Abstract
Astaxanthin (AST) is a powerful antioxidant that occurs naturally in a wide variety of living organisms. We have investigated the role of AST in preventing 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced apoptosis of the substantia nigra (SN) neurons in the mouse model of Parkinson’s disease (PD) and 1-methyl-4-phenylpyridinium (MPP+)-induced cytotoxicity of SH-SY5Y human neuroblastoma cells. In in vitro study, AST inhibits MPP+-induced production of intracellular reactive oxygen species (ROS) and cytotoxicity in SH-SY5Y human neuroblastoma cells. Preincubation of AST (50 μM) significantly attenuates MPP+-induced oxidative damage. Furthermore, AST is able to enhance the expression of Bcl-2 protein but reduce the expression of α-synuclein and Bax, and suppress the cleavage of caspase-3. Our results suggest that the protective effects of AST on MPP+-induced apoptosis may be due to its anti-oxidative properties and anti-apoptotic activity via induction of expression of superoxide dismutase (SOD) and catalase and regulating the expression of Bcl-2 and Bax. Pretreatment with AST (30mg /kg) markedly increases tyrosine hydroxylase (TH)-positive neurons and decreases the argyrophilic neurons compared with the MPTP model group. In summary, AST shows protection from MPP+/MPTP-induced apoptosis in the SH-SY5Y cells and PD model mouse SN neurons, and this effect may be attributable to upregulation of the expression of Bcl-2 protein, downregulation of the expression of Bax and α-synuclein, and inhibition of the activation of caspase-3. These data indicate that AST may provide a valuable therapeutic strategy for the treatment of progressive neurodegenerative disease such as Parkinson’s disease.
Keywords: Astaxanthin, Parkinson’s disease, MPTP, Apoptosis, Substantia nigra neuron
1. Introduction
Parkinson’s disease (PD) is a neurodegenerative disease characterized by the selective loss of dopaminergic neurons in the substantia nigra (SN). The causes of Parkinson’s disease have still been unclear, however, several studies suggest the involvement of mitochondrial dysfunction, the induction of glutamate mediated excitoxicity, and oxidative stress (Mattson, 1990). 1-methyl-4-phenylpyridinium (MPP+), the active metabolite of 1-methyl-4-phenyl-2,3,6-tetrahydropyridine (MPTP), has been shown to selectively and potently inhibit complex I of the mitochondrial electron transport chain (Singer and Ramsay., 1990), inducing a syndrome closely resembling PD models in vitro and in vivo which involves the degeneration of dopaminergic neurons located in the SN and leads to a decline of dopamine (DA) as well as its biosynthetic enzyme, tyrosine hydroxylase (TH). In addition, neuronal cytotoxicity drugs such as MPTP, MPP+, and rotenone induce a pathological hallmark of PD, α-synuclein expression and its aggregation in the dopaminergic neurons of the SN (Lee et al., 2002; Kakimura et al., 2001; Kalivendi et al., 2004). MPP+-induced neuronal cell death is thought to be mediated by the opening of mitochondrial permeability transition (MPT) pores and the collapse of the mitochondrial membrane potential (MMP) (Seaton et al., 1997). This leads to impairment of energy production and increased free radical generation, and eventually causes dopaminergic neuron death (Alcaraz-Zubeldia et al., 2001; Saporito et al., 2000). As reported recently, apoptotic death of DA neurons may be initiated by oxidative stress and neuroinflammation (Zhang et al., 2000; Marchetti and Abbracchio, 2005). Oxidative stress-induced apoptosis is associated with the release of cytochrome c, activation of caspases and cleavage of poly (ADP-ribose) polymerase (PARP) (Nicholson et al., 1995; Slater et al., 1995; O’Brien et al., 2000).
Astaxanthin (3,3′-dihydroxy-β, β′-carotene-4, 4′-dione, AST ; see the chemical structure in Figure 1 is a well-known non-provitamin A carotenoid found in the red pigment of shrimp, crabs, salmon, and asteroideans (Miki et al., 1986; Hussein et al., 2006). AST has been reported to possess anti-oxidant, anti-inflammatory and anti-tumor effects (Tanaka et al., 1995; Kurashige et al., 1990). Recently, AST has been documented to provide important metabolic functions in animals, including conversion to vitamin A (Bendich and Olson, 1989), enhancement of immune response (Jyonouchi et al., 1994), and protection against diseases such as cancer by scavenging of oxygen radicals (Jyonouchi et al., 2000). AST also shows strong activity as an inhibitor of oxygen radical-mediated lipid peroxidation (LPO) (Mortensen et al., 2001). It has been reported that AST exerts a neuroprotective effect on neuronal cell damage by crossing the brain-blood barrier (Liu and Osawa, 2009).
Fig. 1.
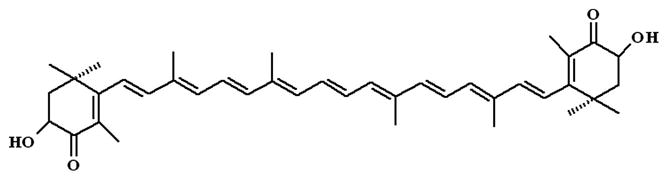
Chemical structure of AST.
Currently, there are no specific or effective therapeutic agents to restrict neuronal damage and neurological dysfunction without undesirable side-effects. Thus, there is a need to develop new protective agents that can prevent the progression of such neuronal apoptosis. Several preclinical and clinical studies reported that several oriental herbs from plants or nutrients from foods have protective activity against apoptosis and are potential therapeutic agents (Park et al., 2004). In the present study, MPTP and MPP+ are used to induce apoptosis in the mouse and human SH-SY5Y neuroblastoma cells, and the protective effect of AST, a food constituent, against MPTP or MPP+-induced damages is investigated to elucidate the mechanism by which AST prevents apoptosis.
In this study, we demonstrate that AST inhibits cell viability loss and ROS elevation caused by MPP+. We also demonstrate that the regulation of MPP+-induced apoptosis by AST involves alteration in the expression of the Bcl-2 family leading to mitochondrial damage, cytochrome c release, and activation of the caspase cascade.
2. Materials and Methods
2.1. Materials
Anti-PARP-1 antibody was obtained from Santa Cruz (Santa Cruz, CA, USA). Cytochrome c antibodies were from Oncogene Research Products (San Diego, CA. USA). Anti-actin antibody was from ICN (Costa Mesa, CA, USA). Anti-Bcl-2, anti-Bax, anti-TH and anti- α-synuclein antibodies were from Cell Signaling (Beverly, MA, USA). 3-(4,5-dimethyl-2-thiazolyl)-2,2,7-dichlorofluorescein diacetate (DCFH-DA), MPTP, and MPP+ were from Sigma (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM) was from Gibco BRL (Gaithersburg, MD, USA).
2.2. Cell culture
Human neuroblastoma SH-SY5Y cells were cultured in DMEM supplemented with 10% (v/v) heat-inactivated fetal calf serum and 100 units/ml penicillin/streptomycin. Cells were kept at 37°C in humidified 5% CO2 and 95% air. All experiments were carried out 24–48 h after cells were seeded. During MPP+ studies, the growth medium was supplemented with 1 mM MMP+, 30 μg/ml adiponectin, 15 mM sodium diethyldithiocarbamate trihydrate, and 13 mM 3-amino-1,2,4-triazole as antioxidant enzyme inhibitors.
2.3. Cell viability assay
SH-SY5Y cells were seeded on 96-well plates at a density of 0.5×105 cells/well. The cultures were grown for 24 h followed by addition of fresh medium containing MPP+. Cell viability was determined by MTT assay. After incubation for 12 h with the desired drug, 30 μl of MTT reagent (0.5 mg/ml MTT in phosphate-buffered saline containing 10 mM HEPES) was added to each well and incubated in a CO2 incubator for 2 h. The medium was aspirated from each well, and the culture plate was dried at 37°C for 1 h. The resulting formazan dye was extracted with 100 μl of 0.04 N HCl in isopropanol, and the absorbance was measured in a micro plate reader (Molecular Device, Sunnyvale, CA, USA) at 570 and 630 nm.
2.4. Measurement of caspase-3 activity
Fluorometric assay of caspase-3 activity was conducted as described [Zheng et al., 2004]. Briefly, after exposure to MPP+ with or without SA treatment, cells were lysed for 10 min in an ice bath, and centrifuged at 14,000 × g for 10 min at 4°C, then the supernatant was incubated with acetyl-Asp-Glu-Val-Asp-aldehyde-AFC, a pseudosubstrate used to measure caspase-3 activity, at 37°C for 1 h. Fluorescence intensity was measured using an F-4500 HITACHI fluorescence spectrophotometer (400 nm excitation and 505 nm emission).
2.5. Measurement of anti-oxidant enzyme activity
SOD activity was measured using assay kits purchased from Dojindo (Kumamoto, Japan). The catalase activity assay was performed as described previously (Beers and Sizer, 1952).
2.6. Measurement of intracellular ROS generation
Intracellular ROS generation was measured by flow cytometry following staining with CMH2DCFDA. Briefly, SH-SY5Y cells (1 × 105 cells per 60 mm plates) were plated, allowed to attach overnight and exposed to DMSO (control) or desired concentrations of AST for specified time periods. The cells were stained with 5 μM CMH2DCFDA for 30 min at 37°C, and the fluorescence was detected by a fluorescence microscope. Alternatively, the fluorescence intensity of dichlorofluorescein in cells was determined usingthe flow cytometer as described previously (Lee et al., 2009). SH-SY5Y cells were seeded in 96-well plates and incubated with increasing concentrations of MPP+ and/or AST for 24 h. Cells were incubated with 10 μM DCFH-DA at 37°C for 30 min and then washed twice with PBS. Intracellular H2O2 or low-molecular-weight peroxides oxidize DCFH-DA to the highly fluorescent compound dichlorofluorscein (DCF). The fluorescence intensity of DCF was measured in a microplate-reader at an excitation wavelength of 485 nm and an emission wavelength of 538 nm.
2.7. Indirect immunofluorescence microscopy
Immunocytochemistry of cells was performed as described (Soeda et al., 2008). The primary antibodies used was anti-cleaved caspase-3 (rabbit pAb, 1:200; Cell-Signaling, Beverly, MA, USA). Alexa Fluor 488 secondary antibody was used (1:1000; Molecular Probes, Eugene, OR, USA). Cells were counterstained with 4′,6-diamidino-2-phenylindole. The following hardware was used: Zeiss Axiovert 200 microscope (Carl Zeiss, Gottingen, Germany), Plan-Neofluar 20 and 40 objectives, AxioCam MrM CCD camera. Axiovision software was used for image acquisition (Carl Zeiss).
2.8. Measurement of mitochondrial membrane potential
The level of MMP was determined by using a cytofluorimetric, lipophilic cationic dye, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimi-dazolylcarbocyanine iodide (JC-1). Green fluorescence signals were measured in the FL1 channel on the Accuri C6 Flow Cytometer. Sample data (10,000 cells) were used to prepare histograms of the Cell Quest data analysis program (Becton Dickinson, Mountain View, CA, USA).
2.9. Flow cytometric analysis using Annexin V and PI
SH-SY5Y cells were centrifuged to remove the medium, washed with PBS and stained with Annexin V-FITC and PI in binding buffer (10 mM Hepes, 140 mM NaCl, 2.5 mM CaCl2). Ten thousand events were collected on each sample. Stained cells were analyzed using a FACScalibur (Becton Dickinson, Mountain View, CA, USA) in the FL1-H and FL2-H channels.
2.10. Animal model and MPTP-administration
Experiments were conducted on 12-week-old C57BL/6 mice, body weight 25–30 g, maintained, under standard animal care conditions, on a 12 h day night cycle and given food and water ad libitum. All studies were carried out in accordance with the protocol of the local animal care and use committee. Animals were divided into five groups (n=8). The treatment group received an i.p. injection of MPTP (10 and 30mg kg−1 day−1) at 24 h intervals for 28 consecutive days, while the sham group in the same paradigm was treated with an equal volume of saline. The pretreatment group was pretreated with AST (10 and 30 mg kg−1 day−1, i.p.) for 28 days and treated with AST 2 h before receiving MPTP (30mg kg−1 day−1) for 28 days.
2.11. Tissue preparation
All animals were killed by decapitation 24 h after the last injection. For each mouse, one of the two substantia nigra was dissected, immediately frozen on dry ice, and stored at -80°C for Western blotting assay. The other hemi-mesencephalon was placed in chilled 4% paraformaldehyde in phosphate buffer (PB, 0.1 mM, pH 7.4), fixed at 4°C for 24 h, and then cryoprotected in 20% glycerol at 4°C for immunohistochemistry assay.
2.12. Immunohistochemistry and quantification
The nigra was serially sectioned, and the section was incubated with 1% bovine serum albumin (BSA)/3% H2O2 in phosphate-buffered saline (PBS, pH 7.4) for 1 h at room temperature, and then incubated with the primary antibodies, which were rabbit anti-mouse polyclonal antibodies, TH (1:1000, Sigma, USA) overnight at 4°C. Sections were washed with PBS, then incubated in sequence with biotinylated anti-rabbit IgG and SABC-reagent (1:400, Vectastain ABC Kit, Vector, USA) for 1 h at room temperature, and finally rinsed with diaminobenzidine (DAB) to produce a brown-yellow precipitate in the plasma of the positive cell. Quantification of the effects in brain tissue sections were performed by counting the TH-positive cell number in SNpc at ×100 magnification and measuring the TH-positive fiber optical density in striatum (ST) at ×40 magnification using Stereo investigator software (MBF Bioscience Inc., Williston, VT, USA); data were presented as a percent of the control group values. The images were photographed under an Axiovert 200 microscope (Carl Zeiss, Inc., Göttingen, Germany).
2.13. Immunoblot analysis
Cells were lysed with 1 x Laemmli lysis buffer (2.4 M glycerol, 0.14 M Tris, pH 6.8, 0.21 M sodium dodecyl sulfate (SDS), 0.3 mM bromophenol blue) and boiled for 7 min. Protein content was measured with BCA Protein Assay Reagent (Pierce, Rockford, IL, USA). The samples were diluted with 1 x lysis buffer containing 1.28 M β-mercaptoethanol, and equal amounts of protein were loaded on 8-15% SDS-polyacrylamide gels. SDS-PAGE analysis was performed according to Laemmli (1970) using a Hoefer gel apparatus. Proteins were separated by SDS-PAGE and electrophoretically transferred to nitrocellulose membrane. The nitrocellulose membrane was blocked with 5% nonfat dry milk in PBS-Tween-20 (0.1%, v/v) for 1 h. The membrane was incubated with primary antibody (diluted according to the manufacturer’s instructions) at 4°C overnight. Horseradish peroxidase conjugated anti-rabbit or anti-mouse IgG was used as the secondary antibody. Immunoreactive protein was visualized by the chemiluminescence protocol (ECL, Amersham, Arlington Heights, IL, USA). To ensure equal protein loading, each membrane was stripped and reprobed with anti-actin antibody to normalize for differences in protein loading. Molecular sizes were determined by the relative mobilities of prestained molecular weight markers. Densitometric analysis was performed with a computer using a gel image analysis program.
2.14. Statistical analysis
Data are expressed as standard error of the mean (SEM). The TH-positive percent was calculated as: (TH-positive cells/total cells) × 100%. Bands of Western blotting were calculated by average densitometric analysis. The statistical significance of differences between groups was determined via one-way analysis of variance (ANOVA). Statistical significance was assumed at P<0.05.
3. Results
3.1. Effect of AST on MPP+-induced cytotoxicity
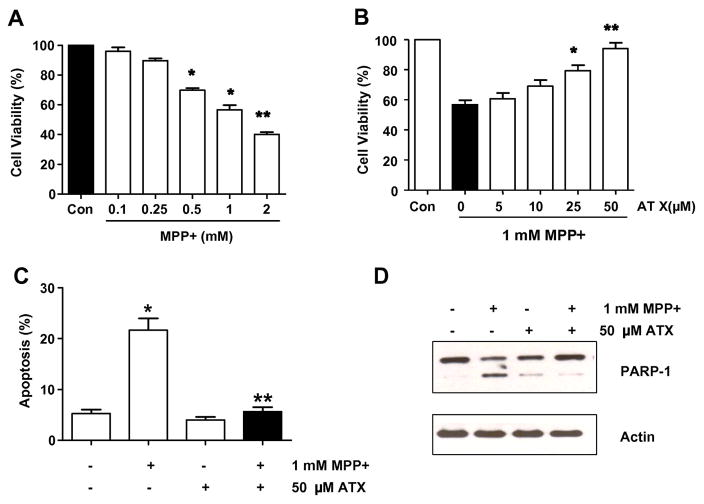
It has been well established that neurotoxic agents such as MPP+ can induce neuronal cell death. In order to investigate the influence of MPP+ on neuronal cell viability, we treated SH-SY5Y cells with MPP+, and examined the effects of MPP+ at various concentrations on cell viability at various concentrations. MPP+-induced cytotoxicity was dependent upon concentrations of the drug (Fig. 2A). Since a final concentration of 1 mM MPP+ and an incubation time of 24 h were previously identified as optimal times and concentrations (data not shown) for the induction of deleterious effects on SH-SY5Y cell viability, these conditions were selected for the rest of experiments. The viability of cells exposed to 1 mM MPP+ for 24 h was 53.2 ± 2.2% that of the control value, while the viability of cells that were pretreated for 2 h with AST at a concentration of 25 and 50 μM prior to exposure to MPP+ increased significantly to 79.6 ± 3.0% and 92.9 ± 2.6% of that of the control value, respectively (Fig. 2B). And, to examine whether AST protects against MPP+-induced apoptosis, cells were treated with 1 mM MPP+ in the absence or presence of 50 μM AST for 24 h and then analyzed with flow cytometric assay. Data from cytometric assay show that AST inhibits MPP+-induced apoptosis in the SH-SY5Y cells (Fig. 2C). We also verified the MPP+-induced apoptotic cell death by examining the cleavage of PARP, which is a 116-kDa nuclear protein that is cleaved to an 89-kDa fragment by activated caspase-3. Treatment with 1 mM MPP+ caused a marked increase in the cleavage of PARP, which was attenuated by treatment with 50 μM AST (Fig. 2D). Taken together, these results indicate that the viability of MPP+-treated cells decreased significantly, but that the AST exerted a protective effect against the MPP+-induced cytotoxicity.
Fig. 2.
Effect of AST on the MPP+-induced decrease in SH-SY5Y cell viability. Cell viability (A and B) or apoptotic death (C and D) was assessed by MTT assay, flow cytometric assay, or immunoblotting assay as described in the Materials and Methods section. (A) Cells were treated with various concentrations (0.1–2 mM) of MPP+ for 24 h. (B) Cells were pretreated with various concentrations (5–50 μM) of AST for 2 h and then treated with 1 mM MPP+ for 24 h in the presence of AST. (C) Cells were treated with either 0.1% DMSO (control) or 1 mM MPP+ in the absence or presence of 50 μM AST for 24 h as described in B. After treatment, cells were stained with fluorescein isothiocyanate (FITC)-Annexin V and propidium iodide (PI). Apoptosis was detected by the flow cytometric assay. In A, B, and C, error bars represent the mean ± SE from three separate experiments. *p < 0.05, compared with control (untreated group); **p < 0.01, compared with the group treated by MPP+ alone. (D) Cells were treated as described C and then harvested. Lysates containing equal amounts of protein (20 μg) were separated by SDS-PAGE and immunoblotted with anti-PARP-1 antibody. Actin was shown as an internal standard.
3.2. AST reduced the MPP+-induced increase of ROS in SH-SY5Y cells
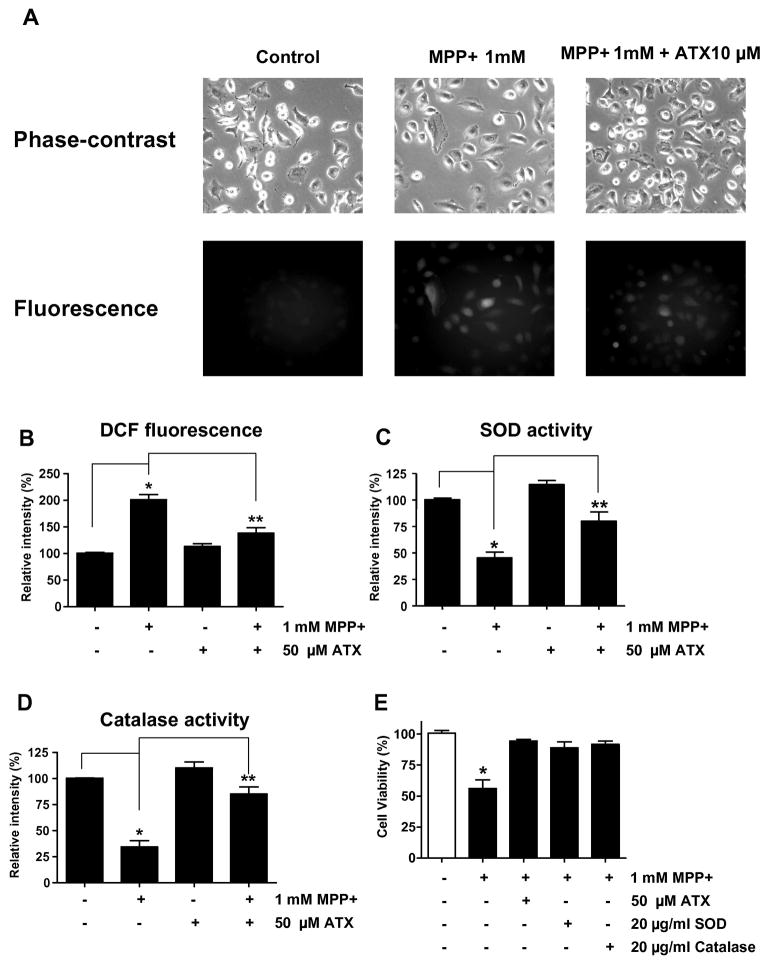
To determine suppression of MPP+-induced ROS production by AST, we measured ROS production in SH-SY5Y under several conditions. ROS generation, which was detected in the MPP+ treated cells, was suppressed by treatment with AST (Figs. 3A and 3B). These results suggest that AST acts as an inhibitor (anti-oxidant) to MPP+-mediated ROS generation. We hypothesized that MPP+ generates ROS via inhibiting anti-oxidant enzymes such as SOD and catalase, and AST suppresses MPP+-induced ROS generation by protecting these anti-oxidant enzymes from MPP+. To examine this possibility, cells were treated with MPP+ in the presence or absence of AST and then SOD activity (Fig. 3C) and catalase activity (Fig. 3D) were determined. As shown in Figs. 3C and 3D, the activities of SOD and catalase decreased to 50% and 37%, respectively, after MPP+ treatment. The inhibitory effect of MPP+ on SOD and catalase activities suggests that the cytotoxic effect of MPP+ (Fig. 2) may have resulted from oxidative stress in SH-SY5Y cells. This possibility was examined by adding SOD or catalase protein during treatment with MPP+. As shown in Fig. 3E, cell viability decreased to 53% when cells were treated with MPP+ alone for 48 h, but this MPP+-induced viability loss was almost fully inhibited by SOD and catalase as well as AST. Based on these findings, we postulate that the anti-oxidative properties of AST may contribute to the protection of SH-SY5Y cells from oxidative stress caused by MPP+.
Fig. 3.
Effect of AST on MPP+-mediated ROS generation in SH-SY5Y cells. (A) and (B) Cells were treated with 1 mM MPP+ in the absence or presence of 50 μM AST for 24 h as described in Fig. 2B. After treatment, ROS generation was determined with 5 μM CMH2DCFDA. Morphological features were analyzed with a phase-contrast microscope and fluorescent signals were detected with fluorescence microscopy (A). DCF fluorescence intensity was determined and plotted (B). (C) and (D) Cells were treated with 1 mM MPP+ in the absence or presence of 50 μM AST for 24 h as described in Fig. 2B. After treatment, SOD activity (C) and catalase activity (D) were measured. (E) Cells were treated with 1 mM MPP+ in the presence/absence of 50 μM AST, 20 μg/ml SOD, or 20 μ/ml catalase for 24 h. Data are expressed as percentage of values in untreated control cultures, and are means ± S.E.M. (n = 4). *p < 0.05, compared with control (untreated group); **p < 0.01, compared with the group treated by MPP+ alone.
3.3. Effects of MPP+ or AST on the protein expression of BCL-2 and BAX
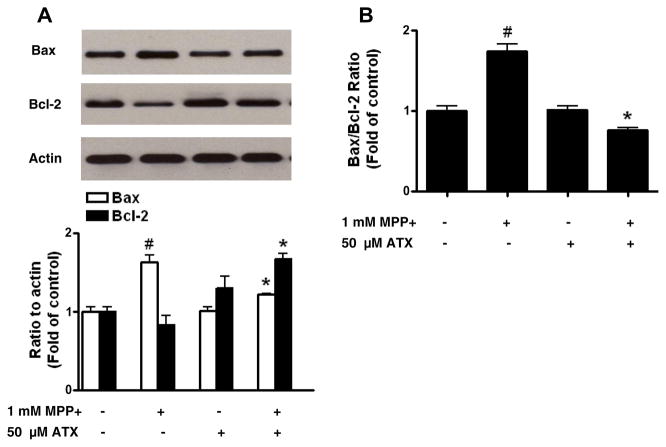
Figs. 2C and 2D show that AST protects cells from MPP+-induced apoptotic death; this may be the result of protecting cells from MPP+-induced alteration of apoptotic-associated gene expression. To examine this possibility, we screened the expression of various anti-apoptotic and pro-apoptotic genes (data not shown). We found that the expression of Bax and Bcl-2 genes was affected by treatment with MPP+ (Fig. 4A). As shown in Fig. 4A, the intracellular level of Bax protein increased significantly in the 1 mM MPP+-treated group compared with that in the control untreated group, whereas the intracellular level of Bcl-2 decreased in the MPP+-treated group. Interestingly, AST treatment (50 μM) prevented MPP+-induced upregulation of Bax and downregulation of Bcl-2. These results were confirmed by determining the Bax/Bcl-2 ratio. The Bax/Bcl-2 ratio increased to 1.6-fold of the control upon treatment with MPP+, while AST prevented the MPP+-induced increase of the Bax/Bcl-2 ratio (Fig. 4B). These results suggest that MPP+ shifts the balance between pro-apoptotic and anti-apoptotic proteins and AST prevents this alteration.
Fig. 4.
Effect of AST on the expression of Bcl-2 and Bax in SH-SY5Y cells. Cells were treated with MPP+ (1 mM) and/or ATS (50 μM) for 24 h, and then cell lysates were subjected to Western blot analysis (upper panel of A). The levels of Bax and Bcl-2 were quantified by densitometric analysis (lower panel of A) and the Bax/Bcl-2 ratio was determined (B). Data are means ± S.E.M. (n = 3). #p < 0.05, compared with control, *p < 0.05, compared with MPP+ alone;
3.4. Effects of AST on MPP+-induced cytochrome c release and caspase-3 activation
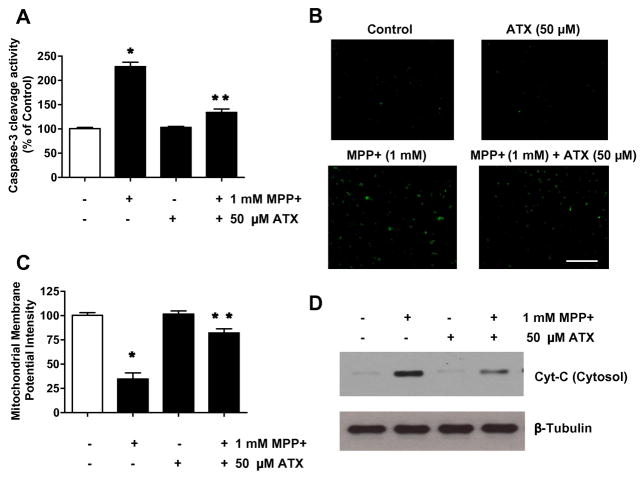
Previous studies showed that apoptosis may occur via a death receptor-dependent (extrinsic) or independent (intrinsic or mitochondrial) pathway. In the extrinsic pathway, TNF family (Fas/APO-1 ligand, TNF, TRAIL) proteins bind to the death receptors and activate caspase cascades via Fas-associated death domain (FADD), an adaptor protein (Kischkel et al., 2000; Thomas et al., 2004). In the intrinsic pathway, mitochondria play a central role in cell death in response to DNA damage, and mediate the interaction(s) of various cytoplasmic organelles, including the endoplasmic reticulum, Golgi apparatus, and lysosomes (Lee et al., 2008; Ferri et al., 2001). The mitochondrial pathway of cell death is mediated by Bcl-2 family proteins, a group of anti-apoptotic and pro-apoptotic proteins that regulate the passage of small molecules, such as cytochrome c, Smac/Diablo, and apoptosis-inducing factor, which activates caspase cascades, through the mitochondrial transition pore (Lee et al., 2008; Shi et al., 2008). We hypothesized that MPP+-induced apoptosis is mediated through the mitochondrial pathway and AST blocks this pathway. To test the hypothesis, we investigated caspase-3 activation, mitochondrial membrane potential alteration, and mitochondrial release of cytochrome c during treatment with MPP+ in the presence/absence of AST. As shown in Figs. 5A and 5B, following 48 h treatment of SH-SY5Y cells with MPP+ (1 mM), we detected a caspase-3 activity increase to 243% of the control level and also observed an increase in the active form of caspase-3 in the cells. Addition of 50 μM AST attenuated MPP+-induced caspase-3 activation and provided 57% suppression (Fig. 5A) and reduced the active form of caspase-3 in the cells (Fig, 5B). However, AST alone did not show a significant effect on the caspase-3 activity in SH-SY5Y cells, which was consistent with its lack of apoptotic response (Figs. 2C and 2D). We next examined the effect of AST on MPP+-induced changes in mitochondrial membrane potential (MMP: Δψm) which was measured by JC-1 staining. The results showed that the Δψm was decreased in the SH-SY5Y cells after MPP+ treatment (Fig. 5C). AST (50 μM) prevented MPP+-induced loss of Δψm. We further examined the effect of AST on MPP+-induced mitochondrial dysfunction. As shown in Fig. 5D, the release of cytochrome c from the mitochondria into the cytosol occurred during treatment with 1 mM MPP+ for 48 h. We also observed a minimal release of cytochrome c from the mitochondria during treatment with 50 μM AST. This may be due to mitochondrial fraction contamination. Nevertheless, the release of cytochrome c was clearly inhibited by pretreatment with 50 μM AST (Fig. 5D). These results suggest that MPP+-induced caspase-3 activation and cytochrome c release from the mitochondria can be effectively blocked by the pretreatment with AST.
Fig. 5.
Effect of AST on MPP+-induced dysfunction of mitochondrial and caspase-3 activity in SH-SY5Ycells. Cells were treated with MPP+ (1 mM) for 24 h in the presence or absence of 50 μM AST. Enzymatic activity of caspase-3 (A), cleaved caspase-3 (active form of caspase-3)-positive cells (B), and mitochondrial membrane potential (C) were determined as described in the Materials and Methods section. Data are from representative experiments repeated at least three times. Data are means ± S.E.M. (n = 3). *p < 0.05, compared with control (untreated cells); **p < 0.05, compared with the group treated by MPP+ alone. (D) Cytochrome c (15 kDa) release into cytosol was determined by immunoblotting for cytochrome c in the cytosolic fraction. Lysates containing equal amounts of protein (20 μg) were separated by SDS-PAGE and immunoblotted with anti-cytochrome c antibody. β-tubulin was shown as an internal standard.
3.5. Tyrosine hydroxylase immunohistochemistry
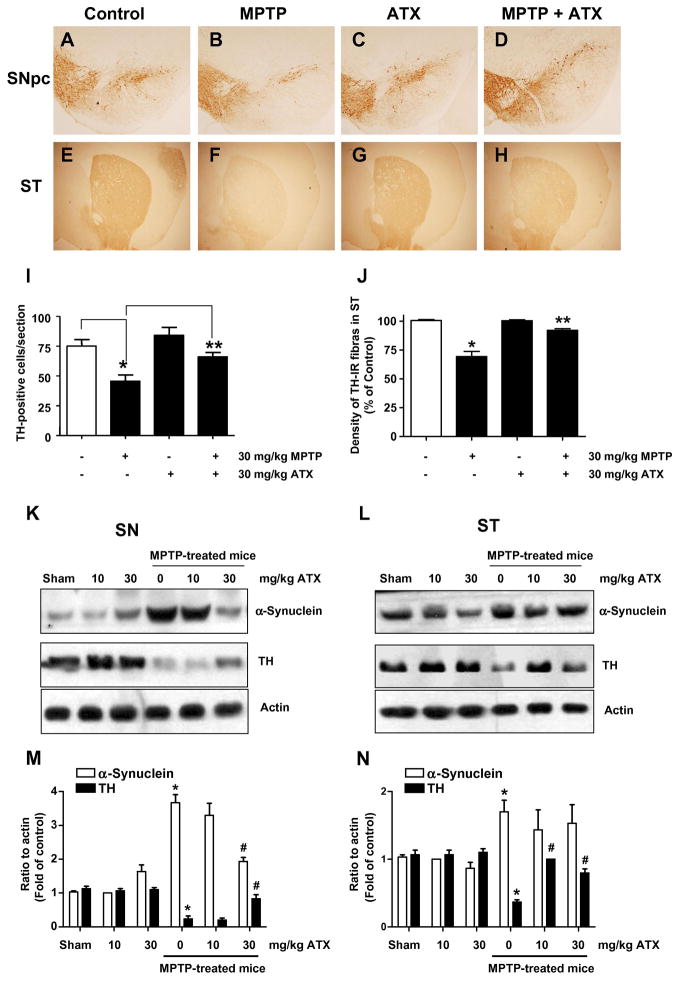
We extended our studies to examine the protective effect of AST on neurotoxin in an animal PD model. For this study, we used MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) which is metabolized into the toxic cation MPP+ by the enzyme monoamine oxidase B (MAO-B) of glial cells and causes permanent symptoms of Parkinson’s disease by destroying certain neurons (Sonsalla et al., 2010; Bi et al., 2008). MPTP treatment impairs tyrosine hydoxylase (TH), which is the rate-limiting enzyme in dopamine biosynthesis. Immunostaining of the SN using an anti-TH antibody followed by a Histostain -SP Kit demonstrated that AST pre-treatment (30 mg/kg) for 28 days significantly reduced MPTP-induced TH-positive dopaminergic neuron loss relative to the MPTP alone group in the SN (Figs. 6A–b and 6A–d). In control mice, the cytoplasm and fibers of dopaminergic neurons were intensively stained and the cellular processes were evident, showing evident immunoreactive positive signals (Fig. 6A–a). In contrast, MPTP treatment resulted in a marked loss of dopamine-containing SN neurons, few immunoreactive positive cells were seen and the cellular processes were absent for most cells (Figs. 6A–b). However, in the AST pre-treated MPTP group, numerous immunoreactive positive cells were evident and the cell processes were easily observed (Fig. 6–d). AST pre-treatment demonstrated a significant attenuation of MPTP-induced loss of dopaminergic immunoreactivety in the SN pars compacta (Fig. 6A–d). In agreement with the above cellular morphological observations, Fig. 6B revealed that MPTP exposure leads to a marked loss of TH positive neurons in the SN pars compacta compared to the saline-treated control mice (38 ± 5 vs 74 ± 6, p < 0.05). AST (30 mg/kg) treatment significantly prevented this reduction of TH immunoreactivity in the SN pars compacta (77 ± 7, p < 0.05). We also examined the change in the levels of TH and α-synuclein in the SN and stria terminalis (ST) using immunoblotting assay (Figs. 6C and 6D). As shown in Figs. 6C and 6D, MPTP treatment markedly reduced the levels of TH protein in the SN and ST compared to the saline-treated control mice (p < 0.05). AST administration prevented the MPTP-induced reduction of TH level in the SN and ST. Interestingly, MPTP treatment significantly increased the level of α-synuclein, the histological hallmark of Parkinson’s disease, in the SN, but not in the ST. Moreover, AST effectively inhibited the MPTP-induced elevation of α-synuclein level in the SN (Fig. 6C). These results suggest that AST can protect dopaminergic neurons from MPTP neurotoxicity in mice.
Fig. 6.
Effects of AST on nigra TH expression and dopaminergic neurons in the mouse MPTP model. (A) Immunohistochemical staining of dopaminergic neurons with an anti-TH antibody in the substantia nigra (SN). Photomicrographs were taken at a magnification of 200×. a, control group. b, MPTP-treated group (30 mg/kg, 1×/day for 28 days). c, AST-treated group (30 mg/kg), d, MPTP + AST-treated group. (B) The immunoreactive cell counts in the SN pars compacta of control group, MPTP-treated group, AST-treated group, and AST + MPTP group. Values represent means ± S.E.M of 4 mice per group. *p <0.05 compared with the control group; and **p <0.01 compared with the MPTP-treated group. (C) and (D) TH and α-synuclein expression was determined in the SN (C) and stria terminalis (ST) (D). Lysates containing equal amounts of protein (20 μg) from SN or ST tissue samples were separated by SDS-PAGE and immunoblotted with anti-α-synuclein or anti-TH antibody. Actin was shown as an internal standard.
4. Discussion
In the present study, AST showed a significant protective effect against MPP+-induced toxicity and showed no/little toxicity to SH-SY5Y cells. Moreover, the present study confirmed the effect of AST in an MPTP-induced animal PD model, which included nigral dopaminergic neuronal loss. Our results clearly demonstrate that AST protects against MPTP/MPP+-induced neuronal mitochondrial damage by ROS in vivo and in vitro, and inhibits ROS generation and Δψm collapse induced by MPP+ in SH-SY5Y cells. Present results suggest that cytoprotection of AST against MPTP/MPP+-induced cell death may be associated with the attenuation of oxidative damage via inhibiting ROS generation and with the prevention of Δψm collapse. These results suggest that the balance between generating and scavenging of free radicals is very important for cell survival.
We used the MTT assay to investigate the protective effects of AST against MPP+-induced neurotoxicity in SH-SY5Y cells. MPP+, a neurotoxin which plays dominant neurotoxic roles in selectively damaging catecholaminergic neurons including dopaminergic neurons, has widely been used in experimental models of PD, and it can operate in extracellular or intracellular oxidation, yielding ROS that lead to toxic downstream molecules and result in neuronal damage (Anantharam et al., 2007). It has been demonstrated that MPP+ is involved in disturbing mitochondrial outer membrane permeability, leading to increased cytosolic cytochrome c and apoptotic proteins, including caspase-3 (Ahn et al., 2009).
Some studies have reported that ROS are involved in the apoptotic mechanism of MPP+-mediated neurotoxicity (Di Monte et al., 1986) and may contribute to the apoptotic processes found in PD (Kehrer et al., 1994). Oxidative stress generated by MPP+ might be, at least in part, responsible for the opening of the mitochondrial permeability transition pore and the loss of MMP (Cassarino et al., 1999) As mentioned previously, data from this study also show that treatment with MPP+ results in a significant increase of ROS (Fig. 3A and 3B). To determine whether suppression of ROS production was effective in preventing apoptosis, we employed antioxidant enzymes (SOD and catalase) to examine their effects on SHSY5Y cell death induced by MPP+. Our results showed that SOD and catalase suppressed MPP+-induced cell death and caspase-3 activation. The protective effects of antioxidant enzymes suggest the involvement of ROS in the cytotoxic effect of MPP+ on SH-SY5Y cells. Some antioxidants prevent apoptotic cell death in the dopaminergic cell lines and SH-SY5Y cells treated with MPP+ (Seaton et al., 1997; Banaclocha et al., 1997). It was reported that MPTP might preferentially target dopaminergic neurons rather than other neurons in the same region. Such toxicity in the dopaminergic nigrostriatal system seems reasonable since the SN is rich in dopamine, which can undergo both enzymatic and non-enzymatic oxidation to produce free radicals (Fahn and Cohen, 1992). However, the antioxidant system in the nigrostriatal tract is severely attenuated in drug-induced PD. For example, MPTP depletes striatal glutathione (GSH) in mice, and this effect may make dopaminergic neurons more susceptible to oxidative stress (Aoyama et al., 2008). Some antioxidants could theoretically prevent, at least in part, the progression of PD. There are several lines of evidence from animal models which imply that a variety of antioxidative strategies, such as overexpression of Cu, Zn superoxide dismutase, Mn superoxide dismutase, or treatment with copper sulfate provide resistance to MPTP (Alcaraz-Zubeldia et al., 2001; Klivenyi et al., 1998; Przedborski et al., 1992).
While there is more than one pathway to apoptosis, the Bcl-2 family members play an important role in MPP+-induced apoptotic cell death (Blum et al., 2001). In addition, O’Malley et al. (2003) reported that the overexpression of Bcl-2 attenuated MPP+-induced cell death. Flow cytometry analysis revealed that AST reduced the number of apoptotic cells evoked by MPP+ (Fig. 2). The effects of AST presented here resemble those of neuroprotective drugs such as green tea polyphenol, epigallocatechin-3-gallate, and rasagilin, which similarly alter Bcl-2 and Bax expression (Moldzio et al., 2001; Nayak et al., 2008). Based on these reports and our observations, we hypothesize that AST, through suppression of ROS generation, inhibits alteration of the Bcl-2 family protein levels in response to MMP+ treatment and then prevents mitochondria-mediated downstream molecular events including cytochrome c release and caspase-3 activation. We believe that the effect of AST on MPP+-induced apoptosis may be, at least partly, mediated by regulating the expression of Bax and Bcl-2. The interplay between these and other pro- and anti-apoptotic Bcl-2 family members may determine the fate of cells by regulating the permeability of the mitochondrial membrane and controlling the release of cytochrome c from the mitochondria (Lee et al., 2008; Cropton, 2000). Once released to the cytosol, cytochrome c could form the apoptosome together with apoptosis-activating factor Apaf-1 and procaspase-9, leading to the activation of capase-9, and then activation of capase-3 (Thornberry and Lazebnik, 1998).
Caspase-3 has been demonstrated to participate in MPP+-induced apoptosis (Blum et al., 2001). Moreover, some previous works showed that reactive oxygen species (ROS) is also implicated in MPP+-induced cytotoxicity including MPT pore opening and cytochrome c release (Kakimura et al., 2001; Di Monte et al., 1986). These previous studies implicated that mitochondrial dysfunction plays an important role in MPP+-induced cytotoxicity, as a result of the decrease of ATP and MPT (Seaton et al., 1997). In this study, caspase-3 activation increased dramatically following 24 h treatment with MMP+. Addition of AST attenuated MPP+-induced caspase-3 activation (Fig. 5A and 5B). Our results showed that AST prevented MPP+-induced loss of MMP and release of cytochrome c from the mitochondria. High levels of MMP are necessary to maintain closure of a multi-protein pore and the MPT pore (Tatton and Olanow, 1999). The opening of the MPT pore causes a release of apoptogenic substances such as cytochrome c from the mitochondria into the cytoplasm (Nicholls and Budd, 2000). Cytochrome c release from the mitochondria was proved to play a critical role in apoptosis (Kluck et al., 1996) and has been observed in MPP+-treated cells (Kakimura et al., 2001). Mitochondrial complex I dysfunction is also believed to be associated with the pathophysiology of PD (Dawson and Dawson, 2003; Schapira et al., 1989). Classical dopaminergic degenerative animal models use neuron-specific neurotoxins such as 6-hydroxydopamine (Glinka et al., 1996), MPTP/MPP+ (Nicklas et al., 1985) or rotenone (Saravanan et al., 2005), which act in part via oxidative stress to induce mitochondrial complex I dysfunction. In addition, several other pathogenic mechanisms contribute to the oxidative stress that causes the degeneration of dopaminergic neurons (Tsang and Chung et al., 2009).
Decreases in dopamine and its metabolites in the striatal nuclei in the brain are associated with the progressive degeneration of dopaminergic neurons, such as in PD (Bernheimer and Hornykiewicz, 1965; Ehringer et al., 1960). TH activity itself is markedly decreased in the SN and ST in the PD brain (Nagatsu et al., 1977), which is one of the mechanisms for the decrease in dopamine. Borges et al. (2002) reported that TH is modified by sulphydryl oxidant and that this post-translational modification results in a decrease in TH catalytic function. Moreover, S-glutathionylation has been suggested to be accelerated by ROS (Giustarini et al., 2004). In fact, it was reported that antioxidants exert a protective effect on TH immunoreactivity (Oyagi et al., 2008). In this study, we demonstrated that dopaminergic neuron death in the SN was significantly reduced in AST plus MPTP-treated animals (Fig. 6A-d). In addition, concurrent with the decrease of TH levels in the striatum, biochemical markers of dopaminergic nigrostriatal proteins such as α-synuclein were increased by MPTP and attenuated by AST treatment (Fig. 6C). It should be noted that, in this study, the animals were treated with AST on the day of MPTP injection. The roles of α-synuclein in normal cell function and in neurodegeneration have not been elucidated, but its potential roles in synaptic plasticity (George et al., 1995), neuronal differentiation (Kholodilov et al., 1999), the up-regulation of dopamine release (Van der Putten et al., 2000), and mitochondrial deficits (Cohen and Duke, 1984; Galon et al., 2002) have been reported. Recently, the dual roles of α-SN in neuroprotection and neurotoxicity were described (Costa et al., 2002; Seo et al., 2002; Zourlidou et al., 2003). Collectively, these findings imply that MPP+-induced α-synuclein aggregation in neuroblastoma cells is probably mediated by oxidants generated from ROS generation. At present, we do not know how MPP+-induced oxidative stress plays a role in up-regulation of α-synuclein, but it has been recently reported that oxidative stress-dependent transcription factors are involved or the 5′- and 3′-untranslated region of α-syn (Leiter et al., 2002). In addition, AST has exhibited neuroprotective effects in other experimental models (Curek et al., 2010; Liu and Osawa, 2010; Chan et al., 2009; Shen et al., 2009).
In conclusion, we show that MPTP/MPP+-induced Δψm loss and cell death occurred following ROS generation. Neuroprotection of AST may be associated with the prevention of Δψm loss via attenuating ROS generation in MPTP/MPP+ experimental models. Our data further support the idea that the mitochondria would be an important target to develop neuroprotective therapeutics for PD, indicating the use of AST for this purpose.
Acknowledgments
This work was supported by the Korean Research Foundation Grant Fund from the Korean Government (MOEHRD, Basic Research Fund, KRF-214-2006-1-E00014) and NIH (CA140554).
Abbreviations used here are
- AST
astaxanthin
- DA
dopamine
- DCFH-DA
3-(4,5-dimethyl-2-thiazolyl)-2,2,7-dichlorofluorescein diacetate
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
Dimethyl sulfoxide
- LPO
lipid peroxidation
- MMP
Δψm, mitochondrial membrane potential
- MPP+
1-methyl-4-phenylpyridinium
- MPT
mitochondrial permeability transition
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide
- PARP
poly (ADP-ribose) polymerase
- PBS
phosphate buffered saline
- PD
Parkinson’s disease
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- SN
substantia nigra
- ST
stria terminalis
- TH
tyrosine hydroxylase
Footnotes
Conflict of interest statement
The authors declare that there are no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn KH, Kim YS, Kim SY, Huh Y, Park C, Jeong JW. Okadaic acid protects human neuroblastoma SH-SY5Y cells from 1-methyl-4-phenylpyridinium ion-induced apoptosis. Neurosci Lett. 2009;449:93–97. doi: 10.1016/j.neulet.2008.10.103. [DOI] [PubMed] [Google Scholar]
- Alcaraz-Zubeldia M, Rojas P, Boll C, Rios C. Neuroprotective effect of acute and chronic administration of copper (II) sulfate against MPP+ neurotoxicity in mice. Neurochem Res. 2001;26:59–64. doi: 10.1023/a:1007680616056. [DOI] [PubMed] [Google Scholar]
- Anantharam V, Kaul S, Song C, Kanthasamy A, Kanthasamy AG. Pharmacological inhibition of neuronal NADPH oxidase protects against 1-methyl-4-phenylpyridinium (MPP+)-induced oxidative stress and apoptosis in mesencephalic dopaminergic neuronal cells. Neurotoxicology. 2007;28:988–997. doi: 10.1016/j.neuro.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama K, Watabe M, Nakaki T. Regulation of neuronal glutathione synthesis. J Pharmacol Sci. 2008;108:227–238. doi: 10.1254/jphs.08r01cr. [DOI] [PubMed] [Google Scholar]
- Banaclocha MM, Hernandez AI, Martinez N, Ferrandiz ML. N-Acetylcysteine protects against age-related increase in oxidized proteins in mouse synaptic mitochondria. Brain Res. 1997;762:256–258. doi: 10.1016/s0006-8993(97)00493-9. [DOI] [PubMed] [Google Scholar]
- Bendich A, Olson JA. Biological actions of carotenoids. FASEB J. 1989;3:1927–1932. [PubMed] [Google Scholar]
- Beers RF, Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- Bernheimer H, Hornykiewicz O. Decreased homovanillic acid concentration in the brain in Parkinsonian subjects as an expression of a disorder of central dopamine metabolism. Klin Wochenschr. 1965;43:711–715. doi: 10.1007/BF01707066. [DOI] [PubMed] [Google Scholar]
- Bi J, Wang XB, Chen L, Hao S, An LJ, Jiang B, Guo L. Catalpol protects mesencephalic neurons against MPTP induced neurotoxicity via attenuation of mitochondrial dysfunction and MAO-B activity. Toxicol In Vitro. 2008;22:1883–1889. doi: 10.1016/j.tiv.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Blum D, Torch S, Lambeng N, Nissou MF, Benabid AL, Sadoul R, Verna JM. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol. 2001;65:135–172. doi: 10.1016/s0301-0082(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Borges CR, Geddes T, Watson JT, Kuhn DM. Dopamine biosynthesis is regulated by S-glutathionylation. Potential mechanism of tyrosine hydroxylast inhibition during oxidative stress. J Biol Chem. 2002;277:48295–48302. doi: 10.1074/jbc.M209042200. [DOI] [PubMed] [Google Scholar]
- Cassarino DS, Parks JK, Parker WD, Jr, Bennett JP., Jr The parkinsonian neurotoxin MPP+ opens the mitochondrial permeability transition pore and releases cytochrome c in isolated mitochondria via an oxidative mechanism. Biochim Biophys Acta. 1999;1453:49–62. doi: 10.1016/s0925-4439(98)00083-0. [DOI] [PubMed] [Google Scholar]
- Chan KC, Mong MC, Yin MC. Antioxidative and anti-inflammatory neuroprotective effects of astaxanthin and canthaxanthin in nerve growth factor differentiated PC12 cells. J Food Sci. 2009;74:225–231. doi: 10.1111/j.1750-3841.2009.01274.x. [DOI] [PubMed] [Google Scholar]
- Cohen JJ, Duke RC. Glucocorticoid activation of a calcium dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984;132:38–42. [PubMed] [Google Scholar]
- Costa CA, Paitel E, Vincent B, Checler F. α-Synuclein lowers p53-dependent apoptotic response of neuronal cells. J Biol Chem. 2002;277:50980–50984. doi: 10.1074/jbc.M207825200. [DOI] [PubMed] [Google Scholar]
- Cropton M. Bax, Bid and the permeabilization of the mitochondrial outer membrane in apoptosis. Curr Opin Cell Biol. 2000;12:414–419. doi: 10.1016/s0955-0674(00)00110-1. [DOI] [PubMed] [Google Scholar]
- Curek GD, Cort A, Yucel G, Demir N, Ozturk S, Elpek GO, Savas B, Aslan M. Effect of astaxanthin on hepatocellular injury following ischemia/reperfusion. Toxicology. 2010;267:147–153. doi: 10.1016/j.tox.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL. Molecular pathways of neuro-degeneration in Parkinson’s disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- Di Monte D, Sandy MS, Elkstrom GM, Smit T. Comparative studies on the mechanisms of paraquat and 1-methyl-4-phenylpyridine (MPP+) cytotoxicity. Biochem Biophys Res Commun. 1986;137:303–309. doi: 10.1016/0006-291x(86)91210-6. [DOI] [PubMed] [Google Scholar]
- Ehringer H, Hornykiewicz O. Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system. Klin Wochenschr. 1960;38:1236–1239. doi: 10.1007/BF01485901. [DOI] [PubMed] [Google Scholar]
- Fahn S, Cohen G. The oxidant stress hypothesis in Parkinson’s disease: evidence supporting it. Ann Neurol. 1992;32:804–812. doi: 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3:255–263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- Galon J, Franchimont D, Hiro N, Frey G, Boettger A, Ehrhart-Bornstein M, O’Shea JJ, Chrousos GP, Bornstein SR. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J. 2002;16:61–71. doi: 10.1096/fj.01-0245com. [DOI] [PubMed] [Google Scholar]
- George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra fish. Neuron. 1995;15:361–372. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- Giustarini D, Rossi R, Milzani A, Colombo R, Dalle-Donne I. S-glutathionylation: from redox regulation of protein functions to human diseases. J Cell Mol Med. 2004;8:201–212. doi: 10.1111/j.1582-4934.2004.tb00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka Y, Tipton KF, Youdim MB. Nature of inhibition of mitochondrial respiratory complex I by 6-Hydroxydopamine. J Neurochem. 1996;66:2004–2010. doi: 10.1046/j.1471-4159.1996.66052004.x. [DOI] [PubMed] [Google Scholar]
- Hussein G, Goto H, Oda S, Sankawa U, Matsumoto K, Watanabe H. Antihypertensive potential and mechanism of action of astaxanthin: III. Antioxidant and histopathological effects in spontaneously hypertensive rats. Biol Pharm Bull. 2006;29:684–688. doi: 10.1248/bpb.29.684. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H, Zhang L, Gross M, Tomita Y. Immunomodulating actions of carotenoids: enhancement of in vivo and in vitro antibody production to T-dependent antigens. Nutr Cancer. 1994;21:47–58. doi: 10.1080/01635589409514303. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H, Sun S, Iijima K, Gross MD. Antitumor activity of astaxanthin and its mode of action. Nutr Cancer. 2000;36:59–65. doi: 10.1207/S15327914NC3601_9. [DOI] [PubMed] [Google Scholar]
- Kakimura J, Kitamura Y, Takata K, Kohno Y, Nomura Y, Taniguchi T. Release and aggregation of cytochrome c and alpha-synuclein are inhibited by the antiparkinsonian drugs, talipexole and pramipexole. Eur J Pharmacol. 2001;417:59–67. doi: 10.1016/s0014-2999(01)00902-5. [DOI] [PubMed] [Google Scholar]
- Kalivendi SV, Cunningham S, Kotamraju S, Joseph J, Hillard CJ, Kalyanaraman B. Alpha-synuclein up-regulation and aggregation during MPP+-induced apoptosis in neuroblastoma cells: intermediacy of transferrin receptor iron and hydrogen peroxide. J Biol Chem. 2004;279:15240–15247. doi: 10.1074/jbc.M312497200. [DOI] [PubMed] [Google Scholar]
- Kehrer JP, Smith CV. Free radicals in biology: sources, reactivities, and roles in the etiology of human diseases. In: Frei B, editor. Natural Antioxidants in Human Health and Disease. Academic Press; San Diego: 1994. pp. 25–62. [Google Scholar]
- Kholodilov NG, Feostat M, Oo TF, Lo SE, Larsen KE, Kulzer D, Burke RE. Increased expression of rat synuclein in the substantia nigra pars compacta identified by mRNA differential display in a model of developmental target injury. J Neurochem. 1999;73:2586–2599. doi: 10.1046/j.1471-4159.1999.0732586.x. [DOI] [PubMed] [Google Scholar]
- Kischkel FC, Lawrence DA, Chuntharapai A, Schow P, Kim KJ, Ashkenazi A. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity. 2000;12:611–620. doi: 10.1016/s1074-7613(00)80212-5. [DOI] [PubMed] [Google Scholar]
- Klivenyi P, St Clair D, Wermer M, Yen HC, Oberley T, Yang L, Flint Beal M. Manganese superoxide dismutase overexpression attenuates MPTP toxicity. Neurobiol Dis. 1998;5:253–258. doi: 10.1006/nbdi.1998.0191. [DOI] [PubMed] [Google Scholar]
- Kluck RM, Martin SJ, Zhou JS, Green DR, Newmeyer DD. Cytochrome c activation of CPP32-like proteolysis plays a critical role in a Xenopus cell-free apoptosis system. EMBO J. 1996;16:4639–4649. doi: 10.1093/emboj/16.15.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurashige M, Okimasu E, Inoue M, Utsumi K. Inhibition of oxidative injury of biological membranes by astaxanthin. Physiol Chem Phys Med NMR. 1990;22:27–38. [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee DH, Rhee JG, Lee YJ. Reactive oxygen species up-regulate p53 and Puma; a possible mechanism for apoptosis during combined treatment with TRAIL and wogonin. British Journal Pharmacology. 2009;157:1189–1202. doi: 10.1111/j.1476-5381.2009.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Szczepanski M, Lee YJ. Role of Bax in quercetin-induced apoptosis in human prostate cancer cells. Biochem Pharmacol. 2008;75:2345–2355. doi: 10.1016/j.bcp.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Szczepanski M, Lee YJ. Role of Bax in quercetin-induced apoptosis in human cancer cells. Biochem Pharmacol. 2008;75:2345–2355. doi: 10.1016/j.bcp.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Shin SY, Choi C, Lee YH, Lee SJ. Formation and removal of alpha-synuclein aggregates in cells exposed to mitochondrial inhibitors. J Biol Chem. 2002;277:5411–5417. doi: 10.1074/jbc.M105326200. [DOI] [PubMed] [Google Scholar]
- Leiter L, McPhee J, Sarang SS, Utsuki T, Greig NH, Lahiri DK, Tanzi RE, Bush AI, Giordano T, Gullans SR. An iron-responsive element type II in the 5′-untranslated region of the Alzheimer’s amyloid precursor protein transcript. J Biol Chem. 2002;277:45518–45528. doi: 10.1074/jbc.M207435200. [DOI] [PubMed] [Google Scholar]
- Liu X, Osawa T. Astaxanthin protects neuronal cells against oxidative damage and is a potent candidate for brain food. Forum Nutr. 2009;61:129–135. doi: 10.1159/000212745. [DOI] [PubMed] [Google Scholar]
- Marchetti B, Abbracchio MP. To be or not to be (inflamed) Is that the question in anti-inflammatory drug therapy of neurodegenerative disorders? Trends Pharmacol Sci. 2005;26:517–525. doi: 10.1016/j.tips.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 1990;1:120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- Miki W, Yamaguchi K, Konosu S. Comparison of carotenoids in the ovaries of marine fish and shellfish. Comp Biochem Physiol B. 1982;71:7–11. doi: 10.1016/0305-0491(82)90167-5. [DOI] [PubMed] [Google Scholar]
- Moldzio R, Radad K, Krewenka C, Kranner B, Duvigneau JC, Wang Y, Rausch WD. Effects of epigallocatechin gallate on rotenone-injured murine brain cultures. J Neural Transm. 2010;117:5–12. doi: 10.1007/s00702-009-0284-z. [DOI] [PubMed] [Google Scholar]
- Mortensen A, Skibsted LH, Truscott TG. The interaction of dietary carotenoids with radical species. Arch Biochem Biophys. 2001;385:13–19. doi: 10.1006/abbi.2000.2172. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Kato T, Numata Y, Ikuta K, Sano M. Phenylethanolamine N-methyltransferase and other enzymes of catecholamine metabolism in human brain. Clin Chim Acta. 1997;75:221–232. doi: 10.1016/0009-8981(77)90193-0. [DOI] [PubMed] [Google Scholar]
- Nayak L, Henchcliffe C. Rasagiline in treatment of Parkinson’s disease. Neuropsychiatr Dis Treat. 2008;4:23–32. [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiol Rev. 2000;80:315–360. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- Nicklas WJ, Vyas I, Heikkila RE. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenylpyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985;36:2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- O’Brien NM, Woods JA, Aherne SA, O’Callaghan YC. Cytotoxicity, genotoxicity and oxidative reactions in cell-culture models: modulatory effects of phytochemicals. Biochem Soc Trans. 2000;28:22–26. doi: 10.1042/bst0280022. [DOI] [PubMed] [Google Scholar]
- O’Malley KL, Liu J, Lotharius J, Holtz W. Targeted expression of BCL-2 attenuates MPP+ but not 6-OHDA induced cell death in dopaminergic neurons. Neurobiol Dis. 2003;14:43–51. doi: 10.1016/s0969-9961(03)00013-5. [DOI] [PubMed] [Google Scholar]
- Oyagi A, Oida Y, Hara H, Izuta H, Shimazawa M, Matsunaga N, Adachi T, Hara H. Protective effects of SUN N8075, a novel agent with antioxidant properties, in in vitro and in vivo models of Parkinson’s disease. Brain Res. 2008;1214:169–176. doi: 10.1016/j.brainres.2008.02.073. [DOI] [PubMed] [Google Scholar]
- Park JH, Lee HJ, Koh SB, Ban JY, Seong YH. Protection of NMDA-induced neuronal cell damage by methanol extract of zizyphi spinosi semen in cultured rat cerebellar granule cells. J Ethnopharmacol. 2004;95:39–45. doi: 10.1016/j.jep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Kostic V, Jackson-Lewis V, Naini AB, Simonetti S, Fahn S, Carlson E, Epstein CJ, Cadet JL. Transgenic mice with increased Cu/Zn-superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J Neurosci. 1992;12:1658–1667. doi: 10.1523/JNEUROSCI.12-05-01658.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saporito MS, Thomas BA, Scott RW. MPTP activates c-Jun NH(2)-terminal kinase (JNK) and its upstream regulatory kinase MKK4 in nigrostriatal neurons in vivo. J Neurochem. 2000;75:1200–1208. doi: 10.1046/j.1471-4159.2000.0751200.x. [DOI] [PubMed] [Google Scholar]
- Saravanan KS, Sindhu KM, Mohanakumar KP. Acute intranigral infusion of rotenone in rats causes progressive biochemical lesions in the striatum similar to Parkinson’s disease. Brain Res. 2005;1049:147–155. doi: 10.1016/j.brainres.2005.04.051. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- Seaton TA, Cooper JM, Schapria AH. Free radical scavengers protect doparminergenic cell line from apoptosis induced by mitochondrial complex I toxins. Brain Res. 1997;777:110–118. doi: 10.1016/s0006-8993(97)01034-2. [DOI] [PubMed] [Google Scholar]
- Seo JH, Rah JC, Choi SH, Shin JK, Min K, Kim HS, Park CH, Kim S, Kim E, Lee SH. α-Synuclein regulates neuronal survival via Bcl-2 family expression and PI3/Akt pathway. FASEB J. 2002;16:1826–1829. doi: 10.1096/fj.02-0041fje. [DOI] [PubMed] [Google Scholar]
- Shen H, Kuo CC, Chou J, Delvolve A, Jackson SN, Post J, Woods AS, Hoffer BJ, Wang Y, Harvey BK. Astaxanthin reduces ischemic brain injury in adult rats. FASEB J. 2009;23:1958–1968. doi: 10.1096/fj.08-123281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Yan SG, Xie ST, Wang HN. Tip30-induced apoptosis requires translocation of Bax and involves mitochondrial release of cytochrome c and Smac/DIABLO in hepatocellular carcinoma cells. Biochim Biophys Acta. 2008;1783:263–274. doi: 10.1016/j.bbamcr.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Singer TP, Ramsay RR. Mechanism of the neurotoxicity of MPTP, an update. FEBS Lett. 1990;274:1–8. doi: 10.1016/0014-5793(90)81315-f. [DOI] [PubMed] [Google Scholar]
- Slater AF, Nobel CS, Maellaro E, Bustamante J, Kimland M, Orrenius S. Nitrone spin traps and a nitroxide antioxidant inhibit a common pathway of thymocyte apoptosis. Biochem J. 1995;306:771–778. doi: 10.1042/bj3060771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeda A, Inagaki A, Oka N, Ikegame Y, Aoki H, Yoshimura S, Nakashima S, Kunisada T, Iwama T. Epidermal growth factor plays a crucial role in mitogenic regulation of human brain tumor stem cells. J Biol Chem. 2008;283:10958–10966. doi: 10.1074/jbc.M704205200. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Wong LY, Winnik B, Buckley B. The antiepileptic drug zonisamide inhibits MAO-B and attenuates MPTP toxicity in mice: clinical relevance. Exp Neurol. 2010;221:329–334. doi: 10.1016/j.expneurol.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Makita H, Ohnishi M, Mori H, Satoh K, Hara A. Chemoprevention of rat oral carcinogenesis by naturally occurring xanthophylls, astaxanthin and canthaxarathin. Cancer Res. 1995;55:4059–4064. [PubMed] [Google Scholar]
- Tatton WG, Olanow CW. Apoptosis in neurodegenerative diseases: the role of mitochondria. Biochim Biophys Acta. 1999;1410:195–213. doi: 10.1016/s0005-2728(98)00167-4. [DOI] [PubMed] [Google Scholar]
- Thomas LR, Henson A, Reed JC, Salsbury FR, Thorburn A. Direct binding of Fas-associated death domain (FADD) to the tumor necrosis factor-related apoptosis-inducing ligand receptor DR5 is regulated by the death effector domain of FADD. J Biol Chem. 2004;279:32780–32785. doi: 10.1074/jbc.M401680200. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Tsang AH, Chung KK. Oxidative and nitrosative stress in Parkinson’s disease. Biochim Biophys Acta. 2009;1792:643–650. doi: 10.1016/j.bbadis.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Van der Putten H, Wiederhold KH, Proust A, Baribieri S, Misty C, Danner S, Kauffmann S, Hoefele K, Spooren WPJM, Ruegg MA. Neuropathology in mice expressing human alpha-synuclein. J Neurosci. 2000;20:6021–6029. doi: 10.1523/JNEUROSCI.20-16-06021.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Dawson VL, Dawson TM. Oxidative stress and genetics in the pathogenesis of Parkinson’s disease. Neurobiol Dis. 2000;7:240–250. doi: 10.1006/nbdi.2000.0319. [DOI] [PubMed] [Google Scholar]
- Zheng XL, Sun HX, Liu XL, Chen YX, Qian BC. Astilbic acid induced COLO 205 cell apoptosis by regulating Bcl-2 and Bax expression and activating caspase-3. Acta Pharmacol Sin. 2004;25:1090–1095. [PubMed] [Google Scholar]
- Zourlidou A, Smith MDP, Latchman DS. Modulation of cell death by α-synuclein is stimulus-dependent in mammalian cells. Neurosci Lett. 2003;340:234–238. doi: 10.1016/s0304-3940(03)00081-8. [DOI] [PubMed] [Google Scholar]