lifespan on the order of weeks, contrasted with cDC turnover within several days (Liu et al., 2007; O'Keeffe et al., 2002). In particular, pDCs show important molecular similarities with B lymphocytes, including shared markers (B220), nucleic acid-sensing TLRs (TLR7, TLR9), transcription factors (SpiB, Bcl11a) and a B cell receptor (BCR)-like signaling pathway that inhibits IFN secretion (Crozat et al., 2010; Gilliet et al., 2008). Moreover, pDCs express B cell-specific pIII isoform of MHC II transactivator CIITA, in contrast to the pI isoform expressed exclusively by cDCs (LeibundGut-Landmann et al., 2004). Finally, pDCs express multiple genes specific for developing T and B lymphocytes, such as human PTCRA and murine Dntt and Ccr9 (Reizis, 2010). Thus, the lineage identity of pDCs remains controversial, and their relationship to cDCs requires further genetic evidence.
Recently, we have shown that class I basic helix-loop-helix transcription factor (E protein) E2-2 (official symbol Tcf4) is a critical regulator of pDC lineage specification in mice and in humans (Cisse et al., 2008). E proteins are important regulators of lymphopoiesis, with other family members E2a and HEB controlling the development of B and T lymphocytes, respectively (D'Cruz et al., 2010; Kee, 2009). The activity of E proteins is antagonized by Id family transcription factors such as Id2, which prevent E protein binding to DNA. Murine and human mature pDCs express high E2-2 but low levels of Id2, whose overexpression blocks pDC development (Cisse et al., 2008; Nagasawa et al., 2008; Spits et al., 2000). E2-2-deficient hematopoietic cells cannot give rise to pDCs, whereas pDCs from mice and from human patients heterozygous for E2-2 manifest aberrant surface phenotype and defective IFN secretion (Cisse et al., 2008). In particular, Tcf4+/- pDCs show reduced expression of the genes that are specific for pDCs (Irf7) or shared between pDCs and lymphocytes (Dntt, Ccr9, Spib).
Based on these results, we hypothesized that E2-2 expression diverts pDCs from the common DC developmental pathway and imparts them with “lymphoid” features (Reizis, 2010). In this scenario, the resulting pDC fate can be either irreversibly “locked” by epigenetic mechanisms, or actively enforced by the presence of E2-2. To test these possibilities, we analyzed the consequences of constitutive or inducible loss of E2-2 in the peripheral pDCs in vivo. We now report that E2-2-deficient mature pDCs spontaneously acquire phenotypic, morphological and functional properties of cDCs. Furthermore, we provide evidence that E2-2 directly activates pDC-specific gene expression program, and might repress genes associated with the alternative cDC lineage. Therefore, continuous E2-2 expression in mature pDCs is required to maintain their unique lineage identity and antagonize the “default” cDC cell fate.
Results
Constitutive deletion of E2-2 in peripheral pDCs generates a cDC-like population
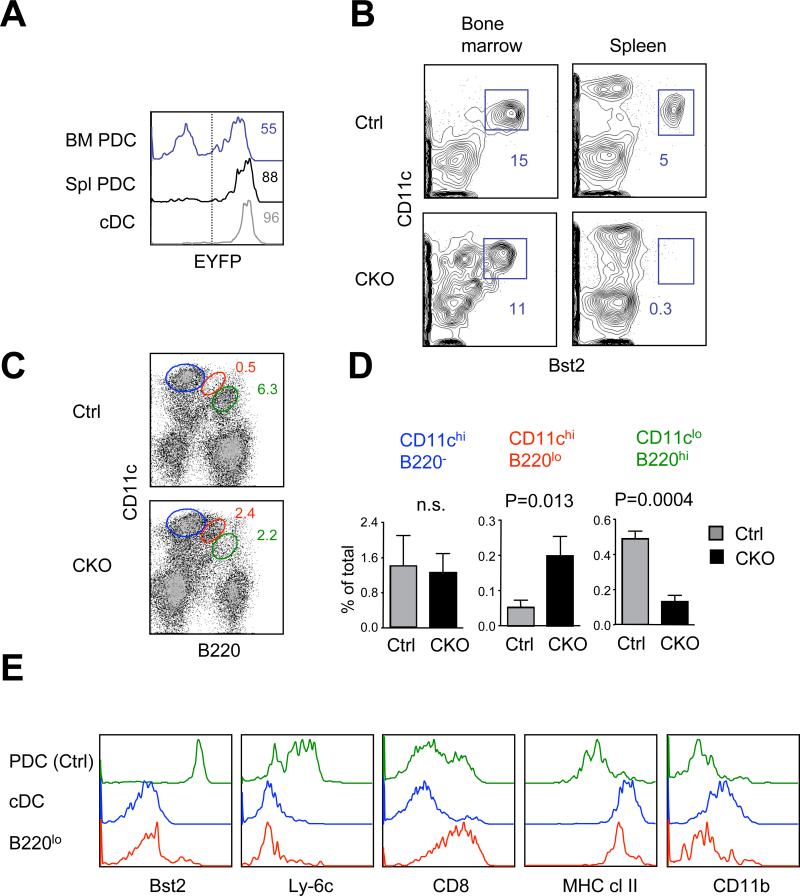
To test the role of E2-2 in pDC maintenance, we performed stage-specific recombination of the conditional E2-2 allele (Tcf4flox) in mature peripheral pDCs. To this end, we used the Itgax-Cre (also known as CD11c-Cre) deleter strain, in which recombination occurs in 50-60% of BM pDCs but increases to ~90% in splenic pDCs (Fig. 1A and ref. (Caton et al., 2007)). To increase the frequency and visibility of pDCs that underwent Cre recombination, we have combined a conditional and a germline null Tcf4 alleles and introduced Cre-inducible enhanced yellow fluorescent protein reporter allele (Gt(ROSA)26Sor-EYFP). In the resulting Itgax-Cre+ Tcf4flox/- Gt(ROSA)26Sor-EYFP+ conditional deletion mice, EYFP+ pDCs were readily detectable in the BM but completely absent from the spleen (Fig. 1B), suggesting that some pDCs develop but do not persist in the periphery. Notably, the analysis of splenic EYFP+ cells revealed a new CD11chigh B220low population appearing as an intermediate between pDCs and cDCs (Fig. 1C,D). These cells lacked pDC markers Bst2 and Ly-6c but expressed high levels of MHC II and CD8, consistent with the cDC phenotype (Fig. 1E).
Figure 1. E2-2 deletion in the periphery causes spontaneous pDC differentiation.
(A) Recombination efficiency in the pDCs from Itgax-Cre deleter strain, as measured by Cre-inducible EYFP reporter expression in Itgax-Cre+ Gt(ROSA)26Sor-EYFP+ mice. Shown are representative histograms of EYFP fluorescence in gated CD11clo Bst2+ pDCs from the BM or spleen, and in splenic CD11chi MHC II+ cDCs as a control. The positive threshold of EYFP fluorescence is indicated by a dashed line.
(B) The pDC population within Cre reporter-positive cells in the Gt(ROSA)26Sor-EYFP+ Itgax-Cre+ Tcf4flox/- conditional knockout (CKO) or Gt(ROSA)26Sor-EYFP+ Itgax-Cre+ control (Ctrl) mice. Shown are staining profiles of EYFP+ cells in the BM or spleen, with the fraction of CD11clo Bst2+ pDCs indicated.
(C) DC populations within Cre reporter-positive splenocytes in control and CKO mice. Shown are staining profiles of EYFP+ cells; highlighted are CD11chi B220- cDCs (blue), CD11clo B220+ pDCs (green) and the CD11chi B220low cDC-like population (red).
(D) The fraction among total splenocytes of the cell populations shown above (mean ± S.D. of 3 animals per genotype). N.s., not significant.
(E) Cell surface phenotype of the B220lo cDC-like population. Shown are staining histograms of EYFP+ splenocyte populations shown in (C), including pDCs from control mice and cDCs and the B220low cDC-like cells from CKO mice. Representative of three CKO animals.
We also analyzed fully conditional Itgax-Cre+ Tcf4flox/flox mice, which demonstrate a partial deletion of splenic pDCs (Cisse et al., 2008). Although less conspicuous without a Cre reporter, the same CD11chigh B220low MHC II+ Bst2- population was markedly elevated in the Itgax-Cre+ Tcf4flox/flox spleens (Fig. S1). On the other hand, this population was not observed in the animals reconstituted with Tcf4-/- hematopoietic progenitors, in which pDC development in the BM is completely blocked (data not shown). Therefore, this population likely represents the progeny of pDCs that escape early E2-2 deletion in the BM and lose E2-2 only in the periphery. These data suggest that the loss of E2-2 in peripheral pDCs causes their spontaneous differentiation into cells with cDC phenotype.
Inducible deletion of E2-2 in pDCs causes acquisition of cDC phenotype
To analyze the fate of mature pDCs after E2-2 deletion, we used Gt(ROSA)26Sor-CreER deleter mice that express tamoxifen (Tmx)-inducible Cre recombinase. High-dose Tmx administration to Gt(ROSA)26Sor-CreER+ Tcf4flox/flox mice leads to rapid pDC loss (Cisse et al., 2008). After the induction with a lower dose of Tmx, splenic pDCs were present in normal numbers at days 2-4 but largely disappeared by day 9 (data not shown). Because pDCs are relatively long-lived, we hypothesized that such rapid depletion may be caused by a defect in peripheral pDCs. We therefore analyzed E2-2-deficient splenic pDCs on day 6 after Tmx treatment, when pDC numbers were reduced by ~50% (Fig. 2A). Notably, pDC survival at this stage was not impaired (Fig. S2), suggesting that pDCs may undergo phenotypic conversion rather than cell death.
Figure 2. Inducible loss of E2-2 in mature pDCs causes differentiation into cDC-like cells.
(A) Reduction of PDC numbers shortly 6 after E2-2 deletion. Tamoxifen (Tmx)-inducible Gt(ROSA)26Sor-CreER+ Tcf4flox/flox CKO mice or Cre-negative littermate controls (Ctrl) were treated with Tmx and analyzed on day 6 after the last administration. Shown are absolute numbers of splenic cDC (CD11chi MHC II+) and PDC (CD11clow Bst2+) in the individual CKO and control mice. N.s., not significant.
(B) BrdU labeling of pre-existing pDCs. Tmx-inducible CKO and control mice were kept on BrdU for 14 days, followed by simultaneous 3-day Tmx treatment and BrdU withdrawal for 8 days. Shown is BrdU incorporation in the splenic CD8+ T cells, CD8+ CD11chi cDCs or CD11clo Bst2+ pDCs from control mice. The threshold of high-level BrdU staining is indicated by the dashed line.
(C) The fraction of pDCs among BrdUhi or BrdU- splenocytes pooled from 3-4 control and CKO animals.
(D) BrdU incorporation and B220 expression in the splenic CD8+ CD11chi MHC II+ cDCs from control and CKO mice. The percentages of B220+ BrdU- and B220+ BrdU+ cells are indicated.
(E) Cell surface phenotype of pDCs after Tmx-induced E2-2 deletion in PTCRA-EGFP reporter mice. Conditional PTCRA-EGFP+ Gt(ROSA)26Sor-CreER+ Tcf4flox/flox (CKO) mice or Cre-negative littermate controls (Ctrl) were treated with Tmx and analyzed 6 days later. Shown are the fractions among total splenocytes and the staining profiles of B220+ EGFP+ pDCs, with the CD11chigh MHC II+ cDC gate indicated. Whereas the fraction of EGFP+ pDCs was variable due to partial EGFP expression in pDCs (Shigematsu et al., 2004), their phenotypic changes are representative of >5 CKO animals.
(F) Staining histograms of gated B220+ EGFP+ pDCs from CKO and control mice (representative of 3 independent experiments).
To test whether acute E2-2 loss depletes pre-existing mature pDCs, Gt(ROSA)26Sor-CreER+ Tcf4flox/flox mice were fed with nucleotide analog bromodeoxyuridine (BrdU) for 2 weeks (“pulse”), followed by BrdU withdrawal (“chase”) at the start of Tmx treatment. In contrast to the CD8+ cDCs that largely turned over after 8 days of chase (corresponding to day 6 after Tmx treatment), a substantial fraction of pDCs retained BrdU (Fig. 2B). Notably, both BrdU+ (i.e. pre-existing) and BrdU- (i.e. newly generated) splenic pDCs were reduced in Gt(ROSA)26Sor-CreER+ Tcf4flox/flox mice at that point (Fig. 2C). Furthermore, the analysis of CD8+ cDCs in CKO spleens revealed a novel B220+ population that was ~50% BrdU+ (Fig. 2D). Because cDCs do not express E2-2 (Cisse et al., 2008) and their proliferation was not changed by E2-2 deletion (Fig. S2), the B220+ BrdU+ cDCs may have developed from pre-existing B220+ cells such as pDCs. Thus, the induction of E2-2 loss from mature splenic pDCs leads to their depletion, likely through differentiation into B220+ cells with CD8+ cDC phenotype.
To trace the fate of splenic pDCs after induced E2-2 deletion, we used human pre-T cell receptor α (PTCRA) transgenic mice that express enhanced green fluorescent protein (EGFP) specifically in pDCs (Shigematsu et al., 2004). Because EGFP protein is very stable without cell division and marks immediate progeny of EGFP+ cells (Boursalian et al., 2004), PTCRA-EGFP expression facilitates the tracing of pDC progeny. In control PTCRA-EGFP+ animals 6 days after Tmx treatment, splenic B220+ EGFP+ pDCs were uniformly Bst2+ CD11clow MHC IIlow (Fig. 2E). In contrast, >30% of B220+ EGFP+ splenocytes in Tmx-treated PTCRA-EGFP+ Gt(ROSA)26Sor-CreER+ Tcf4flox/flox animals lost Bst2 expression; this Bst2- pDC fraction showed a particularly strong upregulation of CD11c and MHC II nearly to the level of cDCs. Furthermore, B220+ EGFP+ E2-2-deficient cells downregulated CCR9 and Ly-6c, and upregulated CD8 in a larger fraction of cells (Fig. 2F and data not shown). The increase in MHC II occurred in both CD8low and CD8hi pDC populations (data not shown), suggesting that it is independent of the heterogeneous CD8 expression on pDCs.
To test whether similar phenotypic conversion may be observed in vitro, E2-2 deletion was induced in Flt3L-supplemented BM cultures. The expansion of CD11chi cDC-like cells at the expense of pDCs was observed as early as 2 days after Tmx addition to PTCRA-EGFP+ Gt(ROSA)26Sor-CreER+ Tcf4flox/flox cultures (Fig. S2). The rapid phenotypic changes suggest that E2-2 protein levels closely follow the transcript levels after deletion. Altogether, these data demonstrate that acute loss of E2-2 from mature pDCs causes their spontaneous conversion into cDC-like cells.
In view of these results, we tested whether pDC abnormalities in Tcf4+/- mice (Cisse et al., 2008) could be intrinsic to peripheral pDCs. Indeed, Tcf4+/- pDCs showed increased expression of CD8, CD11c and MHC II only in the spleen but not in the BM (data not shown). Furthermore, in PTCRA-EGFP+ Tcf4+/- mice EGFP+ pDCs upregulated CD11c and MHC II expression (Fig. S2), as observed after E2-2 deletion (Fig. 2E). Thus, peripheral pDCs in Tcf4+/- mice spontaneously upregulate cDC markers, suggesting that reduced E2-2 expression causes phenotypic conversion to cDC-like cells.
E2-2-deficient pDCs acquire cDC properties
To analyze the morphology of pDCs after acute E2-2 loss, sorted EGFP+ pDCs from Tmx-treated PTCRA-EGFP+ control and Gt(ROSA)26Sor-CreER+ Tcf4flox/flox mice were stained for actin cytoskeleton. As shown in Fig. 3A, the majority (72%) of control pDCs had a characteristic round perinuclear actin staining; the remaining cells showed only minimal changes of cell shape or actin cytoskeleton, and none had dendrites. In contrast, 67% of E2-2-deficient pDCs showed irregular shape and/or prominent actin remodeling, and 17% had overt dendrites similar to those of cDCs (Fig. 3A,B).
Figure 3. E2-2-deficient pDCs acquire morphological and functional properties of cDCs.
Conditional PTCRA-EGFP+ Gt(ROSA)26Sor-CreER+ Tcf4flox/flox (CKO) mice or Cre-negative littermate controls (Ctrl) were treated with Tmx, and 6-7 days later splenic EGFP+ CD11clo/int pDCs and EGFP- CD11chi cDCs were isolated by flow cytometry.
(A) Representative confocal photomicrographs of pDCs stained for actin cytoskeleton (phalloidin, red) and nuclear DNA (DAPI, blue). For control cells, the typical pDC morphology (72% of all cells) and the most extreme example of actin remodeling are shown. For CKO cells, the examples of pDC morphology (33% of all cells), actin remodeling and dendrites are shown, along with sorted cDCs as a positive control.
(B) Quantitative analysis of pDC morphology. Confocal microscopy images shown in panel (A) were scored for irregular cell shape, actin cytoskeleton and dendrites, and cumulative scores of individual control (n=55) and CKO (n=75) cells are shown.
(C) T cell priming capacity of pDCs. The pDCs or cDCs were sorted as above and cultured alone or with purified allogeneic CD4+ T cells, and thymidine incorporation was determined 72 hr later (mean ± S.D. of triplicate cultures).
Next, we tested the Ag presentation capacity of E2-2-deficient pDCs. Consistent with their low MHC II expression, unstimulated splenic pDCs cannot prime CD4+ T cell responses (Young et al., 2008). Indeed, sorted CD11clow EGFP+ pDCs from control PTCRA-EGFP+ animals failed to induce proliferation of naïve allogeneic CD4+ T cells (Fig. 3C). In contrast, CD11clow EGFP+ pDCs from Tmx-treated Gt(ROSA)26Sor-CreER+ Tcf4flox/flox mice induced a significant allogeneic response. A similar increase in T cell proliferation was observed in ovalbumin-specific priming of TCR transgenic CD4+ T cells by E2-2-deficient pDCs from ovalbumin-pulsed animals (data not shown). Thus, E2-2-deficient peripheral pDCs acquire dendritic morphology and capacity for CD4+ T cell priming, the salient features of cDCs.
E2-2-deficient pDCs upregulate cDC-associated genes
To characterize gene expression changes underlying the observed phenotypic and morphological conversion, we examined global expression profile of peripheral pDCs after E2-2 deletion. Control and Gt(ROSA)26Sor-CreER+ Tcf4flox/flox animals were treated with Tmx, and CD11clow Bst2+ pDCs were sorted on days 4 or 6 and analyzed by Affymetrix expression microarrays. The rigorous definition of pDCs as Bst2+ and the additional 4 day time point were used to focus on the earliest and most reliable changes of gene expression. Principal component analysis (PCA) was used to define probe sets that were coordinately induced or decreased in CKO pDCs at both time points (60 and 70 probes, respectively, out of 24,505 total probes; Fig. 4A and Table S1). The upregulated set included 3 probes for Cd8a, consistent with CD8 upregulation in E2-2-deficient pDCs (Table 1). Furthermore, many of the upregulated (16/60) and downregulated (14/70) probes were correspondingly changed in the previous microarray analysis of Tcf4+/- pDCs (Table 1 and Table S1).
Figure 4. E2-2-deficient pDCs upregulate cDC-enriched genes.
(A) Genes coordinately changed in pDCs on days 4 and 6 after E2-2 deletion. The pDCs were sorted from Gt(ROSA)26Sor-CreER+ Tcf4flox/flox (CKO) mice or littermate controls 4 and 6 days after Tmx treatment and subjected to genome-wide expression profiling using microarrays. Principal component analysis (PCA) was used to identify probes up- or downregulated in CKO samples on both days. Top panels show expression graphs of individual probes (grey) and the principal component (red); lower panels show expression graphs for the indicated representative probes. Data represent average log hybridization intensity of duplicate array samples.
(B) Expression profile of the genes upregulated in E2-2-deficient pDCs. For the 60 probes upregulated in pDCs after E2-2 deletion, normal expression values in major immune cell types were derived from GEO dataset GSE9810 and subjected to PCA. Shown are expression graphs for the two major principal components (cDC-enriched and cDC+B cell-enriched probes).
(C) The cDC subset specificity of the upregulated genes. The 60 probes upregulated in pDCs after E2-2 deletion were queried for their expression in the normal CD8- and CD8+ cDC subsets (GEO dataset GSE9810). Shown is log-ratio plot comparison of expression values in CD8- and CD8+ cDCs, with the probes overexpressed >3-fold highlighted in red and green, respectively. The three probes for Cd8a are circled.
(D) Quantitative analysis of gene expression in E2-2-deficient pDCs. The pDCs (CD11clo Bst2+) and cDCs (CD11chi Bst2-) were sorted from control and CKO animals 5 days after Tmx treatment and analyzed by qRT-PCR for microarray target genes (top row), and downregulated (middle row) and upregulated (bottom row) transcription factors. Data represent normalized expression values relative to the control pDC sample (mean ± S.D. of triplicate reactions).
Table 1. Differentially expressed genes in E2-2-deficient pDCs.
Sorted pDCs were analyzed by expression microarrays 4 or 6 days after Tmx-induced E2-2 deletion, and genes coordinately increased or reduced in E2-2-deficient compared to control pDCs were defined (Table S1). Shown are select target genes with the corresponding microarray probes; average fold change by microarray analysis; fold change determined by qRT-PCR in independently sorted pDCs on day 5 after Tmx treatment; >2-fold change, if any, in pDCs from Tcf4+/- mice heterozygous for E2-2 (Cisse et al., 2008); expression pattern as determined by PCA of normal expression values in GSE9810; conserved “expression signature” (Robbins et al., 2008); and high-confidence E2-2 binding in human pDCs as determined by ChIP-on-ChIP (Table S2). n.d., not done.
| Gene | Probe | Fold change | Fold change (qPCR) | Tcf4+/- PDC | Expression pattern (PCA) | Expression signature | E2-2 ChIP target |
|---|---|---|---|---|---|---|---|
|
Reduced | |||||||
| Notch3 | 1421965_s_at | 2.3 | n.d. | down | |||
| Fcrla | 1419907_s_at | 2.1 | 3.8 | down | PDC | yes | |
| Matn2 | 1455978_a_at | 2.3 | 2.2 | down | |||
| Sla2 | 1437504_at | 2.3 | 2.6 | PDC | yes | ||
|
Increased | |||||||
| Cd8a | 1444078_at, 1425335_at, 1440811_x_at | 2.5 | n.d. | up | |||
| H2-DMb2 | 1443686_at, 1443687_x_at | 2.6 | 2.9 | up | cDC+B | yes | |
| H2-Ob | 1440837_at | 2.4 | 4.1 | up | cDC+B | cDC | |
| Fscn1 | 1416514_a_at | 4.1 | 2.9 | cDC | cDC | ||
| S100a4 | 1424542_at | 4.8 | 4.3 | up | CD8- cDC | ||
| Pak1 | 1450070_s_at | 2.7 | 1.8 | cDC | cDC | ||
| Hspa1b | 1427126_at | 2.1 | n.d. | up | cDC | ||
| Tmem176a | 1441811_x_at | 4.0 | 1.7 | cDC | yes | ||
| 1100001H23Rik | 1448786_at | 2.4 | 1.9 | up | cDC | cDC | |
Next, we used PCA to analyze normal expression profiles of the differentially expressed genes. No clear pattern could be established among the downregulated genes, although several of these (Matn2, Fcrla, Sla2, Notch3) are prominently enriched in pDCs. In contrast, the upregulated genes comprised two principal components: enriched in cDCs (16/60 probes) and enriched in cDCs and B cells (9/60 probes) (Fig 4B). Moreover, seven of the upregulated genes belong to the evolutionarily conserved signature that distinguishes cDCs from pDCs (Robbins et al., 2008) (Table 1 and Table S1). Despite the induction of CD8, a substantial fraction of the upregulated genes (14/60) is characteristic of the CD8- rather than CD8+ cDC subset (Fig. 4C and Table S1). Thus, the resulting CD8+ cDC-like cells appear distinct from the bona fide CD8+ cDCs, and instead may resemble the recently described CD8-expressing cDC subset related to CD8- cDCs and pDCs (Bar-On et al., 2010). Functionally, several upregulated genes (Fscn1, Pak1, Dock4) control cytoskeletal organization, including Fscn1 as a known regulator of cDC morphology (Al-Alwan et al., 2001). Furthermore, MHC II presentation pathway genes (H2-DMb2, H2-Ob) were induced, consistent with the acquisition of cDC properties and the corresponding morphological and functional switch.
Next, cDCs and pDCs independently sorted from Gt(ROSA)26Sor-CreER+ Tcf4flox/flox mice 5 days after Tmx treatment were analyzed by quantitative RT-PCR (qPCR). This analysis confirmed microarray results and expression patterns in pDCs and cDCs for the relevant differentially expressed genes (Table 1 and Fig. 4D). In addition, CKO pDCs showed downregulation of pDC-enriched transcription factors Spib and Bcl11a and induction of Id2 and CIITA isoform pI, both of which are highly enriched in cDCs compared to pDCs (Fig. 4D). The downregulation of Bcl11a following E2-2 deletion was confirmed in BM-derived pDC cultures (Fig. S3). Overall, the changes of transcription factor profile are consistent with the onset of conversion to cDC-like cells by E2-2-deficient pDCs.
E2-2 directly controls the expression program of pDCs
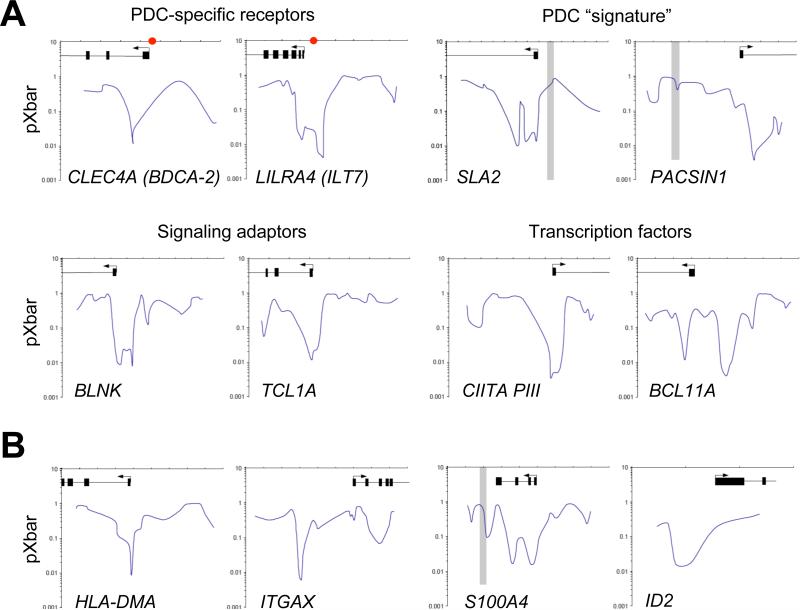
The data so far indicate that E2-2 maintains pDC cell fate and prevents spontaneous conversion into cDC-like cells. To test whether E2-2 mediates these effects through direct binding to the relevant genes, we performed chromatin immunoprecipitation followed by genome-wide promoter microarray hybridization (ChIP-on-chip) in the human pDC cell line CAL-1. The resulting probabilities of probe binding (pXbar) by E2-2-associated chromatin yielded binding profiles of E2-2 across the 5’ regions of genes (Fig. 5A,B). Using stringent criteria (pXbar <0.01, pXbar ratio of E2-2 and control ChIP <10), we have defined high-confidence list of 2,477 genes (corresponding to 4,369 probes, out of ~17,000 genes and 474,392 probes total) as E2-2 binding targets (Table S2). The list included several genes reduced in murine E2-2-deficient and/or E2-2+/- pDCs (SPIB, IRF7, FCRLA, SLA2, DNTT), suggesting these genes as conserved E2-2 targets (Table 2). Notably, the target list included 20 (36%) of 55 human pDC-specific genes compared to 6 (13%) of 47 genes expressed in both pDCs and myeloid cells; furthermore, 23% of conserved pDC “signature” genes (Robbins et al., 2008) represented E2-2 binding targets (Table S3). Indeed, several uniquely pDC-specific genes such as LILRA4 (encoding ILT7; Cao et al., 2006), PACSIN1 (Robbins et al., 2008) and CD2AP (Marafioti et al., 2008) were strongly bound by E2-2 (Table 2 and Fig. 5A). Moreover, E2-2 binding targets (Table 2) included key functional genes in pDCs such as transcription factors (SPIB, IRF7, CIITA pIII), components of TLR signaling pathway (TLR9, TLR7-TLR8, UNC93B1, BTK) and BCR-like signaling cascade that inhibits IFN response (DAP12, BCAP, BLNK) (Gilliet et al., 2008). These data suggest that E2-2 directly controls the specific gene expression program of mature pDCs.
Figure 5. Direct binding of E2-2 to target genes in pDCs.
(A) The binding of E2-2 to the 5’ regions of pDC-specific target genes in the human pDC cell line CAL-1. Chromatin immunoprecipitated with anti-E2-2 or control antibodies was labeled and hybridized to a microarray containing ~10 kb promoter regions of ~17,000 human genes. Shown are graphs of E2-2 binding probability (pXbar) over the 5’ gene regions, highlighting exons (filled boxes) and transcription start sites (arrows). Horizontal axis divisions correspond to 1 kb. Regions showing high background binding in control antibody hybridization (pXbar<0.1) are shaded in grey. Red dots represent the regions of binding determined by qPCR of unamplified chromatin (Cisse et al., 2008).
(B) The binding of E2-2 to the 5’ regions of cDC-enriched target genes in CAL-1, shown as above.
Table 2. Select targets of E2-2 binding in human pDCs.
ChIP-on-Chip with anti-E2-2 or control antibodies was performed in human pDC cell line CAL-1. Shown are select genes among the high-confidence list of E2-2 binding targets (Table S2), with the number of bound probes, rank of the highest signal probe, and binding probability P(Xbar) for E2-2 and control hybridizations. The TLR-mediated IFN induction pathway and pDC-specific IFN inhibitory pathway are reviewed in Gilliet et al. (Gilliet et al., 2008). Expression profiles are derived from the published data (Robbins et al., 2008).
| Gene | No. probes | Rank | P(Xbar) E2-2 | P(Xbar) Ctrl | Notes |
|---|---|---|---|---|---|
|
Pathogen sensing | |||||
| ZBP1 (DAI) | 2 | 10 | 0.002 | 0.9 | |
| PYCARD (ASC) | 3 | 152 | 0.003 | 0.6 | |
| TLR9 | 8 | 172 | 0.003 | 0.7 | IFN induction |
| TLR7-TLR8 * | 3 | 248 | 0.003 | 0.7 | IFN induction |
| UNC93B1 | 8 | 16 | 0.002 | 0.1 | IFN induction |
|
Surface receptors | |||||
| LILRA4 (ILT7) | 3 | 418 | 0.004 | 0.7 | IFN inhibition; PDC-specific |
| FCRLA | 3 | 1271 | 0.006 | 1.0 | |
|
Signal transduction | |||||
| BTK | 4 | 531 | 0.004 | 0.8 | IFN induction |
| TYROBP (DAP12) | 2 | 77 | 0.003 | 0.8 | IFN inhibition |
| PIK3AP1 (BCAP) | 2 | 128 | 0.003 | 0.9 | IFN inhibition |
| BLNK | 3 | 1766 | 0.008 | 0.9 | IFN inhibition |
|
Transcription factors | |||||
| Bcl11a | 3 | 205 | 0.003 | 0.4 | PDC, B cells |
| CIITA PIII | 5 | 209 | 0.003 | 1.0 | PDC, B cells |
| SPIB | 5 | 1065 | 0.006 | 0.3 | PDC, B cells |
| IRF7 | 2 | 453 | 0.004 | 0.3 | IFN induction; PDC-specific |
|
Other PDC-enriched | |||||
| PACSIN1 | 3 | 147 | 0.003 | 0.9 | PDC-specific |
| SLA2 | 1 | 2326 | 0.009 | 0.4 | PDC-enriched |
| CD2AP | 4 | 686 | 0.005 | 0.3 | PDC-enriched |
| PTCRA | 5 | 1295 | 0.006 | 0.5 | PDC (human), immature lymphocytes |
| DNTT | 4 | 178 | 0.003 | 0.7 | PDC (mouse), immature lymphocytes |
| BST2 | 4 | 307 | 0.004 | 0.4 | PDC-specific (mouse) |
Binding peak located in the genomic region between TLR7 and TLR8.
In addition to pDC-specific genes, E2-2 target list included genes associated with the alternative cDC fate, such as ITGAX and HLA-DMA (12.5% of conserved cDC “signature” genes (Robbins et al., 2008)). Furthermore, cDC-enriched genes induced in E2-2-deficient pDCs such as HLA-DMB, S100A4, TMEM176A and ID2 were specifically bound by E2-2 (Fig. 5B and Table S1). Thus, E2-2 may antagonize cDC cell fate in part by directly binding to and repressing cDC-associated genes.
Discussion
Here we demonstrate that the reduction or loss of transcription factor E2-2 from mature pDCs caused their differentiation into cDC-like cells. In Tcf4+/- animals, peripheral pDCs showed increased expression of cDC markers and MHC II, indicating a “drift” towards cDC-like phenotype. This explains the reduced pDC-specific gene expression and IFN secretion capacity in murine and human Tcf4+/- pDCs (Cisse et al., 2008), and suggests that the haploinsufficiency of E2-2 is primarily due to the defective maintenance of peripheral pDCs. The deletion of E2-2 from mature pDCs using constitutive stage-specific or inducible deletion systems resulted in the appearance of a CD8+ cDC-like population that retained some pDC markers such as B220 and PTCRA-EGFP. Further analysis of pDCs shortly after E2-2 deletion confirmed the acquisition of morphological and functional properties of cDCs, and the induction of cDC gene expression program. Because cDCs are very short-lived compared to pDCs, such differentiation accounts for the rapid disappearance of pDCs after E2-2 deletion. Overall, these data suggest that continuous expression of E2-2 is required to maintain the cell fate of mature pDCs and to prevent spontaneous cell fate conversion and eventual loss.
A major implication of the observed pDC conversion into cDC-like cells is that pDCs are genetically closer to cDCs than to any other lineage. The question of pDC origin and lineage identity has been highly controversial ever since the identification of pDCs as a separate cell type. Whereas common BM progenitors and dependence on Flt3 signaling suggest a close relationship with cDCs, pDCs show evidence of lymphoid origin such as D->J rearrangement of IgH and TCRβ loci, and history of Rag1 expression (Corcoran et al., 2003; Pelayo et al., 2005; Welner et al., 2009). Indeed, in almost every aspect of morphology and physiology pDCs resemble lymphocytes (particularly B cells) more than cDCs. To explain this apparent paradox, we proposed that pDCs are genetically and developmentally related to cDCs more than to any other lineage; however, the induction of E2-2 and the ensuing E protein-dependent gene expression program impart the unique lymphoid features upon pDCs (Reizis, 2010). Thus, the cDC cell fate may represent the “default” differentiation pathway of common DC progenitors, which is antagonized by E2-2 to specify pDC lineage. Our current results strongly support this model, and extend it to demonstrate active repression of the “default” cDC cell fate by E2-2.
Although E2-2-deficient pDCs upregulate CD8α, they do not seem to differentiate into the bona fide CD8+ cDCs. Indeed, CD8+ cDC-specific genes such as Tlr3, Xcr1 or Itgae were not increased (data not shown), whereas many CD8- cDC-enriched genes became upregulated. Notably, a similar CD8+ cDC population whose expression profile is related to CD8- cDCs and pDCs was recently identified in the lymphoid organs of naïve mice using the Cx3cr1-EGFP reporter allele (Bar-On et al., 2010). In particular, several genes upregulated in E2-2-deficient pDCs such as S100a4 and Trem176a are prominently expressed in the Cx3cr1-EGFP+ but not in the classical CD8+ cDCs. Moreover, Cx3cr1-EGFP+ CD8+ cDCs express low E2-2 but require E2-2 expression in the BM for their development, suggesting that they arise from committed pDC progenitors that downregulate E2-2. Thus, the loss of E2-2 from mature pDCs and “abortive” pDC development in the steady state yield similar CD8+ cDC subsets, which likely represent a common “default” fate of E2-2-deficient pDCs.
Human pDCs were first identified as efficient precursors of cDCs in the peripheral blood (Grouard et al., 1997), as they acquire functional and morphological cDC properties when cultured in vitro with TLR ligands and/or cytokines (Soumelis and Liu, 2006). On the other hand, virus-induced conversion of pDCs to CD8- cDCs was observed only in the BM but not in the spleen, suggesting that mature pDCs in vivo have lost this capacity (Zuniga et al., 2004). Notably, however, in vitro activated pDCs reduce E2-2 expression by ~75% after 2 days, consistent with the loss of pDC lineage identity (Cisse et al., 2008); furthermore, many E2-2 target genes such as Matn2 and Fcrla are prominently downregulated in activated pDCs (microarray data in Iparraguirre et al. (Iparraguirre et al., 2008)). Our observations suggest that E2-2 downregulation may play an active role after virus-induced pDC activation, e.g. by allowing pDC conversion into cDC-like cells and/or limiting IFN secretion. This hypothesis will be tested once a robust in vivo model of activation-induced pDC differentiation becomes available.
E2-2 is likely to maintain the cell fate of pDCs and oppose that of cDCs through several mechanisms. First, E2-2 binds to a large fraction of pDC-enriched genes by ChIP, including the key transcription factors and functional components of TLR signaling pathway and of inhibitory receptor signaling. These genes appear to be directly activated by E2-2, thereby contributing to pDC gene expression program and functionality. Second, direct targets of E2-2 may include pDC-specific transcriptional repressors that negatively regulate cDC-enriched genes. One attractive candidate is Bcl11a, a transcription factor that is highly expressed in murine and human pDCs and B cells (Marafioti et al., 2008; Pelayo et al., 2005). Moreover, Bcl11a is required for the development of B cells (Liu et al., 2003) as well as of pDCs (P. Tucker, personal communication). We found that BCL11A promoter is a prominent E2-2 binding target, and that Bcl11a expression is reduced after E2-2 deletion in pDCs. This is consistent with the recent identification of Bcl11a as a major direct target of E protein E2a in B lymphocytes (Lin et al., 2010). Because Bcl11a is a bona fide transcriptional repressor, it may at least partially mediate the repression of cDC-associated gene expression by E2-2.
In addition, E2-2 might directly repress at least certain cDC genes, as suggested by E2-2 binding to several such genes in a pDC cell line. This may be particularly relevant for the genes that are expressed in pDCs but at lower levels relative to cDCs, such as Itgax (CD11c) and MHC class II pathway genes. Although E proteins primarily activate transcription, they are also capable of repression in some cases (Kee, 2009). Notably, one apparent target of E2-2-mediated repression is Id2, an antagonist of E proteins. Id2 is abundantly expressed in cDCs and is required for the development of certain cDC subsets such as Langerhans cells and CD8+ cDCs (Hacker et al., 2003); conversely, its overexpression blocks pDC development in vitro (Spits et al., 2000). Thus, the reduction of E2-2 may initiate a feed-forward loop that further inhibits E2-2 through Id2 induction and amplifies the loss of pDC cell fate. These data suggest a dynamic control of pDC and cDC cell fate through the bi-directional inhibition between E2-2 and Id2.
Recent evidence suggests that “terminal” differentiation state of a cell is in fact reversible, as first demonstrated by direct genetic reprogramming of mature B lymphocytes into macrophages (Xie et al., 2004). Furthermore, differentiated state is often maintained by continuous expression of a single transcriptional “master regulator”, and its removal may cause transdifferentiation into a reciprocal cell fate. For instance, deletion of Pax5 from mature B cells leads to de-differentiation into lymphoid progenitors that give rise to T cells (Cobaleda et al., 2007). Likewise, the loss of FoxP3 from mature regulatory T cells abolishes their maintenance and causes autoimmunity (Williams and Rudensky, 2007). Our work provides another example of a postmitotic, long-lived, highly specialized immune cell type whose identity is actively maintained and reversible. These results reveal a close genetic relationship between pDCs and cDCs, and establish a model to elucidate their unique genetic programs and functions.
Supplementary Material
Acknowledgements
We thank D. Holmberg, Y. Zhuang, T. Ludwig and F. Costantini for animal strains, T. Maeda for CAL-1 cells, C. Murre for anti-E2-2 antibody, P. Tucker and S. Jung for communicating unpublished data, and all members of the Reizis lab for help and discussions. Supported by the SPAR-American Asthma Foundation Award (B.R.), NIH awards AI072571, AI072571-S1 and AI085439 (B.R.), and NIH training awards AI007161 (K.L.L.), GM007367 and AI080184 (B.C.).
Methods
Animals
The PTCRA-EGFP (129/SvEvTac, 129) (Shigematsu et al., 2004), Tcf4+/- (129) (Zhuang et al., 1996), Tcf4flox (C57BL/6, B6) (Bergqvist et al., 2000), Itgax-Cre (B6) (Caton et al., 2007), Gt(ROSA)26Sor-CreER (B6) (Cisse et al., 2008) and Gt(ROSA)26Sor-EYFP (mixed B6/129) (Srinivas et al., 2001) mouse strains were used or intercrossed as indicated. For BrdU incorporation, mice were injected with 1 mg BrdU i.p. and then maintained for 2 weeks on drinking water with 0.8 mg/mL BrdU (Sigma-Aldrich). For the induction of Cre recombination in Gt(ROSA)26Sor-CreER+ mice, tamoxifen (Sigma) was administered orally for three consecutive days (1 mg in 0.1 ml sunflower oil per day). Because CreER induction per se does not affect pDC numbers or phenotype (Cisse et al., 2008), Cre-negative Tcf4flox/flox littermates were used as controls for Tcf4flox/flox Gt(ROSA)26Sor-CreER+ animals. All animal studies were performed according to the investigator's protocol approved by the Institutional Animal Care and Use Committee of Columbia University.
Cell analysis
Single-cell suspensions were stained for multicolor analysis with the indicated fluorochrome-conjugated antibodies (BD PharMingen or eBioscience). For analysis and of Gt(ROSA)26Sor-CreER+ animals, DCs and pDCs were enriched by negative selection against CD3+ and CD19+ lymphocytes using MACS columns (Miltenyi Biotech). The samples were acquired using the LSR II flow cytometer or sorted on FACSAria flow sorter (BD Biosciences), and analyzed using FlowJo software (TreeStar). For BrdU incorporation, cells from 3-4 pooled spleens were enriched for DCs and pDCs by negative selection, stained for surface markers, fixed, permeabilized, and stained with an antibody against BrdU according to the BD PharMingen BrdU Flow Kit protocol.
For cell morphology analysis, splenic pDCs (CD11clo EGFP+) and cDCs (CD11chigh EGFP-) from PTCRA-EGFP+ Gt(ROSA)26Sor-CreER+ Tcf4flox/flox mice and Cre-negative littermate controls 7 days after Tmx treatment were sorted onto positively charged glass slides. Cells were fixed with 3% paraformaldehyde for 15 min. at 37°C, washed in PBS and incubated with 100 mM glycine for 10 min at room temperature. Fixed cells were lysed with 0.1% Triton X-100 for 4 min, washed with PBS, blocked with 1% BSA and stained with Rhodamine-phalloidin (Cytoskeleton, Inc.) and DAPI. Images were acquired using LSM 710 NLO confocal microscope and ConfoCor 3 imager (Carl Zeiss). For semi-quantitative analysis of cell morphology, images of individual pDCs in the same central plane were coded and scored by a blinded observer for three categories: irregular shape, actin rearrangement to cell periphery, and dendrites. Each category was scored from 0 (none) to 3 (maximal), and a cumulative score was derived for each cell.
T cell proliferation
Splenic CD11c+ cells from PTCRA-EGFP+ Gt(ROSA)26Sor-CreER+ Tcf4flox/flox mice and Cre-negative littermate controls 7 days after Tmx treatment were enriched by positive selection using MACS columns (Miltenyi Biotech), and pDCs (CD11clow EGFP+) and cDCs (CD11chigh EGFP-) were sorted. Allogeneic CD4+ T cells (>95% purity) were isolated from spleens of Balb/c mice using negative selection of CD11c, B220, Ter119, CD8, DX5 and CD11b-expressing cells on MACS columns. Cells were cultured for 72 hrs at 1:20 DC to T cell ratio, and [3H]-thymidine incorporation for the last 18 hr of culture was determined using β-counter.
Gene expression analysis
For microarray analysis, Gt(ROSA)26Sor-CreER+ Tcf4flox/flox mice and Cre-negative littermate controls were treated with Tmx, and 4 or 6 days later splenocytes were isolated, pooled and enriched for lineage (CD3, CD19)-negative cells using MACS columns. The pDCs (CD11b- B220+ CD11clo Bst2+) were sorted directly into Trizol reagent (Invitrogen), and total RNA was reverse-transcribed, amplified, labeled, and hybridized in duplicates to Mouse Genome 430 2.0 arrays (Affymetrix) as described (Cisse et al., 2008). Principal component analysis was done using the NIA Array software (Sharov et al., 2005). Normal gene expression profiles were derived from GEO dataset GSE9810 (Robbins et al., 2008).
For qRT-PCR analysis, RNA from pDCs or cDCs (CD11chigh Bst2-) isolated as above on day 5 after Tmx treatment was reverse transcribed and assayed by SYBR Green-based real-time PCR with MX3000P instrument (Stratagene). The expression of all genes was normalized to that of β-actin and expressed relative to the indicated reference sample via the ΔΔCT method. All primers were validated for linear amplification (sequences available upon request).
Chromatin immunoprecipitation
Cells of the human pDC lymphoma line CAL-1 (Maeda et al., 2005) were crosslinked with formaldehyde, sonicated, and subjected to immunoprecipitation with anti-E2-2 mAb (Bain et al., 1993) or mouse IgG control as described (Cisse et al., 2008). After crosslink reversal, the isolated chromatin was amplified, labeled and hybridized to Human Promoter ChIP-on-chip Microarray Set (Agilent Technologies) according to manufacturer's instructions. Hybridized microarrays were scanned and analyzed using DNA Analytics software (Agilent Technologies).
Statistical analysis
Statistical significance was estimated using unpaired, two-tailed Student's t-test.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession Numbers
Microarray data for gene expression and chromatin immunoprecipitation analysis have been deposited in the GEO database under accession number GSE24785.
References
- Al-Alwan MM, Rowden G, Lee TD, West KA. Fascin is involved in the antigen presentation activity of mature dendritic cells. J Immunol. 2001;166:338–345. doi: 10.4049/jimmunol.166.1.338. [DOI] [PubMed] [Google Scholar]
- Bain G, Gruenwald S, Murre C. E2A and E2-2 are subunits of B-cell-specific E2-box DNA-binding proteins. Mol Cell Biol. 1993;13:3522–3529. doi: 10.1128/mcb.13.6.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-On L, Birnberg T, Lewis KL, Edelson BT, Bruder D, Hildner K, Buer J, Murphy KM, Reizis B, Jung S. CX3CR1+ CD8{alpha}+ dendritic cells are a steady-state population related to plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2010;107:14745–14750. doi: 10.1073/pnas.1001562107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergqvist I, Eriksson M, Saarikettu J, Eriksson B, Corneliussen B, Grundstrom T, Holmberg D. The basic helix-loop-helix transcription factor E2-2 is involved in T lymphocyte development. Eur J Immunol. 2000;30:2857–2863. doi: 10.1002/1521-4141(200010)30:10<2857::AID-IMMU2857>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J Immunol. 2006;177:3260–3265. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nat Immunol. 2004;5:418–425. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- Cao W, Bover L, Cho M, Wen X, Hanabuchi S, Bao M, Rosen DB, Wang YH, Shaw JL, Du Q, et al. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med. 2009;206:1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Rosen DB, Ito T, Bover L, Bao M, Watanabe G, Yao Z, Zhang L, Lanier LL, Liu YJ. Plasmacytoid dendritic cell-specific receptor ILT7-Fc epsilonRI gamma inhibits Toll-like receptor-induced interferon production. J Exp Med. 2006;203:1399–1405. doi: 10.1084/jem.20052454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisse B, Caton ML, Lehner M, Maeda T, Scheu S, Locksley R, Holmberg D, Zweier C, den Hollander NS, Kant SG, et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobaleda C, Jochum W, Busslinger M. Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors. Nature. 2007;449:473–477. doi: 10.1038/nature06159. [DOI] [PubMed] [Google Scholar]
- Corcoran L, Ferrero I, Vremec D, Lucas K, Waithman J, O'Keeffe M, Wu L, Wilson A, Shortman K. The lymphoid past of mouse plasmacytoid cells and thymic dendritic cells. J Immunol. 2003;170:4926–4932. doi: 10.4049/jimmunol.170.10.4926. [DOI] [PubMed] [Google Scholar]
- Crozat K, Guiton R, Guilliams M, Henri S, Baranek T, Schwartz-Cornil I, Malissen B, Dalod M. Comparative genomics as a tool to reveal functional equivalences between human and mouse dendritic cell subsets. Immunol Rev. 2010;234:177–198. doi: 10.1111/j.0105-2896.2009.00868.x. [DOI] [PubMed] [Google Scholar]
- D'Cruz LM, Knell J, Fujimoto JK, Goldrath AW. An essential role for the transcription factor HEB in thymocyte survival, Tcra rearrangement and the development of natural killer T cells. Nat Immunol. 2010;11:240–249. doi: 10.1038/ni.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008 doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185:1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker C, Kirsch RD, Ju XS, Hieronymus T, Gust TC, Kuhl C, Jorgas T, Kurz SM, Rose-John S, Yokota Y, Zenke M. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat Immunol. 2003;4:380–386. doi: 10.1038/ni903. [DOI] [PubMed] [Google Scholar]
- Iparraguirre A, Tobias JW, Hensley SE, Masek KS, Cavanagh LL, Rendl M, Hunter CA, Ertl HC, von Andrian UH, Weninger W. Two distinct activation states of plasmacytoid dendritic cells induced by influenza virus and CpG 1826 oligonucleotide. J Leukoc Biol. 2008;83:610–620. doi: 10.1189/jlb.0807511. [DOI] [PubMed] [Google Scholar]
- Kee BL. E and ID proteins branch out. Nat Rev Immunol. 2009;9:175–184. doi: 10.1038/nri2507. [DOI] [PubMed] [Google Scholar]
- LeibundGut-Landmann S, Waldburger JM, Reis e Sousa C, Acha-Orbea H, Reith W. MHC class II expression is differentially regulated in plasmacytoid and conventional dendritic cells. Nat Immunol. 2004;5:899–908. doi: 10.1038/ni1109. [DOI] [PubMed] [Google Scholar]
- Lin YC, Jhunjhunwala S, Benner C, Heinz S, Welinder E, Mansson R, Sigvardsson M, Hagman J, Espinoza CA, Dutkowski J, et al. A global network of transcription factors, involving E2A, EBF1 and Foxo1, that orchestrates B cell fate. Nat Immunol. 2010;11:635–643. doi: 10.1038/ni.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev. 2010;234:45–54. doi: 10.1111/j.0105-2896.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat Immunol. 2007;8:578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- Liu P, Keller JR, Ortiz M, Tessarollo L, Rachel RA, Nakamura T, Jenkins NA, Copeland NG. Bcl11a is essential for normal lymphoid development. Nat Immunol. 2003;4:525–532. doi: 10.1038/ni925. [DOI] [PubMed] [Google Scholar]
- Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- Maeda T, Murata K, Fukushima T, Sugahara K, Tsuruda K, Anami M, Onimaru Y, Tsukasaki K, Tomonaga M, Moriuchi R, et al. A novel plasmacytoid dendritic cell line, CAL-1, established from a patient with blastic natural killer cell lymphoma. Int J Hematol. 2005;81:148–154. doi: 10.1532/ijh97.04116. [DOI] [PubMed] [Google Scholar]
- Marafioti T, Paterson JC, Ballabio E, Reichard KK, Tedoldi S, Hollowood K, Dictor M, Hansmann ML, Pileri SA, Dyer MJ, et al. Novel markers of normal and neoplastic human plasmacytoid dendritic cells. Blood. 2008;111:3778–3792. doi: 10.1182/blood-2007-10-117531. [DOI] [PubMed] [Google Scholar]
- Merad M, Manz MG. Dendritic cell homeostasis. Blood. 2009;113:3418–3427. doi: 10.1182/blood-2008-12-180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa M, Schmidlin H, Hazekamp MG, Schotte R, Blom B. Development of human plasmacytoid dendritic cells depends on the combined action of the basic helix-loop-helix factor E2-2 and the Ets factor Spi-B. Eur J Immunol. 2008;38:2389–2400. doi: 10.1002/eji.200838470. [DOI] [PubMed] [Google Scholar]
- Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, Carotta S, O'Keeffe M, Bahlo M, Papenfuss A, et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol. 2007;8:1217–1226. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- O'Keeffe M, Hochrein H, Vremec D, Caminschi I, Miller JL, Anders EM, Wu L, Lahoud MH, Henri S, Scott B, et al. Mouse plasmacytoid cells: long-lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8(+) dendritic cells only after microbial stimulus. J Exp Med. 2002;196:1307–1319. doi: 10.1084/jem.20021031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- Pelayo R, Hirose J, Huang J, Garrett KP, Delogu A, Busslinger M, Kincade PW. Derivation of 2 categories of plasmacytoid dendritic cells in murine bone marrow. Blood. 2005;105:4407–4415. doi: 10.1182/blood-2004-07-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizis B. Regulation of plasmacytoid dendritic cell development. Curr Opin Immunol. 2010;22:206–211. doi: 10.1016/j.coi.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins SH, Walzer T, Dembele D, Thibault C, Defays A, Bessou G, Xu H, Vivier E, Sellars M, Pierre P, et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9:R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid MA, Kingston D, Boddupalli S, Manz MG. Instructive cytokine signals in dendritic cell lineage commitment. Immunol Rev. 2010;234:32–44. doi: 10.1111/j.0105-2896.2009.00877.x. [DOI] [PubMed] [Google Scholar]
- Sharov AA, Dudekula DB, Ko MS. A web-based tool for principal component and significance analysis of microarray data. Bioinformatics. 2005;21:2548–2549. doi: 10.1093/bioinformatics/bti343. [DOI] [PubMed] [Google Scholar]
- Shigematsu H, Reizis B, Iwasaki H, Mizuno S, Hu D, Traver D, Leder P, Sakaguchi N, Akashi K. Plasmacytoid dendritic cells activate lymphoid-specific genetic programs irrespective of their cellular origin. Immunity. 2004;21:43–53. doi: 10.1016/j.immuni.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- Soumelis V, Liu YJ. From plasmacytoid to dendritic cell: morphological and functional switches during plasmacytoid pre-dendritic cell differentiation. Eur J Immunol. 2006;36:2286–2292. doi: 10.1002/eji.200636026. [DOI] [PubMed] [Google Scholar]
- Spits H, Couwenberg F, Bakker AQ, Weijer K, Uittenbogaart CH. Id2 and Id3 inhibit development of CD34(+) stem cells into predendritic cell (pre-DC)2 but not into pre-DC1. Evidence for a lymphoid origin of pre-DC2. J Exp Med. 2000;192:1775–1784. doi: 10.1084/jem.192.12.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Idoyaga J. Features of the dendritic cell lineage. Immunol Rev. 2010;234:5–17. doi: 10.1111/j.0105-2896.2009.00888.x. [DOI] [PubMed] [Google Scholar]
- Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev. 2010;234:142–162. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29:352–361. doi: 10.1016/j.immuni.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Welner RS, Esplin BL, Garrett KP, Pelayo R, Luche H, Fehling HJ, Kincade PW. Asynchronous RAG-1 expression during B lymphopoiesis. J Immunol. 2009;183:7768–7777. doi: 10.4049/jimmunol.0902333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol. 2007;8:277–284. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- Xie H, Ye M, Feng R, Graf T. Stepwise reprogramming of B cells into macrophages. Cell. 2004;117:663–676. doi: 10.1016/s0092-8674(04)00419-2. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wilson NS, Schnorrer P, Proietto A, ten Broeke T, Matsuki Y, Mount AM, Belz GT, O'Keeffe M, Ohmura-Hoshino M, et al. Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nat Immunol. 2008;9:1244–1252. doi: 10.1038/ni.1665. [DOI] [PubMed] [Google Scholar]
- Zhuang Y, Cheng P, Weintraub H. B-lymphocyte development is regulated by the combined dosage of three basic helix-loop-helix genes, E2A, E2-2, and HEB. Mol Cell Biol. 1996;16:2898–2905. doi: 10.1128/mcb.16.6.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga EI, McGavern DB, Pruneda-Paz JL, Teng C, Oldstone MB. Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection. Nat Immunol. 2004;5:1227–1234. doi: 10.1038/ni1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.