Abstract
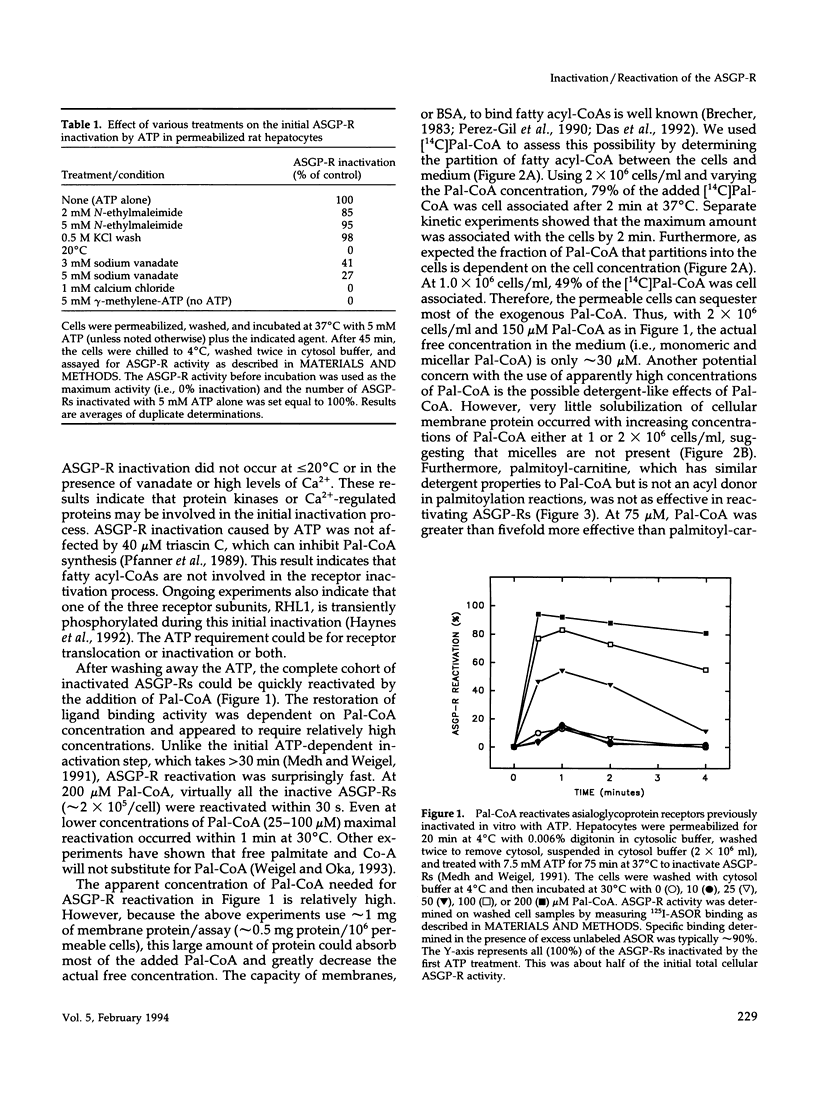
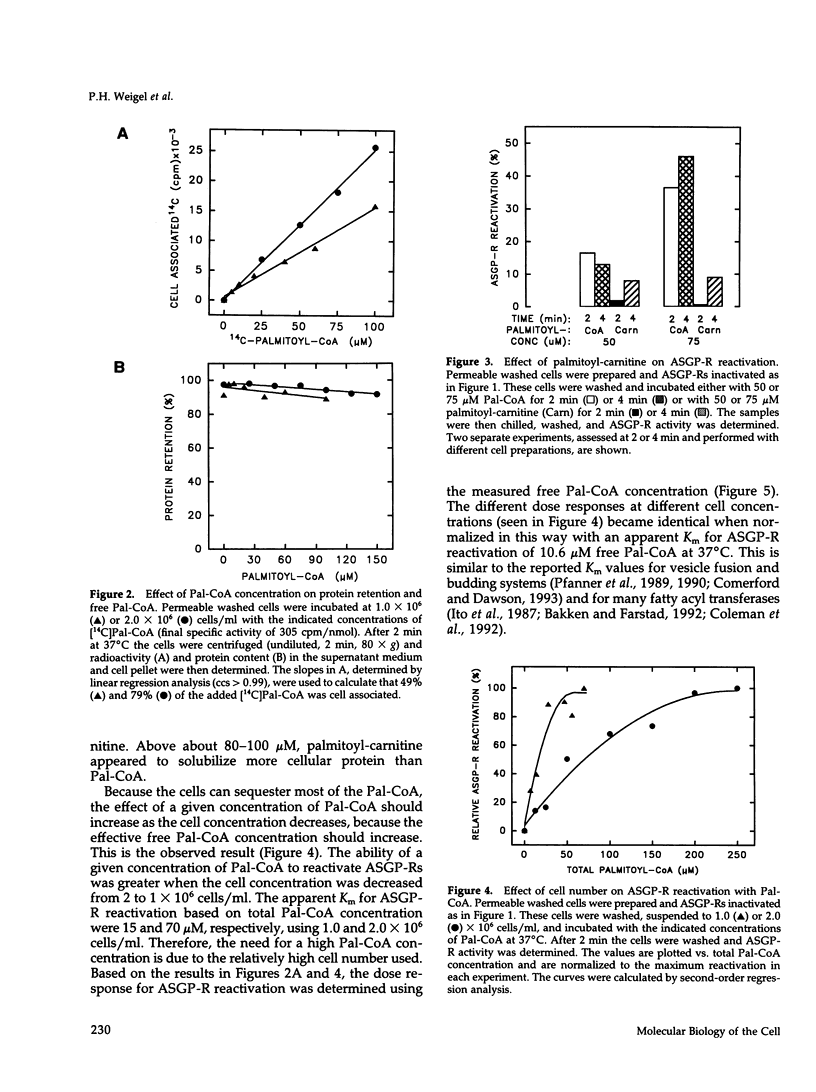
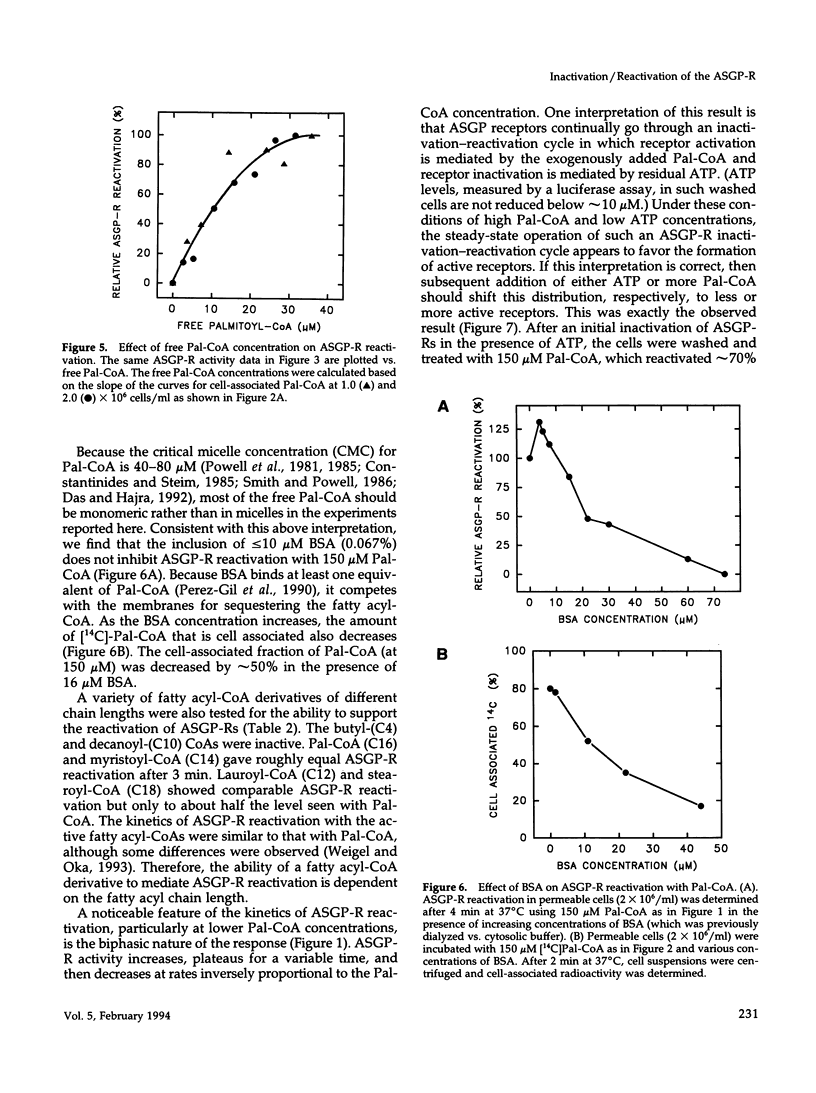
Asialoglycoprotein receptors (ASGP-Rs) in permeable rat hepatocytes can be inactivated in the absence of ligand. This cytosol-independent effect is relatively slow (t1/2 approximately 12 min) and is temperature and ATP dependent. Here we show that in the absence of cytosol, the addition of palmitoyl-CoA (Pal-CoA) rapidly (t1/2 < 0.4 min) and quantitatively reactivates the inactivated receptors. Receptor reactivation was half-maximal at approximately 10-12 microM free Pal-CoA at 37 degrees C. Although substantially higher total concentrations were used, much of the added Pal-CoA was cell associated and not free. The effects of Pal-CoA were eliminated by bovine serum albumin at concentrations sufficient to bind all free monomeric fatty acyl-CoA, suggesting that micellar effects are not responsible for the ability to reactivate ASGP-Rs. Also, palmitoyl-carnitine did not substitute for Pal-CoA. The initial ASGP-R inactivation is not affected by treating cells with N-ethylmaleimide or by a KCl wash but is inhibited by sodium orthovanadate or high Ca2+ levels. Myristoyl-CoA (C14) was also able to reactivate inactive ASGP-Rs about as well as Pal-CoA. Fatty acyl-CoAs with chain lengths of C12 (lauroyl) or C18 (steroyl) were < 50% as active. The ligand binding activity of these receptors can subsequently be modulated within minutes by the further addition of ATP or Pal-CoA to achieve additional rounds of ASGP-R inactivation or reactivation, respectively. These in vitro data demonstrate the occurrence of a novel asialoglycoprotein receptor inactivation-reactivation cycle that could regulate receptor activity during endocytosis and receptor recycling.
Full text
PDF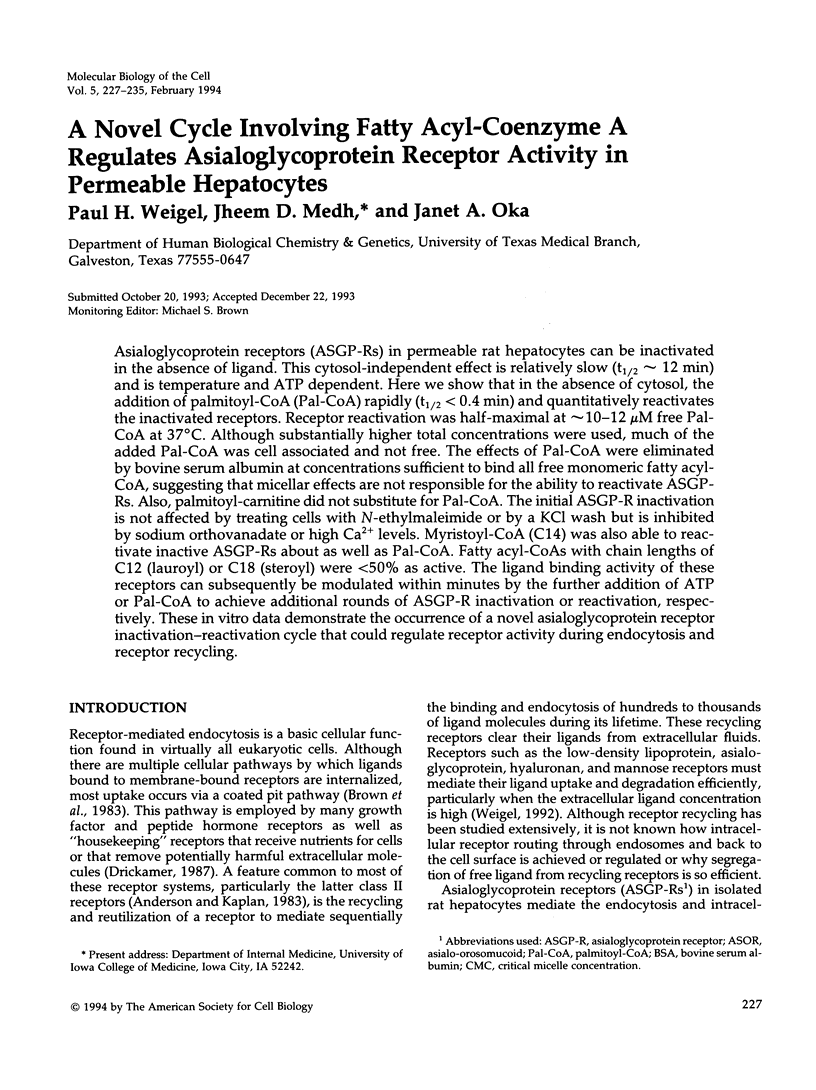




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam M., Turbide C., Johnstone R. M. Incorporation of myristate and palmitate into the sheep reticulocyte transferrin receptor: evidence for identical sites of labeling. Arch Biochem Biophys. 1988 Aug 1;264(2):553–563. doi: 10.1016/0003-9861(88)90321-9. [DOI] [PubMed] [Google Scholar]
- Bakken A. M., Farstad M. The activities of acyl-CoA:1-acyl-lysophospholipid acyltransferase(s) in human platelets. Biochem J. 1992 Dec 15;288(Pt 3):763–770. doi: 10.1042/bj2880763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Anderson R. G., Brown M. S. Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell. 1981 May;24(2):493–502. doi: 10.1016/0092-8674(81)90340-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brecher P. The interaction of long-chain acyl CoA with membranes. Mol Cell Biochem. 1983;57(1):3–15. doi: 10.1007/BF00223520. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Anderson R. G., Goldstein J. L. Recycling receptors: the round-trip itinerary of migrant membrane proteins. Cell. 1983 Mar;32(3):663–667. doi: 10.1016/0092-8674(83)90052-1. [DOI] [PubMed] [Google Scholar]
- Clarke B. L., Weigel P. H. Recycling of the asialoglycoprotein receptor in isolated rat hepatocytes. ATP depletion blocks receptor recycling but not a single round of endocytosis. J Biol Chem. 1985 Jan 10;260(1):128–133. [PubMed] [Google Scholar]
- Clary D. O., Griff I. C., Rothman J. E. SNAPs, a family of NSF attachment proteins involved in intracellular membrane fusion in animals and yeast. Cell. 1990 May 18;61(4):709–721. doi: 10.1016/0092-8674(90)90482-t. [DOI] [PubMed] [Google Scholar]
- Coleman R. A., Rao P., Fogelsong R. J., Bardes E. S. 2-Bromopalmitoyl-CoA and 2-bromopalmitate: promiscuous inhibitors of membrane-bound enzymes. Biochim Biophys Acta. 1992 Apr 23;1125(2):203–209. doi: 10.1016/0005-2760(92)90046-x. [DOI] [PubMed] [Google Scholar]
- Comerford J. G., Dawson A. P. Effects of CoA and acyl-CoAs on GTP-dependent Ca2+ release and vesicle fusion in rat liver microsomal vesicles. Biochem J. 1993 Jan 15;289(Pt 2):561–567. doi: 10.1042/bj2890561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides P. P., Steim J. M. Physical properties of fatty acyl-CoA. Critical micelle concentrations and micellar size and shape. J Biol Chem. 1985 Jun 25;260(12):7573–7580. [PubMed] [Google Scholar]
- Das A. K., Hajra A. K. Critical micellar concentrations of palmitoyl dehydroxyacetone phosphate and 1-palmitoyl-rac-glycerol 3-phosphate. J Biol Chem. 1992 May 15;267(14):9731–9731. [PubMed] [Google Scholar]
- Das A. K., Horie S., Hajra A. K. Biosynthesis of glycerolipid precursors in rat liver peroxisomes and their transport and conversion to phosphatidate in the endoplasmic reticulum. J Biol Chem. 1992 May 15;267(14):9724–9730. [PubMed] [Google Scholar]
- Davoust J., Gruenberg J., Howell K. E. Two threshold values of low pH block endocytosis at different stages. EMBO J. 1987 Dec 1;6(12):3601–3609. doi: 10.1002/j.1460-2075.1987.tb02691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeney J. T., Tornheim K., Korchak H. M., Prentki M., Corkey B. E. Acyl-CoA esters modulate intracellular Ca2+ handling by permeabilized clonal pancreatic beta-cells. J Biol Chem. 1992 Oct 5;267(28):19840–19845. [PubMed] [Google Scholar]
- Grand R. J. Acylation of viral and eukaryotic proteins. Biochem J. 1989 Mar 15;258(3):625–638. doi: 10.1042/bj2580625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg D. F., Wager R. E., Farrell D. C., Hildreth J., 4th, Quesenberry M. S., Loeb J. A., Holland E. C., Drickamer K. Major and minor forms of the rat liver asialoglycoprotein receptor are independent galactose-binding proteins. Primary structure and glycosylation heterogeneity of minor receptor forms. J Biol Chem. 1987 Jul 15;262(20):9828–9838. [PubMed] [Google Scholar]
- Harford J., Bridges K., Ashwell G., Klausner R. D. Intracellular dissociation of receptor-bound asialoglycoproteins in cultured hepatocytes. A pH-mediated nonlysosomal event. J Biol Chem. 1983 Mar 10;258(5):3191–3197. [PubMed] [Google Scholar]
- Hubbard A. L., Wilson G., Ashwell G., Stukenbrok H. An electron microscope autoradiographic study of the carbohydrate recognition systems in rat liver. I. Distribution of 125I-ligands among the liver cell types. J Cell Biol. 1979 Oct;83(1):47–64. doi: 10.1083/jcb.83.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Fukui T., Saito T., Tomita K. Inhibition of acetoacetyl-CoA synthetase from rat liver by fatty acyl-CoAs. Biochim Biophys Acta. 1987 Dec 14;922(3):287–293. [PubMed] [Google Scholar]
- Kaplan J., Keogh E. A. Analysis of the effect of amines on inhibition of receptor-mediated and fluid-phase pinocytosis in rabbit alveolar macrophages. Cell. 1981 Jun;24(3):925–932. doi: 10.1016/0092-8674(81)90118-5. [DOI] [PubMed] [Google Scholar]
- Kindberg G. M., Gudmundsen O., Berg T. The effect of vanadate on receptor-mediated endocytosis of asialoorosomucoid in rat liver parenchymal cells. J Biol Chem. 1990 Jun 5;265(16):8999–9005. [PubMed] [Google Scholar]
- Malhotra V., Orci L., Glick B. S., Block M. R., Rothman J. E. Role of an N-ethylmaleimide-sensitive transport component in promoting fusion of transport vesicles with cisternae of the Golgi stack. Cell. 1988 Jul 15;54(2):221–227. doi: 10.1016/0092-8674(88)90554-5. [DOI] [PubMed] [Google Scholar]
- McAbee D. D., Weigel P. H. ATP depletion causes a reversible redistribution and inactivation of a subpopulation of galactosyl receptors in isolated rat hepatocytes. J Biol Chem. 1987 Feb 15;262(5):1942–1945. [PubMed] [Google Scholar]
- Medh J. D., Weigel P. H. Reconstitution of galactosyl receptor inactivation in permeabilized rat hepatocytes is ATP-dependent. J Biol Chem. 1991 May 15;266(14):8771–8778. [PubMed] [Google Scholar]
- Oka J. A., Weigel P. H. Monensin inhibits ligand dissociation only transiently and partially and distinguishes two galactosyl receptor pathways in isolated rat hepatocytes. J Cell Physiol. 1987 Nov;133(2):243-52, 257. doi: 10.1002/jcp.1041330207. [DOI] [PubMed] [Google Scholar]
- Olson E. N. Modification of proteins with covalent lipids. Prog Lipid Res. 1988;27(3):177–197. doi: 10.1016/0163-7827(88)90012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S. Covalent binding of fatty acid to the transferrin receptor in cultured human cells. J Biol Chem. 1981 May 25;256(10):4715–4718. [PubMed] [Google Scholar]
- Pfanner N., Glick B. S., Arden S. R., Rothman J. E. Fatty acylation promotes fusion of transport vesicles with Golgi cisternae. J Cell Biol. 1990 Apr;110(4):955–961. doi: 10.1083/jcb.110.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N., Orci L., Glick B. S., Amherdt M., Arden S. R., Malhotra V., Rothman J. E. Fatty acyl-coenzyme A is required for budding of transport vesicles from Golgi cisternae. Cell. 1989 Oct 6;59(1):95–102. doi: 10.1016/0092-8674(89)90872-6. [DOI] [PubMed] [Google Scholar]
- Powell G. L., Grothusen J. R., Zimmerman J. K., Evans C. A., Fish W. W. A re-examination of some properties of fatty acyl-CoA micelles. J Biol Chem. 1981 Dec 25;256(24):12740–12747. [PubMed] [Google Scholar]
- Pérez-Gil J., Estrada P., Acebal C., Arche R. Effect of albumin on acyl-CoA: lysolecithin acyltransferase, lysolecithin: lysolecithin acyltransferase and acyl-CoA hydrolase from rabbit lung. Mol Cell Biochem. 1990 May 10;94(2):167–173. doi: 10.1007/BF00214123. [DOI] [PubMed] [Google Scholar]
- Schwartz A. L., Strous G. J., Slot J. W., Geuze H. J. Immunoelectron microscopic localization of acidic intracellular compartments in hepatoma cells. EMBO J. 1985 Apr;4(4):899–904. doi: 10.1002/j.1460-2075.1985.tb03716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Buss J. E. The covalent modification of eukaryotic proteins with lipid. J Cell Biol. 1987 Jun;104(6):1449–1453. doi: 10.1083/jcb.104.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. H., Powell G. L. The critical micelle concentration of some physiologically important fatty acyl-coenzyme A's as a function of chain length. Arch Biochem Biophys. 1986 Jan;244(1):357–360. doi: 10.1016/0003-9861(86)90124-4. [DOI] [PubMed] [Google Scholar]
- Spiess M., Schwartz A. L., Lodish H. F. Sequence of human asialoglycoprotein receptor cDNA. An internal signal sequence for membrane insertion. J Biol Chem. 1985 Feb 25;260(4):1979–1982. [PubMed] [Google Scholar]
- Tietze C., Schlesinger P., Stahl P. Mannose-specific endocytosis receptor of alveolar macrophages: demonstration of two functionally distinct intracellular pools of receptor and their roles in receptor recycling. J Cell Biol. 1982 Feb;92(2):417–424. doi: 10.1083/jcb.92.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tycko B., Keith C. H., Maxfield F. R. Rapid acidification of endocytic vesicles containing asialoglycoprotein in cells of a human hepatoma line. J Cell Biol. 1983 Dec;97(6):1762–1776. doi: 10.1083/jcb.97.6.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel P. H., Oka J. A. Endocytosis and degradation mediated by the asialoglycoprotein receptor in isolated rat hepatocytes. J Biol Chem. 1982 Feb 10;257(3):1201–1207. [PubMed] [Google Scholar]
- Weigel P. H., Oka J. A. Regulation of asialoglycoprotein receptor activity by a novel inactivation/reactivation cycle. Receptor reactivation in permeable rat hepatocytes is mediated by fatty acyl coenzyme A. J Biol Chem. 1993 Dec 25;268(36):27186–27190. [PubMed] [Google Scholar]
- Weigel P. H., Oka J. A. The surface content of asialoglycoprotein receptors on isolated hepatocytes is reversibly modulated by changes in temperature. J Biol Chem. 1983 Apr 25;258(8):5089–5094. [PubMed] [Google Scholar]
- Weigel P. H., Ray D. A., Oka J. A. Quantitation of intracellular membrane-bound enzymes and receptors in digitonin-permeabilized cells. Anal Biochem. 1983 Sep;133(2):437–449. doi: 10.1016/0003-2697(83)90106-9. [DOI] [PubMed] [Google Scholar]


