Abstract
Objectives
We quantified the benefits (life expectancy gains) and harms (efavirenz-related teratogenicity) associated with using efavirenz in HIV-infected women of childbearing age in the United States.
Methods
We used data from the Women’s Interagency HIV Study in an HIV disease simulation model to estimate life expectancy in women who receive an efavirenz-based initial antiretroviral regimen compared with those who delay efavirenz use and receive a boosted protease inhibitor-based initial regimen. To estimate excess risk of teratogenic events with and without efavirenz exposure per 100,000 women, we incorporated literature-based rates of pregnancy, live births, and teratogenic events into a decision analytic model. We assumed a teratogenicity risk of 2.90 events/100 live births in women exposed to efavirenz during pregnancy and 2.68/100 live births in unexposed women.
Results
Survival for HIV-infected women who received an efavirenz-based initial antiretroviral therapy regimen was 0.89 years greater than for women receiving non-efavirenz-based initial therapy (28.91 vs. 28.02 years). The rate of teratogenic events was 77.26/100,000 exposed women, compared with 72.46/100,000 unexposed women. Survival estimates were sensitive to variations in treatment efficacy and AIDS-related mortality. Estimates of excess teratogenic events were most sensitive to pregnancy rates and number of teratogenic events/100 live births in efavirenz-exposed women.
Conclusions
Use of non-efavirenz-based initial antiretroviral therapy in HIV-infected women of childbearing age may reduce life expectancy gains from antiretroviral treatment, but may also prevent teratogenic events. Decision-making regarding efavirenz use presents a tradeoff between these two risks; this study can inform discussions between patients and health care providers.
Keywords: HIV/AIDS, efavirenz, women, teratogenicity
INTRODUCTION
In March 2005, Bristol-Myers Squibb issued a “Dear Health Care Provider” letter informing physicians that the Food and Drug Administration (FDA) pregnancy category for efavirenz was changed from Category C (Risk of Fetal Harm Cannot Be Ruled Out) to Category D (Positive Evidence of Fetal Risk).1, 2 Efavirenz, a non-nucleoside reverse transcriptase inhibitor (NNRTI), was originally classified as FDA pregnancy category C after pre-clinical animal studies showed malformations in 3 of 20 monkey fetuses exposed to efavirenz (versus 0 of 20 concomitant controls).2 The impetus for the category upgrade stemmed from four retrospectively reported cases of neural tube defects in human fetuses exposed to efavirenz during the first trimester of pregnancy.3-5
Based on comparative clinical studies and safety data,6-9 the current Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents recommend the use of efavirenz as the preferred NNRTI component of initial antiretroviral therapy (ART) regimens.10 The exception to this is in women who are pregnant (especially during the first trimester), planning to conceive, or not using effective and consistent contraception. Despite these recommendations, anecdotal evidence suggests that some physicians resist prescribing efavirenz to any woman of childbearing age without a definitive form of birth control (e.g., hysterectomy, tubal ligation).
To inform treatment decision-making for HIV-infected women of childbearing age in the United States, we sought to quantify the tradeoff between a potential loss of maternal life expectancy due to use of a non-efavirenz-based initial ART regimen and the anticipated risk of excess teratogenic events from efavirenz use in HIV-infected women who may become pregnant unintentionally.
METHODS
Analytic Overview
To quantify the benefits (life expectancy gains) and harms (efavirenz-related teratogenicity) associated with using efavirenz in HIV-infected women of childbearing age in the U.S., we conducted this analysis in two parts. First, we used a previously published computer simulation model of HIV disease and treatment11-14 informed by data from the Women’s Interagency HIV Study (WIHS)15 and the published literature from the modern ART era to estimate survival in HIV-infected women given the following two efavirenz prescription policies: 1) an efavirenz-based regimen is available and prescribed as a component of first-line therapy regardless of childbearing potential, and 2) an alternate first-line ART regimen is prescribed and efavirenz use is delayed due to concerns related to unintended pregnancy. We then incorporated reported rates of pregnancy, live birth, and teratogenicity among HIV-infected women into a separate decision analytic model to estimate the risk of teratogenic events per 100,000 women exposed to efavirenz compared with those unexposed to efavirenz.
Estimating Long-term Survival in HIV-infected Women
The Cost-Effectiveness of Preventing AIDS Complications (CEPAC) Model is a previously published computer simulation model of HIV infection which incorporates natural history, disease progression, and state-of-the-art therapeutic interventions.11-14 Patients in the model are divided into “health states,” including chronic HIV disease, acute clinical events (e.g., opportunistic infections, drug toxicities), and death, which reflect HIV disease progression. In the model, stratified CD4 counts determine the absolute risk of opportunistic infections and HIV-related mortality while stratified HIV RNA levels determine the rate of CD4 count decline in the absence of therapy. While the efficacies of the ART regimens modeled are reported in rates of virologic suppression and CD4 benefit, we use the model to project these results over the long term. To achieve stability in estimates, a cohort of 1,000,000 patients is simulated one at a time, from model entry until death. Model outcomes include mean survival time and mean exposure time for all antiretroviral treatment regimens.
HIV-infected women in the model are at risk for the following HIV-related comorbidities: Pneumocystis jirovecii pneumonia (PCP), Mycobacterium avium complex (MAC), toxoplasmosis, cytomegalovirus (CMV), fungal infections, bacterial infections, invasive cervical cancer, and other illnesses (e.g., lymphoma and wasting). Primary and secondary prophylaxis against PCP, toxoplasmosis, and MAC is provided at recommended CD4 thresholds and at efficacy rates reported in the literature.16-24
Antiretroviral therapy functions in the model to suppress HIV RNA with a concomitant increase in CD4 count as reported in treatment trials from the modern ART era.25-30 The model allows for numerous sequential drug treatment regimens after failure. However, subsequent therapy regimens generally result in diminishing capacity to suppress virus due to previous drug exposure and development of resistance. Treatment failure, resulting in a switch to the next available regimen, may occur either as a result of virologic failure (defined as a one-log increase in HIV RNA over two consecutive months) or immunologic failure (defined as a decrease in CD4 count over two consecutive months). For cases of nucleoside reverse transcriptase inhibitor (NRTI)-related toxicity, the model has the capacity to incorporate a single NRTI switch with an associated quality of life decrement, without including a switch of the entire regimen. The model provides six sequential ART regimens to indicate possible treatment sequences for a patient who fails multiple therapies. Due to the efficacy of the regimens, approximately one-third of simulated patients die of unrelated causes and neither progress through all six regimens nor die as a result of sequential ART failure. If all regimens are exhausted, an optimized background regimen is maintained to capitalize on the independent effect of antiretroviral therapy in averting opportunistic infections, despite apparent virologic or immunologic treatment failure.31
Simulation Model Input Data
The Women’s Interagency HIV Study (WIHS) Cohort
The WIHS is a longitudinal cohort of HIV-infected women in 6 U.S. locations (Bronx/Manhattan, NY; Washington DC; San Francisco/Bay Area; Los Angeles/Southern California/Hawaii; Chicago, IL; and Brooklyn, NY). Cohort enrollment from October 1994 through November 1995 resulted in inclusion of 2,056 HIV-infected women.32 Details on the WIHS study have been published elsewhere;15 resulting studies have informed issues related to the natural history of HIV disease in women,33 tuberculin positivity,34 disease progression in women on antiretroviral therapy,35, 36 and HIV effects on cervical dysplasia.37
For this analysis, cohort characteristics and natural history data used as model inputs for disease progression in the absence of treatment were provided based on all non-pregnant, ART-naive WIHS participants enrolled between 1994 and 1995 and followed through 2002 (Table 1; data provided by collaborating WIHS investigators). At baseline, this cohort of women had a mean CD4 count of 520/μl (standard deviation 418/μl) and a mean HIV RNA of 4.4 log10 copies/ml (standard deviation 0.9 copies/ml). The rate of CD4 count decline in the absence of treatment varied by HIV RNA and ranged between 2.48 cells/μl/month (HIV RNA <3,000 copies/ml) to 2.93 cells/μl/month (HIV RNA >100,000 copies/ml).
Table 1.
Selected Model Input Parameters
| Parameter | Base Case | Source |
|---|---|---|
| Mean age (SD) | 36.1 (8.0) years | WIHS* |
| Mean CD4 cell count (SD) | 520 (417) cells/μl | WIHS |
| Distribution of initial HIV RNA (copies/ml) | ||
| > 100,000 | 0.26 | WIHS |
| 30,001–100,000 | 0.20 | |
| 10,001–30,000 | 0.18 | |
| 3,001–10,000 | 0.25 | |
| 501–3,000 | 0.07 | |
| 0–500 | 0.05 | |
| Mean monthly CD4 cell decline (cells/μl) by HIV RNA stratum (copies/ml) | ||
| > 100,000 | 2.93 | WIHS |
| 30,001–100,000 | 2.61 | |
| 3,001–30,000 | 2.59 | |
| ≤3,000 | 2.48 | |
| Chronic AIDS mortality, % monthly risk by CD4 stratum (cells/μl)† | WIHS | ||||||
|---|---|---|---|---|---|---|---|
| 0-50 | 51-100 | 101-200 | 201-300 | 301-500 | >500 | ||
| Without prior OIs | 1.65 | 0.42 | 0.42 | 0 | 0 | 0 | |
| With prior OIs | 6.14 | 3.45 | 2.39 | 0.83 | 0.50 | 0.25 | |
| Severe opportunistic diseases, % monthly risk by CD4 stratum (cells/μl) | WIHS | ||||||
|---|---|---|---|---|---|---|---|
| 0-50 | 51-100 | 101-200 | 201-300 | 301-500 | >500 | ||
| PCP | 1.49 | 1.00 | 0.42 | 0.25 | 0.08 | 0.08 | |
| MAC | 1.14 | 0.00 | 0.04 | 0.04 | 0.02 | 0.02 | |
| Toxoplasmosis | 0.18 | 0.25 | 0.04 | 0.04 | 0.00 | 0.01 | |
| Cytomegalovirus | 0.37 | 0.25 | 0.04 | 0.02 | 0.03 | 0.02 | |
| Fungal infections | 2.47 | 1.08 | 0.33 | 0.33 | 0.17 | 0.08 | |
| Bacterial infections | 0.66 | 0.17 | 0.33 | 0.42 | 0.25 | 0.17 | |
| Invasive cervical cancer | 0.18 | 0.26 | 0.04 | 0.02 | 0.08 | 0.04 | |
| Other | 1.90 | 1.65 | 1.08 | 0.58 | 0.50 | 0.58 | |
| Efficacy of antiretroviral therapy regimens | |||
|---|---|---|---|
| Efavirenz as a component of first-line ART | HIV RNA suppression (<400 copies/ml) |
CD4 Benefit (cells/μl) |
|
| 1st-line: EFV + 2 NRTIs | 86.0% at 24 weeks | 190 at 48 weeks | 28, 50 |
| 2nd-line: ATV/RTV + 2 NRTIs‡ | 79.0% at 24 weeks | 110 at 48 weeks | 29, 51 |
| 3rd-line: RAL + DRV/RTV + OBR | 90.0% at 16 weeks§ | 86 at 16 weeks | 52 |
| 4th-line: T20 + OBR or MVC + OBR +/− T20∥ | 40.0% at 24 weeks | 117 at 48 weeks | 25, 27 |
| 5th-line: OBR (2 PIs +2 NRTIs) | 15.0% at 24 weeks | 45 at 48 weeks | 27 |
| Alternate initial regimen with delayed use of efavirenz | |||
| 1st-line: ATZ/RTV + 2 NRTIs | 78.0% at 48 weeks | 190 at 48 weeks# | 53 |
| 2nd-line: RAL + DRV/RTV + OBR | 90.0% at 16 weeks | 86 at 16 weeks | 52 |
| 3rd-line: LPV/RTV + EFV** | 72.5% at 24 weeks | 110 at 48 weeks | 9 |
| 4th-line: T20 + OBR or MVC + OBR +/− T20∥ | 40.0% at 24 weeks | 117 at 48 weeks | 25, 27 |
| 5th-line: OBR (2 PIs +2 NRTIs) | 15.0% at 24 weeks | 45 at 48 weeks | 27 |
| Sensitivity Analysis: Nevirapine (substitution for efavirenz in first-line) | |||
| 1st-line: NVP + 2 NRTIs | 65.4% at 24 weeks | 190 at 48 weeks†† | 54 |
| 2nd-line: ATV/RTV + 2 NRTIs† | 79.0% at 24 weeks | 110 at 48 weeks | 29, 51 |
| 3rd-line: RAL + DRV/RTV + OBR | 90.0% at 16 weeks | 86 at 16 weeks | 52 |
| 4th-line: T20 + OBR or MVC + OBR +/− T20∥ | 40.0% at 24 weeks | 117 at 48 weeks | 25, 27 |
| 5th-line: OBR (2 PIs +2 NRTIs) | 15.0% at 24 weeks | 45 at 48 weeks | 27 |
Abbreviations: WIHS, Women’s Interagency HIV Study; OI, opportunistic infection; PCP, Pneumocystis jeroveci pneumonia; MAC, Mycobacterium avium complex; EFV, efavirenz; NRTI, nucleoside reverse transcriptase inhibitor; ATV, atazanavir; RTV, ritonavir; LPV, lopinavir; RAL, raltegravir; DRV, darunavir; T20, enfuvirtide; MVC, maraviroc; NVP, nevirapine; OBR, optimized background regimen; PI: protease inhibitor; SD: standard deviation
Data provided by collaborating WIHS investigators.
For CD4 cell counts >200 cells/μl, we used the upper bound of the 95% confidence interval for chronic AIDS mortality data provided by the WIHS because these estimates produced a better match between model-estimated life expectancy and observed long-term patient survival.
ATV/r arm, <4 PI mutations
Although the virologic suppression for this regimen was reported at 16 weeks, the model treats it as 24 week data.
We assumed that 50% of the cohort received T20 + OBR (HIV RNA 32.7% suppressed at 24 weeks; CD4 benefit of 119 cells at 48 weeks27) and 50% received MVC + OBR +/− T20 (BID arm: HIV RNA 60.4% suppressed at 24 weeks and a CD4 benefit of 111 cells at 24 weeks25; 46% of these patients received T20). The viral suppression rate and the CD4 benefits reported in this table are weighted averages of these two regimens.
The CD4 gain of the first regimen of the “efavirenz delayed” scenario was originally reported as 203 cells/μl at 48 weeks. To ensure comparability of the “efavirenz as a component of first-line ART” and “delayed efavirenz” scenarios, we assumed that the CD4 gains in the first and third regimens in the “delayed efavirenz” scenario matched the CD4 gains in the first and second regimens of the “efavirenz as a component of first-line ART” scenario. In addition, we matched the CD4 gain due to the first regimen of the nevirapine strategy (160 cells/μl at 48 weeks) with the CD4 gain of the “efavirenz as a component of first-line ART” (190 cells/μl at 48 weeks).
LPV/r arm, all patients; published CD4 benefit: 273 cells/μl at 96 weeks
Published CD4 benefit: 160 cells/μl at 48 weeks.
Opportunistic infection incidence increased with decreasing CD4 count (Table 1). For CD4 counts >200 cells/μl, we used the upper bound of the 95% confidence interval for AIDS mortality risks provided by the WIHS because these estimates produced a better match between model-estimated life expectancy and observed long-term patient survival.
Antiretroviral treatment efficacy
Antiretroviral therapy is initiated according to current guidelines at a CD4 count of <350 cells/μl and an HIV RNA of >100,000 copies/ml.10 Table 1 provides treatment efficacy data for two possible regimen sequences – one assuming use of efavirenz as a component of first-line ART, and the other assuming use of an alternative boosted protease inhibitor-based initial ART regimen that delays efavirenz use. Treatment efficacy data for a first-line regimen in which nevirapine replaces efavirenz are also included.
To ensure comparability of regimen sequences given the heterogeneity of published ART efficacy reports, we assumed that the CD4 gains in the first and third regimens in the delayed efavirenz use scenario matched the CD4 gains in the first and second regimens of the efavirenz as a component of first-line ART scenario. In addition, we matched the CD4 gain due to the first regimen of the nevirapine strategy (160 cells/μl at 48 weeks) with the CD4 gain of the efavirenz as a component of first-line ART scenario (190 cells/μl at 48 weeks). These assumptions were examined in sensitivity analysis.
Using the simulation model, we assessed the impact of parameter variations on model-estimated survival using sensitivity analyses. Specifically, we conducted one-way sensitivity analyses on AIDS mortality, virologic suppression and CD4 gains due to first-line ART, the CD4 count threshold for ART initiation, and the discount rate. We varied AIDS mortality between the lower and upper limits of 95% confidence intervals and the discount rate from 0% (basecase) to 5%. For first-line ART (with and without efavirenz), we varied CD4 gains by 50%. We also decreased the percentage of patients with HIV RNA <400 copies/ml by 20% and increased this percentage by 20%, up to an absolute maximum of 95%. We conducted an additional analysis in which a nevirapine-based ART regimen was used in place of the recommended efavirenz-based regimen as first-line treatment. To do so, we accounted for the warning regarding hepatoxicity and the CD4 restrictions in women by initiating the regimen at CD4 counts <250 cells/μl.
Estimating the Risk of Teratogenic Events
For women eligible to receive antiretroviral therapy, we constructed a decision analytic model using TreeAge Pro decision modeling software (TreeAge Pro 2009; Williamstown, MA), incorporating literature-based rates of pregnancy,38 live births,38 and teratogenic events39, 40 for HIV-infected women to calculate the risk of teratogenic events per 1,000 women. The decision analytic model simulates pregnancy risk for HIV-infected women, as well as live birth rates conditional on pregnancy and teratogenic event risk conditional on live birth. Simulations are conducted for women receiving an efavirenz-based antiretroviral therapy regimen and women receiving a non-efavirenz-based regimen. The primary outcome of the model is teratogenic events per 100,000 HIV-infected women.
For the base case decision model analysis, we used pregnancy and live birth rates reported by the WIHS (Table 2).38 The Antiretroviral Pregnancy Registry provided data on rates of teratogenic events in women receiving efavirenz during pregnancy (Table 2).39 This is a voluntary, prospective registry which enrolls approximately 1,300 pregnant women in the United States exposed to antiretroviral drugs each year, representing approximately 15% of the 8,650-8,900 HIV-positive women41 who give birth to live infants annually in the United States. As of January 31, 2009, the Registry had enrolled 579 pregnant women exposed to efavirenz during the first trimester, resulting in 477 live births. Fourteen of these 477 live births (2.9%, 95% confidence interval: 1.6-4.9%) experienced a teratogenic event.39 For women not receiving efavirenz during pregnancy, the Metropolitan Atlanta Congenital Defects Program (MACDP) provided a population-based estimate of the rate of teratogenic events (2.72%, 95% confidence interval: 2.68-2.76%).39, 40, 42 Since the rate of teratogenic events with efavirenz reported by the Antiretroviral Pregnancy Registry is not statistically different from the population-based rate, we conducted a sensitivity analysis using the upper 95% confidence limit (4.9% of the rate) in women who received efavirenz.
Table 2.
Epidemiologic Data Used in Decision Model
| Epidemiologic Data | Base Case | Range | Source |
|---|---|---|---|
| Pregnancies (per 100 person-years) in HIV-infected women |
7.40 | 1.40-18.10* | 38, 43 |
| Pregnancy outcomes among HIV-infected women | |||
| Live births per pregnancy | 36.00% | 27.00-45.00% | 38 |
| Probability of teratogenic events among HIV-infected women | |||
| Prevalence of teratogenic events, no efavirenz exposure† |
2.72% | 95% CI: 2.68-2.76% | 39, 42 |
| Prevalence of teratogenic events with efavirenz exposure |
2.90% | 95% CI: 1.60-4.90% | 39 |
The range for this sensitivity analysis was derived using age-stratified pregnancy rates.43
The confidence intervals reported around the general population rate of teratogenic events from the Metropolitan Atlanta Congenital Defects Program (MACDP) used for the prevalence of teratogenic events with no efavirenz exposure were calculated by the Antiretroviral Pregnancy Registry.39 MACDP does not publish confidence intervals because population-based surveillance does not involve sampling.
In addition, since pregnancy rates for HIV-infected women vary substantially with age,43 disease state, and treatment status, we varied these rates widely in sensitivity analyses to ascertain the impact of fertility and child-bearing decision-making on the incidence of teratogenic events. Specifically, we conducted a sensitivity analysis using age-group-specific pregnancy rates for women aged 15-24 years, 25-34 years, and 35-44 years. For women aged 15-24 years, we used a pregnancy rate of 18.1 pregnancies per 100 person-years, while for women aged 25-34 and 35-44 years, we used pregnancy rates of 6.5 and 1.4 pregnancies per 100 person-years, respectively.43
To project a possible range for the risk of teratogenic events, we used one-way and two-way sensitivity analyses to vary all uncertain parameters. The plausible range for each parameter was based on 95% confidence intervals when available, published data, or expert opinion.
RESULTS
Simulation model survival estimates
In the base case simulation model analysis, mean projected life expectancy for women receiving an efavirenz-based first-line ART regimen starting at CD4 <350 cells/μl regardless of childbearing potential was 28.91 life years (Table 3). In comparison, mean life expectancy for women who delayed efavirenz use and were treated with an alternative initial ART regimen which did not contain efavirenz was 28.02 years. The life expectancy gain attributable to using an efavirenz-based initial antiretroviral regimen was 0.89 years. For women receiving an efavirenz-based initial regimen, mean total exposure time to efavirenz was 4.07 years per woman. For women delaying efavirenz use and receiving alternate first-line therapy, mean exposure time to efavirenz was 3.37 years per woman.
Table 3.
Survival Estimates
| Estimated Survival (years) |
Change in Survival from Same Strategy in the Base Case (years) |
Incremental Gain in Life Expectancy (years) |
|
|---|---|---|---|
| Base Case (Start ART at CD4<350 cells/μl) | |||
| No ART | 11.45 | --- | --- |
| PI-based first-line ART | 28.02 | --- | 16.57 |
| EFV as first-line ART | 28.91 | --- | 0.89 |
|
| |||
| Sensitivity Analyses | |||
|
| |||
| Viral Suppression Rate of First-line Antiretroviral Therapy | |||
|
20% Increase in Viral Suppression Rate for First-line Antiretroviral Therapy
to a Maximum of 95% Suppressed | |||
| No ART | 11.45 | 0.00 | --- |
| PI-based first-line ART | 29.07 | 1.14 | 17.62 |
| EFV as first-line ART | 29.51 | 0.60 | 0.44 |
| 20% Decrease in Viral Suppression Rate for First-line Antiretroviral Therapy | |||
| No ART | 11.45 | 0.00 | --- |
| PI-based first-line ART | 26.97 | −1.06 | 15.52 |
| EFV as first-line ART | 27.75 | −1.16 | 0.78 |
| CD4 Benefit of First-line Antiretroviral Therapy | |||
| 50% Increase in CD4 Benefits for First-line Antiretroviral Therapy | |||
| No ART | 11.45 | 0.00 | --- |
| PI-based first-line ART | 28.99 | 0.97 | 17.54 |
| EFV as first-line ART | 29.88 | 0.97 | 0.89 |
| 50% Decrease in CD4 Benefits for First-line Antiretroviral Therapy | |||
| No ART | 11.45 | 0.00 | --- |
| PI-based first-line ART | 25.49 | −2.53 | 14.04 |
| EFV as first-line ART | 26.16 | −2.75 | 0.67 |
| CD4 Benefits Increased to 203 cells/μl * | |||
| No ART | 11.45 | 0.00 | --- |
| PI-based first-line ART | 28.74 | 0.72 | 17.29 |
| EFV as first-line ART | 28.91 | 0.00 | 0.17 |
|
| |||
| Nevirapine-based First-line ART (Start ART at CD4<250 cells/μl) | |||
| No ART | 11.45 | 0.00 | --- |
| NVP as first-line ART | 25.49 | --- | 14.04 |
| PI-based first-line ART | 26.25 | −1.77 | 0.76 |
| EFV as first-line ART | 27.08 | −1.83 | 0.83 |
| Monthly Probability of Chronic AIDS Mortality | |||
| 50% Increase in Monthly Probability of Chronic AIDS Mortality | |||
| No ART | 10.25 | −1.20 | --- |
| PI-based first-line ART | 24.83 | −3.19 | 14.58 |
| EFV as first-line ART | 25.60 | −3.31 | 0.78 |
| 50% Decrease in Monthly Probability of Chronic AIDS Mortality | |||
| No ART | 13.78 | 2.33 | --- |
| PI-based first-line ART | 32.83 | 4.81 | 19.06 |
| EFV as first-line ART | 33.79 | 4.88 | 0.96 |
| Chronic AIDS Mortality, as reported by WIHS † | |||
| No ART | 9.31 | −2.14 | --- |
| PI-based first-line ART | 20.15 | −7.87 | 10.83 |
| EFV as first-line ART | 20.65 | −8.26 | 0.51 |
| Starting ART at CD4<500 cells/μl | |||
| No ART | 11.45 | --- | --- |
| PI-based first-line ART | 29.53 | 1.51 | 18.08 |
| EFV as first-line ART | 30.45 | 1.54 | 0.92 |
| Discount Rate (base case: 0%) | |||
| Discount Rate: 3% | |||
| No ART | 9.01 | −2.44 | --- |
| PI-based first-line ART | 17.51 | −10.51 | 8.49 |
| EFV as first-line ART | 17.86 | −11.05 | 0.35 |
| Discount Rate: 5% | |||
| No ART | 7.86 | −3.59 | --- |
| PI-based first-line ART | 13.73 | −14.29 | 5.86 |
| EFV as first-line ART | 13.93 | −14.98 | 0.21 |
Abbreviations: ART: antiretroviral therapy; EFV: efavirenz; PI: protease inhibitor
The CD4 gain of the first regimen of the “efavirenz delayed” scenario was originally reported as 203 cells/μl at 48 weeks. The CD4 gain of the third regimen of the “efavirenz delayed” scenario was originally reported as 273 cells/μl at 96 weeks.
Chronic AIDS mortality as a % monthly risk by CD4 stratum as reported by the WIHS: 0-50 cells/μl: 1.65; 51-100 cells/μl: 0.42; 101-200 cells/μl: 0.42; 201-300 cells/μl: 0.08; 301-500 cells/μl: 0.08; and >500 cells/μl: 0.83 without a history of opportunistic infections and 0-50 cells/μl: 6.14; 51-100 cells/μl: 3.44; 101-200 cells/μl: 2.39; 201-300 cells/μl: 1.57; 301-500 cells/μl: 1.00; and >500 cells/μl: 066 for women with a history of opportunistic infections. See methods for further details.
In sensitivity analysis, we examined how the life expectancy gains attributed to initial and delayed use of efavirenz varied with changes in selected simulation model input parameters in one-way sensitivity analyses (Table 3). Results were most sensitive to changes in HIV RNA suppression and CD4 count gains due to ART, mortality due to AIDS, and the discount rate. The incremental life expectancy gain with efavirenz-based first-line ART ranged from 0.44 to 0.78 years of life as viral suppression rates of the first-line regimens were increased by 20% to a maximum of 95% and decreased by 20% (Table 3).
When CD4 gains for first-line ART were increased and decreased by 50%, incremental gains in life expectancy due to first-line efavirenz ranged from 0.89 to 0.67 years. For women delaying efavirenz use, estimated life expectancy increased from 28.02 to 28.74 years when the CD4 gains for the first and third regimens in the sequence were increased from 190 cells/μl at 48 weeks to 203 cells/μl at 48 weeks for the first regimen and 86 cells/μl at 16 weeks to 273 cells/μl at 96 weeks for the second regimen, as reported in the literature. This increase in survival for women delaying efavirenz narrowed incremental survival gains due to first-line efavirenz use to 0.17 years (base case: 0.89 years).
When an initial nevirapine-based regimen substituted for the recommended efavirenz-based therapy and ART was initiated at CD4<250 cells/μl, the mean life expectancy for women receiving the nevirapine-based therapy was 25.49 years. For an efavirenz-based first-line ART regimen starting at CD4<250 cells/μl, estimated survival was 27.08 years. Time on initial treatment using a nevirapine-based regimen was 3.02 years compared to 4.00 years with first-line efavirenz.
The incremental gain in life expectancy from efavirenz-based first-line ART ranged from 0.78 to 0.96 years when the monthly probability of chronic AIDS mortality was increased or decreased by 50%. Use of mean chronic AIDS mortality risks for CD4 cells counts >200 cells/μl, rather than the upper bound of the 95% confidence interval used in the base case analysis, decreased the incremental gain in life expectancy due to first-line efavirenz use to 0.51 years.
Mean projected life expectancy for women receiving an efavirenz-based first-line ART regimen starting at CD4 <500 cells/μl was 30.45 life years, while mean life expectancy for women who delayed efavirenz use and were treated with an alternative initial ART regimen which did not contain efavirenz was 29.53 life years. The life expectancy gain attributable to using an efavirenz-based initial antiretroviral regimen was 0.92 years.
Increasing the discount rate from 0% (base case) to 5% lowered incremental life expectancy gains due to use of an efavirenz-based first-line ART regimen from 0.89 to 0.21 years, a difference of 0.68 years.
Decision Model Calculations of Excess Teratogenic Events
For women without efavirenz exposure during pregnancy, the rate of teratogenic events was 72.46 events per 100,000 women (Table 4). For women exposed to efavirenz during pregnancy, the rate was 77.26 events per 100,000 women.
Table 4.
Estimated Teratogenic Events per 1,000 HIV-infected Women
| Teratogenic Events per 100,000 HIV-infected Women |
Excess Events per 100,000 HIV-infected Women |
|
|---|---|---|
| Base Case | ||
|
| ||
| Age-adjusted Pregnancy Rate: 7.4 pregnancies per 100 person-years | ||
| No efavirenz exposure | 72.46 | |
| With efavirenz exposure | 77.26 | 4.80 |
|
| ||
| Sensitivity Analysis Results | ||
|
| ||
| Age-specific Pregnancy Rates | ||
| Ages 15-24 years: 18.1 pregnancies per 100 person-years | ||
| No efavirenz exposure | 177.24 | |
| With efavirenz exposure | 188.96 | 11.73 |
| Ages 25-34 years: 6.5 pregnancies per 100 person-years | ||
| No efavirenz exposure | 63.65 | |
| With efavirenz exposure | 67.86 | 4.21 |
| Ages 35-44 years: 1.4 pregnancies per 100 person-years | ||
| No efavirenz exposure | 13.71 | |
| With efavirenz exposure | 14.62 | 0.91 |
| Live Birth Rate (base case: 36%) | ||
|
| ||
| Live birth rate: 27% | ||
| No efavirenz exposure | 54.35 | |
| With efavirenz exposure | 57.94 | 3.60 |
| Live birth rate: 45% | ||
| No efavirenz exposure | 90.58 | |
| With efavirenz exposure | 96.57 | 5.99 |
| Sensitivity Analysis on Rates of Teratogenic Events Among HIV-Infected Women | ||
| Prevalence of Teratogenic Events with Efavirenz (base case: 2.9%) | ||
| Lower Bound of the 95% Confidence Interval for the Mean (1.6%) | ||
| No efavirenz exposure | 72.46 | |
| With efavirenz exposure | 42.62 | −29.84 |
| Upper Bound of the 95% Confidence Interval for the Mean (4.9%) | ||
| No efavirenz exposure | 72.46 | |
| With efavirenz exposure | 130.54 | 58.08 |
|
Prevalence of Teratogenic Events without Efavirenz (base case: 2.72%)
| ||
| Lower Bound of the 95% Confidence Interval for the Mean (2.68%) | ||
| No efavirenz exposure | 71.40 | |
| With efavirenz exposure | 77.26 | 5.86 |
| Upper Bound of the 95% Confidence Interval for the Mean (2.76%) | ||
| No efavirenz exposure | 73.53 | |
| With efavirenz exposure | 77.26 | 3.73 |
We conducted a sensitivity analysis using age-group-specific pregnancy rates for women aged 15-24 years, 25-34 years, and 35-44 years. Using a pregnancy rate of 18.1 pregnancies per 100 person-years for women aged 15-24 years, the number of teratogenic events with use of efavirenz was 188.96 events per 100,000 women (11.73 excess events per 100,000 women). In contrast, using a pregnancy rate of 1.4 pregnancies per 100 person-years for women aged 35-44 years, the risk of excess teratogenic events decreased to 0.91 events per 100,000 women.
Results of other one-way sensitivity analyses on the rate components of the decision model are summarized in Table 4. When the live birth rate was varied from 27%-45% (base case rate: 36%), the excess risk of teratogenic events attributable to efavirenz use ranged from 3.60 to 5.99 events per 100,000 women. When the rate of teratogenic events with efavirenz was varied from 1.60% to 4.90%, the excess teratogenicity risk ranged from −29.84 to 58.08 events per 100,000 women. Here, a negative risk of excess teratogenic events suggests that efavirenz use confers no excess teratogenicity risk beyond the background risk.
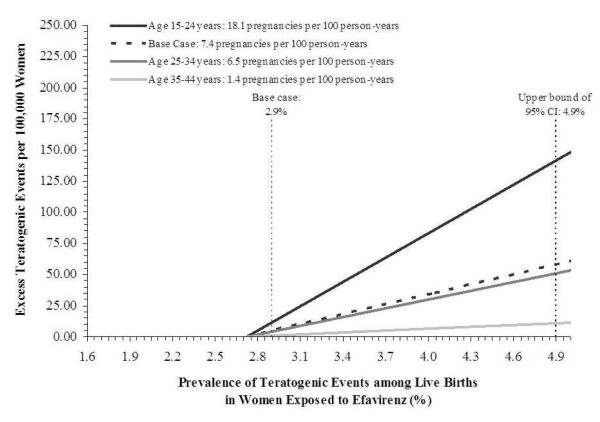
Figure 1 shows the results of a two-way sensitivity analysis on the prevalence of teratogenic events with efavirenz use and the pregnancy rate. For women aged 15-24 years with the highest pregnancy rate (18.1 pregnancies per 100 person-years) and the highest teratogenicity risk (4.9%; the upper bound of the 95% confidence interval for the mean rate of teratogenicity with efavirenz), the estimated number of excess teratogenic events was 142.05 events per 100,000 women. For women aged 35-44 years with the lowest pregnancy rate (1.4 pregnancies per 100 person-years) and lowest teratogenicity risk (1.6%; the lower bound of the 95% confidence interval for the mean rate of teratogenicity with efavirenz), the estimated number of excess teratogenic events was −5.65 events per 100,000 women (not shown in figure).
Figure. Two-way sensitivity analysis on age-specific pregnancy rates and the prevalence of teratogenic events with exposure to efavirenz.
Prevalence of teratogenic events among live births to women exposed to efavirenz during pregnancy is on the horizontal axis. These values range from the lower to the upper bound of the 95% confidence interval of the mean prevalence of teratogenic events with efavirenz exposure reported by the Antiretroviral Pregnancy Registry.39 The mean prevalence of teratogenic events for women exposed to efavirenz (2.9%; used for base case analysis) and the upper bound of the 95% confidence interval for this estimate (4.9%) are indicated by the dashed vertical lines. The vertical axis shows the excess number of teratogenic events per 100,000 women for efavirenz-exposed women compared to unexposed women as estimated by the decision model. The 4 diagonal lines represent different age-specific pregnancy rates. All 4 diagonal lines cross the horizontal axis at 2.72%, the point at which the prevalence of teratogenic events among women exposed and unexposed to efavirenz are equal.
DISCUSSION
Whether to use efavirenz in women of childbearing age remains controversial. In the context of existing options for antiretroviral therapy, limiting efavirenz use as a component of first-line therapy in HIV-infected women of childbearing age may lead to reductions in projected life expectancy from antiretroviral therapy, but may also prevent teratogenic events. In this analysis, we found that projected survival for HIV-infected women receiving an efavirenz-based initial antiretroviral therapy regimen 0.89 years greater than for women delaying efavirenz use and using an alternate first-line regimen (28.91 vs. 28.02 years), but efavirenz exposure was associated with a small (4.80 per 100,000 women) increased risk of teratogenic events. These life expectancy gains are larger than those associated with the use of both PCP and MAC prophylaxis (2.6 months, or 0.22 years).14 The number of excess teratogenic events per 100,000 women ranged from 0.91 events in women aged 35-44 years to 11.73 events in women aged 15-24 years. The higher rate of excess teratogenic events in younger women is attributable to their increased rate of pregnancy (18.1 versus 1.4 pregnancies per 100 person-years).
Sensitivity analyses demonstrated that estimates of life expectancy and risk of excess teratogenic events are influenced by several important parameters. In the estimate of the risk of excess teratogenic events, the pregnancy rate and the teratogenicity risk with efavirenz exposure were the most influential parameters. Not surprisingly, the risk of excess teratogenic events attributable to efavirenz use was greater for women who are more likely to become pregnant. Data on pregnancy rates and outcomes in the modern ART era are limited. Due to the paucity of these data, we used pregnancy rates and outcomes reported in both the modern ART and pre-ART eras. Because more potent regimens have become available since these data were reported, we varied the rates widely in sensitivity analysis to allow for changes in fertility and child-bearing decisions made by HIV-infected women.
In sensitivity analysis, the greatest impact on life expectancy occurred when the discount rate was increased from 0% (base case) to 5%. Changing the discount rate changes the relative attractiveness of treatment strategies that accrue benefits along different timelines. This is a way of giving more weight to events which occur immediately compared to those in the distant future. Changes in first-line ART viral suppression rates and CD4 benefits yielded less dramatic effects on life expectancy. However, sensitivity analysis does demonstrate variation in the efavirenz-related survival benefit.
This analysis has several limitations. First, since the computer simulation model excludes pregnancy status and specific changes in immunity associated with pregnancy, life expectancy estimates are based on natural history and treatment data from non-pregnant HIV-infected women. While pregnancy rates in the base case are initially drawn from WIHS data reported in 2004, we recognize these may not be fully representative of rates seen in the more modern ART era.38 We therefore varied such rates widely in sensitivity analyses using additional ART-era data.43 In addition, the model does not allow simulated patients to switch ART regimens based on pregnancy. As such, this analysis focuses on risk of teratogenic events for women who have not proactively switched antiretroviral regimens in anticipation of becoming pregnant. Although the analysis is specific to women, some data used in the computer simulation model are derived from clinical trials that also include men. However, consistent with literature reporting comparable virologic and immunologic responses to antiretroviral therapy between men and women,44 it is likely that women will benefit equally from these regimens. Third, the evidence for a reduced life expectancy in women treated with non-efavirenz-based regimens comes primarily from cross-trial comparisons. These results should be interpreted with caution, as patients recruited across trials may differ in sociodemographic characteristics. The trials themselves may also vary in study design, which could ultimately result in differences in reported outcomes. As new ART regimens become approved for first-line use, the relative attractiveness of efavirenz-based first-line ART may decline, as evidenced by recently reported results of a study showing equivalent virologic suppression and CD4 gains in patients randomized to boosted atazanavir compared to efavirenz.45-47 Finally, we assume no effect of HIV status or treatment with ART agents other than efavirenz on rates of teratogenicity (i.e., we assume that HIV status itself has no teratogenic effect, and we assume that efavirenz is the only agent which has a teratogenic effect beyond that of the US population risk).
By assessing the tradeoff between gains in maternal life expectancy with the use of efavirenz and the risk of teratogenic events in children born to mothers receiving efavirenz during pregnancy, this analysis does not position the health of the mother and the child in equal terms (i.e., it does not consider survival time for both mothers and children). It does, however, enumerate that the life expectancy benefits achievable for thousands of women may result in putting a very small number of unborn children at risk. These benefits, and risks, discussed by HIV-infected women and clinicians considering options for antiretroviral therapy may well be articulated as a tradeoff between maternal survival and teratogenic events in children.
While considerable discussion has been dedicated to the use of efavirenz in women of childbearing age,48 it is important to note the potential teratogenicity risks of other drugs. For efavirenz (and several other antiretroviral medications), sufficient numbers of first trimester exposures have been monitored by the Antiretroviral Pregnancy Registry to detect at least a two-fold increase in risk of overall birth defects compared with the overall population risk, but no such increase has been detected.39 However, the rates of efavirenz teratogenicity reported by the US-based Antiretroviral Pregnancy Registry are consistent with those reported internationally. For example, a recent publication by Bera et al. reports experience with 818 HIV-infected pregnant women at a regional South African hospital exposed to efavirenz during pregnancy.49 In the 807 live births, they found a teratogenicity rate of 3.3% (95% CI: 1.2-7.0%) for first trimester exposures to efavirenz and 2.6% (95% CI: 1.5-4.2%) for second and third trimester exposures. These rates are similar to the baseline rate used in this analysis (2.72%).
In our analysis, the estimated range of the rate of teratogenic events with efavirenz used in sensitivity analysis (1.6%-4.9%) extends above and below the U.S. background rate of 2.72%. As such, estimates of excess teratogenic events compared to background number of events includes both negative and positive values (range: −72.98 to 142.05 events per 100,000 women), depending on the rates of pregnancy and teratogenicity of efavirenz. This suggests that use of efavirenz may have less of an impact on teratogenicity compared to background rates than this analysis predicts. More data are needed to better estimate the true risk of teratogenic events in pregnant women receiving efavirenz as well as other antiretroviral medications.
The benefits and risks -- both known and unknown -- of antiretroviral therapy should be discussed with HIV-infected women of childbearing age.48 These discussions should address not only the potential survival advantage for the infected woman and the potential for reduction of mother-to-child transmission, but also the possible risks with respect to toxicity, pregnancy outcomes, and the health of the fetus or infant. Clinical decisions about using efavirenz-based treatment present a potential trade-off between life expectancy gains in women and risk of teratogenicity; these results should inform discussions between women and their health care providers.
ACKNOWLEDGEMENTS
This research was supported in part by the National Institute of Allergy and Infectious Diseases (K24AI062476, R37AI42006).
The Cost-Effectiveness of Preventing AIDS Complications (CEPAC) investigators include: Massachusetts General Hospital, Boston, MA: John J. Chiosi, Sarah Chung, Andrea L. Ciaranello, Kenneth A. Freedberg, Heather E. Hsu, Elena Losina, Zhigang Lu, Caroline Sloan, Stacie Waldman, Rochelle P. Walensky, Bingxia Wang, Angela Wong, Hong Zhang; Brigham and Women’s Hospital, Boston, MA: Paul E. Sax; Harvard School of Public Health, Boston, MA: Sue J. Goldie, April D. Kimmel, Kara L. Cotich, Marc Lipsitch, Chara E. Rydzak, George R. Seage III, Milton C. Weinstein; Yale School of Medicine, New Haven, CT: A. David Paltiel; Weill Cornell Medical College, New York City, NY: Bruce R. Schackman.
Data in this manuscript were collected by the Women’s Interagency HIV Study with centers: New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Analysis Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases with supplemental funding from the National Cancer Institute, and the National Institute on Drug Abuse (U01-AI-35004, U01-AI-31834, U01-AI-34994, U01-AI-34989, U01-AI-34993, and U01-AI-42590). Funding is also provided by the National Institute of Child Health and Human Development (U01-HD-32632) and the National Center for Research Resources (M01-RR-00071, M01-RR-00079, M01-RR-00083).
REFERENCES
- 1.“Dear Health Care Provider” letter. Re: Important Change in SUSTIVA® (efavirenz) Package Insert — Change from Pregnancy Category C to D [T4-D0005 02/05] Bristol Myers Squibb; [Accessed April 13, 2006]. 2005. at http://www.fda.gov/medwatch/SAFETY/2005/Sustiva_DHCPletter-061005.pdf. [Google Scholar]
- 2. [Accessed February 10, 2008];Supplement: Safety and toxicity of individual antiretroviral agents in pregnancy. Public Health Service Task Force Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States - November 2, 2007. 2007 at http://www.aidsinfo.nih.gov/contentfiles/PerinatalGLSafetyTox_Sup.pdf.
- 3.De Santis M, Carducci B, De Santis L, Cavaliere AF, Straface G. Periconceptional exposure to efavirenz and neural tube defects. Arch Intern Med. 2002;162:355. doi: 10.1001/archinte.162.3.355. [DOI] [PubMed] [Google Scholar]
- 4.Fundaro C, Genovese O, Rendeli C, Tamburrini E, Salvaggio E. Myelomeningocele in a child with intrauterine exposure to efavirenz. AIDS. 2002;16:299–300. doi: 10.1097/00002030-200201250-00025. [DOI] [PubMed] [Google Scholar]
- 5.Saitoh A, Hull AD, Franklin P, Spector SA. Myelomeningocele in an infant with intrauterine exposure to efavirenz. J Perinatol. 2005;25:555–6. doi: 10.1038/sj.jp.7211343. [DOI] [PubMed] [Google Scholar]
- 6.Robbins GK, De Gruttola V, Shafer RW, et al. Comparison of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349:2293–303. doi: 10.1056/NEJMoa030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staszewski S, Morales-Ramirez J, Tashima KT, et al. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. Study 006 Team. N Engl J Med. 1999;341:1865–73. doi: 10.1056/NEJM199912163412501. [DOI] [PubMed] [Google Scholar]
- 8.Madero J Sierra, Villasis A, Mendez P, et al. A prospective, randomized, open label trial of efavirenz versus lopinavir/ritonavir based HAART among antiretroviral therapy naïve, HIV infected individuals presenting for care with CD4 cell counts <200/mm3 [Abstract TUAB0104]. 17th International AIDS Conference; Mexico City, Mexico. 2008. [Google Scholar]
- 9.Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095–106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents – A Working Group of the Office of AIDS Research Advisory Council (OARAC); [Accessed February 10, 2008]. 2008. at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 11.Freedberg KA, Scharfstein JA, Seage GR, 3rd, et al. The cost-effectiveness of preventing AIDS-related opportunistic infections. JAMA. 1998;279:130–6. doi: 10.1001/jama.279.2.130. [DOI] [PubMed] [Google Scholar]
- 12.Freedberg KA, Losina E, Weinstein MC, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001;344:824–31. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- 13.Paltiel AD, Walensky RP, Schackman BR, et al. Expanded HIV screening in the United States: effect on clinical outcomes, HIV transmission, and costs. Ann Intern Med. 2006;145:797–806. doi: 10.7326/0003-4819-145-11-200612050-00004. [DOI] [PubMed] [Google Scholar]
- 14.Walensky RP, Paltiel AD, Losina E, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006;194:11–9. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]
- 15.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–25. [PubMed] [Google Scholar]
- 16.Bozzette SA, Finkelstein DM, Spector SA, et al. A randomized trial of three antipneumocystis agents in patients with advanced human immunodeficiency virus infection. NIAID AIDS Clinical Trials Group. N Engl J Med. 1995;332:693–9. doi: 10.1056/NEJM199503163321101. [DOI] [PubMed] [Google Scholar]
- 17.Ioannidis JP, Cappelleri JC, Skolnik PR, Lau J, Sacks HS. A meta-analysis of the relative efficacy and toxicity of Pneumocystis carinii prophylactic regimens. Arch Intern Med. 1996;156:177–88. [PubMed] [Google Scholar]
- 18.Leport C, Chene G, Morlat P, et al. Pyrimethamine for primary prophylaxis of toxoplasmic encephalitis in patients with human immunodeficiency virus infection: a double-blind, randomized trial. ANRS 005-ACTG 154 Group Members. Agence Nationale de Recherche sur le SIDA. AIDS Clinical Trial Group. J Infect Dis. 1996;173:91–7. doi: 10.1093/infdis/173.1.91. [DOI] [PubMed] [Google Scholar]
- 19.Lidman C, Berglund O, Tynell E, Lindback S, Elvin K. Aerosolized pentamidine as primary prophylaxis for Pneumocystis carinii pneumonia: efficacy, mortality and morbidity. AIDS. 1994;8:935–9. doi: 10.1097/00002030-199407000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Payen MC, De Wit S, Sommereijns B, Clumeck N. A controlled trial of dapsone versus pyrimethamine-sulfadoxine for primary prophylaxis of Pneumocystis carinii pneumonia and toxoplasmosis in patients with AIDS. Biomed Pharmacother. 1997;51:439–45. doi: 10.1016/s0753-3322(97)82322-0. [DOI] [PubMed] [Google Scholar]
- 21.Girard PM, Landman R, Gaudebout C, et al. Dapsone-pyrimethamine compared with aerosolized pentamidine as primary prophylaxis against Pneumocystis carinii pneumonia and toxoplasmosis in HIV infection. The PRIO Study Group. N Engl J Med. 1993;328:1514–20. doi: 10.1056/NEJM199305273282102. [DOI] [PubMed] [Google Scholar]
- 22.Nightingale SD, Cameron DW, Gordin FM, et al. Two controlled trials of rifabutin prophylaxis against Mycobacterium avium complex infection in AIDS. N Engl J Med. 1993;329:828–33. doi: 10.1056/NEJM199309163291202. [DOI] [PubMed] [Google Scholar]
- 23.Oldfield EC, 3rd, Fessel WJ, Dunne MW, et al. Once weekly azithromycin therapy for prevention of Mycobacterium avium complex infection in patients with AIDS: a randomized, double-blind, placebo-controlled multicenter trial. Clin Infect Dis. 1998;26:611–9. doi: 10.1086/514566. [DOI] [PubMed] [Google Scholar]
- 24.Pierce M, Crampton S, Henry D, et al. A randomized trial of clarithromycin as prophylaxis against disseminated Mycobacterium avium complex infection in patients with advanced acquired immunodeficiency syndrome. N Engl J Med. 1996;335:384–91. doi: 10.1056/NEJM199608083350603. [DOI] [PubMed] [Google Scholar]
- 25.Lalezari J, Goodrich J, DeJesus E, et al. Efficacy and safety of maraviroc plus optimized background therapy in viremic ART-experienced patients infected with CCR5-tropic HIV-1: 24-week results of a phase 2b/3 study in the US and Canada [abstract 104bLB]. 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. 2007. [Google Scholar]
- 26.Malan N, Krantz E, David N, et al. Efficacy and safety of atazanavir-based therapy in antiretroviral naive HIV-1 infected subjects, both with and without ritonavir: 48-week results from AI424-089 [abstract 107LB]. 13th Conference on Retroviruses and Opportunistic Infections; Denver, CO. 2006. [Google Scholar]
- 27.Nelson M, Arasteh K, Clotet B, et al. Durable efficacy of enfuvirtide over 48 weeks in heavily treatment-experienced HIV-1-infected patients in the T-20 versus optimized background regimen only 1 and 2 clinical trials. J Acquir Immune Defic Syndr. 2005;40:404–12. doi: 10.1097/01.qai.0000185314.56556.c3. [DOI] [PubMed] [Google Scholar]
- 28.Gallant JE, DeJesus E, Arribas JR, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354:251–60. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 29.Johnson M, Grinsztejn B, Rodriguez C, et al. Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failures. AIDS. 2005;19:685–94. doi: 10.1097/01.aids.0000166091.39317.99. [DOI] [PubMed] [Google Scholar]
- 30.Grinsztejn B, Nguyen BY, Katlama C, et al. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet. 2007;369:1261–9. doi: 10.1016/S0140-6736(07)60597-2. [DOI] [PubMed] [Google Scholar]
- 31.Cole SR, Hernan MA, Robins JM, et al. Effect of highly active antiretroviral therapy on time to acquired immunodeficiency syndrome or death using marginal structural models. Am J Epidemiol. 2003;158:687–94. doi: 10.1093/aje/kwg206. [DOI] [PubMed] [Google Scholar]
- 32.Investigator Information: Women’s Interagency HIV Study. Department of Epidemiology, Johns Hopkins University Bloomberg School of Public Health; [Accessed February 11, 2008]. 2008. at http://statepiaps.jhsph.edu/wihs/index-invest-info.htm. [Google Scholar]
- 33.Farzadegan H, Hoover DR, Astemborski J, et al. Sex differences in HIV-1 viral load and progression to AIDS. Lancet. 1998;352:1510–4. doi: 10.1016/S0140-6736(98)02372-1. [DOI] [PubMed] [Google Scholar]
- 34.Anastos K, Kalish LA, Palacio H, et al. Prevalence of and risk factors for tuberculin positivity and skin test anergy in HIV-1-infected and uninfected at-risk women. Women’s Interagency HIV Study (WIHS) J Acquir Immune Defic Syndr. 1999;21:141–7. [PubMed] [Google Scholar]
- 35.Anastos K, Barron Y, Miotti P, et al. Risk of progression to AIDS and death in women infected with HIV-1 initiating highly active antiretroviral treatment at different stages of disease. Arch Intern Med. 2002;162:1973–80. doi: 10.1001/archinte.162.17.1973. [DOI] [PubMed] [Google Scholar]
- 36.Anastos K, Barron Y, Cohen MH, et al. The prognostic importance of changes in CD4+ cell count and HIV-1 RNA level in women after initiating highly active antiretroviral therapy. Ann Intern Med. 2004;140:256–64. doi: 10.7326/0003-4819-140-4-200402170-00007. [DOI] [PubMed] [Google Scholar]
- 37.Davis AT, Chakraborty H, Flowers L, Mosunjac MB. Cervical dysplasia in women infected with the human immunodeficiency virus (HIV): a correlation with HIV viral load and CD4+ count. Gynecol Oncol. 2001;80:350–4. doi: 10.1006/gyno.2000.6104. [DOI] [PubMed] [Google Scholar]
- 38.Massad LS, Springer G, Jacobson L, et al. Pregnancy rates and predictors of conception, miscarriage and abortion in US women with HIV. AIDS. 2004;18:281–6. doi: 10.1097/00002030-200401230-00018. [DOI] [PubMed] [Google Scholar]
- 39.Antiretroviral pregnancy registry international interim report for 1 January 1989 through 31 January 2009. Registry Coordinating Center; [Accessed July 22, 2009]. 2009. at http://www.apregistry.com/forms/interim_report.pdf. [Google Scholar]
- 40.Correa-Villasenor A, Cragan J, Kucik J, O’Leary L, Siffel C, Williams L. The Metropolitan Atlanta Congenital Defects Program: 35 years of birth defects surveillance at the Centers for Disease Control and Prevention. Birth Defects Res A Clin Mol Teratol. 2003;67:617–24. doi: 10.1002/bdra.10111. [DOI] [PubMed] [Google Scholar]
- 41.Whitmore SK, Zhang X, Taylor AW. Estimated number of births to HIV-positive women, United States, 2006 [abstract 924]. Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 2009. [Google Scholar]
- 42.Correa A, Cragan JD, Kucik ME, et al. Reporting birth defects surveillance data 1968-2003. Birth Defects Res A Clin Mol Teratol. 2007;79:65–186. doi: 10.1002/bdra.20350. [DOI] [PubMed] [Google Scholar]
- 43.Blair JM, Hanson DL, Jones JL, Dworkin MS. Trends in pregnancy rates among women with human immunodeficiency virus. Obstetrics & Gynecology. 2004;103:663–8. doi: 10.1097/01.AOG.0000117083.33239.b5. [DOI] [PubMed] [Google Scholar]
- 44.Moore AL, Kirk O, Johnson AM, et al. Virologic, immunologic, and clinical response to highly active antiretroviral therapy: the gender issue revisited. J Acquir Immune Defic Syndr. 2003;32:452–61. doi: 10.1097/00126334-200304010-00017. [DOI] [PubMed] [Google Scholar]
- 45.Lennox J, DeJesus E, Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009;374:764–6. doi: 10.1016/S0140-6736(09)60918-1. [DOI] [PubMed] [Google Scholar]
- 46.Ortiz R, Dejesus E, Khanlou H, et al. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS. 2008;22:1389–97. doi: 10.1097/QAD.0b013e32830285fb. [DOI] [PubMed] [Google Scholar]
- 47.Daar E, Tierney C, Fischl M, et al. ACTG 5202: final results of ABC/3TC or TDF/FTC with either EFV or ATV/r in treatment-naive HIV-infected patients [abstract 59LB]. 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA. 2010. [Google Scholar]
- 48.Perinatal HIV Guidelines Working Group. Public Health Service Task Force [Accessed July 15, 2009];Recommendations for use of antiretroviral drugs in pregnant HIV-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. 2009 at http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf.
- 49.Bera E, McCausland K, Nonkwelo R, Mgudlwa B, Chacko S, Majeke B. Birth defects following exposure to efavirenz-based antiretroviral therapy during pregnancy: a study at a regional South African hospital. AIDS. 2010;24:283–9. doi: 10.1097/QAD.0b013e328333af32. [DOI] [PubMed] [Google Scholar]
- 50.Pozniak AL, Gallant JE, DeJesus E, et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz versus fixed-dose zidovudine/lamivudine and efavirenz in antiretroviral-naive patients: virologic, immunologic, and morphologic changes--a 96-week analysis. J Acquir Immune Defic Syndr. 2006;43:535–40. doi: 10.1097/01.qai.0000245886.51262.67. [DOI] [PubMed] [Google Scholar]
- 51.Johnson M, Grinsztejn B, Rodriguez C, et al. 96-week comparison of once-daily atazanavir/ritonavir and twice-daily lopinavir/ritonavir in patients with multiple virologic failures. AIDS. 2006;20:711–8. doi: 10.1097/01.aids.0000216371.76689.63. [DOI] [PubMed] [Google Scholar]
- 52.Steigbigel R, Kumar P, Eron J, et al. Results of BENCHMRK-2, a Phase III study evaluating the efficacy and safety of MK-0518, a novel HIV-1 integrase inhibitor, in patients with triple-class resistant virus [abstract 105bLB]. 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. 2007. [Google Scholar]
- 53.Molina J, Andrade-Villanueva J, Echevarria J, et al. Efficacy and safety of once-daily atazanavir/ritonavir compared to twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine in ARV-naive HIV-1-infected subjects: The CASTLE study, 48-week results [abstract 37]. Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2008. [Google Scholar]
- 54.van Leth F, Phanuphak P, Ruxrungtham K, et al. Comparison of first-line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open-label trial, the 2NN Study. Lancet. 2004;363:1253–63. doi: 10.1016/S0140-6736(04)15997-7. [DOI] [PubMed] [Google Scholar]