Abstract
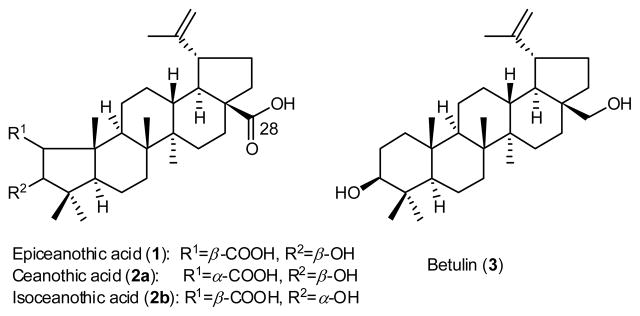
Epiceanothic acid (1) is a naturally occurring, but very rare pentacyclic triterpene with a unique pentacyclic triterpene (PT) structure. An efficient synthesis of 1 starting from betulin (3) has been accomplished in 12 steps with a total yield of 10% in our study. Compound 1 and selected synthetic intermediates were further evaluated as anti-HIV-1 agents, inhibitors of glycogen phosphorylase (GP), and cytotoxic agents. Compound 1 exhibited moderate HIV-1 inhibition. Most importantly, compound 5, with an opened A-ring, showed significant GP inhibitory activity with an IC50 of 0.21 μM, suggesting a potential for development as an anti-diabetic agent. On the other hand, compound 12, with a closed A-ring, showed potent cytotoxicity against A549 and MCF-7 human tumor cell lines, with IC50 values of 0.89 and 0.33 μM, respectively. These results suggest that the A-ring of PTs is an important pharmacophore that could be modified to involve different biological activities.
Keywords: epiceanothic acid, pentacyclic triterpene, anti-HIV agents, glycogen phosphorylase inhibitors, cytotoxic agents
Pentacylic triterpenes (PTs), a group of widespread natural compounds, possess several intriguing biological activities, such as anti-HIV, antitumor, anti-diabetic, anti-inflammatory, antibacterial, antiviral, antiparasitic, hepatoprotective, wound healing, antioxidant, antipruritic, antiangiogenic, antiallergic, and immunomodulatory activities.1–5 In recent years, PTs have been the focus of much interest due to their significant therapeutic potentials. The anti-HIV and antitumor activities of PTs have received the most attention, as several synthetic PT derivatives have advanced into clinical trials [e.g. PA-457 (DSB, Bevirimat, MPC-4326)6,7 and PA-1050040 for AIDS therapy, and betulinic acid, CDDO, and CDDO-Me for cancer therapy). Our previous investigation also showed that PTs represent a new class of glycogen phosphorylase (GP) inhibitors, which may be a key contributing mode of action in their anti-diabetic activity.8–10
Epiceanothic acid (EA, 1) (Figure 1) is a naturally occurring ceanothane-type PT isolated from the seeds of the traditional Chinese medicine Ziziphus jujuba var. spinosa (Bunge) Hu. and the stings of Gleditsia sinensis Lam.11–13 It is reported to possess strong anti-HIV-1 replication activity in HIV-1IIIB infected C8166 cell lines (EC50<0.064 μg/mL).12,13 Compound 1 has two natural configurational isomers, ceanothic acid (2a)14–18 and isoceanothic acid (2b).19 Their structures differ from that of 1 only in the orientations of the 2-carboxylic acid (2a) and 3-hydroxy group (2b) in the A-ring. Compound 2a was reported to possess anti-microbial and cytotoxic activity,20–22 and its derivatives were found to be potent cancer chemopreventive agents.23
Figure 1.
Structures of epiceanothic acid (1) and related PT compounds (2a, 2b, 3).
Despite its obvious potential, only limited research has been reported on 1, because it is very rare in nature. Therefore, it is highly desirable to establish a reliable access to 1-analogs for biological evaluation. Herein, we report an efficient synthetic route to 1 in 12 steps with a total yield of 10% starting from betulin (3), which is easily available at a low price. Compound 1 and the pentacylic triterpene intermediates24 were then evaluated for anti-HIV-1, GP inhibitory, and cytotoxic activities.
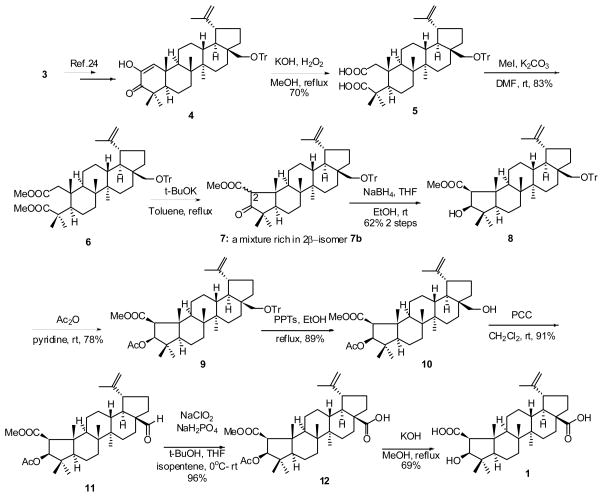
As shown in Scheme 1, crude dione compound 4, which was readily prepared from 3 in 67% yield,25 was treated with KOH and H2O2 in MeOH26 to give the dicarboxylate compound 5 (70%). Methylation of 5 with iodomethane afforded compound 6 (83%). Dieckmann condensation of 6 (t-BuOK/toluene)27 gave crude 5-membered keto ester 7 as a mixture of 2α-ester (7a) and 2β-ester (7b). This mixture could not be separated by column chromatography, as isomerization could be observed during the purification process. The major component was likely 7b, based on a comparison with our related work on oleanane-type PTs (unpublished data) and identification of the reduction product 8. Reduction of the crude 7 in the presence of NaBH4 in THF and EtOH gave 2β-methoxycarbonyl-3β-hydroxy compound 8 as the only product (62% over two steps). Acetylation of the 3β-hydroxy group was accomplished with acetic anhydride in pyridine in 78% yield. Deprotection of 9 with pyridinium p-toluenesulfonate (PPTs) in EtOH gave primary alcohol 10 (89%). Oxidation of 10 with pyridinium chlorochromate (PCC) gave aldehyde 11 in good yield (91%), which was further oxidized with NaClO2 and NaH2PO4 in a mixture of t-BuOH/THF/2-methyl-2-butene25 to afford carboxylic acid 12 in high yield (96%). Hydrolysis of 12 afforded epiceanothic acid (1) in 69% yield.
Scheme 1.
Synthesis of epiceanothic acid (1) from betulin (3).
Compound 1, betulin (3), four selected pentacylic triterpene intermediates (9–12) and PA-457 (as a positive control) were tested in acutely HIV-1NL4-3 infected MT-4 cells, according to the literature methods.28–31 However, 3 and 9–12 did not exhibit significant antiviral activity in our assay. In addition, despite the previous report, compound 1 demonstrated only moderate anti-HIV activity with an EC50 value of 15.6 μM and a therapeutic index (TI) of 2.49 (Table 1). Esterification or acylation of the free carboxylic acid and hydroxy functionalities in A-ring may be a contributing factor to the loss of potency, as seen with 1 vs. 9, 10, 11, and 12. Interestingly, 2a, which has a 1α-COOH rather than the 1β-COOH in 1, showed no anti-HIV-1 activity (unpublished data), suggesting that the configuration of the 2-carboxylic acid group has an impact on the anti-HIV-1 activity. Compared with the reported data,12,13 our results also suggested that different HIV viral strains may have different sensitivity to 1. The molecular mechanisms underlying this phenomenon remain to be elucidated.
Table 1.
Anti-HIV-1 replication activity of PT compounds in HIV-1NL4-3 infected MT-4 cell lines
Results are averaged from two experiments. Compounds 3 and 9–12 were also tested, but were not active.
Type 2 diabetes mellitus is a severe disease with great economic consequences. Hepatic glucose output is elevated in type 2 diabetic patients, and GP is the enzyme that catalyzes glycogenolysis (release of monomeric glucose from the glycogen polymer storage form, resulting in abnormally high glucose production). GP inhibitors lower glucose level acutely and chronically in diabetic animal models, representing promising new hypoglycemic agents for the treatment of type 2 diabetes mellitus.
In our continuing efforts to find potent GP inhibitors from PT compounds, all of the related compounds (1, 3, 5–6, and 8–12) in our study were evaluated for inhibitory activity against rabbit muscle glycogen phosphorylase a (RMGPa).32 As described previously, the activity of RMGPa was measured by detecting the amount of phosphates released from glucose-1-phosphates in the direction of glycogen synthesis.33 The assay results are summarized in Table 2. Most of the newly synthesized PTs exhibited inhibitory activity against RMGPa.
Table 2.
Inhibition of RMGPa by synthesized PT compounds
| Compound | IC50a (μM) ±SD | Compound | IC50a (μM) ±SD |
|---|---|---|---|
| 3 | 41.5±3.2 | 10 | NIb |
| 5 | 0.21±0.1 | 11 | 53.8±4.9 |
| 6 | 2.87±0.1 | 12 | 46.4±3.6 |
| 8 | 20.1±1.1 | 1 | 194.1±17.5 |
| 9 | 15.2±0.7 | Caffeinec | 75.3±6.6 |
Values are means of three experiments.
NI = no inhibition.
Caffeine was used as a positive control.
As shown in Table 2, opening of the A-ring of lupine-type PTs significantly improved the GP inhibitory potency. The A-ring opened compound 6 showed potent GP inhibitory activity with an IC50 value of 2.87 μM, and was 14-fold more potent than betulin (3, IC50 41.5 μM). Another A-ring opened compound 5 showed the most potent GP inhibitory activity with an IC50 value of 0.21 μM. Compound 5 was 14-fold more potent than its methylated parent compound 6 and almost 200-fold more potent than 3. The trityl ether compounds 8 and 9 were slightly more potent than 3, and compounds 11 and 12 showed comparable potency to 3. However, 1 exhibited only weak GP inhibitory activity with an IC50 value of 194.1 μM. Overall, the A-ring was proven to be a very important pharmacophore for modifying PTs’ GP inhibition activity, and the preliminary SAR analysis showed that opening of the A-ring of lupine-type PTs may enhance GP inhibition. The most potent GP inhibitor, 5 (IC50 0.21 μM), merits further development as a potential anti-diabetic agent.
The cytotoxic activity of 1 and its synthetic intermediates (5, 6, 8–12) was tested in vitro using the MTT cytotoxicity assay,34–35 and the results are summarized in Table 3. Five different cancer cell lines were used including PC3 (human prostate cancer), A549 (human lung carcinoma), MCF-7 (human breast cancer), HeLa (human epithelial carcinoma), and BGC-823 (human gastric carcinoma). Adriamycin was used as the reference standard.
Table 3.
Cytotoxic activity of synthesized PT compounds
| Compound | IC50a(μM)±SD for cancer cell lines |
||||
|---|---|---|---|---|---|
| PC3 | A549 | MCF-7 | HeLa | BGC-823 | |
| 5 | 6.75±0.57 | 136.1±10.1 | 559.6±45.5 | N/Ab | N/Ab |
| 6 | 5.32±0.43 | 996.5±80.7 | 373.6±23.7 | 17.1±0.9 | 9.54±0.78 |
| 8 | NAb | 770.1±65.6 | 397.5±15.8 | NAb | 19.3±1.66 |
| 9 | NAb | 892.5±46.8 | 565.3±22.4 | NAb | NAb |
| 10 | 66.3±5.56 | 62.9±5.69 | 603.5±44.5 | 63.1±2.8 | 25.9±1.49 |
| 11 | 8.51±0.73 | 194.2±10.6 | 32.5±2.79 | 105.4±11.2 | 12.7±0.85 |
| 12 | 10.8±0.08 | 0.89±0.07 | 0.33±0.04 | 56.7±4.9 | 7.64±0.19 |
| 1 | 19.7±1.55 | 16.8±2.13 | 87.2±8.46 | 19.1±2.1 | 2.41±0.22 |
| Adriamycinc | 0.68±0.07 | 0.54±0.04 | 1.65±0.11 | 1.35±0.08 | 1.33±0.09 |
Values are means of three experiments.
NA = no activity.
Adriamycin was used as a positive control.
Overall, different cancer cell lines showed different sensitivity to the PT compounds. The trityl ether compounds 8 and 9 were inactive against the PC3 cell line, while aldehyde 11 (IC50 = 8.51 μM) and carboxylic acid 12 (IC50 = 10.8 μM) were more cytotoxic than the corresponding primary alcohol 10 (IC50 = 66.3 μM). With a free carboxylic acid and hydroxy group in the A-ring, 1 (IC50 = 19.7 μM) showed slightly decreased activity compared with 12. The A-ring opened compounds 5 and 6 exhibited more potent cytotoxicity against PC3 with IC50 values of 6.75 and 5.32 μM, respectively.
Noticeably, natural product 1 showed the greatest cytotoxicity against gastric carcinoma BGC-823 with an IC50 value of 2.41 μM. The potency against this cell lines was at least 10-fold higher than against all the remaining four tested cancer cell lines, thus, demonstrating selective sensitivity. Compound 12, with ester-protected carboxylic acid and hydroxy groups in the A-ring relative to 1, showed three-fold decreased activity with an IC50 of 7.64 μM. All of the remaining PTs showed moderate to little cytotoxic activity against BGC-823.
Generally, the A549, MCF-7, and Hela cancer cell lines were insensitive to the tested PT analogs. However, compound 12 showed selective potent cytotoxicity against A549 and MCF-7 with IC50 values of 0.89 and 0.33 μM, respectively. Interestingly, the free carboxylic acid and hydroxy groups in the A-ring of 1 decreased its cytotoxic activity against A549 and MCF-7 by more than 20-fold compared with 12, which is opposite to the activity profile against BGC-823. The activity of 12 against MCF-7 was slightly better than that of adriamycin, suggesting that it merits further SAR and mechanism of action study.
In summary, an efficient access to epiceanothic acid (1) starting from betulin (3) has been developed in 12 steps with an overall yield of 10%. Because 3 is readily available, this preparation gives practical access to the very rare natural PT epiceanothic acid and enables further pharmacological research and drug development.
The synthesized PT derivatives were evaluated biologically as anti-HIV-1 agents, inhibitors of glycogen phosphorylase, and cytotoxic agents. The results showed that 1 has moderate potency against HIV-1NL-43 virus strains. In addition, compound 5 with two free carboxylic acids in an opened A-ring showed potent GP inhibitory activity with an IC50 value of 0.21 μM. To our knowledge, it is the most potent PT derived GP inhibitor thus far. On the other hand, compound 12, with a closed A-ring and protected carboxylic acid and hydroxy groups, exhibited potent cytotoxic activity against A549 and MCF-7 cancer cell lines with IC50 values of 0.89 and 0.33 μM, respectively, which were comparable or better than those of adriamycin. These results suggest that the A-ring is an important pharmacophore for both GP inhibitory and cytotoxic activity of PTs. Different modifications on the A-ring could change the bioactivity of epiceanothic acid derivatives. Further mechanistic and pharmacologic studies of these compounds are currently ongoing.
Acknowledgments
H.B. Sun acknowledges the following agencies for funding: European Foundation for the Study of Diabetes (2007 Grant Awards for Collaborative Diabetes Research between China and Europe), National Natural Science Foundation of China (grants 30672523 and 90713037) and Ministry of Education of China (grants 706030, 20050316008 and NCET-05-0495). Thanks are due to Dr. Chin-Ho Chen at Duke University for anti-HIV replication screening. This research was also partially supported by Grant AI-077417 from the National Institute of Allergies and Infectious Diseases awarded to K. H. Lee.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Ovesna Z, Vachalkova A, Horvathova K, Tothova D. Neoplasma. 2004;51:327. [PubMed] [Google Scholar]
- 2.Sun H, Fang WS, Wang WZ, Hu C. Bot Stud. 2006;47:339. [Google Scholar]
- 3.Sultana N, Ata A. J Enzyme Inhib Med Chem. 2008;23:739. doi: 10.1080/14756360701633187. [DOI] [PubMed] [Google Scholar]
- 4.Laszczyk MN. Planta Med. 2009;75:1549. doi: 10.1055/s-0029-1186102. [DOI] [PubMed] [Google Scholar]
- 5.Petronelli A, Pannitteri G, Testa U. Anticancer Drugs. 2009;20:880. doi: 10.1097/CAD.0b013e328330fd90. [DOI] [PubMed] [Google Scholar]
- 6.Qian K, Nitz TJ, Yu D, Allaway GP, Morris-Natschke SL, Lee KH. Natural Product Chemistry for Drug Discovery, RSC Series. 2009;13:374. [Google Scholar]
- 7.Yu D, Wild CT, Martin DE, Morris-Natschke SL, Chen CH, Allaway GP, Lee KH. Expert Opin Investig Drugs. 2005;14:681. doi: 10.1517/13543784.14.6.681. [DOI] [PubMed] [Google Scholar]
- 8.Wen XA, Zhang P, Liu J, Zhang LY, Wu XM, Ni PZ, Sun HB. Bioorg Med Chem Lett. 2006;16:722. doi: 10.1016/j.bmcl.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Wen XA, Sun HB, Liu J, Cheng KG, Zhang P, Zhang LY, Hao J, Ni PZ, Zographos SE, Leonidas DD, Alexacou KM, Gimisis T, Hayes JM, Oikonomakos NG. J Med Chem. 2008;51:3540. doi: 10.1021/jm8000949. [DOI] [PubMed] [Google Scholar]
- 10.Zhang P, Hao J, Liu J, Lu Q, Sheng H, Zhang L, Sun H. J Nat Prod. 2009;72:1414. doi: 10.1021/np9002367. [DOI] [PubMed] [Google Scholar]
- 11.Li LM, Liao X, Peng SL, Ding LS. J Integr Plant Biol. 2005;47:494. [Google Scholar]
- 12.Li, W., Zhao, W. 101062936, 2007; 15pp.
- 13.Li WH, Zhang XM, Tian RR, Zheng YT, Zhao WM, Qiu MH. J Asian Nat Prod Res. 2007;9:551. doi: 10.1080/10286020600883419. [DOI] [PubMed] [Google Scholar]
- 14.Boyer JP, Eade RA, Locksley H, Simes JJH. Aust J Chem. 1958;11:236. [Google Scholar]
- 15.Birch AJ, Ritchie E, Speake RN. J Chem Soc. 1960:3593. [Google Scholar]
- 16.Ikram M, Tomlinson H. Planta Med. 1976;29:289. doi: 10.1055/s-0028-1097664. [DOI] [PubMed] [Google Scholar]
- 17.Roitman JN, Jurd L. Phytochemistry. 1978;17:491. [Google Scholar]
- 18.Sharma SC, Kumar R. Pharmazie. 1983;38:65. [Google Scholar]
- 19.Merkuza VM, Mascaretti OA, Crohare R, Ruveda EA. Phytochemistry. 1971;10:908. [Google Scholar]
- 20.Lee SS, Chen WC, Huang CF, Su Y. J Nat Prod. 1998;61:1343. doi: 10.1021/np9800856. [DOI] [PubMed] [Google Scholar]
- 21.Jou SJ, Chen CH, Guh JH, Lee CN, Lee SS. J Chin Chem Soc (Taipei, Taiwan) 2004;51:827. [Google Scholar]
- 22.Suksamrarn S, Panseeta P, Kunchanawatta S, Distaporn T, Ruktasing S, Suksamrarn A. Chem Pharm Bull. 2006;54:535. doi: 10.1248/cpb.54.535. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa-Goto K, Yamada K, Taniguchi M, Tokuda H, Lee KH. Bioorg Med Chem Lett. 2009;19:3378. doi: 10.1016/j.bmcl.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.All newly synthesized compounds provided satisfactory MS, 1H NMR, and 13C NMR spectra without any discernable impurities. Selected analytical and spectroscopic data are shown as follows. Compound 5: [α]D +25.0 (c 0.18, CH2Cl2); IR (film, cm−1): 2950, 2869, 1714, 1693, 1560, 1489, 1449, 1265, 1228, 1063, 883, 743, 706, 632; 1H NMR (CDCl3) δ 0.51 (3H, s), 0.86 (3H, s), 0.89 (3H, s), 1.16 (3H, s), 1.24 (3H, s), 1.64 (3H, s), 0.96–2.63 (23H, m), 2.92 (1H, d, J = 8.9 Hz), 3.13 (1H, d, J = 8.8 Hz), 4.52 (1H, s), 4.57 (1H, s), 7.21–7.33 (9H, m), 7.48–7.51 (6H, m); 13C NMR (CDCl3) δ 14.8, 15.9, 19.1, 19.3, 20.7, 21.2, 25.0, 27.0, 29.4, 29.9, 30.1, 33.7, 35.2, 37.1, 40.5, 40.9, 41.8, 42.9, 45.7, 47.6, 47.8, 48.4, 48.9, 59.5, 85.9, 109.5, 126.8, 127.7, 128.8, 144.5, 150.8, 178.3, 187.3; ESI-MS m/z: 753.5 [M+Na]+; HRMS for C49H62O5+Na calcd 753.44895, found 753.4505. Compound 6: [α]D −5.0 (c 0.14, CH2Cl2); IR (film, cm−1): 2947, 2868, 1726, 1597, 1449, 1275, 1151, 1064, 764, 706, 632; 1H NMR (CDCl3) δ 0.52 (3H, s), 0.83 (3H, s), 0.92 (3H, s), 1.21 (3H, s), 1.22 (3H, s), 1.62 (3H, s), 0.76–1.74 (16H, m), 2.11–2.40 (7H, m), 2.90 (1H, d, J = 8.8 Hz), 3.12 (1H, d, J = 8.9 Hz), 3.59 (3H, s), 3.61 (3H, s), 4.50 (1H, s), 4.56 (1H, s), 7.19–7.32 (9H, m), 7.46–7.49 (6H, m); 13C NMR (CDCl3) δ 14.7, 15.9, 19.1, 19.6, 20.8, 21.8, 23.8, 25.3, 27.0, 27.8, 29.9, 30.0, 33.1, 35.2, 37.5, 40.5, 41.6, 41.8, 41.9, 42.9, 46.3, 47.6, 47.7, 48.4, 48.8, 50.7, 51.7, 59.6, 85.9, 109.3, 126.8, 127.7, 128.8, 144.5, 150.8, 171.9, 179.9; ESI-MS m/z: 781.5 [M+Na]+; HRMS for C51H66O5+Na calcd 781.48025, found 781.48348. Compound 8: [α]D −12.3 (c 0.105, CH2Cl2); IR (film, cm−1): 3456, 2945, 2866, 1733, 1632, 1597, 1449, 1375, 1160, 1063, 1030, 775, 741, 706, 632; 1H NMR (CDCl3) δ 0.48 (3H, s), 0.88 (3H, s), 0.91 (3H, s), 1.00 (3H, s), 1.05 (3H, s), 1.63 (3H, s), 0.79–1.68 (18H, m), 2.17–2.32 (4H, m), 2.69 (1H, brs), 2.91 (1H, d, J = 8.9 Hz), 3.11 (1H, d, J = 8.8 Hz), 3.65 (3H, s), 4.01 (1H, d, J = 7.3 Hz), 4.51 (1H, s), 4.57 (1H, s), 7.19–7.32 (9H, m), 7.46–7.49 (6H, m); 13C NMR (CDCl3) δ 13.9, 14.7, 16.5, 18.0, 18.8, 19.2, 23.4, 24.8, 27.1, 30.0, 30.2, 31.7, 34.1, 35.2, 37.0, 41.6, 42.7, 43.0, 47.6, 47.7, 48.9, 49.3, 50.2, 51.4, 59.5, 60.1, 62.0, 82.4, 85.8, 109.4, 126.8, 127.7, 128.8, 144.5, 150.7, 174.2; HRMS for C50H64O4+Na calcd 751.46968, found 751.47246. Compound 9: [α]D −16.7 (c 0.06, CH2Cl2); IR (film, cm−1): 3382, 2946, 2866, 1747, 1449, 1375, 1240, 1153, 1063, 764, 705, 632; 1H NMR (CDCl3) δ 0.50 (3H, s), 0.80 (3H, s), 0.88 (3H, s), 1.08 (3H, s), 1.11 (3H, s), 1.62 (3H, s), 1.99 (3H, s), 0.76–1.67 (18H, m), 2.16–2.23 (3H, m), 2.40 (1H, d, J = 7.7 Hz), 2.90 (1H, d, J = 8.8 Hz), 3.12 (1H, d, J = 8.8 Hz), 3.54 (3H, s), 4.50 (1H, s), 4.56 (1H, s), 5.14 (1H, d, J = 7.5 Hz), 7.19–7.32 (9H, m), 7.46–7.49 (6H, m); 13C NMR (CDCl3) δ 13.3, 14.7, 16.2, 17.8, 19.0, 19.1, 20.8, 23.7, 24.7, 27.1, 29.9, 30.2, 31.1, 34.0, 35.2, 37.0, 41.4, 42.3, 42.6, 47.5, 47.7, 47.8, 48.9, 50.2, 51.1, 59.5, 59.8, 62.1, 83.6, 85.8, 109.4, 126.8, 127.7, 128.8, 144.5, 150.7, 170.5,171.6; ESI-MS m/z: 793.7 [M+Na]+; HRMS for C52H66O5+Na calcd 793.48025, found 793.47867. Compound 10: [α]D −0.67 (c 0.06, CH2Cl2); IR (film, cm−1): 3355, 2945, 2866, 1749, 1458, 1376, 1242, 1159, 1026, 873, 739; 1H NMR (CDCl3) δ 0.76 (3H, s), 0.97 (3H, s), 1.02 (3H, s), 1.11 (3H, s), 1.18 (3H, s), 1.67 (3H, s), 2.00 (3H, s), 0.76–1.67 (18H, m), 2.32–2.41 (1H, m), 2.44 (1H, d, J = 7.7 Hz), 3.33 (1H, d, J = 10.8 Hz), 3.57 (3H, s), 3.79 (1H, d, J = 10.7 Hz), 4.58 (1H, d, J = 1.4 Hz), 4.67 (1H, d, J = 1.8 Hz), 5.17 (1H, d, J = 7.6 Hz); 13C NMR (CDCl3) δ 13.4, 14.8, 16.4, 17.8, 19.0, 19.1, 20.8, 23.8, 24.8, 27.3, 29.3, 29.8, 31.1, 34.0, 34.1, 37.1, 41.7, 42.4, 42.9, 47.76, 47.81, 47.85, 48.8, 50.3, 51.2, 59.9, 60.6, 62.2, 83.6, 109.7, 150.4, 170.5, 171.7; HRMS for C33H52O5+Na calcd 551.37070, found 551.37012. Compound 11: [α]D −4.67 (c 0.03, CH2Cl2); IR (film, cm−1): 2946, 2867, 1747, 1452, 1377, 1360, 1241, 1061, 1042, 738; 1H NMR (CDCl3) δ 0.81 (3H, s), 0.91 (3H, s), 0.97 (3H, s), 1.10 (3H, s), 1.18 (3H, s), 1.69 (3H, s), 2.00 (3H, s), 0.85–2.11 (20H, m), 2.44 (1H, d, J = 7.5 Hz), 2.79–2.89 (1H, m), 3.58 (3H, s), 4.63 (1H, s), 4.75 (1H, s), 5.17 (1H, d, J = 7.5 Hz) 9.68 (1H, s); 13C NMR (CDCl3) δ 13.5, 14.3, 16.3, 17.8, 19.00, 19.04, 20.9, 23.7, 25.1, 29.0, 29.3, 30.0, 31.1, 33.4, 34.2, 38.5, 41.7, 42.4, 42.8, 47.6, 47.9, 48.2, 50.4, 51.2, 59.3, 60.0, 62.2, 83.6, 110.3, 149.6, 170.5, 171.6, 206.6; ESI-MS m/z: 549.0 [M+Na]+; HRMS for C33H50O5+Na calcd 549.35505, found 549.35424. Compound 12: [α]D −10.67 (c 0.09, CH3OH); IR (film, cm−1): 3346, 2948, 2868, 1748, 1701, 1457, 1377, 1241, 1194, 1158, 1029, 736, 638; 1H NMR (C5H5N) δ 0.87 (3H, s), 1.03 (3H, s), 1.06 (3H, s), 1.15 (3H, s), 1.37 (3H, s), 1.76 (3H, s), 2.03 (3H, s), 1.19–1.95 (16H, m), 2.23–2.28 (2H, m), 2.60–2.76 (2H, m), 2.80 (1H, d, J = 7.7 Hz), 3.46–3.53 (1H, m), 3.58 (3H, s), 4.73 (1H, s), 4.91 (1H, s), 5.47 (1H, d, J = 7.7 Hz); 13C NMR (C5H5N) δ 13.8, 14.6, 16.5, 17.8, 19.0, 19.2, 20.4, 24.2, 25.4, 30.2, 30.7, 31.0, 32.7, 34.3, 37.3, 38.1, 41.6, 42.3, 42.8, 47.6, 47.9, 49.5, 50.4, 50.8, 56.3, 59.9, 62.0, 83.8, 109.7, 150.9, 170.1, 171.5, 178.5; ESI-MS m/z: 565.0 [M+Na]+; HRMS for C33H50O6-H calcd 541.35346, found 541.35249. Compound 1 (Epiceanothic acid): [α]D −14.5 (c 0.076, CH3OH) [lit.4, [α]D −16.3 (c 0.08, CH3OH)]; IR (film, cm−1): 3476, 2951, 2868, 1696, 1643, 1459, 1377, 1320, 1237, 1187, 1058, 884; 1H NMR (C5H5N) δ 1.08 (3H, s), 1.13 (3H, s), 1.15 (3H, s), 1.20 (3H, s), 1.67 (3H, s), 1.74 (3H, s), 1.13–2.22 (18H, m), 2.60–2.64 (1H, m), 2.73–2.80 (1H, m), 2.89 (1H, d, J = 7.2 Hz), 3.43–3.49 (1H, m), 4.66 (1H, d, J = 7.4 Hz), 4.69 (1H, s), 4.86 (1H, s); 13C NMR (C5H5N) δ14.6, 15.0, 16.9, 18.4, 19.5, 19.9, 24.6, 25.9, 30.5, 31.2, 32.1, 33.0, 34.8, 37.6, 38.5, 42.0, 42.9, 43.1, 47.8, 48.1, 49.8, 51.1, 56.5, 62.7, 63.1, 83.1, 110.0, 151.1, 175.7, 178.8; ESI-MS m/z: 485.0 [M−H]−; HRMS for C30H46O5-H calcd 485.32725, found 485.32683.
- 25.Hao J, Zhang P, Wen X, Sun H. J Org Chem. 2008;73:7405. doi: 10.1021/jo801232s. [DOI] [PubMed] [Google Scholar]
- 26.Urban M, Sarek J, Klinot J, Korinkova G, Hajduch M. J Nat Prod. 2004;67:1100. doi: 10.1021/np049938m. [DOI] [PubMed] [Google Scholar]
- 27.Konoike T, Takahashi K, Kitaura Y, Kanda Y. Tetrahedron. 1999;55:14901. [Google Scholar]
- 28.HIV-1NL4-3 replication inhibition assay in MT-4 lymphocytes: A previously described HIV-1 infectivity assay was used.29–31 A 96-well microtiter plate was used to set up the HIV-1NL4-3 replication screening assay. NL4-3 variants at a multiplicity of infection (MOI) of 0.01 were used to infect MT-4 cells. Culture supernatants were collected on day 4 post-infection for the p24 antigen capture using an ELISA kit from ZeptoMetrix Corporation (Buffalo, NY).
- 29.Huang L, Ho P, Lee KH, Chen CH. Bioorg Med Chem Lett. 2006;14:2279. doi: 10.1016/j.bmc.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 30.Yu DL, Sakurai Y, Chen CH, Chang FR, Huang L, Kashiwada Y, Lee KH. J Med Chem. 2006;49:5462. doi: 10.1021/jm0601912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian K, Yu D, Chen CH, Huang L, Morris-Natschke SL, Nitz TJ, Salzwedel K, Reddick M, Allaway GP, Lee KH. J Med Chem. 2009;52:3248. doi: 10.1021/jm900136j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enzyme assay: The inhibitory activity of the test compounds against rabbit muscle glycogen phosphorylase a (GPa) was monitored using microplate reader (BIO-RAD) based on the published method1. In brief, GPa activity was measured in the direction of glycogen synthesis by the release of phosphate from glucose-1-phosphate. Each test compound was dissolved in DMSO and diluted at different concentrations for IC50 determination. The enzyme was added into 100 L of buffer containing 50 mM Hepes (pH = 7.2), 100 mM KCl, 2.5 mM MgCl2, 0.5 mM glucose-1-phosphate, 1 mg/mL glycogen and the test compound in 96-well microplates (Costar). After the addition of 150 L of 1 M HCl containing 10 mg/mL ammonium molybdate and 0.38 mg/mL malachite green, reactions were run at 22 °C for 25 min, and then the phosphate absorbance was measured at 655 nm. The IC50 values were estimated by fitting the inhibition data to a dose-dependent curve using a logistic derivative equation.
- 33.Martin WH, Hoover DJ, Armento SJ, Stock IA, McPherson RK, Danley DE, Stevenson RW, Barrett EJ, Treadway JL. Proc Natl Acad Sci USA. 1998;95:1776. doi: 10.1073/pnas.95.4.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MTT assay: The MTT assay was carried out as described previously.31 Cells were seeded in 96-well plates and incubated in the CO2 incubator at 37 °C. When the cells adhered, compounds at different concentrations (0.0001, 0.0005, 0.001, 0.005, 0.01, 0.05 or 0.1mmol·L−1) were added to every well. After incubation for another 48 h, 20 μL MTT (5%) was added to each well and incubated for an additional 4 h. The viable cells were stained with MTT and scanned with an electrophotometer at 570 nm. Each concentration treatment was done in triplicate wells. The IC50 values were estimated by fitting the inhibition data to a dose-dependent curve using a logistic derivative equation.
- 35.INVITTOX. Protocol. Vol. 17. 1980. [Google Scholar]