1. Introduction
Schizophrenia is broadly characterized by the presence of positive symptoms (e.g. delusions and hallucinations) negative symptoms (e.g. social withdrawal, and poor volition) and cognitive deficits (e.g. memory, attention, and reasoning and problem solving deficits) (Crow 1980;Nuechterlein et al. 2004). Research on the pathophysiology of schizophrenia has implicated decreases in glutamate signaling and NMDA receptor hypofunctionality as a causative factor in this disease (Laruelle et al. 2003). A number of studies have demonstrated that treatment with phencyclidine (PCP), ketamine, or dizocilpine (MK-801) can produce symptoms similar to the positive, negative and cognitive symptoms of schizophrenia when given to healthy control subjects, and can exacerbate these symptoms in patients with schizophrenia (Siegel 1978;Snyder 1973). Unlike amphetamine, a dopamine (DA) agonist that is able to mimic the positive symptoms of schizophrenia, only PCP and MK-801 can induce key negative symptoms such as social withdrawal (Guy and Gardner 1985;Lisman et al. 2008;Rung et al. 2005;Sams-Dodd 1996). MK-801, an analogue of PCP, is one of the most potent non-competitive antagonists of the NMDA receptor, binding to a site located with the NMDA receptor ion channel and blocking Ca2+ flow, thus disturbing glutamatergic neurotransmission (Rung et al, 2005). Treatment with MK-801 has been shown to induce a variety of symptoms reflective of schizophrenia, including disrupted sensorimotor gating (prepulse inhibition), increased locomotor activity and disrupted cognitive function, including deficits in rule acquisition and attentional set-shifting (Bast et al. 2000;BELL 1965;Malhotra et al. 1997;Manahan-Vaughan et al. 2008;Rung et al. 2005;Sams-Dodd 1996;Sams-Dodd 1998;Snyder 1973;Stefani and Moghaddam 2005). Furthermore, MK801 induces social withdrawal (Rung et al, 2005, Sams-Dodd et al, 2004, Snigdha et al, 2008), serving as a strong model of the negative symptoms of schizophrenia.
Previous research from our lab has demonstrated that the endogenous brain tripeptide PLG and its analog PAOPA (Figure 1) modify dopaminergic neurotransmission by acting as allosteric modulators of the DA D2 receptor (Johnson et al. 1986;Mishra 1983;Mishra et al. 1983;Mishra et al. 1997;Verma et al. 2005;Chiu et al. 1981;Chiu et al. 1983;Raghavan et al. 2009;Srivastava et al. 1988;Verma et al. 2005). These compounds have been shown to increase agonist binding to DA D2 receptors without affecting antagonist binding, and prevent conversion of high-affinity state DA receptors (D2High) to their low-affinity state (D2Low) (Mishra et al. 1990;Srivastava et al. 1988;Verma et al. 2005). Furthermore, PLG and PAOPA have been shown to potentiate rotational behaviour in the 6-hydroxy dopamine lesion rat (Mishra et al. 1997;Ott et al. 1996;Smith and Morgan 1982), and inhibit neuroleptic drug-induced vacuous chewing in rat models of human tardive dyskinesia (Castellano et al. 2007;Sharma et al. 2003). Given the interaction between PAOPA and the DA D2 receptor, and the effects of PAOPA in preclinical animal models, the objective of this study was to investigate whether this potent analog of PLG has an effect on MK-801-induced social withdrawal in the rat.
Figure 1.
Schematic structure diagram of PLG and its analogue, PAOPA.
2. Methods
2.1 Animals
Age-matched male Sprague Dawley rats (225 – 272 g), Charles River Canada, St. Constant, QC, Canada) were tested in accordance with the Canadian Council for Animal Care guidelines. Animals were housed individually in standard cages on a 12 hr light cycle in a room maintained at 22°C with 50% humidity with access to food and water ad libitum.
2.2 Drugs and Administration Schedule
(+)-MK-801 ((+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate salt) was purchased from Sigma-Aldrich (Oakville, ON, Canada). PAOPA was custom synthesized at the University of Minnesota as previously described (Yu et al. 1988). All drugs were dissolved in 0.9% saline. MK-801 was injected at 0.5 mg/kg, and PAOPA was injected at 1 mg/kg. Four groups of rats were utilized and received daily injections intraperitoneally (I.P.) for 7 days as follows: Group A (n=10) served as a control and was injected with saline; Group B (n=10) received MK-801; Group C (n=10) received PAOPA; and Group D (n=10) received PAOPA followed 30 minutes later by MK-801.
2.3 Social Interaction
The following method was adopted from File (File 1980) and Sams-Dodd (Sams-Dodd 1998). Animals were tested 24 hrs after the last drug injection during the light cycle. Social behavior was recorded in a black Plexiglass arena with dimensions 100 cm × 100 cm × 40 cm for a 5 min period via a ceiling mounted video camera. One rat was randomly marked with non-toxic black marker for identification during analysis. Total time spent in interaction was recorded (ms) for each rat and subdivided into active interaction (sniffing, following, crawling under or over, grooming, and aggressive behaviour) or passive interaction (close proximity). Members of each pair were not familiar with one another, with each pair used only once per test. Recordings were analyzed by a blinded observer.
2.4 Statistical Analysis
The amount of time spent in total interaction, in active or passive interaction, and the number of active and passive interaction episodes, was recorded and analyzed by means of one way analyses of variance (ANOVAs) with Tukey's post-hoc test.
3. Results
3.1 Sub-chronic treatment with MK-801 decreases social interaction in rats
Sub-chronic (7 day) treatment with MK-801 significantly decreased social behavior, as reflected by decreases in total time spent in interaction (F(3,26) = 21.92, ***p < 0.0001; post-hoc, ***p < 0.001), time spent in passive interaction (F(3,25) = 8.464, ***p < 0.004; post-hoc, ***p < 0.001), and the number of passive interaction episodes (F(3,26) = 7.648, ***p = 0.009; post-hoc, *p < 0.05) (FIGURE 2A-E). Interestingly, no significant reductions in the amount of time spent in active interaction (F(3,27) = 10.29, ***p 0.0001; post-hoc, p > 0.05) or the number of active interaction episodes were observed (F(3,28) = 8.621, ***p = 0.0003; post-hoc, p > 0.05) (FIGURE 2B, D). This evidence further validates that sub-chronic treatment with MK-801 reliably models negative symptoms of schizophrenia as demonstrated through social withdrawal.
Figure 2.
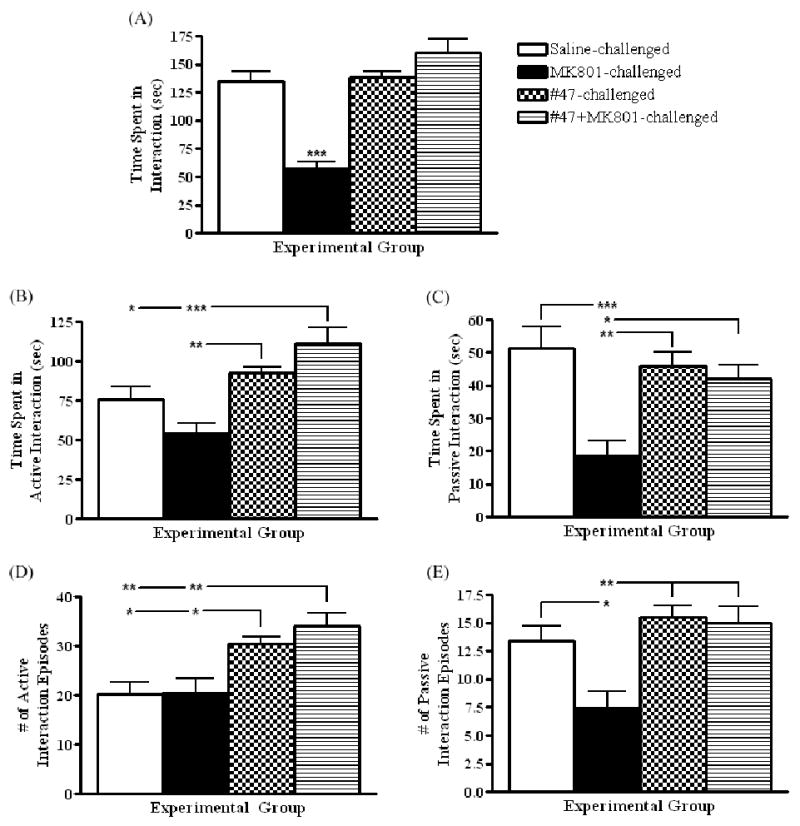
Graphs depicting social behaviour (mean ± SEM) in saline-, MK-801-, MK-801+PAOPA (#47)-, and PAOPA (#47)-injected animals after 7 days of drug challenge. (A) Total time spent in interaction: F(3,26) = 21.92, ***p < 0.0001; (B) time spent in active interaction: F(3,27) = 10.29, ***p 0.0001; (C) time spent in passive interaction: F(3,25) = 8.464, ***p < 0.004; (D) number of active interaction episodes: F(3,28) = 8.621, ***p = 0.0003; and (E) number of passive interaction episodes: F(3,26) = 7.648, *** p = 0.009. Post-hoc: *p < 0.05, **p < 0.01, ***p < 0.001. N=10/group.
3.2 Administration of PAOPA attenuates the effects of MK-801 on social behaviour
When administered 30 minutes before MK-801 challenge, PAOPA attenuated the deficits in social interaction induced by the NMDA receptor antagonist (FIGURE 2A-E). More specifically, animals spent significantly more time in total interaction (***p < 0.001), active interaction (***p < 0.001), and passive interaction (*p < 0.05), and had significantly higher numbers of active (**p < 0.01) and passive (**p < 0.01) interaction episodes relative to rats challenged with MK801. Furthermore, PAOPA alone significantly increased the number of active interaction episodes (*p < 0.05) when compared to saline-challenged animals (FIGURE 2D).
4. Discussion
Sub-chronic (7 day) treatment with the non-competitive NMDA receptor antagonist MK-801 induced deficits in social interaction, including decreased total time spent in interaction, decreased time spent in active and passive interaction, and decreased number of active and passive interaction episodes. Pre-treatment with PAOPA 30 minutes before MK-801 challenge attenuated these effects, such that animals displayed normal social behaviour. Furthermore, treatment with PAOPA alone increased the number of passive interaction episodes when compared to control animals challenged with saline. These results are consistent with previous reports on the ability of sub-chronic MK-801 challenge to interrupt normal social behaviour (Rung et al. 2005;Sams-Dodd 2004), and suggest that PAOPA may serve as a novel compound to treat the negative symptoms associated with schizophrenia.
The ability of non-competitive NMDA receptor antagonists, such as MK-801, to induce schizophrenic-like behavior provides evidence for dysregulated glutamatergic neurotransmission in schizophrenia, which involves hypofunctionality of NMDA receptors (Lahti et al, 2001) (Farber 2003;Laruelle et al. 2003). Pertinent to NMDA receptor activity, a high degree of interaction exists between glutamate and DA neurotransmission. Striatal D2 receptor stimulation results in NMDA deficiency and decreased glutamate transmission, whereas striatal D1 receptor stimulation increases glutamate transmission (Laruelle et al. 2003). In addition, a significant reduction in DA transmission has been observed in rat prefrontal cortex following sub-chronic administration of MK-801 (Jentsch et al, 1998). Ketamine and PCP have also been shown to possess similar levels of affinity for NMDA receptors and DA D2 receptors (Kapur and Seeman 2002).
Current treatment of schizophrenia with antipsychotic drugs (APDs), the majority of which exert their action via dopamine D2 receptor antagonism, is largely targeted toward treating positive symptoms but has limited ability to treat the negative symptoms of this disorder (Blin 1999). Interestingly, treatment with dopamine agonists have been shown to be effective in treating negative symptoms (Benkert et al. 1995;Olbrich and Schanz 1988;Wetzel et al. 1994). PAOPA allosterically binds the DA D2 receptor and has been shown to increase the proportion of D2High states by preventing conversion to the D2Low state (Mishra et al. 1990;Verma et al. 2005). Our results demonstrate that treatment with PAOPA prevents deficits in social interaction induced by MK-801 and increases social behavior when administered alone. While the exact mechanism by which these behavioural changes occur remains unknown, PAOPA's interaction with the D2 receptor may induce increased stimulation of this receptor by endogenous DA. Upon increased agonist stimulation, most receptors are translocated from the cellular membrane to the cytosol by endocytosis as a compensatory mechanism to prevent receptor overstimulation (Namkung et al. 2009). This has been demonstrated for D2 ligands such as quinpirole (Guo et al. 2010) and 2-methoxy-N-propylnorapomorphine (Skinbjerg et al. 2009). Thus, PAOPA's mechanistic action may involve internalization of D2 receptors due to increased agonist stimulation, thereby compensating for excess endogenous DA in the MK-801-induced schizophrenic-like state.
In conclusion, PAOPA caused significant increases in social interaction and attenuated MK-801-induced social deficits. These results suggest that peptidomimetics such as PAOPA may improve the negative symptoms in schizophrenia.
Acknowledgments
We would like to thank all members of the laboratory group for assisting in the preparation and proof-reading of the manuscript. With regard to these results, Ram Mishra, Rodney Johnson, Bailee Dyck and Dipannita Basu are involved in an intellectual property patent (US patent no. 61/378,599).
5. Role of Funding Source: Funding for this study was provided by the National Institutes of Health Research Grant 2R01-NS020036-20; NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
7. Conflict of Interest: All authors declare that they have no conflicts of interest.
6. Contributors: Dr. Ram K. Mishra and Dr. Rodney Johnson designed the study and wrote the protocol, and Dr. Ram K. Mishra and Dr. Bailee Dyck managed the literature searches and analyses. Dr. Bailee Dyck, Kelly Guest, Christal Sookram, and Dipannita Basu undertook the statistical analyses and wrote the first draft of the manuscript. Dr. Bailee Dyck, Kelly Guest and Christal Sookram contributed to running the experiments. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bast T, Zhang W, Feldon J, White IM. Effects of MK801 and neuroleptics on prepulse inhibition: re-examination in two strains of rats. Pharmacol Biochem Behav. 2000;67:647–658. doi: 10.1016/s0091-3057(00)00409-3. [DOI] [PubMed] [Google Scholar]
- Bell DS. Comparison of Amphetamine Psychosis and Schizophrenia. Br J Psychiatry. 1965;111:701–707. doi: 10.1192/bjp.111.477.701. [DOI] [PubMed] [Google Scholar]
- Benkert O, Muller-Siecheneder F, Wetzel H. Dopamine agonists in schizophrenia: a review. Eur Neuropsychopharmacol. 1995 5:43–53. doi: 10.1016/0924-977x(95)00022-h. [DOI] [PubMed] [Google Scholar]
- Blin O. A comparative review of new antipsychotics. Can J Psychiatry. 1999;44:235–244. doi: 10.1177/070674379904400303. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Batrynchuk J, Dolbeare K, Verma V, Mann A, Skoblenick KJ, Johnson RL, Mishra RK. MIF-1 and its peptidomimetic analogs attenuate haloperidol-induced vacuous chewing movements and modulate apomorphine-induced rotational behavior in 6-hydroxydopamine-lesioned rats. Peptides. 2007;28:2009–2015. doi: 10.1016/j.peptides.2007.07.026. [DOI] [PubMed] [Google Scholar]
- Chiu S, Paulose CS, Mishra RK. Neuroleptic drug-induced dopamine receptor supersensitivity: antagonism by L-prolyl-L-leucyl-glycinamide. Science. 1981;214:1261–1262. doi: 10.1126/science.6117947. [DOI] [PubMed] [Google Scholar]
- Chiu S, Wong YW, Wan YP, Chiu P, Mishra RK. Are the pharmacological effects of L-prolyl-L-leucyl-glycinamide (PLG) mediated through specific receptor mechanisms? Prog Neuropsychopharmacol Biol Psychiatry. 1983;7:739–742. doi: 10.1016/0278-5846(83)90056-8. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Positive and negative schizophrenic symptoms and the role of dopamine. Br J Psychiatry. 1980;137:383–386. [PubMed] [Google Scholar]
- Farber NB. The NMDA receptor hypofunction model of psychosis. Ann N Y Acad Sci. 2003;1003:119–130. doi: 10.1196/annals.1300.008. [DOI] [PubMed] [Google Scholar]
- File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- Guo N, Guo W, Kralikova M, Jiang M, Schieren I, Narendran R, Slifstein M, Abi-Dargham A, Laruelle M, Javitch JA, Rayport S. Impact of d2 receptor internalization on binding affinity of neuroimaging radiotracers. Neuropsychopharmacology. 2010;35:806–817. doi: 10.1038/npp.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy AP, Gardner CR. Pharmacological characterisation of a modified social interaction model of anxiety in the rat. Neuropsychobiology. 1985;13:194–200. doi: 10.1159/000118187. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Rajakumar G, Mishra RK. Dopamine receptor modulation by Pro-Leu-Gly-NH2 analogues possessing cyclic amino acid residues at the C-terminal position. J Med Chem. 1986;29:2100–2104. doi: 10.1021/jm00160a051. [DOI] [PubMed] [Google Scholar]
- Kapur S, Seeman P. NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implications for models of schizophrenia. Mol Psychiatry. 2002;7:837–844. doi: 10.1038/sj.mp.4001093. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Kegeles LS, Abi-Dargham A. Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann N Y Acad Sci. 2003;1003:138–158. doi: 10.1196/annals.1300.063. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, Breier A. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17:141–150. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, von Haebler D, Winter C, Juckel G, Heinemann U. A single application of MK801 causes symptoms of acute psychosis, deficits in spatial memory, and impairment of synaptic plasticity in rats. Hippocampus. 2008;18:125–134. doi: 10.1002/hipo.20367. [DOI] [PubMed] [Google Scholar]
- Mishra RK. Modulation of CNS dopamine receptors by peptides. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7:437–442. doi: 10.1016/0278-5846(83)90008-8. [DOI] [PubMed] [Google Scholar]
- Mishra RK, Chiu S, Chiu P, Mishra CP. Pharmacology of L-prolyl-L-leucyl-glycinamide (PLG): a review. Methods Find Exp Clin Pharmacol. 1983;5:203–233. [PubMed] [Google Scholar]
- Mishra RK, Marcotte ER, Chugh A, Barlas C, Whan D, Johnson RL. Modulation of dopamine receptor agonist-induced rotational behavior in 6-OHDA-lesioned rats by a peptidomimetic analogue of Pro-Leu-Gly-NH2 (PLG) Peptides. 1997;18:1209–1215. doi: 10.1016/s0196-9781(97)00147-2. [DOI] [PubMed] [Google Scholar]
- Mishra RK, Srivastava LK, Johnson RL. Modulation of high-affinity CNS dopamine D2 receptor by L-pro-L-leu-glycinamide (PLG) analogue 3(R)-(N-L-prolylamino)-2-oxo-1-pyrrolidineacetamide. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14:821–827. doi: 10.1016/0278-5846(90)90054-k. [DOI] [PubMed] [Google Scholar]
- Namkung Y, Dipace C, Javitch JA, Sibley DR. G protein-coupled receptor kinase-mediated phosphorylation regulates post-endocytic trafficking of the D2 dopamine receptor. J Biol Chem. 2009;284:15038–15051. doi: 10.1074/jbc.M900388200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Olbrich R, Schanz H. The effect of the partial dopamine agonist terguride on negative symptoms in schizophrenics. Pharmacopsychiatry. 1988;21:389–390. doi: 10.1055/s-2007-1017021. [DOI] [PubMed] [Google Scholar]
- Ott MC, Mishra RK, Johnson RL. Modulation of dopaminergic neurotransmission in the 6-hydroxydopamine lesioned rotational model by peptidomimetic analogues of L-prolyl-L-leucyl-glycinamide. Brain Res. 1996;737:287–291. doi: 10.1016/0006-8993(96)00927-4. [DOI] [PubMed] [Google Scholar]
- Raghavan B, Skoblenick KJ, Bhagwanth S, Argintaru N, Mishra RK, Johnson RL. Allosteric modulation of the dopamine D2 receptor by Pro-Leu-Gly-NH2 peptidomimetics constrained in either a polyproline II helix or a type II beta-turn conformation. J Med Chem. 2009;52:2043–2051. doi: 10.1021/jm801575w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rung JP, Carlsson A, Ryden MK, Carlsson ML. (+)-MK-801 induced social withdrawal in rats; a model for negative symptoms of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:827–832. doi: 10.1016/j.pnpbp.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F. Phencyclidine-induced stereotyped behaviour and social isolation in rats: a possible animal model of schizophrenia. Behav Pharmacol. 1996;7:3–23. [PubMed] [Google Scholar]
- Sams-Dodd F. Effects of continuous D-amphetamine and phencyclidine administration on social behaviour, stereotyped behaviour, and locomotor activity in rats. Neuropsychopharmacology. 1998;19:18–25. doi: 10.1016/S0893-133X(97)00200-5. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F. (+) MK-801 and phencyclidine induced neurotoxicity do not cause enduring behaviours resembling the positive and negative symptoms of schizophrenia in the rat. Basic Clin Pharmacol Toxicol. 2004;95:241–246. doi: 10.1111/j.1742-7843.2004.pto950507.x. [DOI] [PubMed] [Google Scholar]
- Sharma S, Paladino P, Gabriele J, Saeedi H, Henry P, Chang M, Mishra RK, Johnson RL. Pro-Leu-glycinamide and its peptidomimetic, PAOPA, attenuate haloperidol induced vacuous chewing movements in rat: A model of human tardive dyskinesia. Peptides. 2003;24:313–319. doi: 10.1016/s0196-9781(03)00045-7. [DOI] [PubMed] [Google Scholar]
- Siegel RK. NIDA Res Monogr. 1978. Phencyclidine and ketamine intoxication: a study of four populations of recreational users; pp. 119–147. [PubMed] [Google Scholar]
- Skinbjerg M, Namkung Y, Halldin C, Innis RB, Sibley DR. Pharmacological characterization of 2-methoxy-N-propylnorapomorphine's interactions with D2 and D3 dopamine receptors. Synapse. 2009;63:462–475. doi: 10.1002/syn.20626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JR, Morgan M. The effects of prolyl-leucyl-glycine amide on drug-induced rotation in lesioned rats. Gen Pharmacol. 1982;13:203–207. doi: 10.1016/0306-3623(82)90090-8. [DOI] [PubMed] [Google Scholar]
- Snyder SH. Amphetamine psychosis: a “model” schizophrenia mediated by catecholamines. Am J Psychiatry. 1973;130:61–67. doi: 10.1176/ajp.130.1.61. [DOI] [PubMed] [Google Scholar]
- Srivastava LK, Bajwa SB, Johnson RL, Mishra RK. Interaction of L-prolyl-L-leucyl glycinamide with dopamine D2 receptor: evidence for modulation of agonist affinity states in bovine striatal membranes. J Neurochem. 1988;50:960–968. doi: 10.1111/j.1471-4159.1988.tb03005.x. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Transient N-methyl-D-aspartate receptor blockade in early development causes lasting cognitive deficits relevant to schizophrenia. Biol Psychiatry. 2005;57:433–436. doi: 10.1016/j.biopsych.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Verma V, Mann A, Costain W, Pontoriero G, Castellano JM, Skoblenick K, Gupta SK, Pristupa Z, Niznik HB, Johnson RL, Nair VD, Mishra RK. Modulation of agonist binding to human dopamine receptor subtypes by L-prolyl-L-leucyl-glycinamide and a peptidomimetic analog. J Pharmacol Exp Ther. 2005;315:1228–1236. doi: 10.1124/jpet.105.091256. [DOI] [PubMed] [Google Scholar]
- Wetzel H, Hillert A, Grunder G, Benkert O. Roxindole, a dopamine autoreceptor agonist, in the treatment of positive and negative schizophrenic symptoms. Am J Psychiatry. 1994;151:1499–1502. doi: 10.1176/ajp.151.10.1499. [DOI] [PubMed] [Google Scholar]
- Yu KL, Rajakumar G, Srivastava LK, Mishra RK, Johnson RL. Dopamine receptor modulation by conformationally constrained analogues of Pro-Leu-Gly-NH2. J Med Chem. 1988;31:1430–1436. doi: 10.1021/jm00402a031. [DOI] [PubMed] [Google Scholar]


