Abstract
We developed a series of near infrared (NIR) cyanine dyes to study dichromic fluorescence phenomenon, which provides new protocols for in vivo optical imaging. Preliminary spectroscopic studies show that dichromic fluorescence correlates with structural symmetry. This feature suggests the potential use of dichromic fluorescent molecules to study biological processes that can alter the structural symmetry of the molecular probes.
Keywords: NIR, Cyanine dye, Contrast agent, Optical imaging, Dual fluorescence
The development of near-infrared (NIR) dyes as fluorescence labels and sensors has gained increasing interest over the last decade because of their potential to advance and facilitate the translation of optical imaging to humans. Compared to blue-shifted dyes, NIR fluorescent probes offer several advantages: (a) NIR light is poorly absorbed by hemoglobin, water and lipids, resulting in deep tissue penetration1,2; (b) background autofluorescence is negligible; (c) light scattering in tissue is relatively low because scattering decreases as wavelength increases. Overall, NIR fluorescent dyes provide enormous opportunities for non-invasive in vivo applications.
Most NIR dyes used for in vivo optical imaging belong to the carbocyanine dye family. These dyes represent one of the most prominent classes of optical imaging agents with adjustable optical properties and high extinction coefficients. In addition, carbocyanine dyes can be conjugated with antibodies, peptides, or small targeting ligands to impart molecular specificity3–12. Using low quantities of these NIR agents, high specificity and good tissue penetration can be achieved. However, relatively low signal-to-background ratio is sometimes observed due to the signal from un-bound NIR agents.
Quenched NIR agents that become detectable upon enzyme activation can increase signal-to-background ratios up to several hundred folds13. The quenching can be either based on self-quenching or fluorescence resonance energy transfer (FRET) 14,15. These so called “smart NIR agents” have been developed to target diagnostic enzymes such as cathepsin B, K, D and H; caspase 1 and 3; matrix metalloproteinases (MMP) 2, 9, and 13; urokinase and other proteases for cancer imaging16–18. Optical imaging based on fluorescence lifetime is also made possible to image biological activities19. This technique is unique because it allows monitoring target site with less dependence on variations in fluorescence intensity or probe concentration. For example, a NIR dye-labeled hexapeptide, Cyp-GRD, was developed to image lung cancer20. In another study, small level of protein phosphorylation was monitored by combining fluorescence lifetime imaging and FRET21.
Despite the recent advancement of NIR optical imaging, NIR probes and signaling protocols that are suitable for in vivo biological studies are still limited. Recently, dichromic fluorescence phenomenon in NIR cyanine dyes was discovered22. It was reported that upon excitation, some carbocyanine dyes exhibit a second emission band at 700 nm, other than the commonly observed emission peak at 800 nm. Interestingly, dichromic fluorescence seems to be favored by structurally nonsymmetrical dyes (Figure 1). Therefore, this technique can be potentially used to monitor enzyme activities by transforming a probe from a symmetrical to a nonsymmetrical form.
Figure 1.
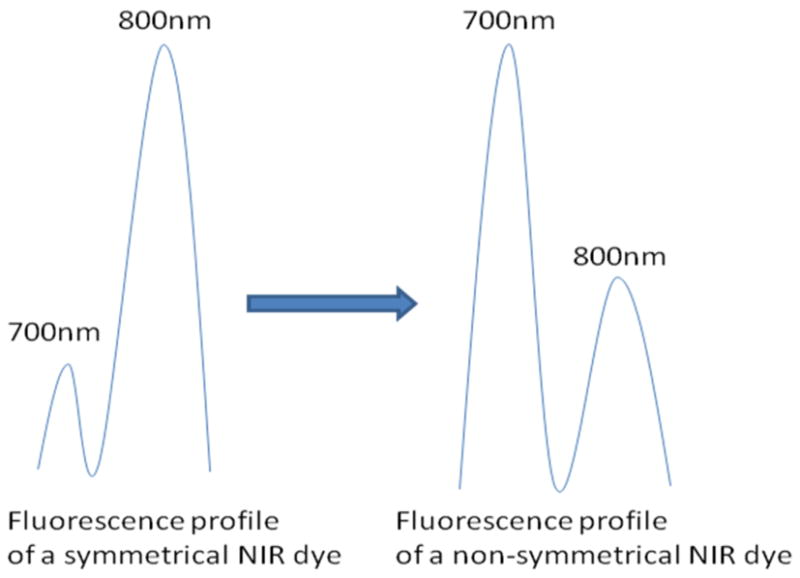
Illustration of fluorescence profiles of symmetrical and non-symmetrical NIR dyes. Non-symmetrical NIR dyes appear to have relatively higher emission at 700nm channel but lower emission at 800nm channel than symmetrical NIR dyes.
Unfortunately, dichromic fluorescence was studied on only a few molecules and the correlation between dichromic fluorescence and molecule symmetry needs to be further verified. Accordingly, we have developed a number of NIR dyes to study dichromic fluorescence at a larger scope using the method shown in scheme 1.
Scheme 1.
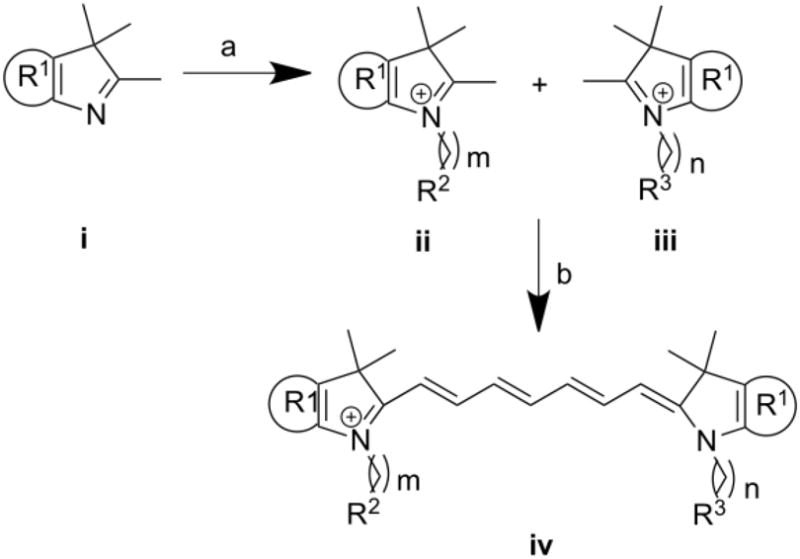
Reagents and conditions: (a) Dichlorobenzene, 110°C; (b) N-(5-anilino-2,4-pentadienylidene) anilinehydrochloride, acetic anhydride, DCM, MeOH, sodium acetate
As illustrated in scheme 1, compounds with structures ii and iii were synthesized from indolium or benzoindolium compound i by refluxing in dichlorobenzene with bromo-alkyl molecules. Subsequent condensation of ii and iii in the presence of sodium acetate and N-(5-anilino-2,4-pentadienylidene) anilinehydrochloride yielded the dichromic fluorescent dyes. The products were purified by semi-preparative HPLC and characterized by LCMS and spectroscopic methods. Detailed structural information and spectroscopic data are summarized in Table 1.
Table 1.
Dichromic fluorescent molecules
| # | R1 | R2 | R3 | m | n | Abs (nm) | Emi 1 (nm) | Emi 2 (nm) | 700/800 nm |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 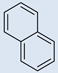 |
CO2H | SO3H | 2 | 4 | 782 | 698 | 809 | 0.67 |
| 2 | 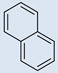 |
CO2H | 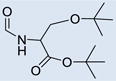 |
2 | 2 | 782 | 698 | 809 | 1.71 |
| 3 | 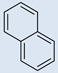 |
CO2H | SO3H | 5 | 4 | 783 | 699 | 808 | 2.82 |
| 4 | 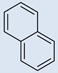 |
CO2H | CO2H | 5 | 5 | 784 | 698 | 808 | 0.25 |
| 5 | 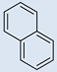 |
CH3 | CH3 | 0 | 0 | 777 | 694 | 800 | 7.04 |
| 6 | 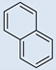 |
CH3 | CH3 | 0 | 5 | 781 | 697 | 799 | 11.9 |
| 7 | CO2H | SO3H | 2 | 4 | 745 | 661 | 770 | 3.78 | |
| 8 | CO2H | CO2H | 2 | 2 | 745 | 661 | 770 | 0.85 | |
| 9 | 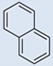 |
CO2H | SO3H | 2 | 3 | 783 | 695 | 809 | 1.34 |
| 10 | 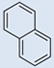 |
SO3H | SO3H | 3 | 3 | 783 | 695 | 810 | 1.51 |
Abs: maximum absorption, Emi 1: maximum emission at short wavelength channel, Emi 2: maximum emission at long wavelength channel.
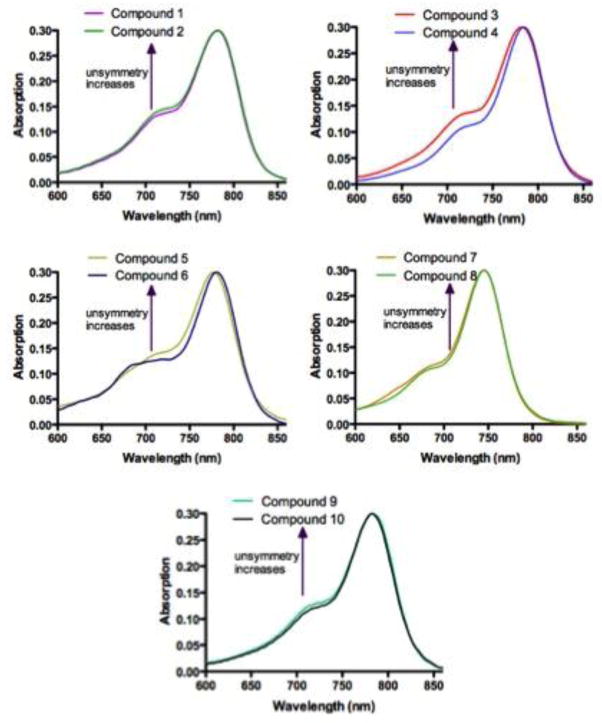
Absorption spectra of compounds 1–10 are shown in Figure 2. Due to various vibrational and rotational states, these molecules have broad absorption peaks, typical to carbocyanine dyes. Compounds 1–6, 9 and 10 with benzoindolium structure have maximum absorption at around 780 nm, whereas compounds 7 and 8 with indolium structure have maximum absorption at 745 nm.
Figure 2.
Absorption spectra of compounds 1–10, normalized at maximum absorption wavelength.
In addition, each absorption curve possesses a blue-shifted shoulder, which increases with the level of structural asymmetry22. For example, compound 3, a nonsymmetrical molecule, has a higher shoulder than the symmetrical compound 4. Similar results were found when nonsymmetrical 2, 7, and 9, are compared to 1 (nonsymmetrical), 8 (symmetrical) and 10 (symmetrical), respectively. Although both 1 and 2 are nonsymmetrical molecules, compound 2 is considered as a more structurally “nonsymmetrical” molecule due to the large protected serine group. Although it is difficult to compare compounds 5 and 6 due to the “biphasic” nature of compound 5’s shoulder, it is clear that the shoulder for the nonsymmetrical 6 at 690 nm is higher than the symmetrical 5. Overall, nonsymmetrical molecules seem to have higher absorption shoulder than symmetrical molecules. This observation matches what have been reported for dichromic fluorescent NIR cyanine dyes22.
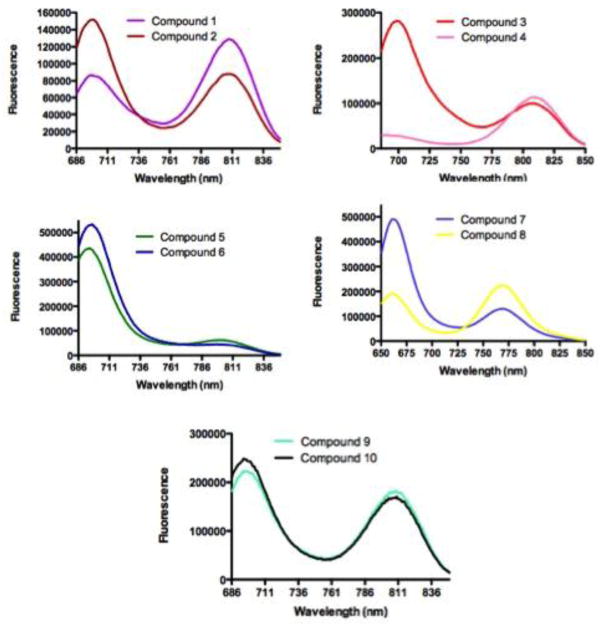
Upon excitation at 640 nm (compounds 7 and 8) or 675 nm (compounds 1–6, 9 and 10), all dyes exhibit dual fluorescence at 660/770 nm (compound 7 and 8) or 700/800 nm (compounds 1–6, 9 and 10). It has been reported that the shorter wavelength emission is favored by nonsymmetrical molecules22.
Our fluorescence profiles support this hypothesis. As shown in Figure 3, the symmetrical dyes, such as 4 and 8, have relatively small contributions of the low wavelength emission in the overall fluorescence spectra. In contrast, the nonsymmetrical compounds 2, 3, 6, 7, and 9 have smaller emission peak at 700 nm but higher emission peak at 800 nm. For example, the 700/800nm emission ratio changed from 2.82 (compound 3) to 0.25 (compound 4), as shown in Table 1. Conversely, compound 8 has higher 770 nm emission peak than compound 7, with a corresponding change in the 661/770 nm emission ratio from 0.85 to 0.3.78. In some cases, the dichromic fluorescence does not always correlate with the two dimensional structure depicted in Scheme 1 and Table 1. As the distance of substituents from the N-heterocyclic atom becomes shorter (m, n; Table 1), the positively charged N atom appears to influence the dichromic pattern. Here, even structurally symmetrical dyes such as 5, 8, and 10 show higher emission at the shorter than longer emission peaks. We did not expect the high 700 nm emission for compound 10 since the reverse is the case with indocyanine green (ICG), which is one carbon longer than compound 10 (i.e. m/n = 4/4 for ICG and m/n = 3/3 for compound 10). Even in this case, we see the same trend where the compound possessing shorter N-alkyl chain has higher emission peak at the shorter wavelength region. It is likely that these molecules exist as distinct species in the ground state due to charge localization. Another distinct possibility is presence of fluorescent dimmers in the ground state of the dyes molecules. However, in a previous study22, dissolution of the dyes in different solvents or at higher than room temperatures did not significantly alter the original spectra. All together, the trend showing that most nonsymmetrical dyes have higher emission peaks at shorter wavelength range is valid for the cases we examined. A future study will focus on using computational models and spectroscopic methods to elucidate the principles of dichromic fluorescence.
Figure 3.
Emission spectra of compounds 1–10. Compound 7 and 8 were excited at 640 nm, others at 675 nm.
In conclusion, we developed a library of structurally symmetrical and nonsymmetrical NIR fluorescent molecules to further verify the dichromic fluorescence phenomenon. The preliminary absorption and emission data show that the 700 nm absorption shoulder and 700/800nm emission ratio correlate with the structural symmetry of these molecules. These findings support the dichromic fluorescence phenomenon for NIR carbocyanine dyes, which provide new protocols for NIR optical imaging. Evaluation of the potential biological applications is in progress.
Supplementary Material
Acknowledgments
This work was supported in part by the US National Institutes of Health grants NIBIB R01 EB00811, R01 EB007276, and NCI R33 CA123537
Footnotes
Details of experimental section
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tromberg BJ, Shah N, Lanning R, Cerussi A, Espinoza J, Pham T, Svaasand L, Butler J. Neoplasia. 2000;2:26. doi: 10.1038/sj.neo.7900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klohs J, Wunder A, Licha K. Basic Research in Cardiology. 2008;103:144. doi: 10.1007/s00395-008-0702-7. [DOI] [PubMed] [Google Scholar]
- 3.Neri D, Carnemolla B, Nissim A, Leprini A, Querze G, Balza E, Pini A, Tarli L, Halin C, Neri P, Zardi L, Winter G. Nat Biotechnol. 1997;15:1271. doi: 10.1038/nbt1197-1271. [DOI] [PubMed] [Google Scholar]
- 4.Bai M, Rone MB, Papadopoulos V, Bornhop DJ. Bioconjugate Chem. 2007;18:2018. doi: 10.1021/bc700251e. [DOI] [PubMed] [Google Scholar]
- 5.Bai M, Wyatt SK, Han Z, Papadopoulos V, Bornhop DJ. Bioconjugate Chem. 2007;18:1118. doi: 10.1021/bc060381r. [DOI] [PubMed] [Google Scholar]
- 6.Achilefu S, Dorshow RB, Bugaj JE, Rajagopalan R. Invest Radiol. 2000;35:479. doi: 10.1097/00004424-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Licha K, Hessenius C, Becker A, Henklein P, Bauer M, Wisniewski S, Wiedenmann B, Semmler W. Bioconjugate Chem. 2001;12:44. doi: 10.1021/bc000040s. [DOI] [PubMed] [Google Scholar]
- 8.Licha KRB, Ntziachristos V, Becker A, Chance B, Semmler W. Photochemistry and Photobiology. 2000;72:392. doi: 10.1562/0031-8655(2000)072<0392:hcdaca>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Kovar JL, Volcheck WM, Chen JY, Simpson MA. Anal Biochem. 2007;361:47. doi: 10.1016/j.ab.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovar JL, Johnson MA, Volcheck WM, Chen JY, Simpson MA. Am J Pathol. 2006;169:1415. doi: 10.2353/ajpath.2006.060324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Backer MV, Patel V, Jehning BT, Backer JM. Bioconjugate Chem. 2006;17:912. doi: 10.1021/bc060037u. [DOI] [PubMed] [Google Scholar]
- 12.Petrovsky A, Schellenberger E, Josephson L, Weissleder R, Bogdanov A. Cancer Res. 2003;63:1936. [PubMed] [Google Scholar]
- 13.Kamiya M, Kobayashi H, Hama Y, Koyama Y, Bernardo M, Nagano T, Choyke PL, Urano Y. Journal of the American Chemical Society. 2007;129:3918. doi: 10.1021/ja067710a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matz MV, Lukyanov KA, Lukyanov SA. Bioessays. 2002;24:953. doi: 10.1002/bies.10154. [DOI] [PubMed] [Google Scholar]
- 15.Zaccolo M. Circ Res. 2004;94:866. doi: 10.1161/01.RES.0000123825.83803.CD. [DOI] [PubMed] [Google Scholar]
- 16.Weissleder R, Mahmood U. Radiology. 2001;219:316. doi: 10.1148/radiology.219.2.r01ma19316. [DOI] [PubMed] [Google Scholar]
- 17.Danthi SN, Pandit SD, Li KCP. Acad Radiol. 2004;11:1047. doi: 10.1016/j.acra.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Weissleder RMU. Radiology. 2001;219:316. doi: 10.1148/radiology.219.2.r01ma19316. [DOI] [PubMed] [Google Scholar]
- 19.Festy F, Ameer-Beg SM, Ng T, Suhling K. Molecular Biosystems. 2007;3:381. doi: 10.1039/b617204k. [DOI] [PubMed] [Google Scholar]
- 20.Bloch S, Lesage F, McIntosh L, Gandjbakhche A, Liang KX, Achilefu S. J Biomed Opt. 2005;10 doi: 10.1117/1.2070148. [DOI] [PubMed] [Google Scholar]
- 21.Treanor B, Lanigan PMP, Kumar S, Dunsby C, Munro I, Auksorius E, Culley FJ, Purbhoo MA, Phillips D, Neil MAA, Burshtyn DN, French PMW, Davis DM. Journal of Cell Biology. 2006;174:153. doi: 10.1083/jcb.200601108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang ZR, Berezin MY, Kao JLF, d’Avignon A, Bai MF, Achilefu S. Angewandte Chemie-International Edition. 2008;47:3584. doi: 10.1002/anie.200800475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.