Abstract
Objective
To investigate the relationship between cerebellar hemorrhage in preterm infants seen on MRI but not ultrasound and neurodevelopmental outcome.
Study design
MR images from a cohort study of MRI in preterm newborns were reviewed for cerebellar hemorrhage. Children were assessed at mean 4.8 years with neurological examination and developmental testing using the Wechsler Preschool and Primary Scale of Intelligence (WPPSI-III).
Results
Of 131 preterm newborns, cerebellar hemorrhage was seen on both ultrasound and MRI in 3 newborns; smaller hemorrhages seen only on MRI in 10 (total of incidence of 10%). Adjusting for gestational age at birth, intraventricular hemorrhage, and white matter injury, cerebellar hemorrhage detectable only by MRI was associated with 5.0-fold increased odds of abnormal neurological examination compared with those without hemorrhage (outcome data in 74%). No association was found with scores on WPPSI-III testing.
Conclusions
Cerebellar hemorrhage is not uncommon in preterm newborns. Although associated with neurologic abnormalities, hemorrhage seen only on MRI is associated with much more optimistic outcomes than that visible by ultrasound.
Keywords: preterm, neuroimaging, neurological outcome, developmental outcomes
Cerebellar hemorrhage in preterm infants has received increasing recognition in recent years, particularly with the more frequent use of MRI and posterolateral fontanelle views on cranial ultrasound.[1–4] Previous reports of cerebellar hemorrhage primarily concerned infants with notable perinatal risk factors including traumatic delivery and coagulopathy; these reported high rates of mortality or severe neurologic devastation. Indeed, the hemorrhages were commonly noted on post-mortem studies.[5–7]
Although routine use of posterolateral fontanelle views in cranial ultrasound has improved the detection of cerebellar hemorrhage, MRI has increased the identification of smaller cerebellar hemorrhages not detectable by cranial ultrasound. Recent data now suggest that cerebellar hemorrhage in preterm infants may be much more common than previously thought, particularly among extremely low birth weight babies (<750g). In one cohort of preterm infants with larger cranial ultrasound-diagnosed cerebellar hemorrhage, 66% were found to have neurological abnormalities at 2.5 years, as well as increased risk for cognitive, learning, and behavioural dysfunction.[8] In another cohort of preterm infants with serial MRI studies, cerebellar hemorrhage was seen in 7% of infants, with increased risk for mortality and significant developmental delay.[9] However, size of hemorrhage was not considered in either analysis. As a result, the neurodevelopmental effect of small cerebellar hemorrhages is not well understood. The objective of the present study is to assess the long-term neurodevelopmental outcome of preterm infants with cerebellar hemorrhage, focusing on infants with smaller cerebellar hemorrhages seen only on MRI scans.
Methods
Preterm newborns admitted to the intensive care nursery at the University of California San Francisco were recruited from 1998 to 2003 as part of an ongoing prospective cohort study of brain injury and development using sequential MRI scans. Inborn and outborn neonates born at <34 weeks gestational age were eligible for the study. Exclusion criteria for study enrollment were (1) clinical evidence of a congenital malformation or syndrome, (2) congenital TORCH infection, and (3) newborns too clinically unstable for transport to the MRI scanner. Parental consent was obtained for all cases following a protocol approved by the UCSF Committee on Human Research.
All subjects were imaged using repeated cranial ultrasounds and MRI scans. Two MRI scans – one soon after birth (mean 32.2 ± 2.1 weeks postmenstrual age) and another near term-equivalent age (mean 36.9 ± 2.3 weeks postmenstrual age) – were performed on each newborn. Cranial ultrasounds were routinely obtained at 7 days of life and again at 4 weeks of life. In case of additional risk factors for intracranial bleeding such as acute decreases in blood pressure or hematocrit, cranial ultrasounds were also obtained at 3 days of life. If abnormalities were found, ultrasounds were repeated weekly until findings stabilized.
A custom MR-compatible incubator with a specialized neonatal head coil was used to provide a quiet, well-monitored environment for the neonate, minimizing patient movement and improving the signal-to-noise ratio.[10] MR imaging of the brain was performed on a 1.5-Tesla Signa EchoSpeed System (GE Medical Systems, Milwaukee, WI), including: 1) T1 weighted sagittal spin echo images (4 mm thickness) using repetition time (TR) = 500 ms, echo time (TE) = 11 ms, 1 excitation, and 192 x 256 acquisition matrix, 2) T2 weighted conventional spin echo (CSE, 4 mm thickness) with TR = 3000 msec, TE = 60 and 120 msec, and 192 x 256 acquisition matrix, and 3) coronal 3D SPGR T1 weighted images (partition size=1.5 mm) with TR=36 ms, TE=min, Flip angle = 35°, NEX=2, FOV=18 cm, as described previously.[11]
MRIs were reviewed for the presence of intraventricular hemorrhage (IVH), white matter injury (WMI), and cerebellar hemorrhage by a pediatric neuroradiologist (AJB) blinded to clinical history and ultrasound findings. Classification of IVH was performed using the grading system of Papile.[12] Classification of WMI was performed using a scoring system previously shown to be associated with neurodevelopmental outcomes at 12–18 months of age.[11] Cerebellar hemorrhage was identified by the presence of hypointensity on the T2 weighted sequences that “bloomed” on the second echo. T2 weighted CSE sequences are very sensitive to the detection of blood in the unmyelinated neonatal brain. [13] The location, number, and size of cerebellar hemorrhages were recorded.
Cranial ultrasounds were performed as clinically indicated. Siemens Sequoia Ultrasound equipment (Mountain View, California) with 8–10 mHz sector transducers was used for views through both the anterior and posterolateral fontanelles. Clinical reports from the electronic health records were reviewed for the presence of intracranial hemorrhage (including IVH and cerebellar hemorrhage), and cerebellar hemorrhage was confirmed by a pediatric ultrasonologist (RG) blinded to the clinical history and MRI findings. For purposes of classification of IVH, due to repeated imaging by multiple modalities, the highest injury score was recorded as the true IVH score for each subject.
Neurodevelopmental outcome
After discharge from hospital, children enrolled in this prospective cohort received repeated assessments in the UCSF intensive care nursery follow-up clinic. At each visit, the child was assessed by a physician (either a pediatrician specialized in newborn follow-up or a pediatric neurologist) and a developmental psychologist blinded to the child’s neonatal course.
A standardized neurological examination was performed by the physician and recorded. If the assessing physician was not a pediatric neurologist, the reports were reviewed by a pediatric neurologist. Abnormalities of the neurological examination were scored using a validated neuromotor score, which scores subjects on the basis of tone, deep tendon reflexes, primitive reflexes, power, and cranial nerve involvement.[14, 15] For the purposes of analysis, the score was dichotomized to the presence or absence of abnormalities on the neurological examination.
Between the ages of 3 and 6 years, follow-up visits also included formal assessments in development by a psychologist using the Wechsler Preschool and Primary Scale of Intelligence (WPPSI-III, The Psychological Corporation, 2002). Scores recorded include composite verbal IQ, performance IQ, and the full scale IQ. The WPPSI is standardized and validated and the mean of each composite score is 100 with a standard deviation of 15 points.
Statistical analysis
Statistical analysis was performed using Stata 10 (Stata Corporation, College Station, Texas). Descriptive statistics were used to look at distribution of neurological and developmental outcomes in children with cerebellar hemorrhage on ultrasound or MRI. To assess the relationship between cerebellar hemorrhage seen on MRI but not cranial ultrasound and neurological outcome, logistic regression analysis was used. Linear regression analysis was used to assess developmental outcome via WPPSI scores. The analysis was performed adjusting for other known risk factors for adverse outcome, including gestational age at birth, and other common preterm brain pathologies including severity of IVH and WMI.
Results
A cohort of 131 preterm newborns was enrolled, and all subjects had both cranial ultrasound and MRI scans during their hospital stay. Cerebellar hemorrhage was seen on cranial ultrasound in 3 newborns. All hemorrhages on ultrasound were confirmed on MRI, and an additional 10 cerebellar hemorrhages that were not detected by ultrasound were seen on MRI. Of the cohort, 3 newborns died during their admission in the nursery, 2 of whom had ultrasound-detected cerebellar hemorrhages. Of the 128 survivors, 94 children continued to have neurodevelopmental assessments until 3–6 years of age (mean 4.8±0.1 years for those without cerebellar hemorrhage, mean 4.9±0.2 years for those with cerebellar hemorrhage). As a result, outcome data were available in 74% of the original cohort, including 1 child with ultrasound-detected cerebellar hemorrhage and 8 children with MRI only cerebellar hemorrhages. No statistical differences in patient characteristics were seen between those with and without follow-up data regarding gestational age at birth, birth weight, sex, severity of IVH, or severity of WMI (P>0.3) (Table I).
Table 1.
Subject demographics in those with follow-up to 3–6 years
| No cerebellar hemorrhage (n=85) | Cerebellar hemorrhage (n=9) | P-value | |
|---|---|---|---|
| Gestational age at birth (mean ± s.d.) | 28.0 ± 2.4 weeks | 27.4 ± 2.4 weeks | 0.5 |
| Birth weight (mean ± s.d.) | 1064 ± 383 grams | 974 ± 262 grams | 0.5 |
| Male sex | 40 (47%) | 6 (67%) | 0.3 |
| IVH | |||
| - mild (grade 1 & 2) | 29 (34%) | 3 (33%) | 0.95 |
| - severe (grade 3 & 4) | 8 (9%) | 1 (11%) | 0.84 |
| WMI | |||
| - mild | 26 (31%) | 1 (11%) | 0.2 |
| - severe | 19 (22%) | 2 (22%) | 1.0 |
IVH intraventricular hemorrhage, WMI white matter injury. Mild WMI is categorized as having ≤ 3 signal abnormalities on T1-weighted MRI imaging of <2mm diameter. Severe WMI is categorized as having >3 signal abnormalities or >5% of the cerebral hemisphere involved. The P-value arises from a t-test comparing two means or from a Fisher exact test comparing two proportions.
Distribution of cerebellar hemorrhage
All three newborns with cerebellar hemorrhage detectable on cranial ultrasound had large hemorrhages ranging from 7 x 8 mm in size to bilateral extensive hemorrhages filling the posterior fossa. Of the 10 newborns with cerebellar hemorrhages seen only on MRI, hemorrhage sizes ranged from 1 to 3 mm in diameter, and were presumed to be germinal matrix hemorrhages by their close proximity to cerebellar cortex. Three of the infants had only a single hemorrhage, one had two hemorrhages in the same cerebellar hemisphere, and the remaining 6 had multiple hemorrhages in both cerebellar hemispheres. Examples of hemorrhages seen on ultrasound and MRI are shown in the Figure.
Figure.
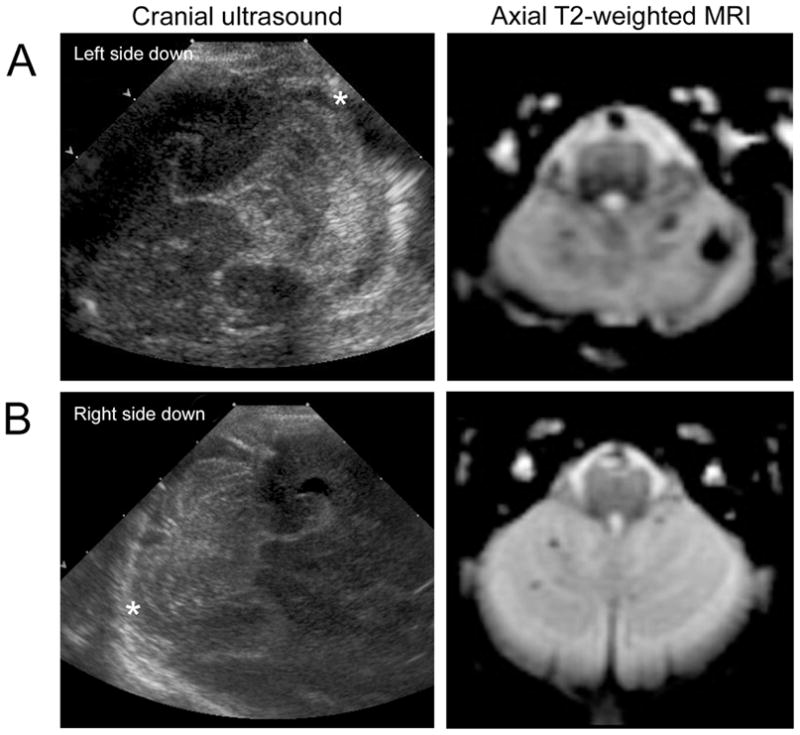
Cerebellar hemorrhage appearances on MRI and cranial ultrasound in (A) a newborn with ultrasound-visible cerebellar hemorrhage and (B) a newborn with cerebellar hemorrhage detected only by MRI. The patient in (A) died during the perinatal period, whereas the patient in (B) had normal neurodevelopmental outcome at 4.5 years. The right cerebellar hemisphere on the sonograms are indicated with a *.
Neurological outcome
Of the 3 newborns with ultrasound-visible cerebellar hemorrhage, 2 died during initial hospitalization. The one survivor had no deficits on neurological examination in follow-up at 4.7 years. Of the 10 newborns with cerebellar hemorrhage seen only on MRI, all children survived their initial neonatal course. Of the 8 who were assessed between the ages of 3–6 years, 4 (50%) had abnormalities on neurological examination. Abnormalities included hypertonia and hyperreflexia (Table II), with no functional impairments in ambulation and, although this was not specifically tested, no specific observations of ataxia or impaired coordination were noted. Of the 85 remaining children without cerebellar hemorrhage assessed in follow-up, 14 (16%) had abnormalities on neurological examination. Abnormalities in neurological examination included truncal hypotonia, lower limb hypertonia, and lower limb hyperreflexia.
Table 2.
Severity of brain injury and neurological deficits in the 8 children with MRI-only cerebellar hemorrhages with outcome data available
| Subject | IVH grade | WMI grade | Location of cerebellar hemorrhage | Distribution of hypertonia and hyperreflexia |
|---|---|---|---|---|
| 1 | 2 | 0 | Right single | None |
| 2 | 2 | 0 | Bilateral multiple | None |
| 3 | 1 | 0 | Left multiple | Bilateral lower limbs |
| 4 | 3 | 2 | Left single | Right upper and lower limbs |
| 5 | 2 | 0 | Bilateral multiple | Bilateral lower limbs |
| 6 | 0 | 2 | Bilateral multiple | Bilateral lower limbs |
| 7 | 2 | 0 | Bilateral multiple | None |
| 8 | 1 | 1 | Bilateral multiple | None |
IVH is scored using the grading system of Papile. For WMI, 0 is no injury, 1 is ≤ 3 T1 signal abnormalities <2mm, 2 is >3 T1 abnormalities <2mm, 3 is >5% hemisphere involvement.
Using logistic regression analysis, the surviving children with cerebellar hemorrhage seen on MRI but not cranial ultrasound had a 5.1-fold increased odds of abnormalities on neurological examination during the following-up period compared with those without cerebellar hemorrhage (95% CI 1.1 to 22.7, P=0.03). Of the 8 children who returned to follow-up with cerebellar hemorrhage seen only on MRI, one had mild and two had severe WMI. Two had mild and one had severe IVH. Four had no IVH or WMI detected. After adjusting for gestational age, IVH, and WMI, the odds ratio was 5.0 (95% CI 1.1 to 23.1, P=0.04).
Developmental outcome
Using the WPPSI-III test, the children without cerebellar hemorrhage demonstrated a mean performance IQ of 99±2, verbal IQ of 98±2, and full scale IQ of 99±2. The children with MRI-only cerebellar hemorrhage demonstrated a mean performance IQ of 100±5, verbal IQ of 95±7, and full scale IQ of 98±6. Using linear regression analysis, adjusting for gestational age, IVH, and WMI, the surviving children with cerebellar hemorrhage seen on MRI but not cranial ultrasound did not have significantly different performance IQ (+2, 95% CI −11 to 14, P=0.8), verbal IQ (−1, 95% CI −14 to 12, P=0.9), or full scale IQ (0, 95% CI −13 to 14, P=0.9) scores compared with those without cerebellar hemorrhage.
Discussion
Although large cerebellar hemorrhages result in increased risk for mortality and severe neurodevelopmental deficits,[8] the effects of smaller cerebellar hemorrhages seen on MRI and not cranial ultrasound have not been previously reported. This prospective cohort study suggests that smaller cerebellar hemorrhages seen only on MRI are associated with an increased risk for abnormalities on neurological examination. However, the presence of these hemorrhages is not associated with abnormalities on developmental testing at ages 3–6 years.
This study of neuroimaging in preterm newborns demonstrated a 2% incidence of ultrasound-detected cerebellar hemorrhage and an 8% incidence of cerebellar hemorrhage detected only on MRI. These rates are comparable with those reported by other groups.[1, 16] In agreement with previous studies,[8, 9] large cerebellar hemorrhages seen on ultrasound were associated with a high risk for adverse outcome – 67% mortality was seen during the initial hospitalization. However, due to the few cases of such hemorrhage in survivors in this cohort, we were unable to assess neurodevelopmental outcome.
This study highlights the power of MRI to detect cerebellar hemorrhages of 1 to 3mm in diameter – hemorrhages previously not detected on cranial sonograms. With increasing use of MRI in brain injury diagnosis in preterm newborns and a resulting increase in identification of these smaller hemorrhages, it is thus important to understand the neurodevelopmental consequences of such findings to guide parental counseling and patient therapy. Of note, the MRI sequences used in this study used a scan thickness of 4mm, and thus may have missed some incidences of these small hemorrhages. The actual incidence of these hemorrhages may be even more common than reported here.
Higher rates of abnormalities on neurological examination were seen in children with MRI-only cerebellar hemorrhages, with a 5-fold increased odds compared with those without cerebellar hemorrhage. Due to the variability of the physician examining the subjects in follow-up, there may be nondifferential misclassification of this outcome measure. As a result, this study may have underestimated the magnitude of the association between MRI-only cerebellar hemorrhages and abnormal neurological examination. Abnormalities tend to include hypertonia and hyperreflexia normally associated with spastic diplegic cerebral palsy. These associations were not dampened after adjusting for the severity of IVH and WMI. Notably, however, these deficits are less severe than those previously reported in children with larger ultrasound-detected cerebellar hemorrhages, and do not prevent ambulation.
That the neurological deficits observed with cerebellar hemorrhage are the same as those classically described with IVH and WMI raises new questions as to the neural pathways that may be involved in these findings. One possibility is that cerebellar injury directly results in changes in tone and reflexes. Another possibility is that limitations in cerebellar function magnify the deficits associated with IVH and WMI, resulting in increased recognition of these findings by the evaluating clinician.
In contrast with previous studies showing cognitive and language impairment associated with cerebellar hemorrhages, this study did not show an association between MRI-only cerebellar hemorrhages and deficits in performance or verbal domains using the WPPSI-III test. This suggests a dramatic difference between the effects of these smaller MRI-only hemorrhages on cognitive outcome compared with previous reports in larger ultrasound-visible hemorrhages. A wide range of ages at developmental testing may be a limitation in this study, although there was no difference between the ages of assessment between those with and without cerebellar hemorrhage, and the variability in scores were small. In addition, more subtle neurocognitive deficits that might result from these cerebellar hemorrhages may only become evident at a later age than tested for this study.
Although we were able to study the effect of overall cerebellar injury on outcome, there were not enough cases of cerebellar hemorrhage to compare effects of bilateral versus unilateral cerebellar hemorrhage or left hemisphere versus right hemisphere hemorrhages, or to look at the association between particular regions of injury and specific neurological findings. Larger studies of preterm newborns with localized cerebellar hemorrhage on MRI would help elucidate whether the location of cerebellar hemorrhage can further inform us regarding the specific deficits that would ensue.
The finding of increased risk of neurological deficits after cerebellar hemorrhage not visible on ultrasound adds to the growing body of evidence that shows additional benefit of the use of MRI to screen for brain injury in preterm newborns in addition to the routine use of cranial ultrasound.[17–19] Considering that preterm newborns with cerebellar hemorrhage not visible on cranial ultrasound have 5-fold increased odds of abnormal neurological examination by school age, this would provide additional information to aid in deciding which children require closer follow-up after hospital discharge and earlier interventions.
With an increasing awareness of the risk of cerebellar hemorrhage in preterm newborns and its associated risk for mortality and severe developmental delay, it has become important to understand the range of cerebellar hemorrhage which can occur and the associated outcomes. Compared with large cerebellar hemorrhages previously identified by cranial ultrasound, small 1 to 3 mm hemorrhages seen by MRI in the preterm cerebellum are associated with an increased risk of abnormalities on neurological examination, but not with cognitive impairment. These findings will be important to aid physicians in counseling parents regarding potential outcomes in their newborn children, and highlight the utility of MRI scans in the assessment of preterm cerebellar injury.
Acknowledgments
Supported by NIH R01 NS346432, NIH/NCRR UCSF-CTSI (grant UL1 RR024131), NIH/NCRR/OD UCSF-CTSI (grant KL2 RR024130 to H.G.). E.T. is a Cerebral Palsy International Research Foundation Ethel & Jack Hausman Clinical Research Scholar.
Abbreviations
- IVH
intraventricular hemorrhage
- WMI
white matter injury
Footnotes
Reprints: None requested
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Merrill JD, Piecuch RE, Fell SC, Barkovich AJ, Goldstein RB. A new pattern of cerebellar hemorrhages in preterm infants. Pediatrics. 1998;102:E62. doi: 10.1542/peds.102.6.e62. [DOI] [PubMed] [Google Scholar]
- 2.Steggerda SJ, Leijser LM, Wiggers-de Bruine FT, van der Grond J, Walther FJ, van Wezel-Meijler G. Cerebellar injury in preterm infants: incidence and findings on US and MR images. Radiology. 2009;252:190–9. doi: 10.1148/radiol.2521081525. [DOI] [PubMed] [Google Scholar]
- 3.Sehgal A, El-Naggar W, Glanc P, Asztalos E. Risk factors and ultrasonographic profile of posterior fossa haemorrhages in preterm infants. J Paediatr Child Health. 2009;45:215–8. doi: 10.1111/j.1440-1754.2008.01456.x. [DOI] [PubMed] [Google Scholar]
- 4.Limperopoulos C, Benson CB, Bassan H, Disalvo DN, Kinnamon DD, Moore M, et al. Cerebellar hemorrhage in the preterm infant: ultrasonographic findings and risk factors. Pediatrics. 2005;116:717–24. doi: 10.1542/peds.2005-0556. [DOI] [PubMed] [Google Scholar]
- 5.Rorke LB. Pathology of perinatal brain injury. New York: Raven Press; 1982. [Google Scholar]
- 6.Grunnet ML, Shields WD. Cerebellar hemorrhage in the premature infant. J Pediatr. 1976;88:605–8. doi: 10.1016/s0022-3476(76)80019-4. [DOI] [PubMed] [Google Scholar]
- 7.Martin R, Roessmann U, Fanaroff A. Massive intracerebellar hemorrhage in low-birth-weight infants. J Pediatr. 1976;89:290–3. doi: 10.1016/s0022-3476(76)80470-2. [DOI] [PubMed] [Google Scholar]
- 8.Limperopoulos C, Bassan H, Gauvreau K, Robertson RL, Jr, Sullivan NR, Benson CB, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. 2007;120:584–93. doi: 10.1542/peds.2007-1041. [DOI] [PubMed] [Google Scholar]
- 9.Dyet LE, Kennea N, Counsell SJ, Maalouf EF, Ajayi-Obe M, Duggan PJ, et al. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics. 2006;118:536–48. doi: 10.1542/peds.2005-1866. [DOI] [PubMed] [Google Scholar]
- 10.Partridge SC, Mukherjee P, Berman JI, Henry RG, Miller SP, Lu Y, et al. Tractography-based quantitation of diffusion tensor imaging parameters in white matter tracts of preterm newborns. J Magn Reson Imaging. 2005;22:467–74. doi: 10.1002/jmri.20410. [DOI] [PubMed] [Google Scholar]
- 11.Miller SP, Ferriero DM, Leonard C, Piecuch R, Glidden DV, Partridge JC, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. Journal of Pediatrics. 2005;147:609–16. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 12.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–34. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 13.Barkovich AJ. Pediatric Neuroimaging. 4. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 14.Miller SP, Latal B, Clark H, Barnwell A, Glidden DV, Barkovich AJ, et al. Clinical signs predict 30-month neurodevelopmental outcome after neonatal encephalopathy. Am J Obstet Gynecol. 2004;190:93–9. doi: 10.1016/s0002-9378(03)00908-6. [DOI] [PubMed] [Google Scholar]
- 15.Hajnal BL, Sahebkar-Moghaddam F, Barnwell AJ, Barkovich AJ, Ferriero DM. Early prediction of neurologic outcome after perinatal depression. Pediatr Neurol. 1999;21:788–93. doi: 10.1016/s0887-8994(99)00101-0. [DOI] [PubMed] [Google Scholar]
- 16.Muller H, Beedgen B, Schenk JP, Troger J, Linderkamp O. Intracerebellar hemorrhage in premature infants: sonographic detection and outcome. J Perinat Med. 2007;35:67–70. doi: 10.1515/JPM.2007.010. [DOI] [PubMed] [Google Scholar]
- 17.Dammann O, Leviton A. Neuroimaging and the prediction of outcomes in preterm infants. N Engl J Med. 2006;355:727–9. doi: 10.1056/NEJMe068123. [DOI] [PubMed] [Google Scholar]
- 18.de Vries LS, Cowan FM. Should cranial MRI screening of preterm infants become routine? Nat Clin Pract Neurol. 2007;3:532–3. doi: 10.1038/ncpneuro0608. [DOI] [PubMed] [Google Scholar]
- 19.Hart AR, Whitby EW, Griffiths PD, Smith MF. Magnetic resonance imaging and developmental outcome following preterm birth: review of current evidence. Dev Med Child Neurol. 2008;50:655–63. doi: 10.1111/j.1469-8749.2008.03050.x. [DOI] [PubMed] [Google Scholar]


