Abstract
“Click-on” fluorogenic reaction: a non-fluorescent benzothiazole with an electron-deficient alkyne group at 2-position reacts with azide containing molecules could form fluorescent adducts.
Keywords: Fluorogenic dye, Benzothiazole, Click-on reaction
To study specific molecules in cells, precise labelling is the key. Ideally, the fluorescent reporter should only tag the interested targets. Fluorescent proteins co-expressed with targeted molecules have been widely used in studying cellular events, and the fluorescent signal makes the real time microscopic visualization of process possible 1, 2. However, the fluorescent proteins are large proteins, which could be too big for many applications. Small peptide tags have also been introduced into protein expressing systems for site-specific labeling 3, 4. For instance, a peptide tag recognized by Texas-red dye has been expressed in cells for fluorescent calcium sensing5. Tetracysteine (-Cys-Cys-Pro-Gly-Cys-Cys-) containing proteins, which could be labeled by fluorogenic biarsenical probes, have been developed for cell labels6, 7. Yet these methods are still protein based approaches, which cannot be applied to investigate non-protein molecules. In order to study other biomolecules, flexible non-protein based labeling approaches are required.
Theoretically, chemical approaches with millions of diversities should be handy for intracellular labeling of biomolecules. Nevertheless, site-specific chemical labeling of biomolecules is not an easy task. Reactions that require non-physiological conditions, such as heat, extreme pH, or organic solvents, cannot be applied to normal cells. Commonly used conjugating groups, including activated ester which react with amino groups and maleimide which react with sulfhydryl groups, these groups are not practical for the site-specific labeling, due to high numbers of amino and sulfhydryl groups in cells. A special chemoselective ligation reaction has to be designed for each type of target. A few chemical ligation approaches using ketone, azide, and alkyne as reactive groups have been reported for cell labels as well8. The typical procedure is started by introducing a small percentage of chemically modified building units together with normal building units, such as amino acids, monosaccharides, or nucleosides, into cell culture. If the appended chemical group was small enough and could be tolerated by natural synthase, the biosynthetic machinery would assemble the biomacromolecules containing those unusual building units. The introduced chemical reactive groups presented on biomacromolecules then would serve as the linking points for subsequent fluorescent tagging.
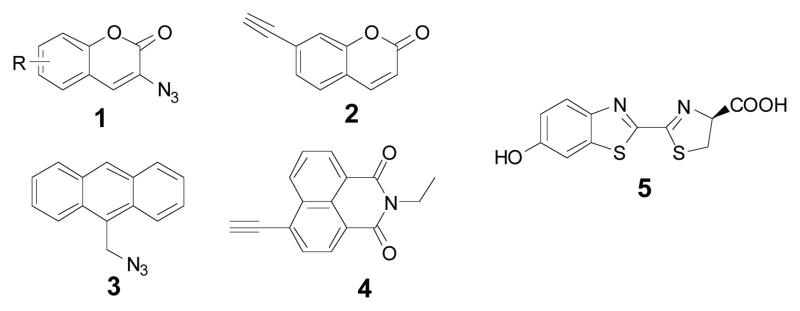
In recent years, “click reaction” has become a handy tool used in drug discovery9–11, combinatorial chemistry12, material science13, and tagging of biomolecules14. The 1,2,3-triazole formation from an azide and an alkyne can be done in aqueous condition, therefore this approach has been applied in cells15, 16 and, most recently, in animals17. Fluorescent reporters have been clicked onto almost all kinds of biomolecules, such as nucleic acids, proteins, carbohydrates, and lipids. However, most fluorescent reporters used in click chemistry are prepared from existing fluorochromes which are functionalized with an alkyne or azide group through a linker. Although the azide/alkynce click reaction is specific, excess fluorochromes are always added to the reaction to ensure good conversion. Since those reporters fluoresce at all time, high background fluorescence signals prohibit real time visualization. Recently, few fluorogenic dyes, such as coumarin 1 and 212,18, anthracene 319 and naphthalimid 4 derivatives20, were reported to have no-fluorescent property until the click reaction occurred. These “click-on” fluorogenic dyes will not have the aforementioned high background issue, because they are designed to be non-fluorescent in their initial structure (Fig. 1). They only fluoresce after the completion of click reaction; therefore, the excess unreacted dyes will not interfere with fluorescence signal.
Figure 1.
Examples for existing fluorogenic dyes and luciferin 5.
Recognizing the usefulness of the switchable dyes, we have developed a new benzothiazole based fluorogenic “click-on” dye and demonstrated its potential usages in nucleic acid and protein labeling.
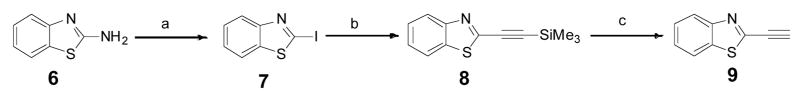
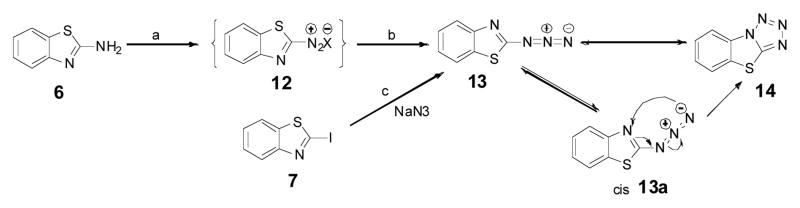
Benzothiazole alkyne 9 was chosen as the profluorophore since its analog luciferin 5 has been shown to have fluorescent property21–24. Based on the chemical structure of benzothiazole ring, it was hypothesized that an electron-deficient alkyne or electron-rich azide groups at the 2-position would diminish the fluorescence property. However, a click reaction which adds a conjugated triazole ring onto the parent benzothiazole ring will convert the chromophore into a fluorophore.
Benzothiazole alkyne 9 was prepared using 6 as the starting material (Scheme 1). Following the protocol developed by Elena et al, 6 was transformed into iodobenzothiazole 7 by a diazotization-iodination in the presence of p-toluenesulfonic acid in acetonitrile25. The protected acetylenic benzothiazole 8 was obtained by the cross-coupling reaction of iodobenzothiazole 7 with a terminal alkyne using catalytic quantities of Pd(PPh3)2Cl2 and CuI under basic conditions26. The prepared 8 was then applied to a silica gel column for further purification; whereas it was found that the protecting trimethylsilanyl group came off automatically during chromatographic purification, resulting in the desired benzothiazole alkyne 9. As expected, 9 whose absorbance maximum is 286 nm (Fig. S1) is non-fluorescent.
Scheme 1.
Reaction and condition: a.p-MeC6H4SO3H, MeCN, RT-10°C, KI, NaNO2, H2O, 10 min, 10–15°C. b.1. CuI, PdCl2(PPh3)2, NEt3, DCM; 2. NaHCO3, H2O c. acid
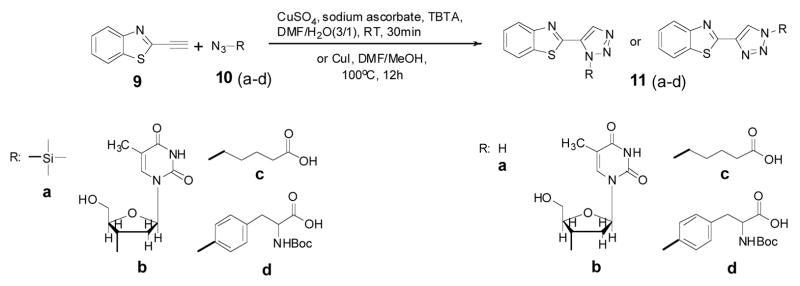
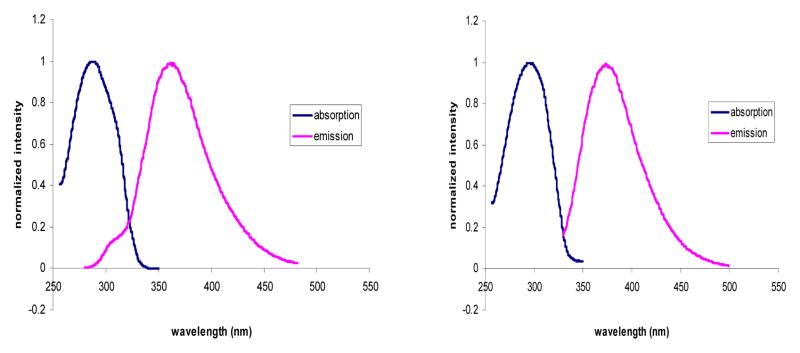
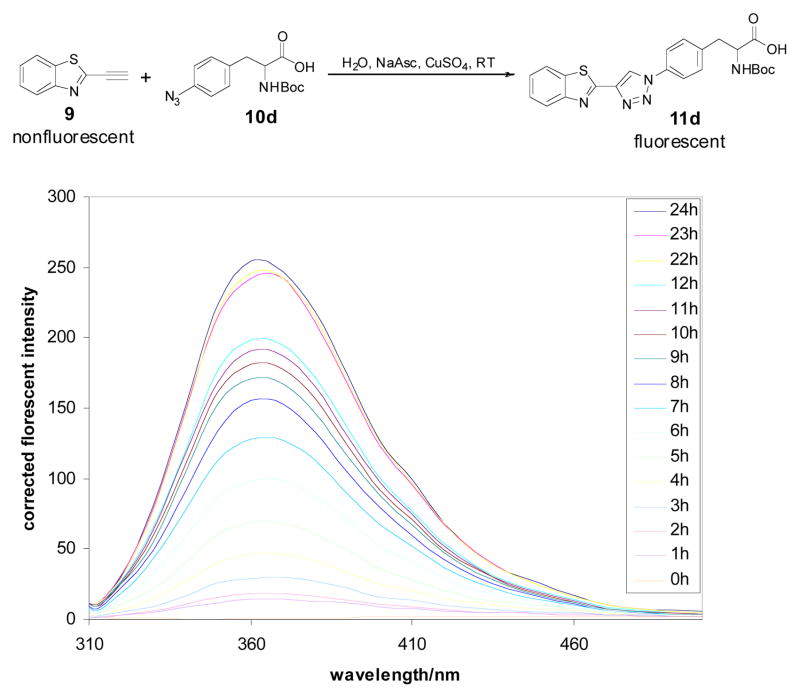
To confirm that the proposed “click-on” reaction can create a fluorophore, the benzothiazole alkyne 9 was first reacted with trimethylsilylazide 10a (according to Jin, T.’s method)27 in DMF/MeOH using CuI as catalyst, at 100°C for 12h (Scheme 2). The reaction conversion was only around 20%. But importantly, the clicked product 11a was fluorescent, having excitation and emission at 285 nm and 363 nm, respectively, in methanol (Fig. 2 (right)). Later, refer to Chan, T. R.’s method28, TBTA (tris-(benzyltriazolylmethyl)amine) was used as the catalyst under room temperature for 1h, the same reactions were tested with other azide containing analogs, including nucleoside AZT (azidothymidine) (10b), 6-azido-hexanoic acid (10c) and Boc-p-azido-phe-OH (10d). Near quantitative conversion was obtained for all reactions (Table S1), and all clicked products are fluorescent as compound 11a. In general, linking different groups to the 2-position of the 1,2,3-triazole ring has little effect on the absorption and emission maxima (Table 1). All “click-on” products (11a–d and 16) showed absorption maximum at around 278–297 nm, emission maximum at around 351–374 nm and stokes’ shift around 70~80 nm in methanol. The fluorescence change of the “click-on” reaction was monitored in real time by reacting compound 9 (φ=0.14% in water, using L-tryptophen as a reference) with 10d (φ=3.5%, in water) catalyzed in an aqueous solution of sodium ascorbate and CuSO4 at RT. The broad emission band was observed from approximately 310 nm to 500 nm, which is characteristic of 11d (Fig. S2), once the CuSO4 was added to the mixture solution of 9 and 10d in 1 mM NaAsc aqueous solution. As the reaction proceeds, the fluorescence emission increases strongly. The fluorescent signal increased from 1.6 a.u. to 254 a.u. within 24 hours, representing a 158-fold increase in fluorescence signal (Fig. 3).
Scheme 2.
Click reaction
Figure 2.
Absorption and fluorescence of dye 16 (left) and 11a (right) in MeOH.
Table 1.
UV absorption and emission spectra of compounds 11a–d and 16.
| 11a | 11b | 11c | 11d | 16 | |
|---|---|---|---|---|---|
| λmax(abs)[nm] | 286 | 278 | 290 | 294 | 297 |
| λmax(em)[nm] | 363 | 351 | 353 | 354 | 374 |
Condition: in MeOH, excitation wavelength: 285 nm.
Figure 3.
Fluorescence emission spectra increase of a 3 ml H2O solution containing 9 (10 μM), 10d (10 μM), NaAsc (1 mM) and CuSO4 (10 μM) (λex=285 nm) within 24h at RT.
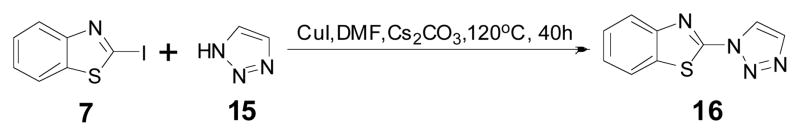
The above results showed that the electron deficient alkyne at position 2 of the benzothiazole ring is critical to the “click-on” reaction. Nevertheless, the “click on” reaction is bi-directional, it is interesting to see if an electron-rich azide group at the same position could have a similar “click-on” effect. Photoinduced electron transfer (PET) effect by lone pair electrons has been widely documented in various fluorochromes29. To synthesize benzothiazole azide 13, two different approaches, direct diazotization of the compound 6 and substitution of an iodol group on benzothiazole 7 with sodium azide, were tried. However none of them could lead to the desired compound 13. The compound 13 seemed not stable20, but forming the byproduct 14 through a spontaneous cyclization as previously suggested (Scheme 3)30. Since the chemical property didn’t allow isolating the “click-on” compound 13, the expected final clicked product was prepared instead to validate the hypothesis. The clicked product 16 was synthesized by reacting iodobenzothiazole (7) with 1,2,3-trizole (15) referencing a reported protocol (Scheme 4) 24. Interestingly the compound 16 excites at 297 nm and emits at 374 nm (Fig. 2 (left)). Comparing the structures of the compound 16 and 11a, the substitution positions on the 1,2,3-trizole ring are different, but the conjugating double bonds inside of 1,2,3-trizole ring of both compounds are extended over the benzothiazole ring, resulting in similar fluorescent property. Although the “click-on” compound 13 was not obtained in this preparation, the compound 16 has suggested that the fluorescent property of an electron rich azide group containing dye could also be activated by an alkyne group through a “click-on” reaction.
Scheme 3.
reaction and condition: a. p-MeC6H4SO3H, MeCN, 0–10°C, NaN3, NaNO2, H2O, 10 min, 10–15°C. b. NaN3. c. NaN3, MeCN
Scheme 4.
Preparation of compound 16
In conclusion, we describe the first development of benzothiazole “click-on” fluorogenic dyes. The click-on dye could have an electron-rich azide group or an electron-deficient alkyne group at the 2-position. The key requisition to achieve the “click-on” fluorescent property is that the newly formed double bonds inside of the 1,2,3-trizole ring have to form conjugate double bonds with the parent fluorophore. Potentially, the same principle could be extended to design other fluorescence dyes. The demonstrated model reactions with functionalized nucleoside and amino acid have indicated that the developed click-on dye could be applied to label various biomolecules, such as nucleic acids, proteins and other molecules.
Supplementary Material
Acknowledgments
We thank the NMR facility at M. D. Anderson Cancer Center for acquiring all NMR data. This research was supported in part by NIH CA135312, and CA114149.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Jarvik JW, Fisher GW, Shi C, Hennen L, Hauser C, Adler S, Berget PB. Biotechniques. 2002;33:852. doi: 10.2144/02334rr02. [DOI] [PubMed] [Google Scholar]
- 2.Giepmans BN, Adams SR, Ellisman MH, Tsien RY. Science. 2006;312:217. doi: 10.1126/science.1124618. [DOI] [PubMed] [Google Scholar]
- 3.Marks KM, Nolan GP. Nat Methods. 2006;3:591. doi: 10.1038/nmeth906. [DOI] [PubMed] [Google Scholar]
- 4.Chen I, Howarth M, Lin W, Ting AY. Nat Methods. 2005;2:99. doi: 10.1038/nmeth735. [DOI] [PubMed] [Google Scholar]
- 5.Marks KM, Braun PD, Nolan GP. Proc Natl Acad Sci U S A. 2004;101:9982. doi: 10.1073/pnas.0401609101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sosinsky GE, Gaietta GM, Hand G, Deerinck TJ, Han A, Mackey M, Adams SR, Bouwer J, Tsien RY, Ellisman MH. Cell Commun Adhes. 2003;10:181. doi: 10.1080/cac.10.4-6.181.186. [DOI] [PubMed] [Google Scholar]
- 7.Gaietta GM, Giepmans BN, Deerinck TJ, Smith WB, Ngan L, Llopis J, Adams SR, Tsien RY, Ellisman MH. Proc Natl Acad Sci U S A. 2006;103:17777. doi: 10.1073/pnas.0608509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prescher JA, Bertozzi CR. Nat Chem Biol. 2005;1:13. doi: 10.1038/nchembio0605-13. [DOI] [PubMed] [Google Scholar]
- 9.Lee LV, Mitchell ML, Huang SJ, Fokin VV, Sharpless KB, Wong CH. J Am Chem Soc. 2003;125:9588. doi: 10.1021/ja0302836. [DOI] [PubMed] [Google Scholar]
- 10.Moorhouse AD, Santos AM, Gunaratnam M, Moore M, Neidle S, Moses JE. J Am Chem Soc. 2006;128:15972. doi: 10.1021/ja0661919. [DOI] [PubMed] [Google Scholar]
- 11.Manetsch R, Krasinski A, Radic Z, Raushel J, Taylor P, Sharpless KB, Kolb HC. J Am Chem Soc. 2004;126:12809. doi: 10.1021/ja046382g. [DOI] [PubMed] [Google Scholar]
- 12.Sivakumar K, Xie F, Cash BM, Long S, Barnhill HN, Wang Q. Org Lett. 2004;6:4603. doi: 10.1021/ol047955x. [DOI] [PubMed] [Google Scholar]
- 13.Joralemon MJ, O’Reilly RK, Hawker CJ, Wooley KL. J Am Chem Soc. 2005;127:16892. doi: 10.1021/ja053919x. [DOI] [PubMed] [Google Scholar]
- 14.Kolb HC, Sharpless KB. Drug Discov Today. 2003;8:1128. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- 15.Link AJ, Tirrell DA. J Am Chem Soc. 2003;125:11164. doi: 10.1021/ja036765z. [DOI] [PubMed] [Google Scholar]
- 16.(a) Jao CY, Salic A. Proc Natl Acad Sci U S A. 2008;105:15779. doi: 10.1073/pnas.0808480105. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kele P, Li X, Link M, Nagy K, Herner A, Lorincz K, Beni S, Wolfbeis OS. Org Biomol Chem. 2009;7:3486. doi: 10.1039/b907741c. [DOI] [PubMed] [Google Scholar]
- 17.(a) Prescher JA, Dube DH, Bertozzi CR. Nature. 2004;430:873. doi: 10.1038/nature02791. [DOI] [PubMed] [Google Scholar]; (b) Chang PV, Prescher JA, Sletten EM, Baskin JM, Miller IA, Agard NJ, Lo A, Bertozzi CR. Proc Natl Acad Sci U S A. 2010;107:1821. doi: 10.1073/pnas.0911116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Z, Fahrni CJ. J Am Chem Soc. 2004;126:8862. doi: 10.1021/ja049684r. [DOI] [PubMed] [Google Scholar]
- 19.Xie F, Sivakumar K, Zeng QB, Bruckman MA, Hodges B, Wang Q. Tetrahedron. 2008;64:2906. [Google Scholar]
- 20.Sawa M, Hsu TL, Itoh T, Sugiyama M, Hanson SR, Vogt PK, Wong CH. Proc Natl Acad Sci U S A. 2006;103:12371. doi: 10.1073/pnas.0605418103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang RJ, Liu D, Xu K, Li JYJ. Photochem Photobiol A-Chem. 2009;205:61. [Google Scholar]
- 22.Saha SK, Purkayastha P, Das AB, Dhara SJ. Photochem Photobiol A-Chem. 2008;199:179. [Google Scholar]
- 23.Orlandini LF, Rodembusch FS, de Luca MA, Jacobi MM, Stefani VJ. Appl Polym Sci. 2008;109:282. [Google Scholar]
- 24.Zhu LB, Guo P, Li GC, Lan JB, Xie RG, You JS. J Org Chem. 2007;72:8535. doi: 10.1021/jo0712289. [DOI] [PubMed] [Google Scholar]
- 25.Krasnokutskaya EA, Semenischeva NI, Filimonov VD, Knochel P. Synthesis. 2007:81. [Google Scholar]
- 26.(a) Van den Hoven BG, Alper H. J Am Chem Soc. 2001;123:1017. doi: 10.1021/ja003085c. [DOI] [PubMed] [Google Scholar]; (b) Schlegel J, Maas G. Synthesis. 1999. p. 100. [Google Scholar]
- 27.Jin T, Kamijo S, Yamamoto Y. Eur J Org Chem. 2004:3789. doi: 10.1021/jo035292b. [DOI] [PubMed] [Google Scholar]
- 28.Chan TR, Hilgraf R, Sharpless KB, Fokin VV. Org Lett. 2004;6:2853. doi: 10.1021/ol0493094. [DOI] [PubMed] [Google Scholar]
- 29.Kiyose K, Aizawa S, Sasaki E, Kojima H, Hanaoka K, Terai T, Urano Y, Nagano T. Chem-Eur J. 2009;15:9191. doi: 10.1002/chem.200900035. [DOI] [PubMed] [Google Scholar]
- 30.Cubero E, Orozco M, Luque FJ. J Am Chem Soc. 1998;120:4723. doi: 10.1021/ja011200t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.