Abstract
The hippocampus is a brain region that is critical for spatial learning, context-dependent memory, and episodic memory. It receives major inputs from the medial entorhinal cortex (MEC) and the lateral entorhinal cortex (LEC). MEC neurons show much greater spatial firing than LEC neurons in a recording chamber with a single, salient landmark. The MEC cells are thought to derive their spatial tuning through path integration, which permits spatially selective firing in such a cue-deprived environment. In accordance with theories that postulate two spatial mapping systems that provide input to the hippocampus—an internal, path-integration system and an external, landmark-based system—it was possible that LEC neurons can also convey a spatial signal, but that the signal requires multiple landmarks to define locations, rather than movement integration. To test this hypothesis, neurons from the MEC and LEC were recorded as rats foraged for food in cue-rich environments. In both environments, LEC neurons showed little spatial specificity, whereas many MEC neurons showed a robust spatial signal. These data strongly support the notion that the MEC and LEC convey fundamentally different types of information to the hippocampus, in terms of their spatial firing characteristics, under various environmental and behavioral conditions.
Keywords: medial entorhinal cortex, medial temporal lobe, parahippocampal, spatial orientation, hippocampus
Introduction
The hippocampus is critically involved in certain forms of spatial learning, context-dependent learning, and declarative memory (O’Keefe and Nadel, 1978; Squire, 1987; Cohen and Eichenbaum, 1993). Despite years of research, elucidation of the precise computations performed by the hippocampus has been hampered by a limited knowledge of its input representations from the entorhinal cortex. The medial entorhinal cortex (MEC) receives major input from the dorsal presubiculum and retrosplenial cortex (Witter and Amaral, 2004), which contain directionally and spatially tuned neurons (Taube et al., 1990; Chen et al., 1994; Cacucci et al., 2004), and from postrhinal cortex, which is connected with visuospatial regions of the neocortex and has been linked to contextual processing (Bucci et al., 2000; Norman and Eacott, 2005). The lateral entorhinal cortex (LEC) receives major input from the perirhinal cortex, which is connected with unimodal sensory areas and appears to be involved in the processing of configurations of objects (Norman and Eacott, 2005).
A major advance in the understanding of hippocampal input representations was the discovery of MEC grid cells, which fire in multiple locations that form a triangular (or hexagonal) grid covering the entire surface of the recording chamber (Hafting et al., 2005). These cells are thought to be part of a path integration system, whereby a population-based representation of location is continuously updated by integrating the animal’s movement velocity (i.e., speed and direction) (Hafting et al., 2005; McNaughton et al., 2006).
LEC neurons show little or no spatial specificity in a simple recording chamber (Hargreaves et al., 2005). In contrast, both MEC and hippocampal neurons show strong spatial selectivity in cue-poor environments, including darkness (Muller and Kubie, 1987; Hafting et al., 2005; Leonard and McNaughton, 1990; Markus et al., 1994; Quirk et al., 1992; Quirk et al., 1990; Hargreaves et al., 2005; Hargreaves et al., 2007). Place cells can be controlled by external stimuli in complex ways that suggest other inputs into the system (O’Keefe and Conway, 1978; Shapiro et al., 1997; Knierim, 2002a). It has been suggested that two navigation systems send input to the hippocampus: an internal navigation system, based on self-motion cues, and an external system, based on configurations of environmental landmarks that define explicit locations (O’Keefe and Nadel, 1978; Touretzky and Redish, 1996). The MEC may be part of the internal navigation system (Hafting et al., 2005; McNaughton et al., 2006; Fuhs and Touretzky, 2006), which provides hippocampal place cells with the ability to maintain spatial selectivity in the absence of many salient, external, sensory cues. In contrast, the LEC may be part of the external navigation system that relies on a complex environment with multiple landmarks rather than path integration. Input from this system might explain the complex responses observed in place cells when external landmarks are manipulated. If this hypothesis is true, the LEC cells may have shown poor spatial selectivity in the cue-impoverished environment of the Hargreaves study (Hargreaves et al., 2005) only because there were not enough spatial landmarks available to define locations precisely. Moreover, it is possible that LEC neurons might display a stronger spatial signal, complementary to the MEC grid signal, under conditions with a greater number of spatial landmarks available. To address this hypothesis, MEC and LEC neurons were recorded in cue-rich environments with landmarks similar to those used in most laboratory experiments of spatial learning in rodents (such as the Morris water maze). Similar to the Hargreaves et al. (2005) study, we found that LEC neurons showed little, if any, spatial tuning in these complex environments. These results not only constitute an important replication of the Hargreaves et al. (2005) study, they generalize this result across complex and simple cue environments and support strongly the notion that the LEC conveys a signal to the hippocampus that is fundamentally different from that of the MEC.
Materials and Methods
Subjects and Surgery
Seven male, Long-Evans rats, aged 5–6 months, were housed individually on a 12:12 h reversed light-dark cycle, with free access to food and water. During behavioral training and recordings, rats were maintained at 80–90% of their free-feeding weights and recordings were performed during the dark portion of the light-dark cycle. Animal care, surgical procedures, and euthanasia were performed in accordance with National Institutes of Health (NIH) and University of Texas Health Science Center at Houston Institutional Animal Care and Use Committee (IACUC) guidelines.
Under surgical anesthesia, a custom-built recording drive (hyperdrive), allowing the independent manipulation of 20 recording probes (18 tetrodes with 2 reference electrodes for differential recording) was implanted over the right hemisphere aimed at either the MEC (n = 4) or LEC (n = 3). For MEC recordings, the most posterior tetrode in the hyperdrive array was positioned 5.0 mm lateral to the midline and 600–800 μm anterior to the transverse sinus, allowing access to neurons along the dorsocaudal to ventral axis of the MEC. For LEC recordings, the center of the hyperdrive array was placed 7.7 mm posterior to bregma and 3.2 to 4.6 mm lateral to the midline, at a lateral cant of 25° in order to access neurons along the lateral to medial axis of LEC.
Training
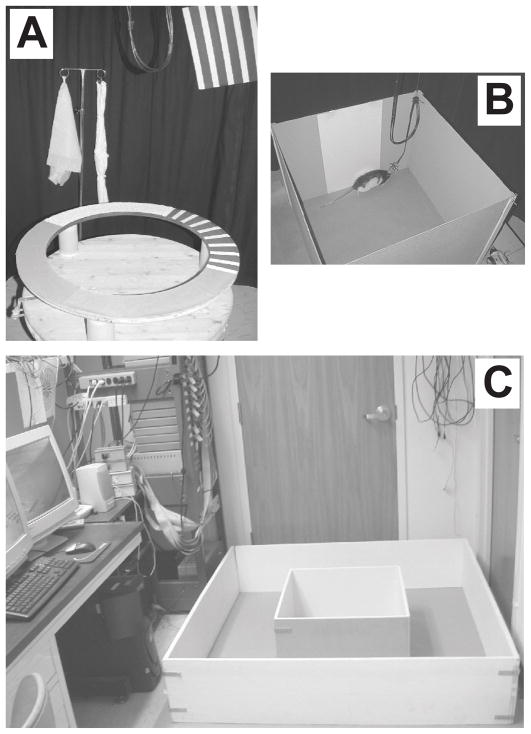
After 5–6 days of recovery, the rats were trained in two behavioral paradigms for food reward (chocolate sprinkles) over a period of 10–14 days. In the first task, the rats ran clockwise on a circular track for 30 min; in the second task, they foraged inside a large box. The circular track (56 cm inner diameter, 76 cm outer diameter) contained four different textured surfaces as proximal visual and tactile cues, each covering one quarter of the track (the textures were a gray rubber mat with a pebbled surface, brown medium-grit sand paper, beige carpet pad material, and gray duct tape with white tape stripes) (Fig. 1a). The track was placed in the center of the room surrounded by a black curtain (275 cm diameter) reaching from ceiling to floor, with three distal cues (a brown cardboard circle, a black and white striped card, and a white card) hanging on the curtain and three distal cues (a white box, an intravenous stand with a lab coat and a blue cloth, and a roll of brown wrapping paper) standing on the floor at the perimeter of the curtain. The ceiling was covered with a black curtain and a single 25 W bulb on the ceiling was centered over the track for illumination. A white-noise generator was placed directly beneath the small table on which the track stood to mask external sounds. The large box (135 × 135 × 30 cm) was placed on the floor on brown wrapping paper in the recording electronics room located adjacent to the behavior room described above. The box was positioned next to the recording system and computer, and a single lamp in the corner of the room provided illumination (Fig 1c).
Figure 1.
Recording environments. (A) Circular track containing four textured surfaces placed in the center of a circular, black curtain. Distal landmarks were located either on the floor next to the curtain or were hung from the curtain. (B) Square box with a cue card on one of the walls used in the Hargreaves et al. (2005) study. The same box was placed in the cue-rich environment shown in C to record LEC neurons from one of the rats in the present study. (C) Small box centered inside the large box on the floor next to the recording system and computer.
During training on the circular track, the rat was carried directly into the room on a pedestal, the recording cables were connected to the headstage, and the animal was placed on the track at a random starting point and allowed to run clockwise. The chocolate sprinkles were placed at random locations by the experimenter, to avoid an association between food rewards and any particular portion of the track. Attempts at counterclockwise movements were blocked by holding a piece of cardboard on the track. During training in the large box, the rat was placed on a pedestal kept right next to the box, connected with recording cables, transferred into the box, and allowed to forage freely, searching for food reward. The chocolate sprinkles were thrown into different locations of the box for complete coverage of the box area.
Recording electronics
After postsurgical recovery, along with training, the tetrodes were slowly advanced by monitoring the EEG and multi-unit activity over the course of several days, while the rat sat quietly on a pedestal next to the recording electronics. Recordings were done with the Cheetah Data Acquisition System (Neuralynx, Bozeman, MT). For unit recordings, neural signals were amplified between 2,000–10,000 times, filtered between 600 Hz-6 kHz, digitized at 32 kHz, and stored on a PC computer. EEG signals were amplified 2,000 times, filtered between 1–475 Hz, and digitized at 1 kHz. The headstage contained a circular array of LEDs (5 red LEDs in front and 5 blue LEDS in back) and a boom arm that extended 2 green LEDs 15 cm behind the headstage. The outputs of a color CCD camera (Model 1300, Cohu, Inc., San Diego, CA), for recordings on the circular track, and a ceiling-mounted camcorder (JVC, Model GR-SXM38u), for recordings in the large box, were captured at 30 Hz by a video frame grabber (DT3120, Data Translation, Inc., Marlboro, MA). The position of the rat at each frame was defined as the center of mass of all blue and red pixels.
Experimental Protocol
Recordings began after the rats were trained to perform both tasks, such that they spent most of the time in motion with few stops, and after the electrodes were lowered into the MEC and LEC areas, by monitoring changes in the neural activity and EEG patterns. The recording locations were estimated by the total distance travelled from the brain surface, passing through areas of neural activity and cell-free zones. Usually, one of the reference tetrodes was advanced to find layer I of MEC or LEC, and then other tetrodes were adjusted relative to these reference tetrodes. Further, the MEC area was localized by the appearance of theta rhythm in the EEG and/or upon observing grid cell like activity in test recordings in a large box. The tetrodes were also lowered after recording on each day to sample different cells on subsequent days. Unit recordings were stopped when all the tetrodes reached layer 1 of MEC or LEC, indicated by lowered brain activity with no recordable cells and theta-phase reversal in the EEG in MEC (Alonso and Garcia-Austt, 1987). During recording on all days, the recording stability was assessed with baseline data collected from the rat during sleep or awake immobility for 20–30 min before and after the behavioral sessions. Each day, five recording sessions were carried out on the circular track, before recordings in the box. During standard sessions (sessions 1, 3, and 5), the configuration between the proximal cues on the track and the distal landmarks was kept identical to that during the training sessions. In mismatch sessions (sessions 2 and 4), the circular track and the distal landmarks were rotated to new configurations (Knierim, 2002a). For the present study, only data from the first standard session (Session 1) were analyzed; data from the mismatch sessions will be presented in a future publication. On completion of the track sessions, recordings were performed in the box in the cue-rich, recording electronics room. In one of the LEC rats, we used the same square box (67 × 67 × 51 cm) with a single cue-card used by (Hargreaves et al., 2005) (Fig 1b). In the remaining animals, the recordings were first done while the rats foraged in a small box (58 × 58 × 30 cm), placed in the center of the large box (Fig 1c). It is possible that LEC cells might have large place fields that would not be apparent in the small box (e.g., if the size of the field was larger than the size of the box). In order to see if the LEC might show spatial specificity in a larger arena, after 6 minutes, the walls of the small box were removed with the rat still in the box, and the rat was allowed to forage in the large box for 34 min.
After the first baseline session (i.e., sleep or awake immobility on a small pedestal), the rat was placed in a covered box for 30 s, taken in the box on a brief walk in the computer room and around the track in the behavioral room, removed from the box, and placed on a pedestal located in the center of the track. The recording cables were connected to the headstage, the rat was placed on the track at a random starting point, and the pedestal was removed. After 15 laps, the rat was placed back on the pedestal, the recording cables were disconnected, the rat was placed back into the covered box and again taken on a brief random walk, and then it was placed on a pedestal in the computer room next to the behavior room. After 5 sessions on the circular track, the small and large boxes were placed on the floor next to the recording system and the computer, the recording cables were connected to the headstage, and the rat was allowed to forage for food reward in the small and/or large boxes (as described above). Upon completion of foraging, a second baseline session was recorded while the rat sat quietly on the pedestal.
Data analysis
Unit isolation
Single units were isolated primarily based on the relative amplitudes of signals recorded simultaneously at the four slightly different locations of the wires of the tetrode. Additional waveform characteristics (such as spike width and “energy”) were also used. Using a custom interactive program running on a PC workstation, these waveform parameters were plotted as a scatter plot of one of the channels versus another and boundaries were drawn around clusters of points formed by the individual units on this scatter plot. Based on the degree of separation of the cluster from background and on the closeness and degree of potential overlap between neighboring clusters, the isolation quality of a cell was rated on a subjective scale of 1 (very well-isolated) to 4 (marginally isolated), independent of the spatial firing characteristics of the cell. All of the cells rated 4 (marginally isolated) were excluded from analysis.
Spatial information
Firing rate maps were created by segmenting the circular track into equal sized position bins and segmenting the large box into 3.2 cm square bins and, for each bin, dividing the number of spikes fired by the amount of time the rat occupied that location. The rate maps were smoothed using an adaptive binning algorithm and the quality of spatial tuning for all cells was quantified with the spatial information measure developed by Skaggs and colleagues (1996), which reflects the amount of information about the rat’s location in the environment conveyed by the firing of a single spike (in bits/spike) from the neuron. As this measure is reliable if a cell fires >50 spikes in a given session (Skaggs et al., 1996), cells that fired <50 spikes were excluded in this study. Further, fast-spiking cells with firing rates >10 Hz were assumed to be interneurons and were also excluded from further analysis (Frank et al., 2001; Hargreaves et al., 2005). The distribution of spatial information scores was analyzed with a Mann-Whitney test. The stability of firing patterns within a session was tested with a pixel-by-pixel correlation of the rate maps from the first half of the session with that of second half and analyzed with a Mann-Whitney test.
Histology
Upon completion of the experiments, marker lesions were made on a subset of the tetrode tips by passing 10 μA current for 10 seconds, a day before the perfusion. The rats were perfused transcardially with 4% formalin, after which the brain was extracted and kept in a 30% sucrose formalin solution until the brain sank. Coronal (for LEC rats) or sagittal (for MEC rats) sections of 40 μm thickness were cut on a microtome, mounted, and stained with 0.1% cresyl violet. Digital photomicrographs were taken for all serial sections and the electrode tracks were then compared with the configuration of the tetrode array and the marker lesions, to identify which track corresponded to which tetrode. After the electrode tracks were identified, a depth reconstruction of the tetrode track was carried out for each recording session, to identify the specific layers of MEC or LEC recorded in each experimental session, assuming 15% shrinkage of the tissue during histological processing. The dorsal-ventral locations of the neurons recorded in the MEC were quantified by measuring the distance from the MEC border with the postrhinal cortex. The medial-lateral locations of LEC neurons were measured as the distance from the rhinal fissure.
Results
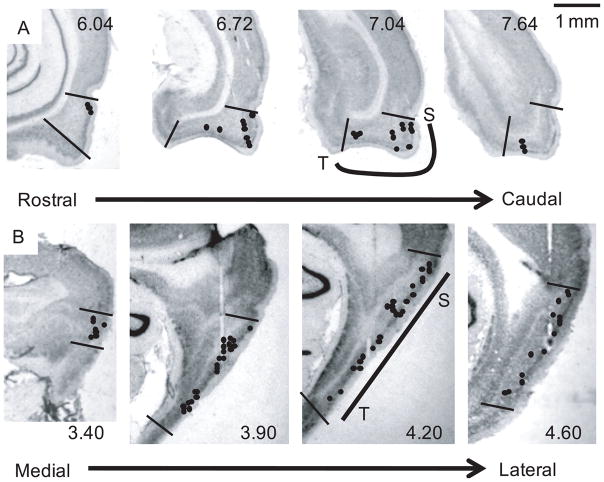
Single units were recorded from the MEC and LEC areas, while the rat ran clockwise on a circular track and then foraged in a small and large box (Figure 1). The circular track had 4 distinct textures on its surface, and the room had 6 salient landmarks in the periphery. The small box was placed inside a larger box in a complex laboratory environment, located next to a rack of electronic equipment and a computer. After foraging for 6 min in the small box, the walls were removed and the rat continued to forage for 34 min in the larger box. Figure 2 shows representative examples of tetrode tracks in the MEC and LEC. Analysis was restricted to cells from superficial layers II and III, which are considered the major input layers to the hippocampus (Witter and Amaral, 2004). The cells were recorded along the dorsocaudal to ventral axis of the MEC and along the lateral to medial axis of the LEC. Samples of 148 cells on the circular track and 120 cells in the small/large box, recorded from 7 rats over several days, were used in the analysis. Most of the cells were recorded in both behavioral paradigms on a given day.
Figure 2.
Cresyl violet stained brain sections showing recording sites in the MEC and LEC. Recording sites from each rat were mapped onto the closest sections from the Paxinos and Watson (1998) atlas and then transferred onto representative histological sections from our data set. Multiple cells were often recorded per site; thus the number of recording sites in the figures is less than the number of cells. (A). Coronal sections showing the recording sites of the LEC. Sections from left to right progress from rostral to caudal. Numbers refer to the distance (mm) posterior to bregma in the corresponding sections from Paxinos and Watson (1998). The black lines indicate approximate boundaries of LEC. As demonstrated on the section at 7.04, the region of LEC that projects to septal hippocampus (S) is near the rhinal sulcus, and more medial regions project to more temporal regions of the hippocampus (T). On the circular track, 20 cells were analyzed from layer II and 19 cells were analyzed from layer III. In the small/large box, 7 cells were analyzed from layer II and 18 cells were analyzed from layer III. Electrode tracks that missed the LEC dorsally are seen in the section at 6.72. (B) Sagittal sections showing the recording sites of the MEC. Sections from left to right progress from medial to lateral. Numbers refer to the distance (mm) lateral to the midline in the corresponding sections from Paxinos and Watson (1998). The black lines indicate approximate boundaries of MEC. As demonstrated on the section at 4.20, the region of MEC that projects to septal hippocampus (S) is in the dorsocaudal part of MEC, and more ventral regions project to more temporal regions of the hippocampus (T). On the circular track, 83 cells were analyzed from layer II and 26 cells were analyzed from layer III. In the small/large box, 68 cells were analyzed from layer II and 27 cells were analyzed from layer III. Electrode tracks are seen in the three rightmost figures. Scale bar = 1 mm.
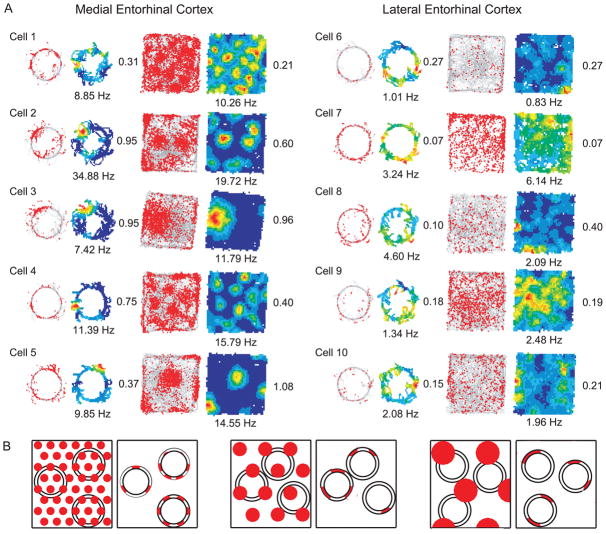
Figure 3a shows examples of MEC and LEC neurons recorded on the circular track and in the large box. Each row shows the firing of a single cell in both environments. For each environment, the left figure shows the location of the rat when each spike fired (red dots), superimposed on the trajectory of the rat (gray lines). The right figure shows the firing rate map for the cell, calculated by dividing the number of spikes in each spatial bin by the amount of time that the rat occupied that bin. Cells 1–3 are putative grid cells recorded along the dorsocaudal to ventral axis of superficial-layer MEC from the same rat (rat 159). Cells 1 and 2 fired in multiple discrete locations in the large box and the spacing between the vertices increased along the dorsocaudal to ventral axis (resulting in a fewer number of vertices appearing on the track and in the box) (Hafting et al., 2005; Brun et al., 2008). The firing fields on the circular track corresponded to the scaling of the grids in the large box, in that the number of fields on the track decreased along with the number of fields in the box. Cells 4 and 5 were recorded from a different rat, also showing spatially specific, grid-like firing in the large box and multiple fields on the circular track. Only a fraction of the MEC cells were grid cells, in agreement with Sargolini et al. (2006). We illustrate only grid cells here; see Savelli et al. (2008) for a description of the firing of non-grid cells from this sample. The quantitative analyses below are based on all cells in the sample, not just the grid cells. Note that, even though the size and number of subfields on the circular track matched qualitatively the grid scale in the box, the fields on the track did not necessarily display the regular, periodic pattern of firing displayed in the box. Figure 3b demonstrates that this irregular firing pattern is precisely what one would expect if a circular track was overlaid on top of a regular, 2-D grid pattern. Thus, the irregular pattern on the circular track is likely caused by the undersampling of the grid as the rat’s movements were confined to the circular ring.
Figure 3.
Examples of MEC and LEC activity on the circular track and in the large box. (A) Each row represents a cell recorded in both circular track and large box sessions. On the left of each pair of diagrams is shown the trajectory of the rat (gray line) and the position of the rat when that cell fired (red dots); on the right is shown the corresponding rate map. Rate maps are color-coded with red indicating peak firing rate and blue indicating no firing. Numbers near the rate maps indicate the spatial information score (right side) and peak firing rate (bottom) in that session. Cells 1–5 are from superficial MEC and cells 6–10 are from superficial LEC. The MEC neurons show spatially specific firing in both circular track and large box sessions, whereas the LEC neurons showed largely nonspecific firing. Cells 1–3 are putative grid cells recorded from the same rat on different tetrodes along the dorsocaudal to ventral axis of MEC, showing a decrease in the number of firing fields in the large box with an increase in spacing between vertices. The same cells recorded on the circular track also showed a change in the number of fields on the track, corresponding to increased grid vertex spacing. (B) Diagram illustrating how circular trajectories on underlying grid-cell patterns of different scales can lead to both periodic and nonperiodic firing patterns on the circular track.
In contrast to the MEC cells, the superficial LEC neurons (cells 6–10) showed nonspecific firing, with weak or no spatial specificity compared to the MEC neurons (Fig. 3a). Some cells fired sparsely throughout the environment (e.g., cells 6 and 8), whereas others fired at a higher rate, but with little spatial specificity (e.g., cells 7 and 9). Cell 7 appeared to fire with a bias near the north and east walls of the box, similar to boundary cells reported previously in the MEC (Savelli et al., 2008; Solstad et al., 2008) (see also the LEC data in Supplementary Figure S1 of Hargreaves et al. [2005]). We did not attempt to quantify boundary-related activity in LEC cells, as the cells are not very specific for boundaries (i.e, cell 7 also fires in the middle of the large box) and we did not perform boundary manipulations on many LEC cells. LEC cells were similarly nonspecific on the track and in the large box. Thus, neither the presence of salient local textures, distal landmarks, and a stereotyped behavior on the track, nor the presence of a highly complex visual environment in an extended recording arena in the large box, were able to produce sharp, spatially selective firing in superficial LEC neurons.
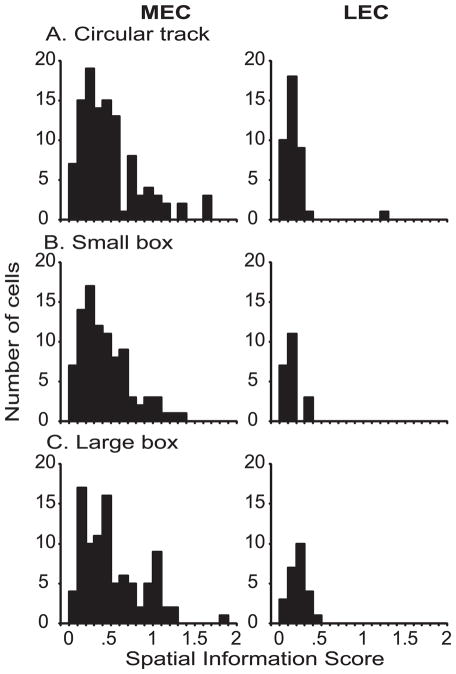
Figure 4 shows the distribution of spatial information scores (Skaggs et al., 1996) of superficial MEC and LEC neurons recorded on the circular track, in the small box inside the large box, and in the large box. In each environment, superficial MEC neurons had significantly higher spatial information scores than superficial LEC neurons (Mann-Whitney, p < .0001). In one rat (rat 151), superficial LEC neurons were recorded in a small, high-walled, gray box with a single cue card in the cue-rich environment (Fig. 1b), rather than the small box inside the large box. The same gray box was used in a cue-poor environment by Hargreaves et al. (2005). Because the floor area of this box was different from the small box inside the large box, which affects the spatial information calculation, the information scores are shown separately in Figure 5. Similar to the values observed in Hargreaves et al. (2005) and the values shown in Figure 4, there were low spatial information scores of LEC neurons in the small, gray box environment. The distributions of MEC spatial information scores in all 3 environments were also qualitatively very similar to the distribution in the cue-poor environment reported by Hargreaves et al. (2005), although differences in the sizes of the recording environments precludes a quantitative assessment of the similarity.
Figure 4.
Comparison of spatial information scores of MEC and LEC neurons. Superficial MEC neurons had significantly higher spatial information scores than did superficial LEC neurons, even in the cue-rich, complex environments of the present study. (A) Circular track (MEC: n = 109, median = 0.391, interquartile range = .22–.59; LEC: n = 39, median = .146, interquartile range = .10–.21; Mann-Whitney p < .0001). (B) Small box inside the large box (MEC: n = 92, median = .367, interquartile range = .21–.60; LEC: n = 21, median = .128, interquartile range = .09–.17; Mann-Whitney p < .0001). (C) Large box (MEC: n = 95, median = .434, interquartile range = .22–.72; LEC: n = 25, median = .212, interquartile range = .16–.28; Mann-Whitney p < .0001).
Figure 5.
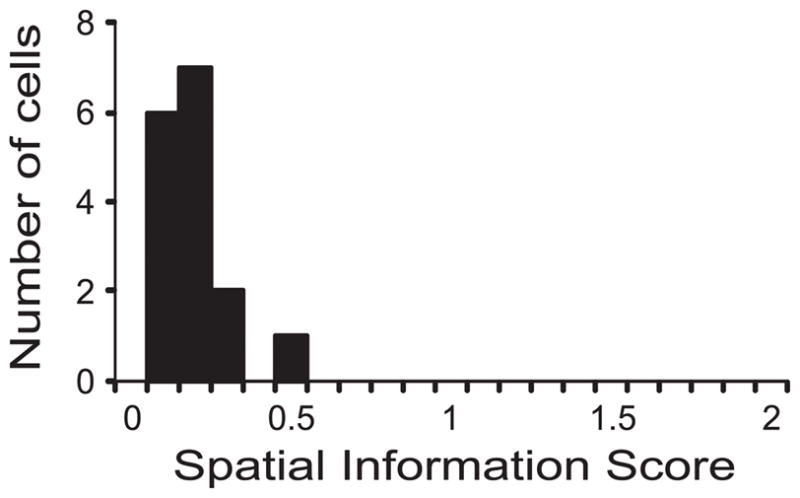
Spatial information scores of superficial LEC neurons in a square box, placed in a cue-rich, complex environment (the same box used in Hargreaves et al., 2005). The distribution of spatial information scores was very similar to that observed by Hargreaves et al. (2005) in a cue-impoverished room, showing that the presence of complex visual landmarks in the room did not improve the spatial tuning of superficial layer LEC neurons.
The MEC and LEC project topographically to the hippocampus, such that the dorsolateral projection band projects to the dorsal (septal) hippocampus and the ventromedial band projects to the ventral (temporal) hippocampus (Ruth et al., 1982; Dolorfo and Amaral, 1998). Place fields of the dorsal hippocampus are more specific than those of the ventral hippocampus (Jung et al., 1994; Kjelstrup et al., 2008), and the scale of grid cells of MEC increases correspondingly from the dorsal to ventral regions of the MEC (Hafting et al., 2005; Brun et al., 2008). It is possible that the observed differences in spatial selectivity in the present study resulted from biases in the anatomical recording sites in the MEC and LEC. That is, if the MEC recordings were biased toward the dorsolateral band and the LEC recordings were biased toward the ventromedial band, this difference in recording locations could account for the differences in spatial information between the two samples. Careful anatomical reconstruction did not reveal any clear biases, as recordings were obtained across the projection bands from both regions (e.g., Figure 2). This issue was addressed quantitatively by dividing the recordings from each area into two halves based on location along the projection zones. For MEC neurons, the recording sites in the sagittal sections were divided into two halves based on dorsal-ventral distance from the dorsal MEC border. For LEC neurons, the recording sites in the coronal sections were divided into two halves based on medial-lateral distance from the rhinal sulcus. There was no significant difference in spatial information scores between dorsocaudal and ventral MEC neurons and between medial and lateral LEC neurons (Figure 6). Thus, the differences between the two regions (MEC and LEC) in the present study cannot be due to biases in the sampling of different projection bands in the two areas.
Figure 6.
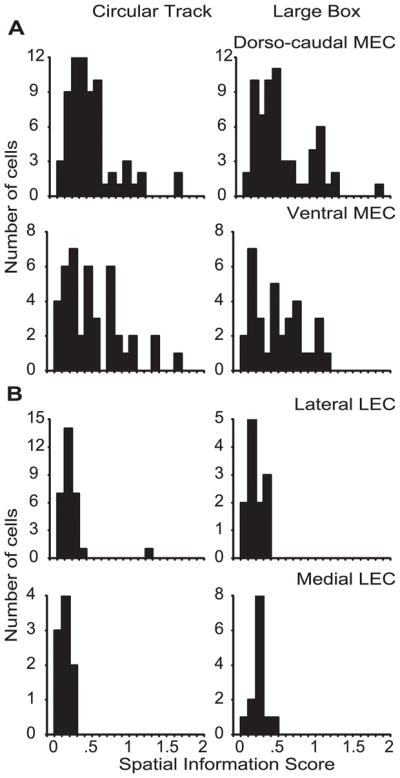
Comparison of spatial information scores between (A) dorsocaudal MEC and ventral MEC and (B) lateral LEC and medial LEC, in circular track and large box sessions. There was no significant difference between spatial information scores of dorsocaudal and ventral MEC neurons (Circular track: dorsocaudal MEC median = .368, interquartile range = .23–.56; ventral MEC median = .414, interquartile range = .22–.75; Mann-Whitney p = .8372. Large box: dorsocaudal MEC median = .420, interquartile range = .23–.73; ventral MEC median = .490, interquartile range = .20–.72; Mann-Whitney p = .9067) or between lateral and medial LEC neurons (Circular track: lateral LEC median = .143, interquartile range = .11–.21; medial LEC median = .148, interquartile range = .10–.18; Mann-Whitney p = .5485. Large box: lateral LEC median = .189, interquartile range = .13–.26; medial LEC median = .220, interquartile range = .20–.28; Mann-Whitney p = .1917). Because there were no differences between projection zones within each area, the observed spatial specificity dissociation between MEC and LEC cannot be due to any possible biases in sampling of neurons from different projection zones of MEC and LEC. [Note that the similarity of information scores between dorsal and ventral MEC neurons is not inconsistent with the increased scaling of grid cells reported along this axis (Hafting et al., 2005). Because both the size of the grid vertices and the intervertex distance increases proportionately, the information score (Skaggs et al., 1996) is not sensitive to the scaling of grid cells. For example, in the hypothetical case of a binary rate map with uniform sampling, if 50% of the pixels in the rate map fire at a high rate and 50% fire at a low rate, the measure does not distinguish whether these pixels form a single field or multiple, punctate fields. The amount of spatial information is the same. See the examples in Figure 3 of the main text.]
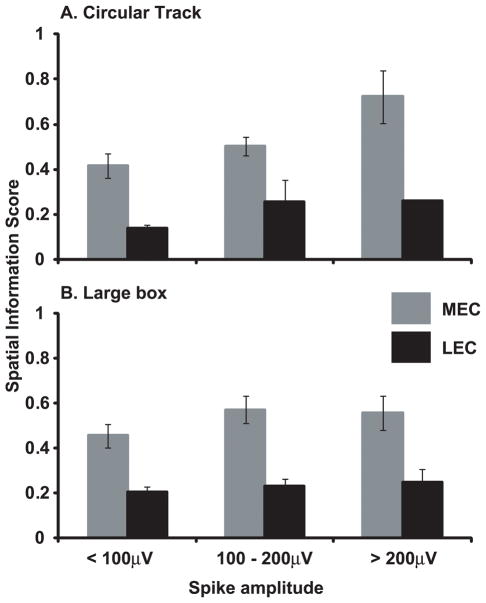
As in Hargreaves et al. (2005), the average spike height of MEC neurons was larger than that of LEC neurons, although this difference was statistically significant only for the circular track data (MEC 119 ± 5 μV, LEC 98 ± 6 μV, t146 = 2.16, p < .05) and not for the large box data (MEC 122 ± 6 μV, LEC 113 ± 9 μV, t118 = 0.71, n.s.). Although all of the cells in the sample met our criteria for isolation quality based on waveform parameters and tetrode spike-sorting, it is possible that the small difference in spike amplitude might account for the differences in spatial information between MEC and LEC, as spike amplitude is correlated with unit isolation quality. To test for this possibility, we sorted the cells into 3 spike-amplitude bins (< 100 μV, 100–200 μV, and 200 μV). As shown in Figure 7, MEC cells had a higher information score than LEC cells regardless of the spike amplitude, and the lowest amplitude MEC cells (< 100 μV) had a greater spatial information score than the highest amplitude LEC cells (> 200 μV). A 2-way ANOVA between brain area and spike amplitude, with spatial information as the dependent variable, showed a significant main effect of brain area for both circular track and large box data (p < .01), with no significant main effect of spike amplitude and no significant interaction between these factors for either data set (see Fig. 7 caption for F values). Thus, as in Hargreaves et al. (2005), it is highly unlikely that potential differences between LEC and MEC in unit isolation quality can account for the differences in spatial information between the two areas.
Figure 7.
Spatial information scores as a function of spike amplitude. For both circular track (A) and large box (B) sessions, the spatial information scores of MEC were larger than LEC regardless of the amplitude of the extracellularly recorded action potentials. The information scores of the smallest MEC spikes were larger than the information scores of the largest LEC spikes, showing that the difference in spatial information between MEC and LEC is unlikely to be an artifact of differences in unit isolation of the MEC and LEC samples. (A) Main effect of brain region: F(1,142) = 7.37, p < 0.01; Main effect of spike amplitude: F(2,142) = 1.80, p = .17; Interaction: F(2,142) = .19, p = 0.83. (B) Main effect of brain region: F(1,114) = 9.43, p < 0.01; Main effect of spike amplitude: F(2,114) = 0.48, p = .62; Interaction: F(2,114) = .17, p = 0.84.
Layer II neurons of MEC and LEC project to the DG and CA3 regions of the hippocampus, whereas Layer III neurons project to the CA1 and subiculum regions (Steward and Scoville, 1976). In addition, Layer II of MEC has a higher proportion of grid cells than Layer III (Sargolini et al., 2006). It was thus of interest to determine whether the differences in spatial tuning between LEC and MEC were restricted to either of these two superficial layers. The spatial information scores were transformed by calculating the log10 of the score, thus making the distributions close to normal, and a 2-way ANOVA was performed on the transformed values. This analysis revealed a significant overall difference between MEC and LEC, but no main effect of the Layer II vs. Layer III factor and no significant interaction (Circular track: Layer F (1,144) = 2.292, p = 0.1322; Area F (1,144) = 45.392, p < 0.0001, Layer × Area F (1,144) = 0.238, p = 0.6266; Large box: Layer F (1,116) = 0.393, p = 0.5321; Area F (1,116) = 12.449, p = 0.001, Layer × Area F (1,116) = 0.706, p = 0.4025). Thus, the differences between LEC and MEC were apparent in both superficial layers.
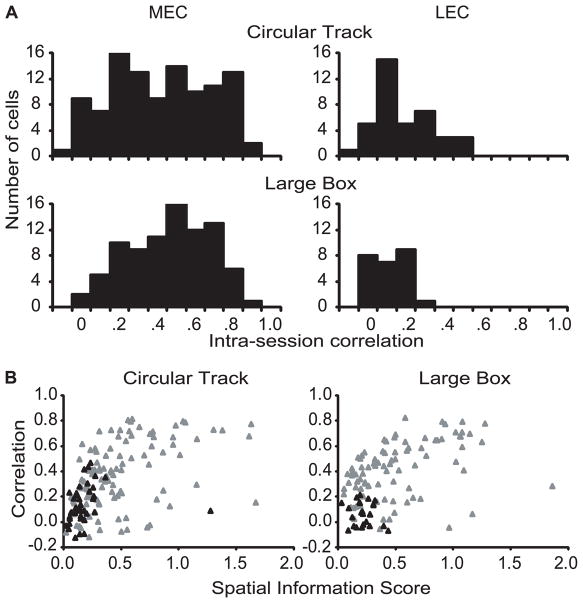
Even though the LEC neurons showed poor spatial selectivity, it was of interest to determine whether the poor spatial firing patterns were at least consistent within a session. That is, if the cells showed the same pattern of firing in the first and second halves of the session, this result would indicate that there was a weak but consistent spatial signal conveyed by these neurons. To address this question, we calculated separate rate maps for the first half and the second half of the recording sessions for each cell, and we performed a spatial correlation between the two rate maps. Figure 8A shows the distribution of the correlations for MEC and LEC cells in both the circular track and the large box. For both environments, the MEC cells showed much more stable spatial firing patterns within the session than the LEC cells (Mann-Whitney p < 0.001 in both cases). Figure 8B shows scatter plots of the intra-session correlation between the first and second halves of the session against the spatial information score of the neuron (calculated across the entire session). For both environments, the intrasession correlation was significantly correlated with spatial information in MEC (gray triangles), but not in LEC (black triangles; see caption for statistics). However, an outlier of the LEC distribution on the circular track had a high spatial information score but a low correlation; this cell fired mainly on the last two laps of the session. When this outlier was removed, the correlation on the circular track became significant for the LEC neurons (r = 0.546, p = 0.0003). This correlation suggests that even though the spatial selectivity on the track was low, the weak selectivity was consistent across the session for those cells with slightly higher information scores, perhaps due to the presence of salient local cues on the circular track.
Figure 8.
Stability of spatial firing pattern within a recording session. (A) Histograms showing the distribution of intra-session, pixel-by-pixel rate map correlations (between the first half and the second half of the session) in superficial MEC and LEC neurons in circular track and large box sessions. Superficial MEC neurons had a significantly higher correlation between the first and second halves of the session than superficial LEC neurons on both circular track (MEC median = .373, interquartile range = .18–.58; LEC median = .093, interquartile range = .04–.23; Mann-Whitney p < .0001) and large box (MEC median = .433, interquartile range = .27–.59; LEC median = .064, interquartile range = −.03–.15; Mann-Whitney p < .0001) sessions. (B) Scatter plots showing the relationship between the spatial information score and the reproducibility of firing patterns between the first and second halves of the session. Superficial MEC neurons (gray triangles), but not superficial LEC neurons (black triangles), showed a strong relationship between spatial information score and intra-session correlations in both circular track (MEC: r = 0.460, p < 0.0001; LEC: r = 0.166, p = 0.3149) and large box (MEC: r = 0.401, p 0.0001; LEC: r = −0.139, p = 0.5105) sessions.
Discussion
Episodic memory is characterized by memory for the content of an event (what), the spatial context of the event (where), and the timing of the event (when) (Clayton and Dickinson, 1998; Tulving, 2002; Eichenbaum and Fortin, 2005). The hippocampus is critically involved in episodic memory (O’Keefe and Nadel, 1978; Vargha-Khadem et al., 1997). The MEC and the LEC are parts of two processing streams that may convey distinct forms of information to the hippocampus in support of its role in episodic memory (Suzuki et al., 1997; Burwell, 2000; Hargreaves et al., 2005; Norman and Eacott, 2005; Manns and Eichenbaum, 2006; Knierim et al., 2006). The MEC, with its connections with the postrhinal cortex, visuospatial cortical areas, and limbic areas (including the dorsal presubiculum, retrosplenial cortex, and parasubiculum, which all contain spatially selective neurons [Knierim, 2006]), appears to convey spatial information to the hippocampus (where). The LEC, with its connections with perirhinal cortex, appears to be part of a stream that processes representation of objects or individual items or configurations of items (what) (Xiang and Brown, 1998; Suzuki et al., 1997; Zhu et al., 1995; Young et al., 1997). The hippocampus may combine these two processing streams to form conjunctive representations of items and the locations/contexts in which the items occurred, thus forming the basis of an episodic representation. The present study shows a dissociation between the MEC and LEC in the spatial information content of neuronal firing in complex environments replete with visual landmarks. MEC neurons displayed significant spatial tuning and grid-cell properties. In contrast, LEC neurons showed little spatial tuning. Thus, the lack of spatial information in LEC neurons cannot be explained by a paucity of spatial landmarks, as was potentially the case in a previous study (Hargreaves et al., 2005).
The lack of spatial selectivity under the cue-rich conditions of the present study was not a necessary or strongly predicted outcome from the prior study of Hargreaves et al. (2005), who showed that LEC cells had poor spatial selectivity in a simple environment with a single cue card as the only salient landmark. Much prior research has shown conclusively that hippocampal place cells can fire robustly in the absence of external sensory cues (O’Keefe and Speakman, 1987; Leonard and McNaughton, 1990; Quirk et al., 1990; Markus et al., 1994; Save et al., 1998) and can completely decouple from salient landmarks under certain conditions (such as disorientation) (Jung and McNaughton, 1993; Knierim et al., 1995). These phenomena were the basis for the idea that place cells either constitute the brain’s path integration system (McNaughton et al., 1996) or received strong input from a path integration system (Touretzky and Redish, 1996). MEC neurons share with place cells the properties described above. They maintain grid-like firing in darkness (Hafting et al., 2005) and can decouple from external landmarks under certain conditions (Hargreaves et al., 2007). Many investigators believe that the grid cells are part of the path integration system, and that the hippocampus receives its path integration input from the medial perforant path (O’Keefe and Burgess, 2005; McNaughton et al., 2006; Hafting et al., 2005; but see Kropff and Treves, 2008). Nonetheless, these properties of place cells and grid cells make no strong predictions about how lateral entorhinal neurons should fire in cue-rich or cue-poor conditions. The lack of spatial firing in cue-poor environments (Hargreaves et al., 2005) argued that it is unlikely that LEC cells are part of a circuit that computes position based on integration of self-motion. However, it was an open question whether LEC cells might display stronger spatial selectivity if there was a rich array of visual landmarks available. O’Keefe and Nadel (1978) postulated that the hippocampus received input from two spatial systems: an internal system that uses dead-reckoning (path integration) to update representations of the animal’s current location and an external system that computes position based on the configurations of external cues that an animal perceives at a given location (O’Keefe and Nadel, 1978, pp. 93–94). If one hypothesizes that these two input systems correspond to the two major input pathways to the hippocampus, the MEC would most certainly correspond to the internal system and the LEC would correspond to the external system. (Lisman [2007] has recently proposed a related dichotomy between MEC and LEC as “self” vs. “non-self” spatial systems, one representing the organism’s location in allocentric coordinates, the other representing the location of external cues in an egocentric coordinate system.) The presence of these two inputs could explain why sometimes place cells acted as if they were path-integration cells (Knierim et al., 1995; Gothard et al., 1996; Sharp et al., 1990; Jeffery and O’Keefe, 1999;Knierim, 2002b) and other times acted as if they responded to configurations of external sensory cues (O’Keefe and Conway, 1978; Shapiro et al., 1997). However, the present results demonstrate that the LEC does not carry a strong spatial signal, even in the presence of multiple, external sensory cues. It is thus unlikely that the LEC acts as the hypothesized external spatial input system, at least under the present recording conditions.
Of course, these experiments cannot rule out the possibility that LEC cells would show a spatial signal under different conditions. For example, it is possible that attention, or some degree of behavioral or emotional significance, is required to drive LEC neurons. This idea has some support from in vitro, in vivo, and anatomical studies. Slice experiments show that simultaneous activation of perirhinal cortex and amygdala are required to overcome the “wall of inhibition” that perirhinal-alone stimulation meets in the LEC (Kajiwara et al., 2003; de Curtis and Pare, 2004). A number of recent place-cell studies raise the possibility that the level of attention to external landmarks modulates the ability of these landmarks to affect the firing of place cells (Kentros et al., 2004; Muzzio et al., 2009; Fenton et al., 2010), an idea that is consistent with the notion that the hippocampal activity represents the “automatic encoding of attended experience” (Morris and Frey, 1997). From considerations of anatomical connectivity patterns, Burwell has argued that the LEC-associated processing stream “might be involved in focussed attentional processing of behaviorally relevant stimuli” (Burwell, 2000) (p. 40). Thus, although the current recording conditions were typical for most place-cell recording studies that show an influence of external stimuli, it is conceivable that LEC cells might fire in a more spatially-consistent manner if the animal was required to attend more to the external stimuli.
Alternatively, the LEC may provide a fundamentally different, nonspatial signal to the hippocampus (Manns and Eichenbaum, 2006; Knierim et al., 2006; Burwell, 2000). An episodic memory consists of the events or stimuli that occur in a particular spatial context. If the MEC is critical for providing the spatial framework required to produce a hippocampal contextual representation (Nadel et al., 1985; Jeffery et al., 2004; Smith and Mizumori, 2006), then the LEC may be the pathway that provides information to the hippocampus about the discrete items (or configurations of items) that make up the nonspatial content of an experience. Modulation of place-cell firing by nonspatial stimuli has been demonstrated from the very first reports of place cells (O’Keefe and Dostrovsky, 1971; O’Keefe, 1976), and more recent studies have shown compelling evidence that the hippocampus represents nonspatial stimuli within the context of an underlying spatial framework (Wiebe and Staubli, 1999; Moita et al., 2003; Manns and Eichenbaum, 2009; Komorowski et al., 2009). It is not known whether this nonspatial input is from the LEC or MEC (or both), but preliminary data from this laboratory has shown that LEC cells, but not MEC cells, fire when the animal investigates discrete objects (Deshmukh and Knierim, 2008). This model of fundamentally different processing functions of LEC and MEC is consistent with the differences in theta modulation of LEC and MEC neurons: MEC cells are strongly modulated by theta, whereas LEC cells show weak modulation (Deshmukh et al., 2010). Because theta is associated with movement and exploratory behaviors in rats, this difference in theta modulation suggests that the MEC and LEC are performing fundamentally different computations. Similar differences in theta modulation have been advanced to support arguments for different computations in the dorsal vs. ventral hippocampus (Royer et al., 2010).
In summary, the present results replicate and extend the results of Hargreaves and colleagues (2005). The lack of spatial tuning in LEC neurons even under conditions in which there are numerous visual landmarks in an environment demonstrates the generality of the dissociation with MEC and disproves the alternative explanation that LEC neurons might show spatial tuning under similar behavioural conditions in a complex visual environment. It remains to be determined what type of information is explicitly represented by the LEC, and how the hippocampus combines these input streams to create representations that are essential for normal episodic memory.
Acknowledgments
Grant sponsor: NINDS Grant number: R01 NS039456
References
- Alonso A, Garcia-Austt E. Neuronal sources of theta rhythm in the entorhinal cortex of the rat. I. Laminar distribution of theta field potentials. Exp Brain Res. 1987;67:493–501. doi: 10.1007/BF00247282. [DOI] [PubMed] [Google Scholar]
- Brun VH, Solstad T, Kjelstrup KB, Fyhn M, Witter MP, Moser EI, Moser MB. Progressive increase in grid scale from dorsal to ventral medial entorhinal cortex. Hippocampus. 2008;18:1200–1212. doi: 10.1002/hipo.20504. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Phillips RG, Burwell RD. Contributions of postrhinal and perirhinal cortex to contextual information processing. Behav Neurosci. 2000;114:882–894. doi: 10.1037//0735-7044.114.5.882. [DOI] [PubMed] [Google Scholar]
- Burwell RD. The parahippocampal region: corticocortical connectivity. Ann N Y Acad Sci. 2000;911:25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x. [DOI] [PubMed] [Google Scholar]
- Cacucci F, Lever C, Wills TJ, Burgess N, O’Keefe J. Theta-modulated place-by-direction cells in the hippocampal formation in the rat. J Neurosci. 2004;24:8265–8277. doi: 10.1523/JNEUROSCI.2635-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL, Lin LH, Green EJ, Barnes CA, McNaughton BL. Head-direction cells in the rat posterior cortex. I. Anatomical distribution and behavioral modulation. Exp Brain Res. 1994;101:8–23. doi: 10.1007/BF00243212. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;395:272–274. doi: 10.1038/26216. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, Amnesia, and the Hippocampal System. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- de Curtis M, Pare D. The rhinal cortices: a wall of inhibition between the neocortex and the hippocampus. Prog Neurobiol. 2004;74:101–110. doi: 10.1016/j.pneurobio.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Deshmukh S, Knierim JJ. 2008 Neuroscience Meeting Planner. Program number 90.5. Society for Neuroscience; 2008. Properties of lateral entorhinal cortex neurons in an environment with discrete objects. Online. [Google Scholar]
- Deshmukh S, Yoganarasimha D, Voicu H, Knierim JJ. Theta modulation in the medial and lateral entorhinal cortex. J Neurophysiol. 2010 doi: 10.1152/jn.01141.2009. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolorfo CL, Amaral DG. Entorhinal cortex of the rat: topographic organization of the cells of origin of the perforant path projection to the dentate gyrus. J Comp Neurol. 1998;398:25–48. [PubMed] [Google Scholar]
- Eichenbaum H, Fortin NJ. Bridging the gap between brain and behavior: cognitive and neural mechanisms of episodic memory. J Exp Anal Behav. 2005;84:619–629. doi: 10.1901/jeab.2005.80-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton AA, Lytton WW, Barry JM, Lenck-Santini PP, Zinyuk LE, Kubik S, Bures J, Poucet B, Muller RU, Olypher AV. Attention-like modulation of hippocampus place cell discharge. J Neurosci. 2010;30:4613–4625. doi: 10.1523/JNEUROSCI.5576-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank LM, Brown EN, Wilson MA. A comparison of the firing properties of putative excitatory and inhibitory neurons from CA1 and the entorhinal cortex. J Neurophysiol. 2001;86:2029–2040. doi: 10.1152/jn.2001.86.4.2029. [DOI] [PubMed] [Google Scholar]
- Fuhs MC, Touretzky DS. A spin glass model of path integration in rat medial entorhinal cortex. J Neurosci. 2006;26:4266–4276. doi: 10.1523/JNEUROSCI.4353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothard KM, Skaggs WE, McNaughton BL. Dynamics of mismatch correction in the hippocampal ensemble code for space: interaction between path integration and environmental cues. J Neurosci. 1996;16:8027–8040. doi: 10.1523/JNEUROSCI.16-24-08027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hargreaves EL, Rao G, Lee I, Knierim JJ. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science. 2005;308:1792–1794. doi: 10.1126/science.1110449. [DOI] [PubMed] [Google Scholar]
- Hargreaves EL, Yoganarasimha D, Knierim JJ. Cohesiveness of spatial and directional representations recorded from neural ensembles in the anterior thalamus, parasubiculum, medial entorhinal cortex, and hippocampus. Hippocampus. 2007;17:826–841. doi: 10.1002/hipo.20316. [DOI] [PubMed] [Google Scholar]
- Jeffery KJ, Anderson MI, Hayman R, Chakraborty S. A proposed architecture for the neural representation of spatial context. Neurosci Biobehav Rev. 2004;28:201–218. doi: 10.1016/j.neubiorev.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Jeffery KJ, O’Keefe JM. Learned interaction of visual and idiothetic cues in the control of place field orientation. Exp Brain Res. 1999;127:151–161. doi: 10.1007/s002210050785. [DOI] [PubMed] [Google Scholar]
- Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3:165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- Jung MW, Wiener SI, McNaughton BL. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J Neurosci. 1994;14:7347–7356. doi: 10.1523/JNEUROSCI.14-12-07347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara R, Takashima I, Mimura Y, Witter MP, Iijima T. Amygdala input promotes spread of excitatory neural activity from perirhinal cortex to the entorhinal-hippocampal circuit. J Neurophysiol. 2003;89:2176–2184. doi: 10.1152/jn.01033.2002. [DOI] [PubMed] [Google Scholar]
- Kentros CG, Agnihotri NT, Streater S, Hawkins RD, Kandel ER. Increased attention to spatial context increases both place field stability and spatial memory. Neuron. 2004;42:283–295. doi: 10.1016/s0896-6273(04)00192-8. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KB, Solstad T, Brun VH, Hafting T, Leutgeb S, Witter MP, Moser EI, Moser MB. Finite scale of spatial representation in the hippocampus. Science. 2008;321:140–143. doi: 10.1126/science.1157086. [DOI] [PubMed] [Google Scholar]
- Knierim JJ. Dynamic interactions between local surface cues, distal landmarks, and intrinsic circuitry in hippocampal place cells. J Neurosci. 2002a;22:6254–6264. doi: 10.1523/JNEUROSCI.22-14-06254.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ. The path-integration properties of hippocampal place cells. In: Sharp PE, editor. The neural basis of navigation: Evidence from single cell recording. New York: Kluwer; 2002b. pp. 41–58. [Google Scholar]
- Knierim JJ. Neural representations of location outside the hippocampus. Learn Mem. 2006;13:405–415. doi: 10.1101/lm.224606. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Kudrimoti HS, McNaughton BL. Place cells, head direction cells, and the learning of landmark stability. J Neurosci. 1995;15:1648–1659. doi: 10.1523/JNEUROSCI.15-03-01648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, Lee I, Hargreaves EL. Hippocampal place cells: parallel input streams, subregional processing, and implications for episodic memory. Hippocampus. 2006;16:755–764. doi: 10.1002/hipo.20203. [DOI] [PubMed] [Google Scholar]
- Komorowski RW, Manns JR, Eichenbaum H. Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens where. J Neurosci. 2009;29:9918–9929. doi: 10.1523/JNEUROSCI.1378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropff E, Treves A. The emergence of grid cells: Intelligent design or just adaptation? Hippocampus. 2008;18:1256–1269. doi: 10.1002/hipo.20520. [DOI] [PubMed] [Google Scholar]
- Leonard B, McNaughton BL. Spatial representation in the rat: Conceptual, behavioral, and neurophysiological perspectives. In: Olton DS, Kesner RP, editors. The Neurobiology of Comparative Cognition. Hillsdale, NJ: Lawrence Erlbaum Associates; 1990. pp. 363–422. [Google Scholar]
- Lisman JE. Role of the dual entorhinal inputs to hippocampus: a hypothesis based on cue/action (non-self/self) couplets. Prog Brain Res. 2007;163:615–625. doi: 10.1016/S0079-6123(07)63033-7. [DOI] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. Evolution of declarative memory. Hippocampus. 2006;16:795–808. doi: 10.1002/hipo.20205. [DOI] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. A cognitive map for object memory in the hippocampus. Learn Mem. 2009;16:616–624. doi: 10.1101/lm.1484509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus EJ, Barnes CA, McNaughton BL, Gladden VL, Skaggs WE. Spatial information content and reliability of hippocampal CA1 neurons: effects of visual input. Hippocampus. 1994;4:410–421. doi: 10.1002/hipo.450040404. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, Gerrard JL, Gothard K, Jung MW, Knierim JJ, Kudrimoti H, Qin Y, Skaggs WE, Suster M, Weaver KL. Deciphering the hippocampal polyglot: the hippocampus as a path integration system. J Exp Biol. 1996;199:173–185. doi: 10.1242/jeb.199.1.173. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the ‘cognitive map’. Nat Rev Neurosci. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- Moita MA, Rosis S, Zhou Y, LeDoux JE, Blair HT. Hippocampal Place Cells Acquire Location-Specific Responses to the Conditioned Stimulus during Auditory Fear Conditioning. Neuron. 2003;37:485–497. doi: 10.1016/s0896-6273(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Morris RG, Frey U. Hippocampal synaptic plasticity: role in spatial learning or the automatic recording of attended experience? Philos Trans R Soc Lond B Biol Sci JID - 7503623. 1997;352:1489–1503. doi: 10.1098/rstb.1997.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7:1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzio IA, Levita L, Kulkarni J, Monaco J, Kentros C, Stead M, Abbott LF, Kandel ER. Attention enhances the retrieval and stability of visuospatial and olfactory representations in the dorsal hippocampus. PLoS Biol. 2009;7:e1000140. doi: 10.1371/journal.pbio.1000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L, Wilner J, Kurtz EM. Cognitive maps and environmental context. In: Balsam PD, Tomie A, editors. Context and learning. Hillsdale, N.J: Earlbaum; 1985. pp. 385–406. [Google Scholar]
- Norman G, Eacott MJ. Dissociable effects of lesions to the perirhinal cortex and the postrhinal cortex on memory for context and objects in rats. Behav Neurosci. 2005;119:557–566. doi: 10.1037/0735-7044.119.2.557. [DOI] [PubMed] [Google Scholar]
- O’Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976;51:78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Burgess N. Dual phase and rate coding in hippocampal place cells: theoretical significance and relationship to entorhinal grid cells. Hippocampus. 2005;15:853–866. doi: 10.1002/hipo.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Conway DH. Hippocampal place units in the freely moving rat: why they fire where they fire. Exp Brain Res. 1978;31:573–590. doi: 10.1007/BF00239813. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map: Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford: Clarendon Press; 1978. [Google Scholar]
- O’Keefe J, Speakman A. Single unit activity in the rat hippocampus during a spatial memory task. Exp Brain Res. 1987;68:1–27. doi: 10.1007/BF00255230. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Muller RU, Kubie JL. The firing of hippocampal place cells in the dark depends on the rat’s recent experience. J Neurosci. 1990;10:2008–2017. doi: 10.1523/JNEUROSCI.10-06-02008.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Muller RU, Kubie JL, Ranck JB., Jr The positional firing properties of medial entorhinal neurons: description and comparison with hippocampal place cells. J Neurosci. 1992;12:1945–1963. doi: 10.1523/JNEUROSCI.12-05-01945.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Sirota A, Patel J, Buzsaki G. Distinct representations and theta dynamics in dorsal and ventral hippocampus. J Neurosci. 2010;30:1777–1787. doi: 10.1523/JNEUROSCI.4681-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruth RE, Collier TJ, Routtenberg A. Topography between the entorhinal cortex and the dentate septotemporal axis in rats: I. Medial and intermediate entorhinal projecting cells. J Comp Neurol. 1982;209:69–78. doi: 10.1002/cne.902090107. [DOI] [PubMed] [Google Scholar]
- Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser MB, Moser EI. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science. 2006;312:758–762. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- Save E, Cressant A, Thinus-Blanc C, Poucet B. Spatial firing of hippocampal place cells in blind rats. J Neurosci. 1998;18:1818–1826. doi: 10.1523/JNEUROSCI.18-05-01818.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savelli F, Yoganarasimha D, Knierim JJ. Influence of boundary removal on the spatial representations of the medial entorhinal cortex. Hippocampus. 2008;18:1270–1282. doi: 10.1002/hipo.20511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro ML, Tanila H, Eichenbaum H. Cues that hippocampal place cells encode: dynamic and hierarchical representation of local and distal stimuli. Hippocampus. 1997;7:624–642. doi: 10.1002/(SICI)1098-1063(1997)7:6<624::AID-HIPO5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Sharp PE, Kubie JL, Muller RU. Firing properties of hippocampal neurons in a visually symmetrical environment: contributions of multiple sensory cues and mnemonic processes. J Neurosci. 1990;10:3093–3105. doi: 10.1523/JNEUROSCI.10-09-03093.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1996;6:149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJ. Hippocampal place cells, context, and episodic memory. Hippocampus. 2006;16:716–729. doi: 10.1002/hipo.20208. [DOI] [PubMed] [Google Scholar]
- Solstad T, Boccara CN, Kropff E, Moser MB, Moser EI. Representation of geometric borders in the entorhinal cortex. Science. 2008;322:1865–1868. doi: 10.1126/science.1166466. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and Brain. Oxford: Oxford Univ. Press; 1987. [Google Scholar]
- Steward O, Scoville SA. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J Comp Neurol. 1976;169:347–370. doi: 10.1002/cne.901690306. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Miller EK, Desimone R. Object and place memory in the macaque entorhinal cortex. J Neurophysiol. 1997;78:1062–1081. doi: 10.1152/jn.1997.78.2.1062. [DOI] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci. 1990;10:420–435. doi: 10.1523/JNEUROSCI.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touretzky DS, Redish AD. Theory of rodent navigation based on interacting representations of space. Hippocampus. 1996;6:247–270. doi: 10.1002/(SICI)1098-1063(1996)6:3<247::AID-HIPO4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Wiebe SP, Staubli UV. Dynamic filtering of recognition memory codes in the hippocampus. J Neurosci. 1999;19:10562–10574. doi: 10.1523/JNEUROSCI.19-23-10562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, Amaral DG. Hippocampal formation. In: Paxinos G, editor. The rat nervous system. 3. Amsterdam: Elsevier; 2004. pp. 635–704. [Google Scholar]
- Xiang JZ, Brown MW. Differential neuronal encoding of novelty, familiarity and recency in regions of the anterior temporal lobe. Neuropharmacol. 1998;37:657–676. doi: 10.1016/s0028-3908(98)00030-6. [DOI] [PubMed] [Google Scholar]
- Young BJ, Otto T, Fox GD, Eichenbaum H. Memory representation within the parahippocampal region. J Neurosci. 1997;17:5183–5195. doi: 10.1523/JNEUROSCI.17-13-05183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XO, Brown MW, Aggleton JP. Neuronal signalling of information important to visual recognition memory in rat rhinal and neighbouring cortices. Eur J Neurosci. 1995;7:753–765. doi: 10.1111/j.1460-9568.1995.tb00679.x. [DOI] [PubMed] [Google Scholar]