Abstract
The isolation of 2-bromo-1-hydroxyphenazine from a marine Streptomyces sp., strain CNS284, and its activity against NFκB, suggested that a short and flexible route for the synthesis of this metabolite and a variety of phenazine analogues be developed. Numerous phenazines were subsequently prepared and evaluated as inducers of quinone reductase 1 (QR1) and inhibitors of quinone reductase 2 (QR2), NF-κB, and inducible nitric oxide synthase (iNOS). Several of the active phenazine derivatives displayed IC50 values vs. QR1 induction and QR2 inhibition in the nanomolar range, suggesting they may find utility as cancer chemopreventive agents.
Introduction
In 2008, cancer accounted for almost 8 millions deaths worldwide1 and it is the second leading cause of death in the United States.2, 3 Moreover, the number of cases is expected to increase by over 45% in the next 20 years.3 Even with the recent advances in chemotherapy, the best strategy for dealing with the cancer problem is simply to prevent it from developing. Chemoprevention aims to prevent, delay, and ultimately reverse cancer development.4 A strategy to avoid carcinogenesis is to avert the metabolic formation of carcinogens.5 This may be achieved by inhibition of phase I enzymes,6 such as aromatase.7 These enzymes modify potential carcinogens, rendering them more active.7, 8 In some cases, quinone reductase 2 (QR2) has behaved like a phase I enzyme by converting quinone substrates into species capable of causing carcinogenesis.9–11 The same end could be achieved by induction of phase II enzymes, such as quinone reductase 1 (QR1)12–15 or glutathione S-transferase (GST),16 which detoxify carcinogens.17 Finally, the transcription factor NF-κB18, 19 and inducible nitric oxide synthase (iNOS)20 are also regarded as potential chemopreventive targets.
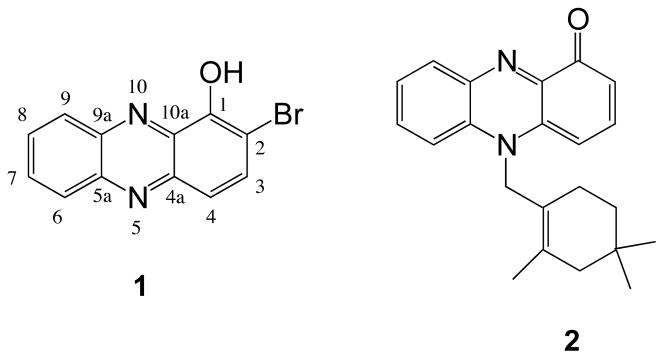
Small molecules that can affect more than one pathway or interact with multiple targets are especially attractive candidates for chemoprevention.21–27 Molecules of the phenazine class have been observed as secondary metabolites from Streptomyces, Pseudomonas, and other marine microorganisms.28 Natural or synthetic phenazines have shown antibiotic, anticancer, antifungal and neuroprotective properties.29–31 For example, 2-bromo-1-hydroxyphenazine (1, Figure 1)32 and lavanducyanin (2, Figure 1)33, 34 present interesting biological activities, including cytotoxicity against cancer cells and antibiotic activity.
Figure 1.
Phenazine Natural Products
As described in the present report, the natural product 1, which was isolated by activity-directed fractionation of a Streptomyces sp., strain CNS284, was highly cancer cell cytotoxic (IC50 = 0.1 μM against HCT-116) and showed weak to moderate activity in the NF-κB-luciferase assay (IC50 = 73 μM). This result suggested further exploration of the chemopreventive activities of analogues of phenazine 1. The phenazines 8, 9, and 10 were obtained during the synthesis of the natural product 1. Examination of these compounds in a panel of chemopreventive targets revealed surprising activities vs. a number of them. Phenazine 8 inhibited inducible nitric oxide synthase activity (IC50 4.2 μM), while compound 9 doubled the concentration of quinone reductase 1 at a concentration of 29.4 μM, and the phenazine 10 inhibited quinone reductase 2 (IC50 18.5 μM). These results suggested the further optimization of these activities through structure manipulation. However, in view of the disparate targets and inadequate knowledge of the mechanisms of action involved with a number of them, and the complete lack of any information about the chemopreventive activities of any phenazines, the initial approach has necessarily been empirical, consisting mainly in synthesis of a random array substituted phenazines that were brominated, chlorinated, methylated, methoxylated, and nitrated in various locations. The activities of the resulting compounds were determined vs. QR1 induction, QR2 inhibition, inducible nitric oxide synthase inhibition, and NF-κB inhibition. Although several reports have evaluated the biological activities of phenazines,28–34 to our knowledge, this is the first report on their chemopreventive properties, including QR1 induction and QR2 inhibition.
Results and Discussion
Chemistry
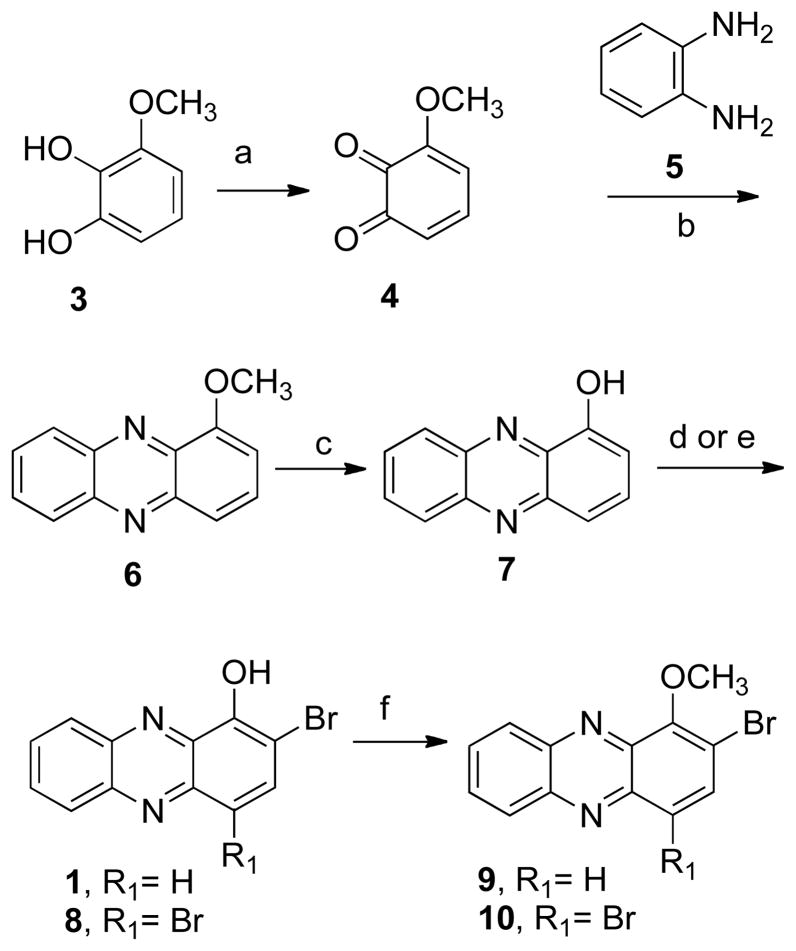
The core structure, 1-methoxyphenazine (6), was prepared following published procedures (Scheme 1).35, 36 Briefly, 1-methoxycatechol (3) was oxidized with o-chloranil and then condensed with o-phenylenediamine (5). Demethylation under standard conditions35 yielded 7. Subsequent bromination with one equivalent of N-bromosuccinimide gave the natural product 2-bromo-1-hydroxyphenazine (1). The spectral properties of the synthetic compound were identical with those of the natural product isolated from the marine Streptomyces sp., strain CNS284. Treatment of compound 7 with two equivalents of N-bromosuccinimide yielded compound 8. This compound showed a good QR1 induction ratio (IR) of 2.8; consequently, the position of the bromine substituent was altered to study the effect on activity.
Scheme 1a.
aReagents and conditions: (a) o-chloranil, ether;
(b) PhH, HOAc; (c) HBr, HOAc, reflux;
(d) 1 eq NBS, PhCH3; (e) 2 eq NBS, PhCH3;
(f) MeI, K2CO3, acetone.
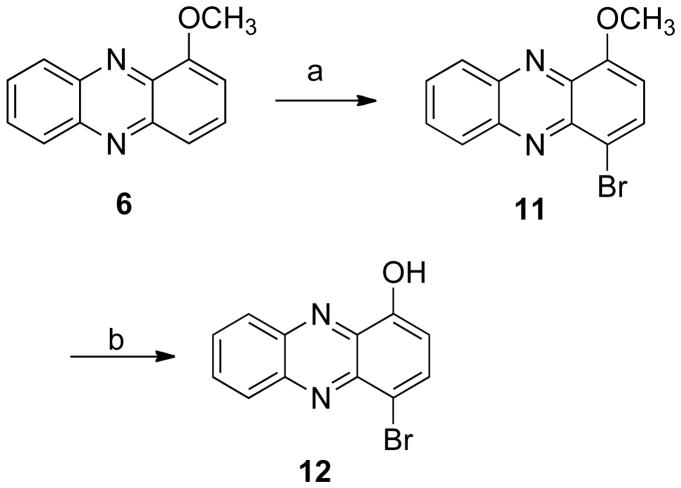
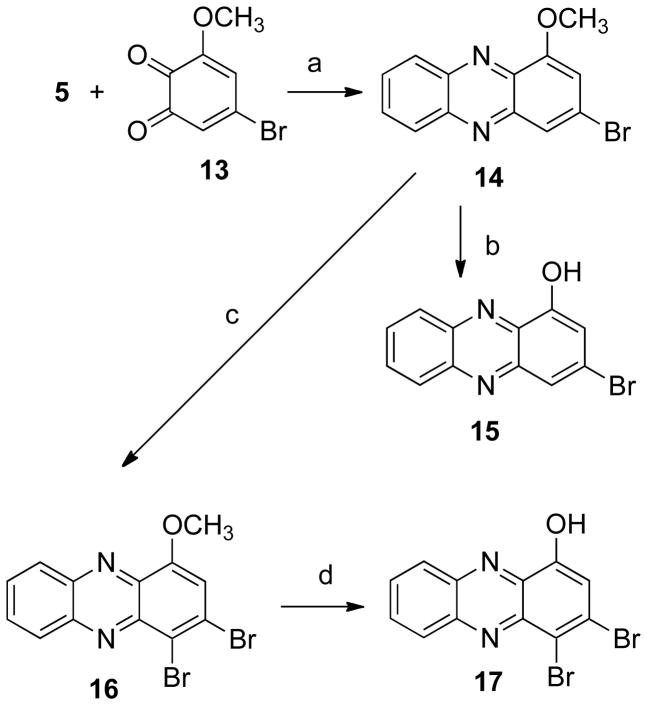
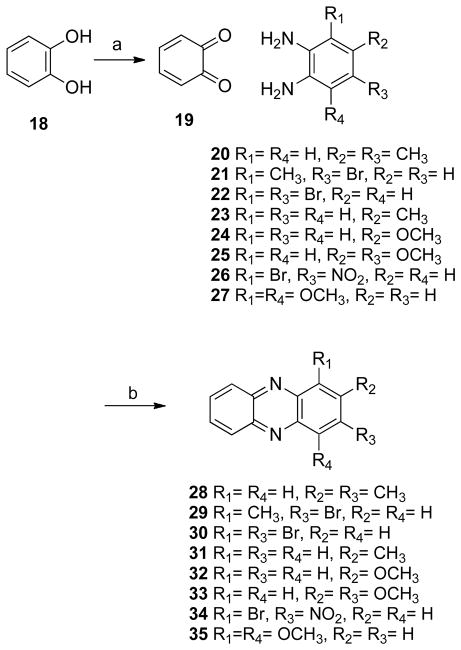
The para-substituted phenazines 11 and 12 were synthesized by bromination and demethylation of compound 6 (Scheme 2). The synthesis of the m-bromo derivative 14 (Scheme 3) was achieved by condensation of the appropriate quinone 13 with o-phenylenediamine (5). Subsequent deprotection, bromination, and demethylation gave the analogues 15–17. In addition, a series of simple mono- and di-substituted phenazines, 28–35, were prepared in order to study the influence that different substitution patterns have on the biological activity (Scheme 4).
Scheme 2a.
aReagents and conditions: (a) 1 eq NBS, PhCH3;
(b) BBr3, CH2Cl2.
Scheme 3a.
aReagents and conditions: (a) PhH, HOAc;
(b) HBr, HOAc; (c) NH4OAc, Br2, HOAc;
(d) BBr3, CH2Cl2.
Scheme 4a.
aReagents and conditions: (a) Ag2O, acetone;
(b) PhH, HOAc.
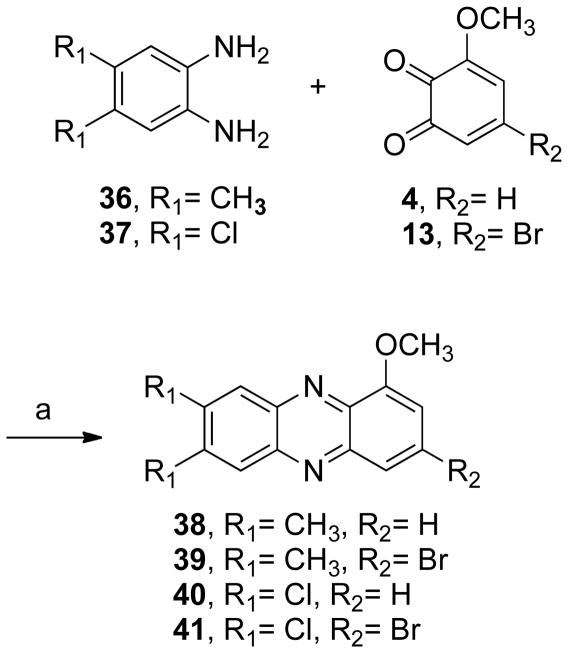
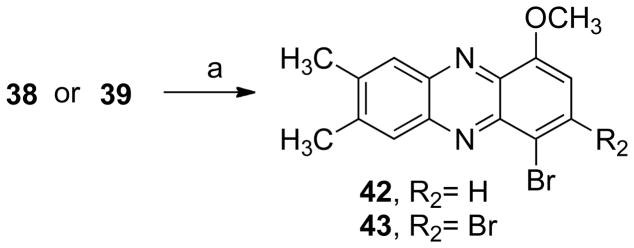
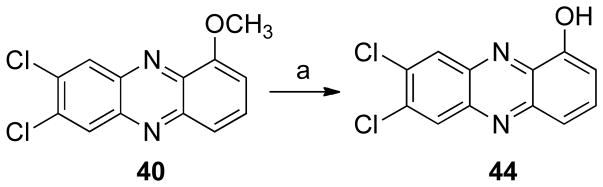
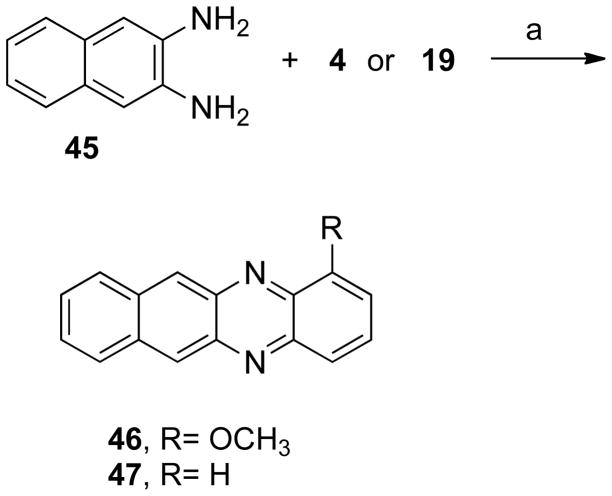
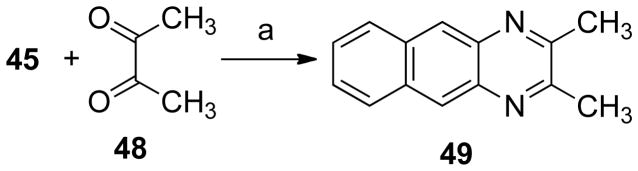
The next step consisted in the addition of methyl groups (38 and 39) or chloride (40 and 41) to the phenazine system (Scheme 5). This series also included some bromide substituents (39, 41–43, Schemes 5 and 6). Compound 40 was demethylated yielding 44 (Scheme 7). In addition, the benzo[b]phenazine analogues 46 and 47 were synthesized (Schemes 8). In compound 49 (Scheme 9), the positions of the nitrogens were changed. Finally, quinoxaline analogues of the phenazines were synthesized using the same condensation reaction (Scheme 10) in order to study the behavior of smaller fragments.
Scheme 5a.
a Reagents and conditions: (a) PhH, HOAc.
Scheme 6a.
a Reagents and conditions: (a) Br2, HOAc, NaOAc.
Scheme 7a.
aReagents and conditions: (a) HBr, HOAc.
Scheme 8a.
aReagents and conditions: (a) PhH, HOAc.
Scheme 9a.
a Reagents and conditions: (a) PhH, HOAc.
Scheme 10a.
a Reagents and conditions: (a) PhH, HOAc
(b) HBr, HOAc (c) 2 eq NBS.
Quinone Reductase 1 Inductive Activity
It is believed that chemoprevention can be achieved by induction of QR1, which deactivates potential carcinogens.37 The effect of phenazines on QR1 induction was tested as described in the Experimental Section.38 Analogues of the phenazine natural product 1 and compound 6 were prepared in order to establish structure-activity relationships for QR1 induction. Both the induction ratio (IR) and the concentration to double (CD) values were determined for each compound, where the IR is the QR1 activity in mouse hepatoma cells in the presence of a 50 μM concentration of the phenazine divided by the activity in the absence of the phenazine, and the CD is the concentration of the drug that doubles the QR1 activity. The experimental results (Table 1) indicate that a bromine substituent para to the oxygen atom at C-1 increases QR1 induction when substituents are absent on the second benzene ring. This effect is apparent in compounds 8 and 11, which present induction ratios (IRs) of 2.8 and 2.7 and concentration to double QR1 activity (CD) values of 27.0 μM and 4.3 μM, respectively. On the other hand, a bromine substituent in the meta position has a negative effect on enzyme induction, i.e. 14 and 15. Methylation of the hydroxyl group has, in general, a positive effect on the activity. For example, compound 11 has an IR of 2.7, which is larger than its hydroxyl analogue 12, which has an IR of 1.5. Furthermore, the methylated compound 16 has an IR of 1.6 versus a 1.0 value for the phenolic analogue 17. This result documents the detrimental effect of a bromine substituent in the meta position, which is also seen with other compounds.
Table 1.
Induction of QR1, and Inhibition of NF-κB, iNOS and QR2
| QR1 | NF-κB-luciferase | Nitrite assay | QR2 | |||||
|---|---|---|---|---|---|---|---|---|
| Compd | IRa,b | CDc,d (μM) | Lucif. act. % inhibb,e | IC50f (μM) | % Max Inhibb,g | IC50f (μM) | % Max Inhib | IC50f,h (μM) |
| 1 | 0.7 | 50.0 ± 0.7 | 73.0 ± 0.0 | 31.5 ± 2.5 | N/Ah | N/A | ||
| 6 | 1.4 | 0 | 2.4 ± 1.1 | 88.6 ± 1.7 | 31.1 ± 1.7 | |||
| 8 | 2.8 | 27.0 | 17.5 ± 4.9 | 70.9 ± 1.1 | 4.2 ± 0.5 | N/A | N/A | |
| 9 | 2.5 | 29.4 | 0 | 44.7 ± 2.2 | 94.9 ± 1.1 | 0.186 ± 0.03 | ||
| 10 | 1.8 | 0 | 24.7 ± 4.0 | 88.7 ± 7.5 | 18.5 ± 4.4 | |||
| 11 | 2.7 | 4.3 | 54.0 ± 8.5 | 47.5 ± 7.7 | 90.4 ± 2.9 | 36.8 ± 2.7 | ||
| 12 | 1.5 | 75.0 ± 5.2 | 5.19 ± 2.4 | 84.7 ± 0.0 | 31.5 ± 0.0 | 50.3 ± 2.7 | 26.9 ± 3.6 | |
| 14 | 1.0 | 0 | 68.6 ± 5.1 | 26.5 ± 1.5 | 88.8 ± 2.24 | 0.69 ± 0.11 | ||
| 15 | 0.7 | 99.8 ± 0.1 | 5.42 ± 0.4 | 86.5 ± 0.4 | 28.4 ± 0.4 | N/A | N/A | |
| 16 | 1.6 | 84.2 ± 2.0j | 70.9 ± 0.4 | 33.7 ± 0.4 | 93.3 ± 0.64 | 0.16 ± 0.02 | ||
| 17 | 1.0 | 91.5 ± 4.8 | 4.74 ± 2.35 | 79.2 ± 3.3 | 17.4 ± 1.2 | 61.5 ± 2.3 | 1.4 ± 0.27 | |
| 28 | 2.4 | 23.0 | 82.0 ± 5.7 | 9.15 ± 3.18 | 49.1 ± 1.8 | 90.2 ± 2.03 | 13.5 ± 0.93 | |
| 29 | 1.2 | 0 | 5.5 ± 1.8 | 89.9 ± 0.5 | 0.45 ± 0.02 | |||
| 30 | 1.9 | 0 | 16.4 ± 0.4 | 42.1 ± 0.78 | 3.3 ± 0.27 | |||
| 31 | 0.8 | 10.5 ± 7.8 | 38.7 ± 1.8 | 109.1 ± 9.1 | 60.8 ± 9.86 | |||
| 32 | 0.8 | 16.0 ± 5.7 | 12.5 ± 4.4 | 90.0 ± 1.51 | 15.3 ± 0.76 | |||
| 33 | 0.8 | 0 | 29.4 ± 3.3 | 80.0 ± 2.47 | 18.2 ± 1.59 | |||
| 34 | 1.6 | 72.5 ± 15.2 | 13.1 ± 0.7 | 79.7 ± 3.3 | 7.2 ± 1.1 | 85.1 ± 0.4 | 0.32 ± 0.02 | |
| 35 | 1.0 | 18.5 ± 9.2 | 5.0 ± 2.6 | 93.6 ± 9.3 | 35.5 ± 8.2 | |||
| 38 | 4.7 | 11.3 | 48.5 ± 9.2 | 53.8 ± 9.9 | 47.0 ± 1.4 | 100.5 ± 8.6 | 11.7 ± 3.17 | |
| 39 | 1.2 | 79.2 ± 5.9 | 6.64 ± 0.76 | 8.8 ± 5.9 | 31.2 ± 2.5 | 10.5 ± 2.7 | ||
| 40 | 4.2 | 9.42 | 93.4 ± 0.2 | 16.17 ± 3.5 | 14.6 ± 9.5 | 74.4 ± 3.81 | 4.8 ± 0.95 | |
| 41 | 3.1 | 0.012 | 77.4 ± 2.0 | 2.46 ± 0.5 | 0.0 ± 3.3 | N/A | N/A | |
| 42 | 6.6 | 1.83 | 67.5 ± 3.5 | 17 ± 0.85 | 51.9 ± 2.9 | 48.9 ± 1.6 | 42.7 ± 3.6 | 11.3 ± 1.0 |
| 43 | 1.4 | 92.4 ± 2.3 | 1.39 ± 1.7 | 77.7 ± 5.5 | 34.5 ± 2.0 | 82.6 ± 1.12 | 4.1 ± 0.22 | |
| 44 | 2.2 | 1.85 | 50.5 ± 3.3 | 51.2 ± 1.8 | 47.2 ± 1.2 | 76.7 ± 1.95 | 0.48 ± 0.09 | |
| 46 | 7.9i,j | 0.0047 | 99.5 ± 9.9 | 2.1 ± 1.3 | 98.9 ± 0.4 | 7.2 ± 1.0 | 64.4 ± 1.21 | 3.5 ± 0.28 |
| 47 | 0.6 j | 4.39 | 84.5 ± 6.4 | 2.48 ± 1.3 | 98.7 ± 3.7 | 5.9 ± 0.5 | 56.1 ± 1.9 | 11.2 ± 1.2 |
| 49 | 3.2 | 16.6 | 77.3 ± 7.9j | 3.4 ± 11.4 | N/A | N/A | ||
| 51 | 1.4 | 52.0 ± 4.24 | 0.8 ± 13.6 | N/A | N/A | |||
| 52 | 1.7 | 52.5 ± 4.95j | 0.0 ± 7.3 | N/A | N/A | |||
| 53 | 1.8 | 91.9 ± 2.4 | 1.93 ± 0.3 | 81.3 ± 0.4 | 45.6 ± 2.6 | 6.2 ± 0.76 | 2.9 ± 1.54 | |
| 54 | 0.23 | |||||||
| 55 | 0.26 | |||||||
IR, induction ratio.
Testing concentration, 50 μM (except for 1, which was tested at 100 μM).
CD is the concentration that doubles the activity.
CD values were determined for phenazines with induction ratios > 2.
NF-κB IC50 calculated when inhibition > 70%.
IC50, median inhibitory concentration.
iNOS IC50 calculated when inhibition > 50%.
N/A; not applicable, no inhibition; compounds 1 and 8 react directly with the NRH substrate, so no inhibition data could be obtained.
Testing concentration, 305 nM.
Cytotoxic at testing concentration.
The next aim was to analyze how various substituents on the unsubstituted benzene ring affect QR1 induction. The parent compound 38 (IR = 4.7, CD = 11.3 μM) was more active than its brominated analogues 39 and 43 (IR’s of 1.2 and 1.4). The positive effect of a bromine substituent para to the methoxy group did hold for the analogue 42, which shows an IR of 6.6 (CD = 1.83 μM). Given that the IR of 38 was also higher than the parent compound 6, it was hypothesized that the better IR values of these compounds were also a consequence of the substituents at C-7 and C-8. This hypothesis was confirmed when other substituents were added to the ring. For example, the chlorinated compounds 40, 41 and 44 have IR values of 4.2, 3.1, and 2.2 (CD’s = 9.42 μM, 0.012 μM, 1.85 μM). The positive effect of the methoxy group on QR1 activity is also observed with these compounds. In addition, the data suggest a positive effect of oxygen substitution on the IR values, i.e. compound 38 has an IR of 4.7, which is higher than the deoxygenated compound 28 with and IR of 2.4. The best inducer of QR1 is compound 46, with an IR value of 7.9 at 305 nM (the IR at the testing concentration of 50 μM was 2.2) and a CD value of only 4.7 nM. This compound was moderately cytotoxic in Hepa 1c1c7 cells, with an IC50 of 9.58 μM, hence the lower IR at the testing concentration. This cytotoxicity is not a concern, since the GI50 value is three orders of magnitude above the dose needed to significantly induce QR1. The chemopreventive index (CI) for this compound (GI50/CD) is 2,038, where the GI50 is the concentration required to inhibit growth 50% relative to control (no drug) after a 48 hour incubation period.
Quinone Reductase 2 Inhibitory Activity
As stated earlier, in some cases QR2 may produce toxic metabolites, and therefore, its inhibition could prevent carcinogenesis.10 Inhibition assays were performed on the synthetic compounds as described in the experimental section. The addition of chlorine atoms increases inhibitory activity, resulting in IC50 values of 4.8 ± 0.95 μM (compounds 40) and 0.48 ± 0.09 μM (44) versus the unsubstituted compound 6, with an IC50 of 31.1 ± 1.7 μM. Compounds with a bromine atom in the meta position, with respect to the oxygen, also showed good inhibition of QR2 with IC50 values of 0.69 ± 0.11 μM (compound 14), 0.16 ± 0.02 μM (16), 1.4 ± 0.27 μM (17), and 4.1 ± 0.22 μM (43). Three other compounds that showed very good inhibitory activity were 9, 29, and 34, with IC50 values of 0.18 ± 0.03 μM, 0.45 ± 0.02 μM, and 0.32 ± 0.02 μM.
NF-κB Inhibitory Activity
Nuclear factor-κB (NF-κB) is an inducible transcription factor that, when activated, promotes cell survival and differentiation. On the other hand, its down regulation renders cells more sensitive to apoptosis.19 This fact makes NF-κB inhibitors good candidates for chemoprevention. Most of the compounds showed modest to good activity against NF-κB. In general, a bromine meta to the oxygen increased activity, i.e. compounds 15–17, 39, 41, and 43. Also, molecules with two bromine atoms, such as 43, were even more reactive than molecules 39 or 42, with only one bromine substituent. Finally, bromine-substituted hydroxyphenazines showed a higher activity than their methylated analogues, i.e. 11 vs. 12 or 16 vs. 17.
Nitrite Assay
Inhibitors of inducible nitric oxide synthase (iNOS) have been considered as potential anti-inflammatory and cancer chemopreventive agents.20 Therefore, the inhibitory activities of phenazines on the production of nitrite, a stable product of nitric oxide (NO), have been evaluated. Various phenazines showed moderate inhibitory iNOS activities. Most of the compounds that contain bromine atoms in the meta the and/or para positions, with respect to the oxygen, were active. The hydroxyl compounds are more active than their methylated analogs, i.e. 8 vs. 10, or 15 and 17 vs. 14 and 16. Also, phenazines with methyl substituents on the non-oxygenated benzene ring were active. Overall, the most active compounds were 8, 34, 46 and 47.
Multiple Activities
Arguably, the most attractive feature of these phenazines is their ability to interact with multiple targets (Table 1). The ability to block multiple pathways may create a synergistic effect that could lead to greater chemopreventive potential.24–27, 39–41 This is critical in chemoprevention because “cancer is a multifactorial disease that requires modulation of multiple pathways and multiple targets.”42 The most notable compounds are 9, 16, 28, 34, 38, and 46. These compounds present QR1 inductive or QR2, iNOS or NF-κB inhibitory activities at low micromolar concentrations. In some cases, the biological results were obtained in the nanomolar concentration range. 1-Methoxybenzo[b]phenazine (46) exhibited the best all-around activity vs. all four targets: QR1 IR 7.9 and CD 0.0047 μM, NF-κB inhibition 2.1 μM, nitrite assay 7.2 μM, and QR2 inhibition 3.5 μM. Out of the thirty-two compounds, it ranked first vs. QR1 induction, third vs. NF-κB inhibition, tied for third vs. iNOS inhibition, and tenth vs. QR2 inhibition. This clearly demonstrates that good activity vs. all four targets can be achieved with a single compound.
Table 2 contains rank-order lists of the twelve most active compounds in each of the four assays. The numbers of the compounds that appear in both the nitrite and NF-κB columns are bolded. Eight of the compounds appear in both the iNOS and NF-κB lists, from which it may be concluded that some of the structural features that confer inhibitory activity vs. iNOS (nitrite assay) also confer inhibitory activity in the NF-κB assay. Five of the eight compounds are brominated phenazines or phenazine analogues having either a phenol or a methyl ether in the same position or an equivalent position. Activity vs. QR1 and QR2 seems to result from independent pharmacophores, with less overlap in their rank order lists.
Table 2.
Rank Order of Compounds in the Nitrite, NF-κB, QR1, and QR2 Assays
| Nitrite | NF-κB | QR1 | QR2 | ||||
|---|---|---|---|---|---|---|---|
| Compb | IC50 (μM) | Compb | IC50 (μM) | Compb | CD | Compb | IC50 (μM) |
| 8 | 4.2 | 43 | 1.4 | 46 | 0.0047 | 16 | 0.16 |
| 47 | 5.9 | 53 | 1.9 | 41 | 0.012 | 9 | 0.19 |
| 46 | 7.2 | 46 | 2.1 | 42 | 1.8 | 34 | 0.32 |
| 34 | 7.2 | 41 | 2.5 | 44 | 1.8 | 29 | 0.45 |
| 17 | 17.4 | 47 | 2.5 | 11 | 4.3 | 44 | 0.48 |
| 14 | 26.5 | 17 | 4.7 | 47 | 4.4 | 17 | 1.4 |
| 15 | 28.4 | 12 | 5.2 | 40 | 9.4 | 53 | 2.9 |
| 12 | 31.5 | 15 | 5.4 | 38 | 11.3 | 30 | 3.3 |
| 16 | 33.7 | 39 | 6.6 | 49 | 16.6 | 46 | 3.5 |
| 43 | 34.5 | 28 | 9.2 | 28 | 23 | 43 | 4.1 |
| 53 | 45.6 | 34 | 13.1 | 8 | 27 | 40 | 4.8 |
| 38 | 47.0 | 42 | 17.0 | 9 | 29.4 | 39 | 10.5 |
The compounds listed are the twelve most active compounds in each of the four assays out of thirty-two compounds tested.
The compound numbers that are bolded appear in both the nitrile and NFκB columns.
Conclusion
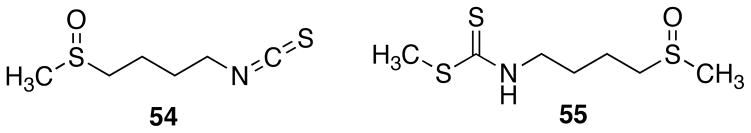
Simple syntheses of the natural product 1 and a series of its analogues have been carried out. The activities of the natural product 1 were significantly optimized vs. QR 1 induction, inhibition of NF-κB-luciferase activity, inhibition of iNOS activity, and inhibition of QR 2, through the synthesis of congeners and analysis of structure-activity relationships. Several of the compounds were active in the nanomolar range, including 38, 40–42 and 46 vs. QR1; and 9, 14, 16, 29 and 34 vs. QR2. These results provide new phenazine leads compounds for the design and synthesis of chemopreventive agents targeting QR1 induction, QR2 inhibition, iNOS inhibition, and NF-κB inhibition. The CD value of 4.7 nanomolar and CI value of 2,038 for compound 46 vs. QR1 are particularly noteworthy. The CD value of 4.7 nanomolar is the lowest CD value ever recorded by our group for any QR1 inducer. For comparison, the standard compound sulforaphane (54, Figure 2), which is a natural product found in broccoli, has a CD value of 0.23 μM and a CI value of 42, while the synthetic compound sulforamate (55) has a CD of 0.26 μM and a CI of 137.43 Sulforaphane has been proven to induce QR1 in rat mammary gland in vivo in a manner that is consistent with its chemopreventive activity.44
Figure 2.
Structures of Sulforaphane (54) and Sulforamate (55)
Various compounds synthesized in the present study inhibit or induce two or more distinct targets, which provides a foundation for the development of effective chemopreventive agents. Multiple targeting offers the likelihood of improved efficacy if the two protein targets operate in a synergistic way.
Experimental Section
NMR spectra were obtained at 300 (1H) and 75 or 125 (13C) MHz in CDCl3 or DMSO-d6 using Bruker ARX300 or Bruker DX-2 500 (QNP probe or BBO probe, respectively) spectrometers unless otherwise indicated. Flash chromatography was performed with 230–400 mesh silica gel. TLC was carried out using commercially available precoated glass silica gel plates of 2.5 mm thickness and compounds were visualized with short-wavelength UV light. Melting points were determined using capillary tubes with a Mel-Temp apparatus and are uncorrected. IR spectra were obtained as films on salt plates with CH2Cl2 as solvent, using a Perkin-Elmer 1600 series FTIR spectrometer. Microanalyses were performed at the Purdue Microanalysis Laboratory or Galbraith Laboratories, Inc. Natural product isolation was performed with Prep Nova-Pak HR C18, 6 μm, 60Å, 300 mm × 40 mm (1st purification) or Phenomenex Luna C8 (2), 10 mm × 250 mm, 5 μm (2nd purification). HPLC analyses were performed on a Waters 1525 binary HPLC pump/Waters 2487 dual λ absorbance detector system using a 5 μM C-18 reverse phase column. All yields refer to isolated compounds. Unless otherwise stated, chemicals and solvents were of reagent grade and used as obtained from commercial sources without further purification. The purities of all of the tested compounds was >95% as determined by HPLC or elemental analysis.
Isolation of 2-Bromo-1-hydroxyphenazine (1) from Streptomyces sp., strain CNS284
The producing strain was cultured in 2.8 L Fernbach flasks (25 × 1 L) containing A1BFe media for 7 days at 25–27 °C on a shaker at 230 rpm. The fermentation broth was then extracted by addition of 20 g/L of XAD-7 resin, which was subsequently collected by filtration and extracted with acetone. The organic residue (20 g) from the resin was subjected to silica normal-phase flash chromatography generating 8 fractions (gradient solvent system: 100% isooctane, 20% ethyl acetate-isooctane, 40% ethyl acetate-isooctane, 60% ethyl acetate-isooctane, 80% ethyl acetate/isooctane, 100% ethyl acetate, 10% methanol-ethyl acetate, 20% methanol-ethyl acetate). The active fractions 6 and 7 were purified by RP-HPLC (detection at 254 nm; 0–10 min, 40% aqueous CH3CN; 10–25 min, linear gradient up to 65% aqueous CH3CN) to afford three sub-fractions. These sub-fractions were then separately purified by RP HPLC (detection of 254 nm; 0–40 min, 30–65% aqueous CH3CN; 40–50 min, 65–100% aqueous CH3CN) giving 3.2 mg of the natural product, 2-bromo-1-hydroxyphenazine (1) as a light yellow amorphous solid; UV (CH3OH) λmax (log ε) 270 (4.43), 378 (2.19) nm; IR (neat) νmax 3653, 2907, 1513, 1470, 1385, 1267, 1174, 1103, 882, 751 cm−1; 1H NMR (500 MHz, CDCl3) and 13C NMR (125 MHz, CDCl3) see Table 3; ESIMS m/z 275 (MH+), 297 (MNa+), 751 (2MNa+); HRESIMS m/z: calcd 275.9814, found 275.9814.
Table 3.
NMR Spectroscopic Data for 2-Bromo-1-hydroxyphenazine (1) in CDCl3 at 500 MHz (Assignments made by analysis of 2D NMR Data).
| C/H # | δH (J Hz) | δC | COSY | HMBC | |
|---|---|---|---|---|---|
| 1 | 149.3 | C | |||
| 2 | 103.9 | C | |||
| 3 | 7.83 d (8.6) | 135.5 | CH | H-4 | C-1, C-2, C-4a |
| 4 | 7.63 d (8.6) | 121.3 | CH | H-3 | C-2, C-3, C-10a |
| 4a | 100.0 | C | |||
| 5 | |||||
| 5a | 141.7 | C | |||
| 6 | 8.18 d (8.4) | 129.1 | CH | H-7. H-8 | C-8, C-9a |
| 7 | 7.82 m | 131.6 | CH | H-6, H-8, H-9 | C-7, C- |
| 8 | 7.82 m | 131.6 | CH | H-6, H-7, H-9 | C-7, C-9a |
| 9 | 8.20 d (8.4) | 129.5 | CH | H-7, H-8 | C-8, C-5a |
| 9a | 141.7 | C | |||
| 10 | |||||
| 10a | 134.2 | C | |||
| 1-OH | 8.52 br s | ||||
General Procedure for the Preparation of Phenazines 6, 38, 40, and 46
3-Methoxycatechol (3, 1.03–2.10 g, 7.5–15.0 mmol, 1 equiv) was dissolved in ethyl ether (20–40 mL) and the mixture was cooled to −78 °C. o-Chloranil (1.84–3.67 g, 7.5–15.0 mmol, 1 equiv) was added to the reaction mixture. The solution was stirred for 4 h, keeping the temperature constant. The reaction mixture was filtered, giving the desired benzoquinone 4, which was used without further purification. Compound 4 (1.03–2.10 g, 7.5–15.0 mmol, 1 equiv) was immediately added to a flask containing the appropriate o-phenylenediamine (1.02–2.67 g, 7.5–15.0 mmol, 1 equiv) dissolved in benzene (25–54 mL) and acetic acid (25–45 mL). The mixture was stirred for 24 h at room temperature. The solvent was removed in vacuo.
General Procedure for the Preparation of Phenazines 14, 39, and 41
5-Bromo-3-methoxycatechol (13, 0.7–1.41 g, 3.05–6.10 mmol, 1 equiv) was dissolved in ether (15–30 mL) and the solution was cooled down to −78 °C. o-Chloranil (0.75–1.50 g, 3.06–6.11 mmol) was added slowly and the reaction mixture stirred at −78 °C for 6 h. The purple solid, benzoquinone 13, was separated by filtration and immediately added to a solution of the appropriated o-phenylenediamine (0.33–0.83 g, 3.01–6.10 mmol, 0.99–1 equiv) in benzene (40–80 mL) and acetic acid (35–70 mL). The reaction mixture was stirred at room temperature for 12 h.
General Procedure for the Preparation of Phenazines 28–35, and 47
Catechol (18, 0.112–0.284 g, 1.01–2.64 mmol) and silver(I) oxide (0.564–1.44 g, 2.43–6.12 mmol, 2.31–2.40 equiv) were dissolved in acetone (20–50 mL) and the reaction mixture stirred for 10 min. The obtained solid was filtered and added immediately, without purification, to a solution of the appropriate o-phenylenediamine (0.215–0.485 g, 0.81–2.89 mmol, 1.0–1.2 equiv) dissolved in benzene (20–30 mL) and acetic acid (17–25 mL). The flask was purged with argon and kept at 0 °C. The reaction mixture was allowed to warm to room temperature and stirred for 24 h. The solvent was removed in vacuo.
2-Bromo-1-hydroxyphenazine (1)
1-Hydroxyphenazine35 (7, 0.51 g, 2.60 mmol) and N-bromosuccinimide (0.51 g, 2.85 mmol) were dissolved in toluene (50 mL) and the reaction mixture was heated at 50 °C for 8 h. The reaction mixture was cooled to room temperature and the toluene was removed in vacuo. The sample was dissolved in dichloromethane (25 mL), silica gel (5 g) was added to the flask, and the solvent removed. The product was purified by silica gel column chromatography, using dichloromethane to dichloromethane-ethyl acetate, 100:5. The product was obtained as a yellow solid (0.24 g, 34%): mp 233–235 °C. IR (film) 3359, 2907, 575, 1551, 1530, 1419, 1385, 1103, 764, 751 cm−1; 1H NMR (CDCl3) δ 8.53 (bs, 1 H), 8.27-8.24 (m, 2 H), 7.91-7.88 (m, 3 H), 7.72 (d, J = 9.5 Hz, 1 H); ESIMS m/z (rel intensity) 275 (MH+, 66), 277 (MH+, 61). Anal. Calcd. for C12H6BrN2O, C, 52.39; H, 2.56; N, 10.18; Br, 29.05. Found: C, 52.05; H, 2.32; N, 10.06; Br, 29.15.
1-Methoxyphenazine (6)
The general procedure provided a solid that was dissolved in ethyl acetate (25 mL) and water was added (10 mL). Sodium bicarbonate was added until the generation of CO2 ceased. The aqueous layer was decanted and the organic solvent was removed in vacuo. The compound was purified by silica gel column chromatography with the column containing 2 cm of neutral alumina on top of the silica gel. The compound was eluted using a gradient of hexane to ethyl acetate-hexane, 1:3 and ethyl acetate-dichloromethane 1:3. The product was obtained as a yellow solid (0.63 g, 42%): mp 166–168 °C (lit.35 mp 168–169 °C). 1H NMR (CDCl3) δ 8.38 (m, J = 10.0 Hz, 1 H), 8.20 (m, J = 10.0 Hz, 1 H), 7.84-7.79 (m, 3 H), 7.72 (t, J1 = 7.0 Hz, J2 = 12.0 Hz, 1 H), 7.04 (d, J = 7 Hz, 1 H); 13C NMR (CDCl3) δ 154.7, 143.8, 143.1, 141.8, 136.4, 130.4, 130.1, 129.8, 128.9, 121.0, 106.0, 56.1; ESIMS m/z (rel intensity) 211 (MH+, 100). Anal. Calcd. for C13H10N2O: C: 74.27, H: 4.79, N: 13.33. Found: C: 74.04, H: 4.62, N: 13.29.
1-Hydroxyphenazine (7)
1-Methoxyphenazine (6, 2.13 g, 10.1 mmol) was dissolved in 48% hydrobromic acid (60 mL) and glacial acetic acid (60 mL) and the reaction mixture was heated at reflux for 8 h. Hydrobromic acid (48%, 30 mL) was added and the reaction mixture was stirred for another 8 h. The aqueous solution was extracted with ethyl acetate (5 × 35 mL). The organic extracts were combined and washed with saturated sodium bicarbonate (2 × 30 mL), brine (1 × 30 mL) and water (2 × 30 mL). The solvent was removed in vacuo and the compound purified by silica gel column chromatography, using hexane-ethyl acetate, 1:1 to 1:10. The product was obtained as a yellow solid (1.35 g, 68%): mp 148–150 °C (lit.16 mp 159–160 °C). 1H NMR (CDCl3) δ 8.23 (m, 2 H), 7.86 (t, J = 4.0 Hz, 1 H), 7.78 (t, J = 3.0 Hz, 1 H), 7.25 (t, J = 3.0 Hz, 1 H); 13C NMR (CDCl3) δ 151.6, 143.9, 143.7, 141.1, 134.5, 131.7, 130.9, 130.6, 130.3, 129.5, 129.0, 119.8, 108.8; ESIMS m/z (rel intensity) 197 (MH+, 100).
2,4-Dibromo-1-hydroxyphenazine (8)
1-Hydroxyphenazine (7, 0.55 g, 2.80 mmol) and N-bromosuccinimide (1.09 g, 6.16 mmol) were dissolved in toluene (50 mL) and the reaction mixture was heated at 50 °C for 8 h. The reaction mixture was cooled to room temperature and the toluene was removed in vacuo. The product was purified by silica gel column chromatography, using dichloromethane to dichloromethane-ethyl acetate, 100:5. The product was obtained as a yellow solid (0.925 g, 93%): mp 196–198 °C. IR (film) 3320, 3068, 1620, 1592, 1514, 1466, 1404, 1371, 948, 758 cm−1; 1H NMR (DMSO-d6) δ 11.55 (bs, 1 H), 8.39 (s, 1 H), 8.35-8.27 (m, 2 H), 8.06-8.01 (m, 2 H); 13C NMR (DMSO-d6) δ 150.9, 142.7, 141.3, 139.4, 136.8, 135.5, 132.2 (2 C), 129.4, 128.9, 111.4, 104.4; ESIMS m/z (rel intensity) 353 (MH+, 100), 355 (MH+, 41). Anal. Calcd. for C12H6Br2N2O, C, 40.71; H,1.71, N, 7.91; Br, 45.14. Found: C, 40.34; H, 1.59; N: 7.77; Br, 45.22.
2-Bromo-1-methoxyphenazine (9)
2-Bromo-1-hydroxyphenazine (1, 30 mg, 0.11 mmol) was dissolved in acetone (5 mL). Potassium carbonate (28 mg, 0.2 mmol) was added to the reaction mixture and the mixture was stirred for 1 min. Methyl iodide (0.1 mL) was added and the reaction mixture was stirred at reflux for 3 h. The solvent was removed and the compound dissolved in chloroform (10 mL). The organic phase was washed with water (3 × 10 mL). The product was purified by preparative TLC, using chloroform as solvent, and obtained as a yellow solid (31 mg, 98%): mp 145–147 °C. IR (film) 1728, 1623, 1588, 1552, 1514, 1474, 1415, 1360, 1302, 929, 753 cm−1; 1H NMR (CDCl3) δ 8.34 (m, 1 H), 8.21 (m, 1 H), 7.92 (m, 2 H), 7.86 (m, 2 H), 4.31 (s, 3 H); 13C NMR (CDCl3) δ 152.9, 143.5, 143.0, 142.6, 138.9, 134.3, 131.0, 130.9, 130.0, 129.4, 125.9, 116.4; EIMS m/z 288 (M+), CIMS m/z (rel intensity) 289 (MH+, 100); HREIMS m/z: calcd 287.9898, found 287.9896. HPLC purity: 95.02% (MeOH, 100); 96.03% (MeOH-H2O, 90:10).
2,4-Dibromo-1-methoxyphenazine (10)
2,4-Dibromo-1-hydroxyphenazine (8, 0.155 g, 0.438 mmol) contained in a high-pressure vessel was dissolved in tetrahydrofuran (10 mL) and dimethylformamide (1 mL), and the reaction mixture was cooled to −78 °C. Sodium hydride (0.100 g, 4.17 mmol) was added. The reaction vessel was purged with argon and the reaction mixture was stirred for 30 min, keeping the atmosphere inert. The reaction mixture was warmed to 0 °C and methyl iodide (0.5 mL) was added. The flask was closed and heated for 8 h at 100 °C. The reaction mixture was allowed to cool to room temperature and diluted with water (10 mL). The aqueous solution was extracted with ethyl acetate (3 × 15 mL). The organic extracts were combined and washed with aqueous ammonium chloride (20 mL), water (3 × 20 mL), and brine (20 mL). The solvent was removed in vacuo and the product was purified by silica gel column chromatography, eluting with hexane-ethyl acetate, 100:1. The product was obtained as a yellow solid (0.149 g, 92%): mp 171–173 °C. IR (film) 3061, 2933, 1617, 1576, 1513, 1467, 1401, 968, 945, 797, 747 cm−1; 1H NMR (CDCl3) δ 8.32-8.27 (m, 2 H), 8.26 (s, 1 H), 7.90-7.88 (m, 2 H), 4.30 (s, 3 H); 13C NMR (CDCl3) δ 152.8, 142.9, 142.6, 140.5, 138.8, 136.3, 131.7, 131.4, 129.7, 129.6, 118.9, 115.7, 62.6; EIMS m/z (rel intensity) 369 (MH+, 100). Anal. Calcd. for C13H8Br2N2O: C, 42.22; H, 2.26; N, 7.36. Found: C, 42.43; H, 2.19; N, 7.61.
4-Bromo-1-methoxyphenazine (11).45, 46
1-Methoxyphenazine (6, 240 mg, 1.14 mmol) was dissolved in toluene (20 mL) and acetonitrile (20 mL). N-Bromosuccinimide (213 mg, 1.19 mmol) was added with stirring and the reaction mixture was heated to 50 °C for 8 h. Toluene was removed in vacuo and the compound was purified by silica gel column chromatography, eluting with chloroform-hexane, 1:1 to chloroform-ethyl acetate, 1:1. The product was obtained as a yellow solid (249 mg, 75%): mp 148–150 °C (lit.45, 46 mp 155 °C). IR (film) 3060, 3004, 2961, 1621, 1600, 1551, 1521, 1476, 1461, 1281, 1095, 917, 754 cm−1; 1H NMR (CDCl3) δ 8.38 (m, 2 H), 8.63 (d, J = 8.2 Hz, 1 H), 7.89 (m, 2 H), 6.94 (d, J = 8.1 Hz, 1 H), 4.15 (s, 3 H); 13C NMR (CDCl3) δ 154.9, 143.4, 142.1, 140.8, 136.9, 133.0, 131.2, 130.9, 129.7, 129.6, 114.0, 106.7, 56.5; EIMS m/z (rel intensity) 290 (M+, 57), 288 (M+, 57), 179 (M+-OCH4Br, 100). Anal. Calcd. for C13H9BrN2O: C, 54.00; H, 3.14; N, 9.69; Br, 27.64. Found: C, 53.79; H, 3.11; N, 9.41; Br, 27.51.
4-Bromo-1-hydroxyphenazine (12)32
4-Bromo-1-methoxyphenazine (11, 400 mg, 1.38 mmol) was dissolved in dry dichloromethane (25 mL). The reaction mixture was purged with argon and cooled to −78 °C. Boron tribromide (1 M solution in dichloromethane, 4.0 mL) was added dropwise to the reaction mixture with stirring. The temperature was maintained at −78 °C and the reaction mixture was stirred for 1 h. The reaction mixture was allowed to warm to room temperature and stirred for 12 h. The reaction mixture was heated at reflux for 1 h. The solution was allowed to cool to room temperature and saturated aqueous sodium bicarbonate (30 mL) at ~ 4 °C was slowly added. The solution was extracted with dichloromethane (3 × 40 mL). The reaction mixture was filtered through a column packed with celite® 545 and basic alumina on top (1 inch) and the solvent was removed in vacuo. The compound was purified by silica gel column chromatography (ethyl acetate-hexane, 1:1). The product was obtained as a yellow powder (322 mg, 85%): mp 199–201 °C. IR (film) 3359, 1731, 1628, 1518, 1472, 1421, 1372, 1343, 1319, 1300, 924, 766, 750 cm−1; 1H NMR (CDCl3) δ 8.41-8.38 (m, 1 H), 8.25-8.21 (m, 1 H), 8.07 (d, J = 8.1 Hz, 1 H), 7.94-7.85 (m 2 H), 7.13 (d, J = 8.1 Hz, 1 H); 13C NMR (CDCl3) δ 151.5, 144.2, 141.1, 140.7, 134.8, 134.6, 131.3, 130.0, 128.7, 112.0, 109.3; CIMS m/z (rel intensity) 277 (MH+, 100), 275 (MH+, 98), HREIMS m/z calcd 273.9742, found 273.9740. HPLC purity: 100.00% (MeOH-H2O, 95:5).
3-Bromo-1-methoxyphenazine (14)
The general procedure was followed. The solvent was removed in vacuo and the compound was purified by silica gel column chromatography (1 inch of basic alumina was added to the top of the silica gel column), dichloromethane-ethyl acetate, 3:1. The product was obtained as a dark-yellow solid (0.41 g, 47%): mp 148–150 °C. IR (film) 3058, 2957, 2932, 1622, 1591, 1555, 1518, 1475, 1398, 1363, 1351, 1311, 1218, 1145, 1111, 864, 826, 755, 736 cm−1; 1H NMR (CDCl3) δ 8.40-8.35 (m, 1 H), 8.25-8.17 (m, 2 H), 8.05 (d, J = 1.9 Hz, 1 H), 7.89-7.83 (m, 2 H), 7.14 (d, J = 1.9 Hz, 1 H), 4.18 (s, 3 H); 13C NMR (CDCl3) δ 155.2, 144.0, 143.7, 141.9, 135.5, 131.3, 130.4, 130.1, 129.2, 124.9, 123.5, 110.9, 56.7; CIMS m/z (rel intensity) 289 (MH+, 100). Anal. Calcd for C13H9N2OBr: C, 54.00; H, 3.14; N, 9.69. Found: C, 53.94; H, 3.11; N, 9.49.
3-Bromo-1-hydroxyphenazine (15)
3-Bromo-1-methoxyphenazine (14, 203 mg, 0.71 mmol) was dissolved in 48% hydrobromic acid (20 mL) and glacial acetic acid (15 mL) and the reaction mixture was heated in an oil bath at 110 °C for 16 h. An extra amount of 48% hydrobromic acid (10 mL) was added and the reaction mixture was heated in an oil bath at 110 °C for 8 h. The reaction mixture was allowed to cool to room temperature and extracted with ethyl acetate (5 × 20 mL). The combined organic layers were washed with aqueous sodium bicarbonate (2 × 25 mL) and water (2 × 30 mL). The solvent was removed and the compound purified by silica gel column chromatography, eluting with ethyl acetate-hexane, 2:1. The product was obtained as a light brown solid (135 mg, 69.9%): mp 200–202 °C. IR (film) 3360, 3070, 2917, 2849, 1624, 1604, 1557, 1517, 1476, 1417, 1380, 1353, 1318, 1162, 1121, 751 cm−1; 1 H NMR (CDCl3) δ 8.25-8.18 (m, 3 H), 7.99 (d, J = 1.7 Hz, 1 H), 7.88-7.86 (m, 2H), 7.34 (d, J = 1.7 Hz, 1 H); 13C NMR (CDCl3) δ 151.9, 149.5, 143.7, 140.9, 133.4, 131.3, 130.7, 129.6, 129.1, 122.0, 113.1; CIMS m/z (rel intensity) 275 (MH+, 100); HREIMS m/z calcd 273.9742, found 273.9748. Anal. Calcd for C12H7BrN2O·0.65 EtOAc: C, 54.00; H, 3.14; N, 9.69. Found: C, 53.94; H, 3.11; N, 9.49. HPLC purity 100.00% (MeOH-H2O, 90:10), 97.85 (MeOH-H2O, 95:5).
3,4-Dibromo-1-methoxyphenazine (16)
3-Bromo-1-methoxyphenazine (14, 270 mg, 0.93 mmol) was dissolved in toluene (20 mL). Bromine (148 mg, 0.93 mmol), ammonium acetate (200 mg, 2.43 mmol) and acetic acid (20 mL) were added. The reaction mixture was heated at reflux for 1 h. The solvent was removed in vacuo and the orange solid dissolved in ethyl acetate (40 mL). The organic phase was washed with brine (1 × 30 mL) and water (2 × 30 mL). The organic phase was removed in vacuo and the compound purified by silica gel column chromatography, eluting with hexane-ethyl acetate, 5:1 to 3:1. The product was obtained as a yellow solid (331 mg, 96%): mp 208–210 °C. IR (film) 3060, 2917, 1619, 1586, 1547, 1515, 1473, 1260, 1145, 750 cm−1; 1H NMR (CDCl3) δ 8.32-8.27 (m, 2 H), 7.88-7.83 (m, 2 H), 7.20 (s, 1 H), 4.13 (s, 3 H); 13C NMR (CDCl3) δ 154.2, 143.7, 141.7, 135.6, 131.7, 131.1, 129.6, 127.9, 117.2, 111.6, 56.8; CIMS m/z (rel intensity) 369 (MH+, 100); HREIMS m/z calcd 365.9003, found 365.9006. Anal. Calcd for C13H8Br2N2O: C, 42.50; H, 2.18; N, 7.46. Found: C, 42.43; H, 2.19; N, 7.61.
3,4-Dibromo-1-hydroxyphenazine (17)
3,4-Dibromo-1-methoxyphenazine (16, 170 mg, 0.46 mmol) was dissolved in freshly distilled anhydrous dichloromethane (30 mL) under argon atmosphere. The reaction mixture was cooled to −78 °C. Boron tribromide (200 μL, 2.07 mmol) was added keeping the temperature constant. The reaction mixture was stirred for 3 h at −78 °C and then for 21 h at room temperature. The reaction mixture was heated at reflux for 1 h. A saturated aqueous solution of sodium bicarbonate (40 mL) at 4 °C was slowly added. The reaction mixture was stirred for 10 min. The aqueous phase was removed and the organic layer was washed with water (2 × 30 mL). The solvent was removed in vacuo and the compound purified by silica gel column chromatography, eluting with dichloromethane-ethyl acetate, 3:1. The product was obtained as a yellow solid (140 mg, 86%): mp 208–210 °C. IR (film) 3347, 3067, 2957, 2922, 2851, 1626, 1606, 1585, 1548, 1512, 1471, 1413, 1349, 1305, 922, 834 cm−1; 1H NMR (CDCl3) δ 8.41 (m, 1 H), 8.24 (m, 2 H), 7.94-7.91 (m, 2 H), 7.50 (s, 1 H); 13C NMR (CDCl3) δ 151.0, 144.8, 141.0, 140.8, 133.9, 131.7, 131.5, 130.0, 129.3, 128.7, 115.1, 114.0; EIMS m/z (rel intensity) 354 (M+, 100), HRCIMS m/z calcd 351.8847, found 351.8851. HPLC purity 99.44 (MeOH-H2O, 95:5).
2,3-Dimethylphenazine (28)
The general procedure was followed. The compound was purified by silica gel column chromatography (1 inch of basic alumina was added to the top of the silica gel column), eluting with hexane-chloroform, 1:1. The product was obtained as a brown solid (62.2 mg, 20.3%): mp 170–172 °C (lit47 mp 174–175 °C). 1H NMR (CDCl3) δ 8.22-8.19 (m, 2 H), 7.98 (s, 2 H), 7.81-7.77 (m, 2 H), 2.21 (s, 6 H); 13C NMR (CDCl3) δ 142.9, 142.8, 141.8, 129.6, 129.4, 127.8, 20.6; EIMS m/z (rel intensity) 208 (M+, 100). HPLC purity 95.46% (MeOH, 100).
3-Bromo-1-methylphenazine (29)
The general procedure was followed. The compound was purified by silica gel column chromatography (1 inch of basic alumina was added to the top of the silica gel column), eluting with hexane-dichloromethane, 2:1. The product was obtained as a yellow powder (59 mg, 19%): mp 132–134 °C. IR (film) 1622, 1556, 1519, 1276, 764, 751 cm −1; 1H NMR (CDCl3) δ 8.20-8.13 (m, 3 H), 7.82-7.78 (m, 2 H), 7.65 (s, 1 H), 2.85 (s, 3 H); 13C NMR (CDCl3) δ 143.6, 143.2, 142.5, 141.5, 139.6, 132.8, 130.8, 130.1, 129.9, 124.7, 17.3; EIMS m/z (rel intensity) 274 (M+, 54), 272 (M+, 54), 179 (M+-Br, 100). Anal. Calcd. for C13H9BrN2: C, 57.17; H, 3.32; N, 10.26. Found: C, 57.01; H, 3.27; N, 10.14.
1,3-Dibromophenazine (30)
The general procedure was followed. The compound was purified by silica gel column chromatography (1 inch of basic alumina was added to the top of the silica gel column), eluting with hexane-dichloromethane, 2:1. The product was obtained as a yellow powder (124 mg, 36%): mp 179–181 °C. IR (film) 3051, 1621, 1582, 1502, 1342, 1129, 956, 862, 757, 748 cm −1; 1H NMR (CDCl3) δ 8.38 (d, J = 2.0 Hz, 1 H), 8.32 (m, 1 H), 8.24 (m, 2 H), 7.89 (m, 2 H); 13C NMR (CDCl3) δ 143.8, 143.5, 143.4, 139.4, 136.4, 131.8, 131.4, 131.3, 130.0, 129.2, 125.2, 123.8; EIMS m/z (rel intensity) 338 (M+, 100). Anal. Calcd for C12H6Br2N2: C, 42.64; H, 1.79; N, 8.29. Found: C, 42.46; H, 1.74; N, 8.20.
2-Methylphenazine (31)48
The general procedure was followed. The compound was purified by silica gel column chromatography, eluting with ethyl acetate-hexane, 1:1. The compound was obtained a light brown solid (49 mg, 9.7%): mp 107–108 °C (lit49 mp 117 °C). IR (film) 3026, 2976, 2920, 1633, 1604, 1511, 1482, 1466, 1437 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.24 (m, 2 H), 8.14 (d, J = 8.9 Hz, 1 H), 8.00 (s, 1 H), 7.81 (m, 2 H), 7.28 (d, J = 8.9 Hz, 1 H), 2.66 (s, 3 H); 13C NMR (75 MHz, CDCl3) δ 143.5, 143.1, 142.8, 142.2, 141.1, 133.4, 130.1, 129.8, 129.5, 129.4, 128.9, 127.5,22.1; EIMS m/z (rel intensity) 194 (M+, 100). HPLC purity 98.48% (MeOH-H2O, 80-20).
2-Methoxyphenazine (32)
The general procedure was followed. The compound was purified by silica gel column chromatography, eluting with ethyl acetate-dichloromethane, 1:1. The product was obtained as light brown solid (26 mg, 6.5%): mp 116–118 °C (lit50 mp 126 °C). 1H NMR (CDCl3) δ 8.14 (m, 2 H), 8.04 (d, J = 9.5 Hz, 1 H), 7.75 (m, 2 H), 7.47 (d, J = 2.7 Hz, 1 H), 7.34 (s, 1 H), 3.97 (s, 1 H); 13C NMR (CDCl3) δ 149.7, 143.3, 142.8, 141.6, 137.4, 135.2, 130.7, 130.4, 129.5, 128.9, 121.9, 111.7, 55.8; EIMS m/z (rel intensity) 210 (M+, 100). HPLC purity 99.40% (MeOH-H2O, 80:20).
2,3-Dimethoxyphenazine (33)
The general procedure was followed. The compound was purified by silica gel column chromatography, eluting with ethyl acetate-dichloromethane, 1.5:1. The product was obtained as a yellow solid (113 mg, 25%): mp 215-127 °C. (lit51 mp 230–231 °C). 1H NMR (CDCl3) δ 8.13-8.09 (m, 2 H), 7.74-7.70 (m, 2 H), 7.34 (s, 2 H), 4.07 (s, 3 H); 13C NMR (CDCl3) δ 154.3, 141.8, 141.7, 128.9, 128.7, 105.1, 56.3; EIMS m/z (rel intensity) 240 (M+, 100). HPLC purity 96.82% (MeOH-H2O, 80-20).
1-Bromo-3-nitrophenazine (34)
The general procedure was followed. The compound was purified by silica gel column chromatography, eluting with ethyl acetate-hexane, 1.5:1. The product was obtained as a dark yellow solid (39 mg, 6.7%): mp 214–216 °C. IR (film) 3089, 1624, 1599, 1558, 1535, 1509, 1461, 1411, 1346, 862, 758 cm−1, 1H NMR (CDCl3) δ 9.16 (d, J = 2.3 Hz, 1 H), 8.90 (d, J = 2.3 Hz, 1 H), 8.41 (dd, J = 7.8 Hz, 2.1 Hz, 1 H), 8.32 (dd, J = 7.8 Hz, 2.0 Hz, 1 H), 8.03-7.98 (m, 2 H); 13C NMR (CDCl3) δ 147.4, 144.8, 142.0, 141.4, 133.2, 132.6, 130.1, 129.6, 126.4, 126.1; ESIMS m/z (rel intensity) 304 (MH+, 100); HRESIMS m/z calcd 302.9643, found 302.9651. HPLC purity: 95.46% (MeOH-H2O, 70:30), 98.05% (MeOH-H2O, 90:10).
1,4-Dimethoxyphenazine (35)
The general procedure was followed. The compound was purified by silica gel column chromatography (with one-half inch of basic alumina on top) eluting with dichloromethane. The product was obtained as a yellow solid (68 mg, 17%): mp 179–181 °C (lit52 mp 185 °C). IR (film) 3056, 2994, 2935, 2835, 1626, 1606, 1485, 1463, 1397, 1383, 1247, 1231, 1092, 764, 752 cm−1, 1H NMR (CDCl3) δ 8.34 (m, 2 H), 7.79 (m, 2 H), 6.88 (m, 2 H), 4.05 (s, 3 H); 13C NMR (CDCl3) δ 148.7, 142.1, 136.9, 130.6, 129.8, 105.7, 56.2; EIMS m/z (rel intensity) 240 (M+, 86), 225 (M+-CH3, 100); CIMS m/z (rel intensity) 241 (MH+, 100); HREIMS m/z calcd 240.0899, found 240.0900. HPLC purity: 95.00% (MeOH, 100), 98.05% (MeOH-H2O, 90:10).
7,8-Dimethyl-1-methoxyphenazine (38)
The general procedure provided a solid that was dissolved in hot ethyl acetate (70 mL). The solution was filtered through celite. The celite was washed with ethyl acetate (~ 200 mL) until the filtered solution was light brown. Water (40 mL) was added and the organic phase was collected. The organic layer was washed with a saturated aqueous solution of sodium bicarbonate (30 mL) and water (2 × 30 mL). The compound was purified by silica gel column chromatography (1 inch of basic alumina on top), using ethyl acetate-hexane, 1:1. The product was obtained as a yellow powder (713 mg, 39.9%): mp 187–189 °C (dec). IR (film) 3043, 2916, 2834, 1636, 1606, 1560, 1515, 1477, 1469, 1442, 1400, 1109, 871, 775, 739 cm−1; 1H NMR (CDCl3) δ 8.12 (s, 1 H), 7.96 (s, 1 H), 7.78 (d, J = 8.8 Hz, 1 H), 7.7 (t, J = 7.6 Hz, 1 H), 7.01 (d, J = 7.3 Hz, 1 H), 4.15 (s, 3 H), 2.53 (s, 6 H); 13C NMR (CDCl3) δ 154.8, 143.5, 142.6, 141.8, 141.3, 141.1, 136.0, 129.4, 128.2, 127.3, 121.1, 105.6, 56.1, 20.4; CIMS m/z (rel intensity) 239 (M+, 100); HREIMS m/z calcd 238.1106, found 238.1108. Anal. Calcd for C15H14N2O·0.2 H2O: C,74.48; H, 6.00; N, 11. 58. Found: C, 74.14; H, 6.00; N, 11.76.
7,8-Dimethyl-3-bromo-1-methoxyphenazine (39)
The general procedure was followed. The solvent was removed in vacuo. The compound was purified by silica gel column chromatography (1 inch of basic alumina on top), eluting with dichloromethane-ethyl acetate, 95:5. The product was obtained as a yellow powder (919 mg, 47.5%); mp 229–231 °C. IR (film) 3054, 2986, 2918, 2849, 1691, 1632, 1593, 1557, 1516, 1465, 1442, 1421, 1265, 748, 705 cm−1; 1H NMR (CDCl3) δ 8.09 (s, 1 H), 7.99 (d, J = 1.6 Hz, 1 H), 7.92 (s, 1 H), 4.16 (s, 3 H), 2.55 (s, 3 H), 2.54 (s, 3 H); 13C NMR (CDCl3) δ 155.2, 144.0, 143.8, 143.5, 142.1, 141.4, 128.4, 127.5, 123.7, 123.4, 110.4, 56.6, 20.6; EIMS m/z (rel intensity) 319 (M+, 87), 317 (M+, 85) 237 (M+-HBr, 100); HREIMS m/z calcd 317.0289, found 317.0291. HPLC purity 97.8% (MeOH-H2O, 95:5), 96.11% (MeOH, 100).
7,8-Dichloro-1-methoxyphenazine (40)
The general procedure provided a solid that was dissolved in hot ethyl acetate (70 mL). The solution was filtered through a column containing celite® 545 (~ 1 inch) and basic alumina on top (~ 1 inch). The celite was washed with ethyl acetate until the filtered solution was light in color. The organic solutions were combined and washed with saturated sodium bicarbonate (50 mL) and water (50 mL). The compound was purified by silica gel column chromatography, eluting with ethyl acetate-hexane, 1:3. The product was obtained as a dark yellow powder (539 mg, 19.4%): mp 245–247 °C. IR (film) 3041, 2964, 1619, 1555, 1518, 1503, 1466, 1413, 1396, 1284, 1254, 1111, 885, 763 cm−1; 1H NMR (CDCl3) δ 8.53 (s, 1 H), 8.35 (s, 1 H), 7.79 (d, 2 H), 7.11 (t, J = 4.33 Hz, 1 H); 13C NMR (CDCl3) δ 155.0, 144.5, 141.8, 140.5, 137.1, 135.7, 135.0, 131.5, 130.2, 129.5, 121.3, 107.1, 56.5; EIMS m/z (rel intensity) 278 (M+, 100); HREIMS m/z calcd 278.0014, found 278.0011. HPLC purity: 96.74% (MeOH-H2O, 95:5).
3-Bromo-7,8-dichloro-1-methoxyphenazine (41)
The general procedure was followed. The solvent was removed under vacuum and the solid purified by silica gel column chromatography (with one-half inch of basic alumina on top) using hexane-ethyl acetate, 1:3. The product was obtained as a yellow solid (331 mg, 18%): mp: 249–251 °C. IR (film) 2962, 2922, 1609, 1578, 1554, 1505, 1452, 1431, 1260, 1099, 801 cm−1; 1H NMR (CDCl3) δ 8.49 (s, 1 H), 8.33 (s, 1 H), 8.00 (s, 1 H), 7.15 (s, 1 H), 4.18 (s, 1 H); 13C NMR (CDCl3) δ 155.1, 144.3, 142.1, 140.3, 136.4, 135.8, 135.5, 130.4, 129.4, 126.2, 123.4, 111.7, 56.9; EIMS m/z (rel intensity) 357 (M+, 100); HRCIMS m/z calcd 356.9197, found 356.9199. HPLC purity 98.45% (MeOH-H2O, 95:5).
4-Bromo-7,8-dimethyl-1-methoxyphenazine (42)
7,8-Dimethyl-1-methoxyphenazine (38, 330 mg, 1.38 mmol) was dissolved in toluene (13 mL) and acetonitrile (1.3 mL). N-Bromosuccinimide (246 mg, 1.38 mmol) was added to the flask and the reaction mixture was heated at reflux for 12 h. The solvent was removed under vacuum and the solid residue purified by silica gel column chromatography, eluting with ethyl acetate-hexane, 2:1. The product was obtained as a yellow powder (223 mg, 51%): mp 194–196 °C. IR (film) 3048, 2931, 2835, 1630, 1600, 1549, 1515, 1472, 1415, 1385, 1343, 1287, 1235, 1123, 1097, 924, 727 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.11 (s, 1 H), 8.09 (s, 1 H), 7.99 (d, J = 8.2 Hz, 1 H), 6.89 (d, J = 8.2 Hz, 1 H), 4.13 (s, 3 H), 2.53 (s, 6 H); 13C NMR (125 MHz, CDCl3) δ 155.0, 142.8, 142.4, 141.6, 140.4, 136.4, 132.2, 128.0, 127.9, 113.9, 106.9, 106.2, 56.4, 20.6, 20.5; CIMS m/z (rel intensity) 397 (MH+, 100), 395 (MH+, 69); HREIMS m/z calcd 393.9316, found 393.9327. HPLC purity 96.50% (MeOH-H2O, 90:10).
7,8-Dimethyl-1-methoxy-3,4-dibromophenazine (43)
7,8-Dimethyl-3-bromo-1-methoxyphenazine (39, 270 mg, 0.93 mmol) was dissolved in toluene (20 mL). Bromine (148 mg, 0.93 mmol), sodium acetate (200 mg, 2.43 mmol) and acetic acid (20 mL) were added. The reaction mixture was heated at reflux for 1 h. The solvent was removed in vacuo and the orange solid dissolved in ethyl acetate (40 mL). The organic phase was washed with brine (1 × 30 mL) and water (2 × 30 mL). The organic phase was removed in vacuo and the compound purified by silica gel column, eluting with hexane-ethyl acetate, 5:1 to 3:1. The product was obtained as a yellow powder (276 mg, 74%): mp 225–227 °C. IR (film) 3047, 2973, 2931, 1633, 1584, 1459, 1315, 1131, 1000, 863, 763, 750 cm−1; 1 H NMR (CDCl3) δ 7.98 (s, 1 H), 7.95 (s, 1 H), 7.1 2 (s, 1 H), 4.08 (s, 3 H), 2.48 (s, 6 H); 13C NMR (CDCl3) δ 154.2, 143.2, 142.9, 142.5, 141.0, 140.9, 134.9, 127.9, 127.8, 126.8, 117.0, 111.0, 56.6, 20.6, 20.5; CIMS m/z (rel intensity) 395 (MH+, 68); HREIMS m/z calcd 393.9316, found 393.9316. Anal. Calcd for C15H12Br2N2O: C, 45.23; H, 2.96; N, 6.91. Found: C, 45.49; H, 3.05; N, 7.07
7,8-Dichloro-1-hydroxyphenazine (44)53
7,8-Dichloro-1-methoxyphenazine (40, 270 mg, 0.96 mmol) was dissolved in dry, freshly distilled dichloromethane (30 mL). The reaction mixture was purged with argon and cooled to −78 °C. Boron tribromide (1 M solution in dichloromethane, 2.5 mL) was added dropwise to the mixture with stirring. After 5 min, more boron tribromide (1 mL, 1 M solution in dichloromethane) was added. The temperature was maintained at −78 °C and the reaction mixture was stirred for 2 h. The reaction mixture was allowed to warm to room temperature and stirred for 16 h. The reaction mixture was heated at reflux for 1 h. The solution was allowed to cool to room temperature and saturated aqueous sodium bicarbonate (40 mL) at 0 °C was slowly added. The solution was extracted with ethyl acetate (4 × 30 mL). The organic extracts were combined and washed with brine (30 mL) and water (30 mL). The solvent was removed and the compound purified by silica gel column chromatography, eluting with ethyl acetate-acetic acid, 100:1. The product was obtained as an orange very sticky solid (191 mg, 75%): mp 270–271 °C (dec). IR (film) 3398, 1630, 1505, 1462, 1450, 1419, 1275, 1260, 764, 750 cm−1; 1 H NMR (DMSO-d6) δ 10.80 (OH, br s, 1 H), 8.56 (s, 1 H), 8.55 (s, 1 H), 7.83 (t, J = 8.4 Hz, 1 H), 7.67 (d, J = 8.7 Hz, 1 H), 7.22 (d, J = 7.4 Hz, 1 H); ESIMS m/z (rel intensity) 265 (MH+, 100); HRESIMS m/z calcd 264.9935, found 264.9937. Anal Calcd. for C12H6Cl2N2O·0.5 CHCl3; C, 46.23; H, 2.02; N, 8.63. Found; C, 46.50; H, 2.07; N, 8.85. HPLC purity: 97.93% (MeOH-H2O, 95:5).
1-Methoxybenzo[b]phenazine (46)
The general procedure provided a solid that was dissolved in hot ethyl acetate (100 mL). The solution was filtered through a column containing celite® 545 (~ 1 inch) with basic alumina (~ 1 inch) on top. The celite was washed with ethyl acetate until the filtered solution was light in color. The organic solutions were combined and washed with saturated sodium bicarbonate (50 mL) and water (50 mL). The compound was purified by silica gel column chromatography, eluting with ethyl acetate-hexane, 1:3.5. The product was obtained as a dark red powder (577 mg, 17%): mp 245–247 °C. IR (film) 2932, 1732, 1582, 1548, 1536, 1463, 1406, 1284, 1254, 1111, 885, 763, 736 cm−1; 1H NMR (CDCl3) δ 9.04 (s, 1 H), 8.83 (s, 1 H), 8.10-8.08 (m, 2 H), 7.78 (d, J = 9 Hz, 1 H), 7.68 (t, J = 7.5 Hz, 1 H), 7.51-7.47 (m, 2 H), 6.96 (d, J = 7.3 Hz, 1 H), 4.17 (s, 3 H); 13C NMR (CDCl3) δ 154.9, 145.0, 139.8, 138.6, 138.4, 134.6, 134.2, 130.7, 128.6, 128.4, 127.1, 126.9, 126.6, 121.6, 105.6, 56.4; EIMS m/z (rel intensity) 260 (M+, 66), 231 (M+-CHO,100); HREIMS m/z calcd 260.0950, found 260.0949. HPLC purity: 96.25% (MeOH-H2O, 95:5).
Benzo[b]phenazine (47)
The general procedure was followed. The compound was purified by silica gel column chromatography (with one-half inch of basic alumina on top) eluting with dichloromethane. The product was obtained as an orange solid (93 mg, 32%): mp 219–221 °C (lit54 mp 233 °C). IR (film) 1686, 1581, 1528, 1456, 1381, 1365, 1326 cm−1; 1H NMR (CDCl3) δ 8.84 (s, 2 H), 8.18 (dd, J = 6.8, J = 3.3 Hz, 2 H), 8.07 (dd, J = 6.6, J = 3.2 Hz, 2 H), 7.77 (dd, J = 6.9, J = 3.1 Hz, 2 H), 7.49 (dd, J = 6.7, J = 3.1 Hz, 2 H); 13C NMR (CDCl3) δ 144.4 (2 C), 139.9 (2 C), 134.4 (2 C), 130.5 (2 C), 129.8 (2 C), 128.4 (2 C), 127.5 (2 C), 126.7 (2 C); EIMS m/z (rel intensity) 230 (M+, 100); HREIMS m/z calcd 230.0844, found 230.0843. HPLC purity 95.18% (MeOH-H2O, 90:10), 97.11% (MeOH-H2O, 95:5).
2,3-Dimethylbenzo[g]quinoxaline (49)55
2,3-Butanedione (48, 0.43 g, 4.95 mmol) in benzene (6 mL) was added dropwise to a solution of 2,3-diaminonaphthalene (45, 0.53 g, 3.35 mmol) in benzene (10 mL) and glacial acetic acid (6 mL). The reaction mixture was stirred for 12 h. The solvent was removed in vacuo and the solid dissolved in ethyl acetate (50 mL). The solution was washed with saturated aqueous sodium bicarbonate (2 × 40 mL), brine (40 mL) and water (40 mL). The product was purified by silica gel column chromatography, eluting with ethyl acetate-hexane, 1:3. The product was obtained as a brown solid (0.31 g, 45%): mp 209–211°C. IR (film) 2990, 2917, 1630, 1580, 1522, 1438, 1411, 1373, 1158, 899, 877, 787 cm−1; 1H NMR (CDCl3) δ 8.46 (s, 2 H), 8.03-7.99 (m, 2 H), 7.50-7.46 (m, 2 H), 2.69 (s, 6 H); 13C NMR (CDCl3) δ 154.3, 137.9, 133.1, 128.2, 126.3, 126.1, 23.5; CIMS m/z (rel intensity) 209 (MH+, 100); HREIMS m/z calcd 208.1000, found 208.0998. HPLC purity: 95.41% (MeOH-H2O, 95:5).
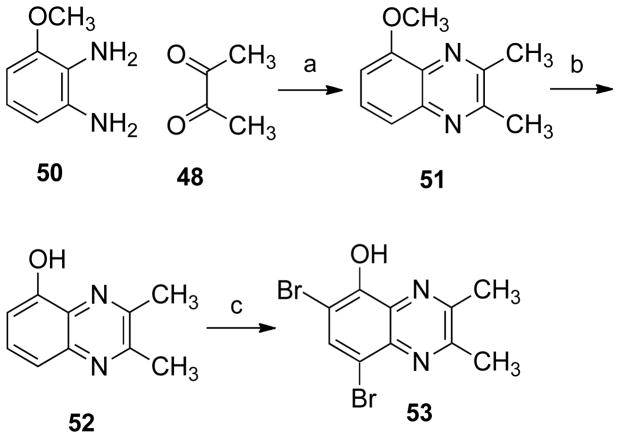
5-Methoxy-2,3-dimethylquinoxaline (51)
A solution of 2,3-butanedione (48, 0.26 g, 3.0 mmol) was added dropwise to a solution of 3-methoxy-o-phenylenediamine (50, 0.41 g, 3.0 mmol) in benzene (30 mL) and glacial acetic acid (25 mL). The reaction mixture was stirred for 12 h at room temperature. The solvent was removed in vacuo and the solid dissolved in ethyl acetate (50 mL). The solution was washed with saturated aqueous sodium bicarbonate (2 × 30 mL), brine (30 mL) and water (2 × 20 mL). The solvent was removed and the compound purified by silica gel column chromatography, using dichloromethane as solvent. The product was obtained as a beige solid (322 mg, 57%): mp 118–119 °C (lit. mp56 118 °C). IR (film) 3088, 3054, 2993, 2948, 1633, 1609, 1571, 1480, 1467, 1446, 1402, 1376, 1196, 1094, 996, 823, 769 cm−1; 1H NMR (CDCl3) δ 7.56 (m, 2 H), 7.00 (t, J = 4.8 Hz, 1 H), 4.06 (s, 3 H), 2.76 (s, 3 H), 2.71 (s, 3 H); 13C NMR (CDCl3) δ 154.5, 153.7, 152.0, 141.8, 132.8, 128.6, 120.1, 107.0, 56.1, 23.2, 23.0. CIMS m/z (rel intensity) 189 (MH+, 100); HRCIMS m/z calcd 188.0950, found 188.0952. HPLC purity: 95.95% (MeOH-H2O, 95:5).
5-Hydroxy-2,3-dimethylquinoxaline (52)57
5-Methoxy-2,3-dimethylquinoxaline (51, 278 mg, 1.47 mmol) was dissolved in dry, freshly distilled dichloromethane (25 mL). The reaction mixture was purged with argon and cooled to −78 °C. Boron tribromide (1 M solution in dichloromethane, 3.0 mL) was added dropwise to the mixture with stirring. The temperature was maintained at −78 °C and the reaction mixture was stirred for 1 h. The reaction mixture was allowed to warm to room temperature and stirred for 12 h. The reaction mixture was heated at reflux for 1 h. The solution was allowed to cool to room temperature and saturated aqueous sodium bicarbonate (30 mL) at ~ 4 °C was slowly added. The solution was extracted with dichloromethane (3 × 35 mL). The solvent was removed and the compound purified by silica gel column chromatography, eluting with ethyl acetate-hexane, 1:1. The product was obtained as a light brown powder (203 mg, 79%): mp 139–140 °C. IR (film) 3358, 3022, 2997, 2954, 2919, 1619, 1576, 1488, 1477, 1442, 1427, 1408, 1383, 1217, 1193, 823, 764, 751 cm−1; 1H NMR (CDCl3) δ 8.01 (s, 1 H), 7.52-7.43 (m, 2 H), 7.07 (dd, J = 7.2, J = 1.3 Hz, 1 H), 2.64 (s, 1 H), 2.59 (s, 1 H); 13C NMR (CDCl3) δ 154.1, 151.3, 151.0, 141.2, 130.7, 129.6, 118.5, 109.9, 22.9, 22.6; EIMS m/z (rel intensity) 174 (M+, 100); HREIMS m/z calcd 174.0793, found 174.0790. HPLC purity: 96.94% (MeOH-H2O, 95:5).
6,8-Dibromo-2,3-dimethylquinoxalin-5-ol (53)
N-Bromosuccinimide (654 mg, 3.68 mmol) and 5-hydroxy-2,3-dimethyl quinoxaline (52, 320 mg, 1.84 mmol) were mixed together and dissolved in toluene (10 mL) and acetonitrile (5 mL). The reaction mixture was heated at reflux for 8 h. The solvent was removed in vacuo and the compound purified by silica gel column chromatography (ethyl acetate-hexane, 1:3). The product was obtained as a brown solid (301 mg, 49.3%): mp 108–109 °C. IR (film) 3408, 3057, 2995, 2956, 2922, 2852, 1713, 1687, 1610, 1560, 1459, 1424, 1401, 1381, 1371, 1203, 937, 734 cm−1; 1H NMR (CDCl3) δ 8.05 (OH br s, 1 H), 7.93 (s, 1 H), 2.74 (s, 1 H), 2.70 (s, 1 H); 13C NMR (CDCl3) δ 155.2, 152.9, 148.5, 137.6, 135.0, 130.9, 111.7, 103.4, 23.2, 22.6; EIMS m/z (rel intensity) 330 (M+, 88); HREIMS m/z calcd 329.9792, found 329.9005. HPLC purity: 98.31% (MeOH-H2O, 90:10).
Quinone Reductase 1 (QR1) Assay
Hepa 1c1c7 (mouse hepatoma) cells were used in the assay. Cells were incubated in a 96-well plate with test compounds at a maximum concentration of 50 μM for 48 h, digitonin was used to permeabilize cell membranes, and enzyme activity was measured by the reduction of 3-(4,5-dimethylthiazo-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to a blue formazan. Production was measured by absorption at 595 nm. A total protein assay using crystal violet staining was run in parallel. The total protein levels were determined using crystal violet staining in parallel to determine the cytotoxicity of test compounds.58 Briefly, the media was discarded after 48 h incubation with test compounds, and cells were stained with 100 μL of 0.2% crystal violet in 2% ethanol for 10 min. After washing cells with tap water to remove excessive crystal violet, 200 μL of 0.5% SDS in 50% ethanol was added to dissolve the stained cells. The optical density was determined at 595 nm. 4′-Bromoflavone (CD = 0.01 μM) was used as a positive control.
Expression and Purification of Human Quinone Reductase 2 (QR2)
Human QR2 was expressed and purified from 3 L of E. coli BL21(DE3) grown in Luria-Bertani medium supplemented with 100 μg/mL ampicillin following our previously reported procedures.9 The concentration of purified QR2 was determined using the Bio-RAD Protein Assay. Frozen QR2, previously stored at −80 °C, was thawed on ice and then adjusted to the desired concentration, typically as a 4 mg/mL stock for enzyme kinetics measurements, by diluting in storage buffer without glycerol.
Steady-State Kinetic Assays and QR2 IC50 Value Determination
The catalytic activity of QR2 was determined using MTT [3-(4,5-dimethyltiazol-2-yl)-2,5-diphenyltetrazolium bromide] and NMeH as substrates. Assays were run in 96-well plates with a final assay volume of 200 μL. Each assay mixture contained approximately 12 nM QR2, 17.5 μM NMeH, and 200 μM MTT in a reaction buffer containing 100 mM NaCl, 50 mM Tris, and 0.1% Triton-X100 (Fisher Biotech 93004). The assays were performed at 23 °C using a SpectraMax Plus 384 UV/Vis microplate reader by monitoring the increase in absorbance at 612 nm, which is due to formation of the product formazan, the reduced form of the substrate MTT. All reactions were initiated by the addition of QR2. The initial slopes of the reaction (ΔAbs/Δtime) were measured and were used to calculate the initial rates of the reaction using a value of 11,300 mM-1 cm−1 for the molar extinction coefficient.
IC50 values were also determined in 96-well plates at 23 °C using the same assay conditions described above except that inhibitor concentrations were varied from 0.1 to 100 μM. Assays at each inhibitor concentration were performed in triplicate, and the average and standard deviations in the rate values were used to determine the final IC50 values by calculating the percent inhibition (%I) at each inhibitor concentration versus the negative control with zero inhibitor. These data were then plotted as the percent inhibition versus inhibitor concentration, [I]. All data were fit to the equation: %I = %Imax/[(1 + [I]/IC50)] using non-linear regression via the Enzyme Kinetics Module of the program SigmaPlot from SPSS Scientific. IC50 values are reported along with their standard error in the fitted parameters.
NF-κB Luciferase Assay
Studies were performed with NF-κB reporter stably-transfected human embryonic kidney cells 293 from Panomics (Fremont, CA). This cell line contains chromosomal integration of a luciferase reporter construct regulated by NF-κB response element. The gene product, luciferase enzyme, reacts with luciferase substrate, emitting light, which is detected with a luminometer. Data were expressed as % inhibition at 50 μM or IC50 values (i.e., concentration of test sample required to inhibit TNF-α activated NF-κB activity by 50%). After incubating treated cells, they were lysed in Reporter Lysis buffer. The luciferase assay was performed using the Luc assay system from Promega, following the manufacturer’s instructions. In this assay, Nα-tosyl-L-phenylalanine chloromethyl ketone (TPCK) was used as a positive control; IC50 = 5.09 μM.
Nitric Oxide (NO) Synthase Assay
RAW 264.7 cells were incubated in a 96-well culture plate for 24 h. The cells were treated with various concentrations of compounds dissolved in phenol red-free DMEM for 30 min followed by 1 μg/mL of LPS treatment for 24 h. NO was oxidized to the stable end product, nitrite, by the addition of Griess reagent [1:1 mixture (v/v) of 1% sulfanilamide in 2.5% H3PO4 and 0.1% N-(1-naphthyl)ethylenediamine], and absorbance was measured at 540 nm. A standard curve was created by using known concentrations of sodium nitrite. The positive control in this assay was Na-L monomethyl arginine (L-NMMA); IC50 = 19.7 μM.
Sulforhodamine B (SRB) Assay
To evaluate the cytotoxicity of test compounds toward NF-κB reporter stably-transfected human embryonic kidney cells 293 and RAW 264.7 cells in the assay condition, SRB assay was performed as describe previously.59 Briefly, after the fixation with 10% trichloroacetic acid, cells were stained with 0.4% SRB solution followed by dissolving bound SRB to cells in 10 mM Tris-buffer. The optical density was determined at 515 nm.
Acknowledgments
This work was facilitated by the National Institutes of Health (NIH) through support with Research Grant P01 CA48112. M.C.S. thanks Dr. Karl Wood, Dr. Huaping Mo, and Dr. Daniel Lee for consultation and valuable discussion.
Abbreviations
- BBO
multinuclear broadband observe
- CD
concentration to double QR1 induction
- CI
chemopreventive index, IC50/CD
- DMEM
Dulbecco’s Modified Eagle Medium
- IC50
half maximal inhibitory concentration
- iNOS
inducible nitric oxide synthase
- IR
QR1 induction ratio
- LPS
lipopolysaccharide
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PS1
phase 1 enzyme
- QNP
quattro nucleus probe
- QR1
quinone reductase 1
- QR2
quinone reductase 2
References
- 1.Seffrin JR, Hill D, Burkart W, Magrath I, Badwe RA, Ngoma T, Mohar A, Grey N. It Is Time to Include Cancer and Other Noncommunicable Diseases in the Millennium Development Goals. Ca-a Can J Clin. 2009;59:282–284. doi: 10.3322/caac.20033. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao YP, Xu JQ, Thun MJ. Cancer Statistics, 2009. Ca-a Can J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of Cancer Incidence in the United States: Burdens Upon an Aging, Changing Nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 4.Hong WK, Sporn MB. Recent Advances in Chemoprevention of Cancer. Science. 1997;278:1073–1077. doi: 10.1126/science.278.5340.1073. [DOI] [PubMed] [Google Scholar]
- 5.Cuendet M, Oteham CP, Moon RC, Pezzuto JM. Quinone Reductase Induction as a Biomarker for Cancer Chemoprevention. J Nat Prod. 2006;69:460–463. doi: 10.1021/np050362q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Georgiadis P, Demopoulos NA, Topinka J, Stephanou G, Stoikidou M, Bekyrou M, Katsouyianni K, Sram R, Autrup H, Kyrtopoulos SA. Impact of Phase I or Phase II Enzyme Polymorphisms on Lymphocyte DNA Adducts in Subjects Exposed to Urban Air Pollution and Environmental Tobacco Smoke. Toxicol Lett. 2004;149:269–280. doi: 10.1016/j.toxlet.2003.12.038. [DOI] [PubMed] [Google Scholar]
- 7.Dietz BM, Kang YH, Liu GW, Eggler AL, Yao P, Chadwick LR, Pauli GF, Farnsworth NR, Mesecar AD, van Breemen RB, Bolton JL. Xanthohumol Isolated from Humulus lupulus Inhibits Menadione-Induced DNA Damage through Induction of Quinone Reductase. Chem Res Toxicol. 2005;18:1296–1305. doi: 10.1021/tx050058x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barillari J, Iori R, Broccoli M, Pozzetti L, Canistro D, Sapone A, Bonamassa B, Biagi GL, Paolini M. Glucoraphasatin and Glucoraphenin, a Redox Pair of Glucosinolates of Brassicaceae, Differently Affect Metabolizing Enzymes in rRats. J Agric Food Chem. 2007;55:5505–5511. doi: 10.1021/jf070558r. [DOI] [PubMed] [Google Scholar]
- 9.Calamini B, Santarsiero BD, Boutin JA, Mesecar AD. Kinetic, Thermodynamic, and X-Ray Structural Insights into the Interaction of Melatonin and Analogs with Qinone Reductase 2. Biochem J. 2008 doi: 10.1042/BJ20071373. Immediate Publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buryanovskyy L, Fu Y, Boyd M, Ma YL, Hsieh TC, Wu JM, Zhang ZT. Crystal Structure of Quinone Reductase 2 in Complex with Resveratrol. Biochemistry. 2004;43:11417–11426. doi: 10.1021/bi049162o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lisztwan J, Pornon A, Chen B, Chen SA, Evans DB. The Aromatase Inhibitor Letrozole and Inhibitors of Insulin-like Growth Factor I Receptor Synergistically Induce Apoptosis in In Vitro Models of Estrogen-Dependent Breast Cancer. Breast Cancer Res. 2008;10:R56. doi: 10.1186/bcr2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang L, Zhang YS. Dietary Isothiocyanates Inhibit the Growth of Human Bladder Carcinoma Cells. J Nutr. 2004;134:2004–2010. doi: 10.1093/jn/134.8.2004. [DOI] [PubMed] [Google Scholar]
- 13.Eggler AL, Gay KA, Mesecar AD. Molecular Mechanisms of Natural Products in Chemoprevention: Induction of Cytoprotective Enzymes by Nrf2. Mol Nutr Food Res. 2008;52:S84–S94. doi: 10.1002/mnfr.200700249. [DOI] [PubMed] [Google Scholar]
- 14.Misico RI, Song LL, Veleiro AS, Cirigliano AM, Tettamanzi MC, Burton G, Bonetto GM, Nicotra VE, Silva GL, Gil RR, Oberti JC, Kinghorn AD, Pezzuto JM. Induction of Quinone Reductase by Withanolides. J Nat Prod. 2002;65:677–680. doi: 10.1021/np0106337. [DOI] [PubMed] [Google Scholar]
- 15.Fu Y, Buryanovskyy L, Zhang ZT. Quinone Reductase 2 Is a Catechol Quinone Reductase. J Biol Chem. 2008;283:23829–23835. doi: 10.1074/jbc.M801371200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talalay P, Fahey JW, Holtzclaw WD, Prestera T, Zhang YS. Chemoprotection Against Cancer by Phase 2 Enzyme Induction. Toxicology Lett. 1995;82–3:173–179. doi: 10.1016/0378-4274(95)03553-2. [DOI] [PubMed] [Google Scholar]
- 17.Angeloni C, Leoncini E, Malaguti M, Angelini S, Hrelia P, Hrelia S. Modulation of Phase II Enzymes by Sulforaphane: Implications for Its Cardioprotective Potential. J Agric Food Chem. 2009;57:5615–5622. doi: 10.1021/jf900549c. [DOI] [PubMed] [Google Scholar]
- 18.Surh YJ. NF-kappa B and Nrf2 as Potential Chemopreventive Targets of Some Anti-inflammatory and Antioxidative Phytonutrients with Anti-inflammatory and Antioxidative Activities. Asia Pac J Clin Nutr. 2008;17:269–272. [PubMed] [Google Scholar]
- 19.Libermann TA, Zerbini LF. Targeting Transcription Factors for Cancer Gene Therapy. Curr Gene Ther. 2006;6:17–33. doi: 10.2174/156652306775515501. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal BB, Shishodia S. Molecular Targets of Dietary Agents for Prevention and Therapy of Cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Spector N, Xia WL, El-Hariry I, Yarden Y, Bacus S. Small Molecule HER-2 Tyrosine Kinase Inhibitors. Breast Cancer Res. 2007;9:205. [Google Scholar]
- 22.Fabian MA, Biggs WH, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, Carter TA, Ciceri P, Edeen PT, Floyd M, Ford JM, Galvin M, Gerlach JL, Grotzfeld RM, Herrgard S, Insko DE, Insko MA, Lai AG, Lelias JM, Mehta SA, Milanov ZV, Velasco AM, Wodicka LM, Patel HK, Zarrinkar PP, Lockhart DJ. A Small Molecule-Kinase Interaction Map for Clinical Kinase Inhibitors. Nat Biotechnol. 2005;23:329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 23.Jin X, Gossett DR, Wang S, Yang D, Cao Y, Chen J, Guo R, Reynolds RK, Lin J. Inhibition of AKT Aurvival Pathway by a Small Molecule Inhibitor in Human Endometrial Cancer Cells. Br J Cancer. 2004;91:1808–1812. doi: 10.1038/sj.bjc.6602214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple Biological Activities of Curcumin: A Short Review. Life Sci. 2006;78:2081–2087. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Thangapazham RL, Sharma A, Maheshwari RK. Multiple Molecular Targets in Cancer Chemoprevention by Curcumin. Aaps Journal. 2006;8:E443–E449. doi: 10.1208/aapsj080352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manson MM, Holloway KA, Howells LM, Hudson EA, Plummer SM, Squires MS, Prigent SA. Modulation of Signal-Transduction Pathways by Chemopreventive Agents. Biochemical Soc Trans. 2000;28:7–12. doi: 10.1042/bst0280007. [DOI] [PubMed] [Google Scholar]
- 27.Cross JV, Deak JC, Rich EA, Qian YY, Lewis M, Parrott LA, Mochida K, Gustafson D, Vande Pol S, Templeton DJ. Quinone Reductase Inhibitors Block SAPK/JNK and NF Kappa B Pathways and Potentiate Apoptosis. J Biol Chem. 1999;274:31150–31154. doi: 10.1074/jbc.274.44.31150. [DOI] [PubMed] [Google Scholar]
- 28.Laursen JB, Nielsen J. Phenazine Natural Products: Biosynthesis, Synthetic Analogues, and Biological Activity. Chem Rev. 2004;104:1663–1685. doi: 10.1021/cr020473j. [DOI] [PubMed] [Google Scholar]
- 29.Kerr JR, Taylor GW, Rutman A, Hoiby N, Cole PJ, Wilson R. Pseudomonas aeruginosa Pyocyanin and 1-Hydroxyphenazine Inhibit Fungal Growth. J Clin Pathol. 1999;52:385–387. doi: 10.1136/jcp.52.5.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinya K, Shimizu S, Kunigami T, Furihata K, Hayakawa Y, Seto H. Novel Neuronal Cell Protecting Substances, Aestivophoenin-a and Aestivophoenin-B, Produced by Streptomyces-Purpeofuscus. J Antibiot. 1995;48:1378–1381. doi: 10.7164/antibiotics.48.1378. [DOI] [PubMed] [Google Scholar]
- 31.Shinya K, Shimizu S, Kunigami T, Furihata K, Seto H. A New Neuronal Cell Protecting Substance, Lavanduquinocin, Produced by Streptomyces Viridochromogenes. J Antibiot. 1995;48:574–578. doi: 10.7164/antibiotics.48.574. [DOI] [PubMed] [Google Scholar]
- 32.Birkofer L, Birkofer A. Bacteriostatic Effect of a Phenazine Derivative of Mycobacterium tuberculosis Type Gallinaceus. Naturwissenschaften. 1949;36:92. [Google Scholar]
- 33.Nakayama O, Arakawa H, Yagi M, Tanaka M, Kiyoto S, Okuhara M, Kohsaka M. Ws-9659 a and B, Novel Testosterone 5-Alpha-Reductase Inhibitors Isolated from a Streptomyces.3. Biological Characteristics and Pharmacological Characteristics. J Antibiot. 1989;42:1235–1240. doi: 10.7164/antibiotics.42.1235. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto M, Seto H. Stimulation of Mammalian-Cell Proliferation by Lavanducyanin. J Antibiot. 1991;44:1471–1473. doi: 10.7164/antibiotics.44.1471. [DOI] [PubMed] [Google Scholar]
- 35.Surrey A. Pyocyanine. Org Syn. 1955;3:753. [Google Scholar]
- 36.Wrede F, Strack E. Synthesis of Pyocyanine and Some of Its Homologues. Ber Desch Chem Ges. 1929;62:2051–2057. [Google Scholar]
- 37.Dinkova-Kostova AT, Talalay P. Persuasive Evidence that Quinone Reductase Type 1 (DT diaphorase) Protects Cells Against the Toxicity of Electrophiles and Reactive Forms of Oxygen. Free Radical Biol Med. 2000;29:231–240. doi: 10.1016/s0891-5849(00)00300-2. [DOI] [PubMed] [Google Scholar]
- 38.Schupp PJ, Kohlert-Schupp C, Whitefield S, Engemann A, Rohde S, Hemscheidt T, Pezzuto JM, Kondratyuk TP, Park EJ, Marler L, Rostama B, Wright AD. Cancer Chemopreventive and Anticancer Evaluation of Extracts and Fractions from Marine Macro- and Micro-organisms Collected from Twilight Zone Waters Around Guam. Nat Prod Commun. 2009;4:1717–1728. [PMC free article] [PubMed] [Google Scholar]
- 39.Lohar MV, Mundada R, Bhonde M, Padgaonkar A, Deore V, Yewalkar N, Bhatia D, Rathos M, Joshi K, Vishwakarma RA, Kumar S. Design and Synthesis of Novel Furoquinoline Based Inhibitors of Multiple Targets in the PI3K/Akt-mTOR Pathway. Bioorg Med Chem Lett. 2008;18:3603–3606. doi: 10.1016/j.bmcl.2008.04.078. [DOI] [PubMed] [Google Scholar]
- 40.Xu Q, Briggs J, Park S, Niu GL, Kortylewski M, Zhang SM, Gritsko T, Turkson J, Kay H, Semenza GL, Cheng JQ, Jove R, Yu H. Targeting Stat3 Blocks Both HIF-1 and VEGF Expression Induced by Multiple Oncogenic Growth Signaling Pathways. Oncogene. 2005;24:5552–5560. doi: 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]
- 41.Fan QW, Knight ZA, Goldenberg DD, Yu W, Mostov KE, Stokoe D, Shokat KM, Weiss WA. A Dual PI3 Kinase/mTOR Inhibitor Reveals Emergent Efficacy in Glioma. Cancer Cell. 2006;9:341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aggarwal BB, Takada Y, Oommen OV. From Chemoprevention to Chemotherapy: Common Targets and Common Goals. Expert Opin Invest Drugs. 2004;13:1327–1338. doi: 10.1517/13543784.13.10.1327. [DOI] [PubMed] [Google Scholar]
- 43.Gerhauser C, You M, Liu JF, Moriarty RM, Hawthorne M, Mehta RG, Moon RC, Pezzuto JM. Cancer Chemopreventive Potential of Sulforamate, a Novel Analogue of Sulforaphane that Induces Phase 2 Drug-metabolizing Enzymes. Cancer Res. 1997;57:272–278. [PubMed] [Google Scholar]
- 44.Cornblatt BS, Ye LX, Dinkova-Kostova AT, Erb M, Fahey JW, Singh NK, Chen MSA, Stierer T, Garrett-Mayer E, Argani P, Davidson NE, Talalay P, Kensler TW, Visvanathan K. Preclinical and Clinical Evaluation of Sulforaphane for Chemoprevention in the Breast. Carcinogenesis. 2007;28:1485–1490. doi: 10.1093/carcin/bgm049. [DOI] [PubMed] [Google Scholar]
- 45.Yosioka I. Phenazines. XXI. Bromination of Phenazine Derivatives. Chem Pharm Bull. 1959;8:581–584. [Google Scholar]
- 46.Ueda K, Yosioka I. Studies on Phenazines. XXX. Bromination of Phenazine Derivatives by N-Bromosuccinimide (2) Chem Pharm Bull. 1968;16:1521–1526. [Google Scholar]
- 47.Teuber HJ, Staiger G. Reactions with Nitrosodisulfonate. 7. Ortho-Benzoquinone and Phenazine. Chem Ber /Recl. 1955;88:802–827. [Google Scholar]
- 48.Shah JR, Shah C, Velingkar SV. Synthesis and Antimicrobial Activity of Some Phenazine aAnalogues. Indian J Heterocycl Chem. 2003;13:175–176. [Google Scholar]
- 49.Vivian DL, Hartwell JL, Waterman HC. Phenazine Syntheses. 3. Miscellaneous Phenazines. J Org Chem. 1954;19:1641–1645. [Google Scholar]
- 50.Tietze M, Iglesias A, Merisor E, Conrad J, Klaiber I, Beifuss U. Efficient Methods for the Synthesis of 2-Hydroxyphenazine Based on the Pd-Catalyzed N-Arylation of Aryl Bromides. Org Lett. 2005;7:1549–1552. doi: 10.1021/ol050198y. [DOI] [PubMed] [Google Scholar]
- 51.Vivian DL. Phenazine Syntheses. 7. Certain Disubstituted Phenazines. J Org Chem. 1956;21:824–825. [Google Scholar]
- 52.King FE, Clark NG, Davis PMH. Hydroxy-Quinoxalines and Hydroxy-Phenazines, and Experiments on the Preparation of Hydroxyquinoxaline Di-N-Oxides. J Chem Soc. 1949:3012–3016. [Google Scholar]
- 53.Issidorides CH, Atfah MA, Sabounji JJ, Sidani AR, Haddadin MJ. Application of 1,2-Diketones in the Synthesis of Phenazine Oxides. Tetrahedron. 1978;34:217–221. [Google Scholar]
- 54.Vanallan JA, Reynolds GA, Adel RE. Polynuclear Heterocycles. 2. Addition Reactions of Benzophenazines. J Org Chem. 1962;27:2873–2878. [Google Scholar]
- 55.Mariaud M, Levillain P. New Quantitative Determination of 2,3-Dioxobutane (Diacetyl) in Alcoholic Beverages by an Indirect Spectrofluorometric Procedure with Use of 2,3-Diaminonaphthalene as Reagent of Derivatization. Talanta. 1994;41:75–79. doi: 10.1016/0039-9140(94)80170-3. [DOI] [PubMed] [Google Scholar]
- 56.Lane ES, Williams C. Organic Complex-Forming Agents for Metals. 2. Preparation of 5-Hydroxy-Quinoxalines and 5-8-Dihydroxy-Quinoxalines and Related Compounds. J Chem Soc. 1956:2983–2986. [Google Scholar]
- 57.Barret R, Daudon M. Oxidation of Phenols to Quinones by Bis(trifluoroacetoxy)iodobenzene. Tetrahedron Lett. 1990;31:4871–4872. [Google Scholar]
- 58.Su BN, Gu JQ, Kang YH, Park EJ, Pezzuto JM, Kinghorn AD. Induction of the Phase II Enzyme, Quinone Reductase, by Withanolides and Norwithanolides from Solanaceous Species. Mini-Rev in Org Chem. 2004;1:115–123. [Google Scholar]
- 59.You M, Wickramaratne DBM, Silva GL, Chai HY, Chagwedera TE, Farnsworth NR, Cordell GA, Kinghorn AD, Pezzuto JM. (−)-Roemerine, an Aporphine Alkaloid from Annona-Senegalensis That Reverses the Multidrug-Resistance Phenotype with Cultured-Cells. J Nat Prod. 1995;58:598–604. doi: 10.1021/np50118a021. [DOI] [PubMed] [Google Scholar]