Abstract
Adherent PAMPs (pathogen-associated molecular patterns) act through Toll-like receptor2 (TLR2) and TLR4 to increase the biological activity of orthopaedic wear particles in cell culture and animal models of implant loosening. This study tested whether this is dependent on TLR association with lipid rafts as reported for the response to soluble TLR ligands. For this purpose, RAW264.7 murine macrophages were activated by exposure to titanium particles with adherent PAMPs, soluble lipopolysaccharide (LPS), soluble lipotecichoic acid (LTA), or heat-killed bacteria that had been extensively washed to remove soluble PAMPs. Lipid rafts were isolated by two independent methods and the location of TLR4 and TLR2 was analyzed by western blotting. The cognate TLRs associated with lipid rafts when the macrophages were activated with soluble LPS and LTA but not after stimulation with either titanium particles with adherent PAMPs or heat-killed bacteria. The lipid raft disruptor, methyl-β-cyclodextrin, dose-dependently inhibited TNFα release in response to LPS but had no affect on TNFα release in response to titanium particles with adherent PAMPs. We conclude, therefore, that titanium particles with adherent PAMPs and heat-killed bacteria activate TLR2 and TLR4 in macrophages without inducing either TLR to associate with lipid rafts. These results have important implications for the mechanisms of orthopaedic implant loosening as well the mechanisms for TLR activation in other inflammatory situations.
Introduction
Total joint replacement is a common procedure performed for the relief of pain from arthritis, trauma and other degenerative conditions of the joint. However, with time, loosening at the bone-implant interface may result in failure of the implant necessitating revision arthroplasty. This process, aseptic loosening, is believed to be triggered by the generation of wear particles from the implant surfaces.(1–2) The wear particles are phagocytosed by cells such as macrophages and stimulate the production of inflammatory cytokines including tumor necrosis factorα (TNFα), interleukin-1 and interleukin-6. The inflammatory cytokines increase osteoclast differentiation primarily by increasing production of RANKL and thereby disrupting the homeostatic balance between osteoclasts and osteoblasts.(3–4)
We and others have proposed that bacterially-derived immunostimulatory molecules known as PAMPs (pathogen-associated molecular patterns) may contribute to aseptic loosening of orthopaedic implants.(5–8) This seemingly paradoxical proposal is supported by a study of over 20,000 implants from the Norway Arthroplasty Register that revealed that systemic antibiotics and antibiotics in cement reduced the rate of aseptic loosening by approximately 50%.(9) It is also supported by extensive cell culture and animal studies showing that the bacterial PAMPs increase the inflammatory and osteolytic effects of the wear particles.(5,8) Moreover, PAMPs likely exist in peri-prosthetic tissue of at least some patients with aseptic loosening.(8) The primary source of these PAMPs is likely to be the bacterial biofilm that is often found on implants retrieved from patients despite the lack of any clinical signs of infection.(5–8) Additional possible sources of bacterial PAMPs during aseptic loosening include the systemic circulation and the implants themselves.(5,8)
One of the best studied PAMPs is lipopolysaccharide (LPS), the classical endotoxin produced by Gram-negative bacteria. Gram-positive bacteria do not produce LPS but produce other PAMPs, such as lipoteichoic acid (LTA), which have similar immunostimulatory effects.(10) The primary mammalian receptors for LPS and LTA are Toll-like receptors 4 and 2 (TLR4 and TLR2) respectively.(11–13) Both TLR4 and TLR2 have recently been shown to be robustly expressed by macrophages in peri-prosthetic tissue of patients with aseptic loosening.(14) Moreover, inactivating mutations in, or genetic deletion of, either TLR4 or TLR2 reduce the inflammatory and osteolytic responses to titanium wear particles with adherent PAMPs in cell culture and in vivo model systems.(4,15) In contrast the biological activity of titanium particles without adherent PAMPs is not dependent on either TLR2 or TLR4.(15) Thus, adherent PAMPs on titanium particles exert their biological activity in these model systems at least partially through interactions with TLR4 and TLR2.
Although the relative importance of PAMPs for induction of osteolysis in aseptic loosening patients remains unknown(8), the studies described in the previous paragraphs demonstrate their potential importance and thereby provide a strong rationale for studies designed to understand the mechanisms by which PAMPs can contribute to the biological activity of orthopaedic wear particles.
Lipid rafts are cholesterol containing microdomains within the cell membrane that act as platforms for the initiation of many intracellular signaling pathways.(16) In response to soluble PAMPs, TLR4 and TLR2 associate with lipid rafts,(17–22) and disruption of the lipid rafts blocks the resulting biological responses.(21–23) Therefore, this study tested the possibility that the biological activity of wear particles with adherent PAMPs also depend on association of TLR4 and TLR2 with lipid rafts. For this purpose, we examined whether disruption of lipid rafts with methyl-β-cyclodextrin blocks TNFα secretion in response to titanium particles with adherent PAMPs. In order to gain a further understanding of the mechanisms involved we also investigated the association of TLR4 and TLR2 with lipid rafts. This was accomplished using two complementary methods for isolation of detergent resistant membrane fractions (DRMs), which are commonly used as models of lipid rafts.(24–26)
Methods
Particle Preparation
Commercially pure titanium (CpTi) particles (catalog #00681, lot #G11G04) were obtained from Johnson Matthey (Ward Hill, MA). Ninety percent of the cpTi particles were less than 3.6 μm in diameter.(27) The particles were sterilized in 70% ethanol and were stored at 4°C in sterile phosphate buffered saline with penicillin (100 U/ml) and streptomycin (100 μg/ml) at a concentration of 2.7×107 particles/ml until use. Adherent endotoxin on the particles (620 EU/ml) was measured using the high sensitivity version of the Limulus Amebocyte Lysate Assay (Biowhittaker, Walkersville, MD) in the presence of a β-glucan blocking reagent (Biowhittaker) as we have previously described.(28)
Cell Culture
Cell culture media used in all experiments was phenol-red free minimum essential media with Earle salts (Hyclone, Logan, UT), supplemented with 2mM L-glutamine (Mediatech, Herndon, VA), non-essential amino acids (Mediatech), 100 U/ml penicillin (Mediatech) and 100 μg/ml streptomycin (Mediatech). Unless otherwise noted, all media also contained 10% fetal bovine serum (Hyclone). Serum-free media contained 0.1% bovine serum albumin (Sigma, St. Louis, MO). Calcium and magnesium-free Dulbecco’s PBS was obtained from Mediatech. All reagents were tested for endotoxin using the high-sensitivity version of the Limulus Amebocyte Lysate assay (Biowhittaker) and were from the lots containing the lowest amounts of endotoxin available.
RAW264.7 murine macrophages (ATCC, Manassas, VA) were cultured in Petri dishes at 37°C and 5% CO2. Prior to experiments, the cells were plated in tissue culture dishes (8.3×104 cells/cm2) and incubated for 18–20 hours. Cells were exposed to cpTi particles (3.3×106 particles/cm2), LPS (200 pg/ml, E. coli, Sigma), LTA (100 μg/ml, Cat# LTA-SA, S. aureus, Invivogen), heat-killed E. coli (3.3×106 particles/cm2, Invivogen), heat-killed S. aureus (3.3×106 particles/cm2, Invivogen) or control medium without stimuli. In selected experiments, RAW264.7 cells were prepared as above and then pre-incubated for 30 minutes with various concentrations of the lipid raft inhibitor methyl-β-cyclodextrin (Sigma) before the addition of either soluble LPS or cpTi particles.
TNFα Release
Media was collected after 90 minutes of incubation with the indicated stimuli and centrifuged to remove cellular debris and titanium particles for 25 minutes at 6400g. Aliquots were stored at −20°C. The samples were measured for TNFα by enzyme-linked immunosorbent assay (ELISA) using capture and detection antibodies (R&D Systems) and polyHRP20-streptavidin conjugate (RDI) as we have done previously.(4)
Isolation of Detergent Resistant Membranes (DRMs)
DRMs were isolated using the sucrose gradient flotation method.(24,26) RAW 264.7 murine macrophages were washed twice with ice cold PBS with 1mM sodium orthovanadate. All successive steps were carried out at 4°C. Cells were lysed by incubation for 10 minutes in 1% Triton X-100, 50mM Hepes-OH, 150mM NaCl, 5mM EDTA, 20mM sodium pyrophosphate, 1mM sodium orthovanadate, and 20mM sodium fluoride. Lysate aliquots (500ul) were then placed at the bottom of 5 ml ultracentrifuge tubes (Beckman-Coulter) and mixed with an equal volume of 90% sucrose. Three ml of 35% sucrose was carefully overlayed above the samples followed by 1 ml of 5% sucrose. The gradients were centrifuged at 150,000g for 20 hours in a SW55Ti ultracentrifuge rotor (Beckman-Coulter). After ultracentrifugation, 500μl fractions were removed sequentially from top to bottom and stored at −80°C until analyzed.
DRMs were also isolated using the successive detergent extraction method.(25) RAW 264.7 murine macrophages were suspended in 150mM NaCl, 25mM MES-OH (pH 6.5) and lysed with an equal volume of 2% Triton X-100, 150mM NaCl, 25mM MES (pH 6.5), 2mM sodium orthovanadate and 2mM phenylmethylsulfonyl fluoride. The lysates were incubated on ice for 30 minutes and then centrifuged at 14,000g at 4°C for 20 minutes. The supernatant was harvested and the pellets were resuspended and lysed in 1% Triton X-100, 60mM n-octylglucoside, 10mM Tris-Cl (pH 7.6), 500mM NaCl, 2mM sodium orthovanadate, and 1mM phenylmethylsulfonyl fluoride on ice for 30 minutes. These lysates were then centrifuged at 14,000g at 4°C for 20 minutes. After centrifugation, the supernatants were stored at −80°C until analyzed.
Western Blotting
Primary antibodies included rabbit anti-mouse TLR-4 antibody (Cat# 24-9004-91, eBioscience, San Diego, CA), rabbit anti-mouse TLR-2 antibody (Cat# 24-9020-91, eBioscience) and rat affinity purified anti-mouse CD14 antibody (Cat# 14-0141-88, eBioscience).
Equal portions of each fraction were loaded onto 10% Tris-glycine gels (Cambrex, North Brunswick, NJ) and resolved by electrophoresis. The electrophoresed proteins were then transferred to nitrocellulose membranes (Bio-Rad) for 1.5 hours at 26mAmp in the presence of transfer buffer (24mM Tris, 192mM glycine, 15% methanol). The membranes were then placed in 25ml of Miser Antibody Extender (Pierce) for 10 minutes followed by rinsing with ultrapure water (Pierce). Nonspecific binding sites were then blocked by incubation in blocking buffer (5% non-fat dry milk, Tris-buffered saline, 0.1% Tween-20) for 1 hour. The membranes were washed with Tris-buffered saline with 0.1% Tween-20 (TBST) and incubated with the appropriate dilution of primary antibody overnight at 4°C. After incubation, the membranes were washed with TBST and incubated for 1.5 hours with the appropriate secondary antibody labeled with horseradish peroxidase at room temperature. The membranes were then washed with TBST and developed using the ECL reagent system (Amersham Biosciences, Buckinghamshire, United Kingdom).
Measurement of Cytotoxicity
Aliquots of medium from inhibitor experiments were stored at 4°C for no more than 3 days and were used for assessment of cytotoxicity using a lactate dehydrogenase (LDH) detection kit (Roche, Mannheim, Germany). Percent cytotoxicity was calculated by comparison to cultures lysed with 1% Triton X-100 as recommended by the manufacturer.
Statistical Analysis
All figures are representative of 3 or more experiments. TNFα data is presented as means ± standard error measurements of triplicate cultures each assayed in triplicate. Statistical analysis was performed using one way analysis of variance with Sigma Stat Software and Bonferroni-Dunn analysis.
Results
The biological activity of the cpTi particles used in this study has previously been shown to be significantly increased by their adherent PAMPs and to depend on both TLR2 and TLR4.(4,15) LPS and LTA are known to induce TLR4 and TLR2 association with DRMs after 60 minutes of exposure.(20–21) Our initial experiments therefore examined the effects of the cpTi particles at this time point. These experiments utilized sucrose gradients to isolate DRMs as this is the “gold-standard” method. As expected,(20–21) this approach showed that exposure to soluble LPS for 60 minutes induces TLR4 to associate with DRMs in RAW264.7 macrophages but does not affect TLR2 (3rd and 6th panels in Figure 1). In contrast, the cpTi particles did not induce either TLR4 or TLR2 to associate with DRMs at this time point (2nd and 5th panels in Figure 1). CD14, a lipid raft marker(17), was, as expected, constitutively associated with DRMs in all conditions (Figure 1, see also bottom panels in Figure 2A, 3, 4 and 6).
Figure 1.
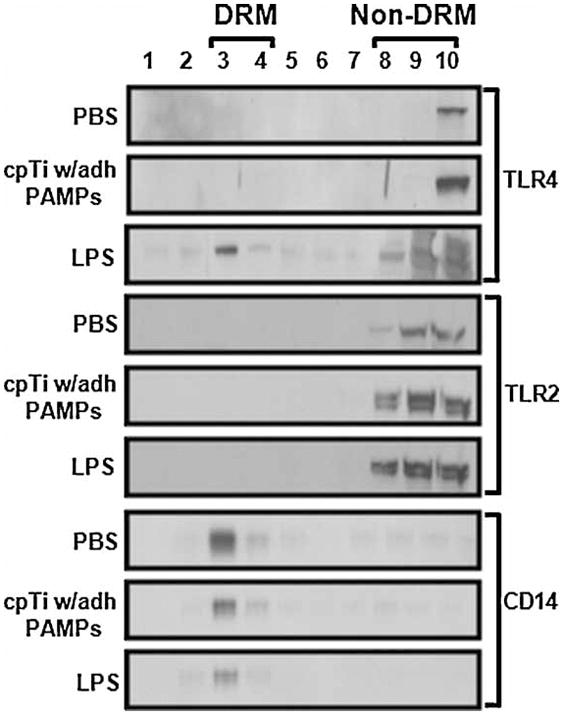
cpTi particles with adherent PAMPs do not induce the TLRs to associate with detergent rich membranes (DRMs) isolated by sucrose gradient flotation. RAW264.7 cells were incubated with PBS, cpTi particles with adherent PAMPs, or LPS for 60 min prior to lysis, sucrose gradient fractionation, and Western blotting for TLR4, TLR2, and the lipid raft marker CD-14.
Figure 2.
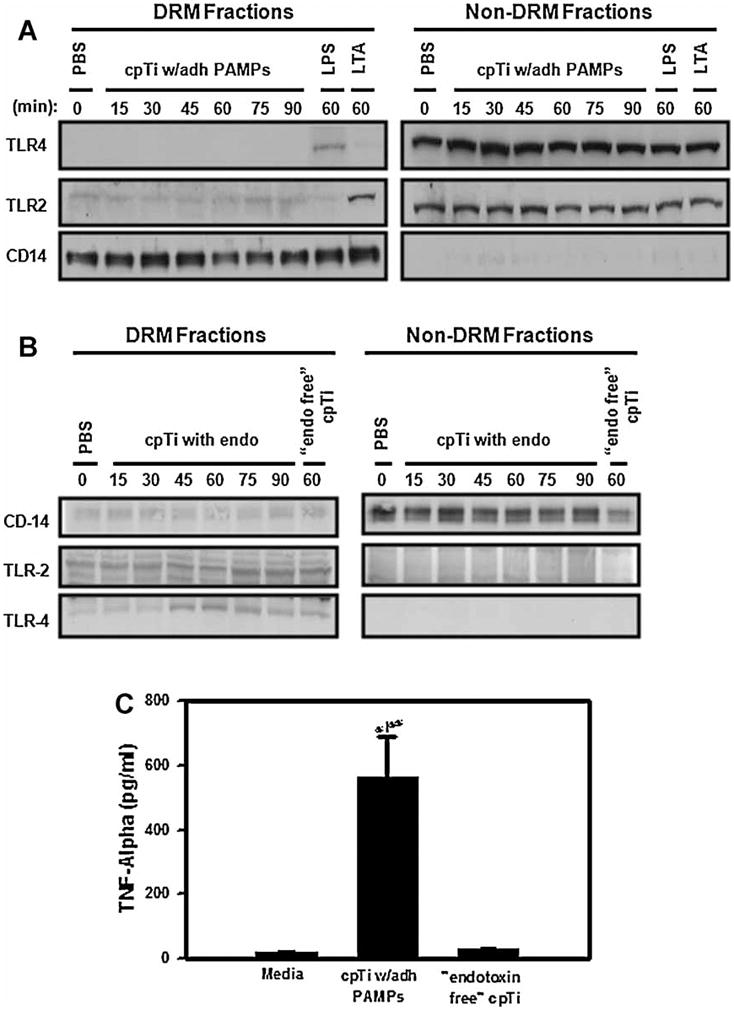
cpTi particles with adherent PAMPs do not induce the TLRs to associate with DRMs isolated by successive detergent extraction. (A) RAW264.7 cells were incubated with PBS, cpTi particles with adherent PAMPs, LPS, or LTA for the indicated times prior to lysis, successive detergent extraction, and Western blotting for TLR4, TLR2, and the lipid raft marker CD-14. (B) RAW264.7 cells were incubated with PBS, cpTi particles with adherent PAMPs or “endotoxin free” cpTi particles for the indicated times prior to lysis, successive detergent extraction and Western blotting for TLR4, TLR2, and CD-14. (C) RAW264.7 cells were incubated with PBS, cpTi particles with adherent PAMPs or “endotoxin free” cpTi particles for 90 min prior to collection of the culture medium for TNF-α measurement by ELISA. Asterisk indicates p < 0.05 compared to media control group. Double asterisk indicates p < 0.05 compared to the “endotoxin free” cpTi group.
Figure 3.
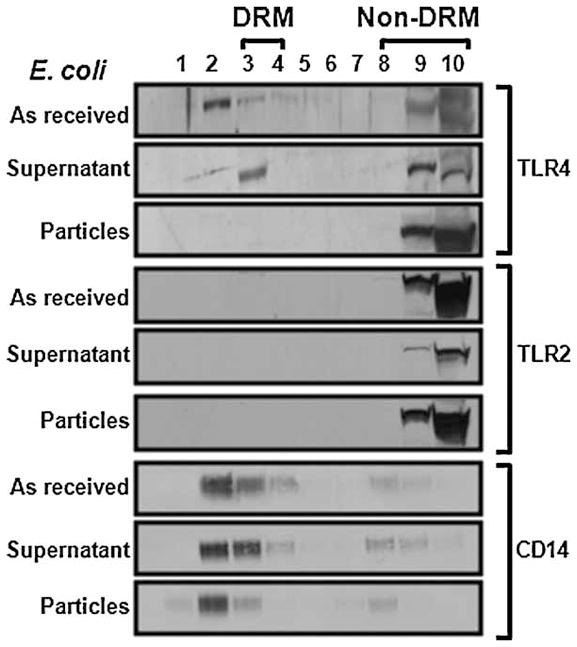
“As received” E. coli and the supernatant obtained by centrifugation induce TLR-4 to associate with DRMs but centrifuged E. coli particles do not. RAW264.7 cells were incubated with the stimuli for 60 min prior to lysis, sucrose gradient fractionation, and Western blotting for TLR4, TLR2, and the lipid raft marker CD-14.
Figure 4.
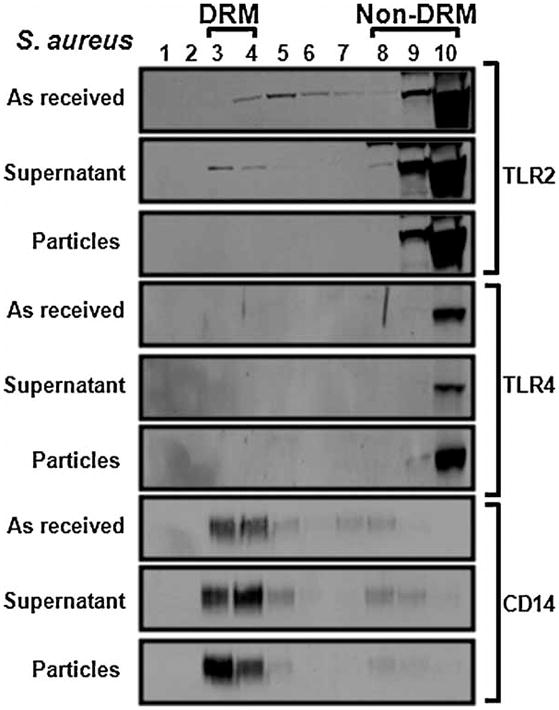
“As received” S. aureus and the supernatant obtained by centrifugation induce TLR-2 to associate with DRMs but centrifuged S. aureus particles do not. RAW264.7 cells were incubated with the stimuli for 60 min prior to lysis, sucrose gradient fractionation, and Western blotting for TLR-4, TLR-2, and the lipid raft marker CD-14.
Figure 6.
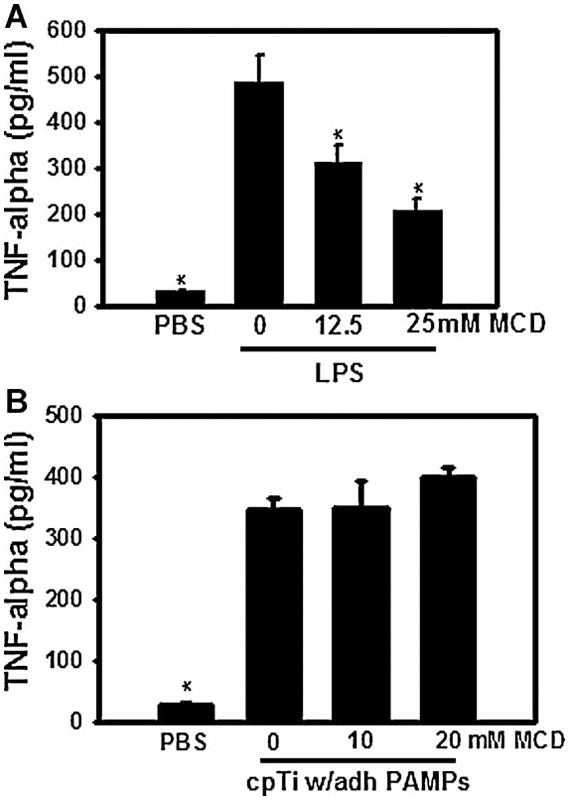
The lipid raft disruptor, methyl-β-cyclodextrin (MCD), inhibits TNF-α secretion induced by LPS but does not affect TNF-α secretion induced by cpTi particles with adherent PAMPs. RAW264.7 cells were pre-incubated with the indicated concentrations of MCD for 30 min prior to incubation with either LPS or cpTi particles with adherent PAMPs. Asterisks indicates p < 0.05 compared to group without MCD but with stimulus.
Having shown that exposure to cpTi particles for 60 minutes does not induce TLR4 or TLR2 to associate with DRMs, we asked whether such an association might occur at other time points. For this purpose, it was necessary to utilize the successive detergent extraction method since the sucrose gradient method is not feasible for experiments with large numbers of samples or multiple time points. As expected(18–22,29), soluble LPS and LTA induced TLR4 and TLR2, respectively, to associate with DRMs (top and middle panels in Figure 2A). In contrast, cpTi particles did not induce either TLR4 or TLR2 to associate with DRMs at any of the tested time points (top and middle panels in Figure 2A) despite robustly activating TNFα release (Figure 2C). “Endotoxin free” cpTi particles also did not induce movement of TLR4 and TLR2 into DRMs at 60 minutes (Figure 2B).
The results described in the previous paragraphs suggested that, in contrast to soluble LPS and LTA, TLR4 and TLR2 are activated independently of lipid rafts by ligands bound to particles. To test this possibility, we compared the effects of soluble LPS with an “as received” suspension of heat-killed E. coli particles and with the same particles following centrifugation to remove soluble LPS that had been released by the bacteria. As expected since E. coli activates TLR4(19,29), the “as received” heat-killed E. coli induced TLR4 to associate with DRMs and did not affect the distribution of TLR2 (1st and 4th panels in Figure 3). The supernatant obtained by centrifugation of the E. coli suspension also induced TLR4 to associate with DRMs (2nd panel in Figure 3) similarly to that shown previously for soluble LPS (Figures 1 and 2). In contrast, the centrifuged E.coli had no effect (3rd panel in Figure 3) similarly to the cpTi particles (Figures 1 and 2). To determine whether TLR2 is also differentially affected by soluble and particle-bound ligands, similar experiments were performed using heat-killed S. aureus, a Gram-positive bacteria that activates TLR2. The “as received” heat-killed S. aureus and the supernatant induced TLR2, but not TLR4, to associate with DRMs (1st and 3rd panels in Figure 4) and the centrifuged S. aureus had no effect (2nd panels in Figure 4). Thus, both TLR4 and TLR2 are induced to associate with soluble ligands but not by particle-bound ligands. All of the bacteria preparations robustly stimulated TNFα release (Figure 5).
Figure 5.
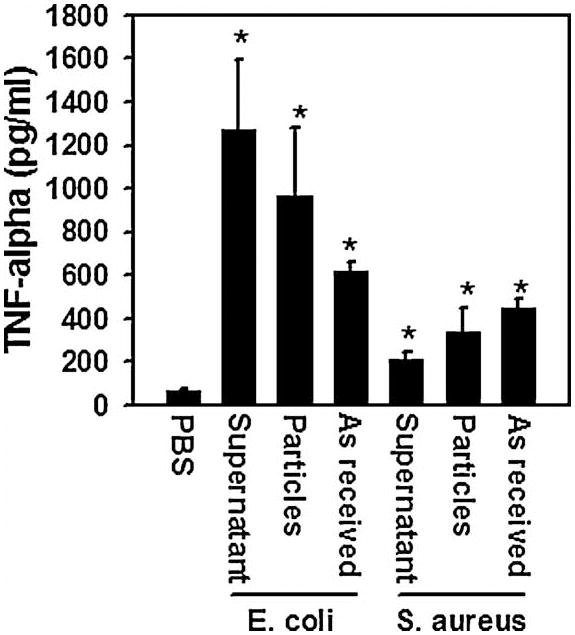
“As received,” supernatants, and centrifuged preparations of E. coli and S. aureus induce abundant TNF-α secretion. RAW264.7 cells were incubated with the stimuli for 90 min prior to collection of the culture media for TNF-α measurement by ELISA. Asterisks indicates p < 0.01 compared to PBS control group.
To further examine the role of lipid rafts in macrophage activation by cpTi particles, we tested whether disruption of lipid rafts alters biological responses induced by the particles. Measurement of TNFα secretion was used for this purpose since TNFα is the pro-inflammatory cytokine with the most evidence demonstrating a role in aseptic loosening and is the first pro-inflammatory cytokine produced in response to wear particles(30). Disruption of lipid rafts with methyl-β-cyclodextrin inhibited TNFα secretion induced by soluble LPS (Figure 6A). In contrast, methyl-β-cyclodextrin (MCD) did not inhibit TNFα secretion induced by the cpTi particles (Figure 6B). All samples were tested for cytotoxicity using a LDH assay and no significant difference was found between control and MCD treated groups demonstrating that the decrease in TNFα secretion was not due to increased cell death. Importantly, these experiments, as well as all other experiments used in this study, utilized concentrations of LPS and the particles that induce similar amounts of TNFα release (Figures 2B, 5 and 6B).
Discussion
We have previously shown that cpTi particles with adherent PAMPs activate macrophages to produce inflammatory cytokines, at least in part, through both TLR2 and TLR4.(4,15) The primary finding of the current study is that this occurs independently of association of TLR2 or TLR4 with lipid rafts and is not blocked by disruption of lipid rafts with methyl-β-cyclodextrin. In contrast, soluble PAMPs, such as LPS and LTA, induce their cognate TLRs to associate with lipid rafts and cytokine secretion induced by these soluble PAMPs is blocked by lipid raft disruption. Thus, our results demonstrate that association with lipid rafts are not necessary for TLR activation and may be more important for other processes, such as TLR internalization.(21–22) Since TLR2 and TLR4 exist and are typically activated at the cell surface,(31) it is likely that orthopaedic wear particles activate them in cell surface domains outside of lipid rafts.
The major limitations of this study are that these experiments were performed with RAW264.7 cells, a transformed murine macrophage cell line, rather than normal human macrophages and that we cannot exclude the possibility that a small number of TLR molecules may have associated with the lipid rafts or that the association was extremely transient and not detected in our time course experiments. Nonetheless, our results show that cpTi particles did not induce detectable association of TLR4 or TLR2 with lipid rafts at any time point tested while LPS and LTA routinely did so. Moreover, disruption of lipid rafts blocked TNFα secretion in response to LPS but had no effect on TNFα secretion in response to cpTi particles. In addition, obtaining similar results with both methods of DRM isolation strengthens the conclusion that cpTi particles trigger cytokine release in a manner independent of lipid rafts.
Our results suggest that particulate bound PAMPs activate TLRs through mechanisms that are different than those used by soluble PAMPs. This conclusion is based on the findings that TLRs were not induced to associate with lipid rafts by cpTi particles or by E. coli or S. aureus that had been centrifuged to remove any soluble PAMPs that had been released by the bacteria. In addition to recognizing PAMPs, TLRs also act as receptors for a variety of endogenous molecules known as danger-associated molecular pathogens (DAMPs) or alarmins(32). One of the most studied DAMPs is heat shock protein 60 (Hsp60). It has been reported that orthopaedic wear particles induce monocytes to release Hsp60(33) and the authors of that study proposed that the released Hsp60 may be responsible for activation of TLRs during aseptic loosening. When assessing that hypothesis, it is important to take into account that at least some of the effects of many DAMPs, including Hsp60, on TLR activation are due either to contamination of the preparations with bacterial PAMPs or to interactions between DAMPs and PAMPs.(34–35) Moreover, we have recently shown that adherent PAMPs are required for activation of TLR2 and TLR4 by orthopaedic wear particles and that endogenous DAMPs are not sufficient(15).
Loosening of orthopaedic implants and the need for revision arthroplasty are concerns with sizeable personal and economic implications in the modern health care system. The process of aseptic loosening is believed to begin with the generation of wear particles from implant surfaces which are phagocytosed by immune cells leading to inflammatory cytokine release and subsequent osteolysis. Together with our previous studies,(4,15) our results show that macrophage activation by cpTi particles with adherent PAMPs is dependent on TLR2 and TLR4 but independent of lipid rafts. Further research is needed to determine the mechanisms responsible for activation of TLRs by cpTi particles with adherent PAMPs and the resultant signaling processes in order to develop possible pharmacological interventions that could reduce the rate of aseptic loosening and its associated morbidity and economic implications.
Acknowledgments
The authors wish to thank Faramarz Ishmail-Beigi, Darrell Rubin and Janet Rubin for guidance with lipid raft isolation as well as Jennifer Nalepka and Joscelyn Tatro for endotoxin measurements. This work was supported by research grant R01 AR43769 to E.M.G. from the National Institutes of Health (NIH). A.S.I. was supported by an Allen Fellowship. M.A.B. was supported by NIH training grant AR07505.
References
- 1.Wilkinson JM, Harner AJ, Stockley I, Eastell R. Polyethylene wear rate and osteolysis: crtitical threshold versus continuous dose-response relationship. J Ortho Res. 2005;23:520–5. doi: 10.1016/j.orthres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Goodman SB, Wright T. 2007 AAOS/NIH osteolysis and implant wear: biological, biomedical engineering, and surgical principles. Introduction. J Am Acad Orthop Surg. 2008;16(Suppl 1):x–xi. [PubMed] [Google Scholar]
- 3.Greenfield EM, Bi Y, Ragab AA, Goldberg VM, Van De Motter RR. The role of osteoclast differentiation in aseptic loosening. J Orthop Res. 2002;20:1–8. doi: 10.1016/S0736-0266(01)00070-5. [DOI] [PubMed] [Google Scholar]
- 4.Bi Y, Seabold JM, Kaar SG, Ragab AA, Goldberg VM, Anderson JM, Greenfield EM. Adherent endotoxin on orthopaedic wear particles stimulates cytokine production and osteoclast differentiation. J Bone Miner Res. 2001;16:2082–91. doi: 10.1359/jbmr.2001.16.11.2082. [DOI] [PubMed] [Google Scholar]
- 5.Greenfield EM, Bi Y, Ragab AA, Goldberg VM, Nalepka JL, Seabold JM. Does endotoxin contribute to aseptic loosening of orthopaedic implants? J Biomed Mater Res B Appl Biomater. 2005;72:179–85. doi: 10.1002/jbm.b.30150. [DOI] [PubMed] [Google Scholar]
- 6.Nelson CL, McLaren AC, McLaren SG, Johnson JJ, Smeltzer MS. Is aseptic loosening truly aseptic? Clin Orthop Rel Res. 2005;437:25–30. doi: 10.1097/01.blo.0000175715.68624.3d. [DOI] [PubMed] [Google Scholar]
- 7.Sundfeldt M, Carlsson LV, Johansson CB, Thomsen P, Gretzer C. Aseptic loosening, not only a question of wear: a review of different theories. Acta Orthop. 2006;77:177–97. doi: 10.1080/17453670610045902. [DOI] [PubMed] [Google Scholar]
- 8.Greenfield EM, Bechtold J. What other biologic and mechanical factors might contribute to osteolysis? J Am Acad Orthop Surg. 2008;16(Suppl 1):S56–62. doi: 10.5435/00124635-200800001-00012. [DOI] [PubMed] [Google Scholar]
- 9.Engesaeter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LL. Antibiotic prophylaxis in total hip arthroplasty. Acta Orthop Scand. 2003;74:644–51. doi: 10.1080/00016470310018135. [DOI] [PubMed] [Google Scholar]
- 10.Hauschildt S, Brabetz W, Schromm AB, Hamann L, Zabel P, Rietschel ET, Muller-Loennies S. Structure and activity of endotoxins. In: Aktories K, Just I, editors. Handbook of experimental pharmacology. Berlin: Springer; 2000. pp. 619–59. [Google Scholar]
- 11.Poltorak A, He X, Smirnova I, Liu M, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Detecting LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in TLR-4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 12.Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in toll-like receptor 4 (TLR4) J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 14.Takagi M, Tamaki Y, Hasegawa H, Takakubo Y, Konttinen L, Tiainen VM, Lappalainen R, Konttinen YT, Salo J. Toll-like receptors in the interface membrane around loosening total hip replacement implants. J Biomed Mater Res A. 2007;81:1017–26. doi: 10.1002/jbm.a.31235. [DOI] [PubMed] [Google Scholar]
- 15.Greenfield EM, Beidelschies MA, Tatro JM, Goldberg VM, Hise AG. Bacterial pathogen-associated molecular patterns are required for activation of toll-like receptors by orthopaedic wear particles: host-derived danger-associated molecular patterns are not sufficient. 2010 doi: 10.1074/jbc.M110.136895. Submitted to J Biol Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–4. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 17.Pfeiffer A, Bottcher A, Orso E, Kapinsky M, Nagy P, Bodnar A, Spreitzer I, Liebisch G, Drobnik W, Gempel K, Horn M, Holmer S, Hartung T, Multhoff G, Schutz G, Schindler H, Ulmer AJ, Heine H, Stelter F, Schutt C, Rothe G, Szollosi J, Damjanovich S, Schmitz G. Lipopolysaccharide and ceramide docking to CD14 provokes ligand-specific receptor clustering in rafts. Eur J Immunol. 2001;31:3153–64. doi: 10.1002/1521-4141(200111)31:11<3153::aid-immu3153>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Soong G, Reddy B, Sokol S, Adamo R, Prince A. TLR2 is mobilized into an apical lipid raft receptor complex to signal infection in airway epithelial cells. J Clin Invest. 2004;113:1482–9. doi: 10.1172/JCI20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci. 2002;115:2603–11. doi: 10.1242/jcs.115.12.2603. [DOI] [PubMed] [Google Scholar]
- 20.Triantafilou M, Manukyan M, Mackie A, Morath S, Hartung T, Heine H, Triantafilou K. Lipoteichoic acid and toll-like receptor 2 internalization and targeting to the Golgi are lipid raft-dependent. J Biol Chem. 2004;279:40882–40889. doi: 10.1074/jbc.M400466200. [DOI] [PubMed] [Google Scholar]
- 21.Triantafilou M, Brandenburg K, Kusumoto S, Fukase K, Mackie A, Seydel U, Triantafilou K. Combinational clustering of receptors following stimulation by bacterial products determines lipopolysaccharide responses. Biochem J. 2004;381:527–536. doi: 10.1042/BJ20040172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Triantafilou M, Gamper FG, Haston RM, Mouratis MA, Morath S, Hartung T, Triantafilou K. Membrane sorting of toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J Biol Chem. 2006;281:31002–11. doi: 10.1074/jbc.M602794200. [DOI] [PubMed] [Google Scholar]
- 23.Zhang AY, Yi F, Zhang G, Gulbins E, Li PL. Lipid raft clustering and redox signaling platform formation in coronary arterial endothelial cells. Hypertension. 2006;47:74–80. doi: 10.1161/10.1161/01.HYP.0000196727.53300.62. [DOI] [PubMed] [Google Scholar]
- 24.Solomon KR, Mallory MA, Finberg RW. Determination of the non-ionic detergent insolubility and phosphoprotein associations of glycosylphosphatidylinositol-anchored proteins expressed on T cells. Biochem J. 1998;334:325–333. doi: 10.1042/bj3340325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solomon KR, Adolphson LD, Wank DA, McHugh KP, Hauschka PV. Caveolae in human and murine osteoblasts. J Bone Miner Res. 2000;15:2391–2401. doi: 10.1359/jbmr.2000.15.12.2391. [DOI] [PubMed] [Google Scholar]
- 26.Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 27.Taki N, Seabold J, Nalepka J, Togawa D, Goldberg V, Rimnac C, Greenfield E. Polyethylene and titanium particles induce osteolysis by similar, lymphocyte-independent mechanisms. J Orthop Res. 2005;23:376–83. doi: 10.1016/j.orthres.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Tatro JM, Taki N, Islam AS, Goldberg VM, Rimnac CM, Doerschuk CM, Stewart MC, Greenfield EM. The balance between endotoxin accumulation and clearance during particle-induced osteolysis in murine calvaria. J Ortho Res. 2007;25:361–9. doi: 10.1002/jor.20289. [DOI] [PubMed] [Google Scholar]
- 29.Hornef MW, Normark BH, Vandewalle A, Normark S. Intracellular recognition of lipopolysaccharide by toll-like receptor 4 in intestinal epithelial cells. J Exp Med. 2003;198:1225–35. doi: 10.1084/jem.20022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beidelschies MA, Huang H, McMullen MR, Smith MV, Islam AS, Goldberg VM, Chen X, Nagy LE, Greenfield EM. Stimulation of macrophage TNFalpha production by orthopaedic wear particles requires activation of the ERK1/2/Egr-1 and NF-kappaB pathways but is independent of p38 and JNK. J Cell Physiol. 2008;217:652–66. doi: 10.1002/jcp.21539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 32.Matzinger P. Friendly and dangerous signals: is the tissue in control? Nat Immunol. 2007;8:11–3. doi: 10.1038/ni0107-11. [DOI] [PubMed] [Google Scholar]
- 33.Hao Q, Hong SH, Maret W. Lipid raft-dependent endocytosis of metallothionein in HepG2 cells. J Cell Physiol. 2007;210:428–35. doi: 10.1002/jcp.20874. [DOI] [PubMed] [Google Scholar]
- 34.Osterloh A, Kalinke U, Weiss S, Fleischer B, Breloer M. Synergistic and differential modulation of immune responses by Hsp60 and lipopolysaccharide. J Biol Chem. 2007;282:4669–80. doi: 10.1074/jbc.M608666200. [DOI] [PubMed] [Google Scholar]
- 35.Tsan MF, Gao B. Heat shock proteins and immune system. J Leukoc Biol. 2009;85:905–10. doi: 10.1189/jlb.0109005. [DOI] [PubMed] [Google Scholar]


