Abstract
Objective
To examine whether differentially targeting physical activity within the context of pilot family-based pediatric weight control treatment results in differential change in abdominal fat, particularly visceral fat.
Method
Twenty-nine overweight children (>85th BMI percentile) and at least one participating parent were randomly assigned to one of two family-based behavioral weight management conditions that either targeted 1) primarily dietary change (STANDARD; n=15) or 2) dietary plus physical activity change (ADDED; n=14). Differences at post-treatment in overall child weight status (e.g., BMI), whole-body composition (measured by dual x-ray absorptiometry), and abdominal fat (measured by waist circumference and magnetic resonance imaging) were assessed using intent-to-treat analyses, as were post-treatment parent BMI and weight circumference. Child and parent physical activity and dietary behavior changes were also evaluated.
Results
At post-treatment, overall child weight status, whole-body composition, and child dietary measures did not differ by condition. Children in the ADDED condition tended to have higher physical activity and lower visceral abdominal fat at post-treatment relative to children in the STANDARD condition.
Conclusions
Increasing physical activity may be important to optimize reductions in abdominal fat, especially visceral fat, among overweight children provided family-based behavioral weight management treatment.
Keywords: pediatric overweight, visceral fat, physical activity, weight management, obesity
INTRODUCTION
The high prevalence of pediatric overweight in the U.S. continues (1). Family-based behavioral treatment for child overweight successfully prevents further increases in child weight status (e.g., body mass index or BMI, BMI z-score), at least during treatment contact and for some in the long-term (2, 3). However, it is not clear whether simply improving child weight status, regardless of magnitude, attenuates health risk or if the level of risk attenuation is directly related to the amount of child weight change (4, 5).
Evidence in adults suggests that body fat distribution confers differential risk at similar levels of body weight or fat (6–9). Abdominal fat in particular conveys more cardiovascular disease risk in adults than overall weight status or whole-body fat (10). Although data are more limited, the same higher cardiovascular risk is evident for abdominal than whole-body fat in children (11–15). Despite offering a more precise estimate of risk, change in fat distribution is rarely measured in pediatric overweight treatment trials. This is perhaps because abdominal fat and subcomponents (e.g., subcutaneous abdominal and intra-abdominal or visceral fat) are most accurately measured by radiologic means (e.g., magnetic resonance imaging or MRI) (16, 17), which are expensive, and it is not clear whether and under what circumstances simple and inexpensive metrics of children’s abdominal fat (i.e., waist circumference) are good proxy measures (18). For example, no detectable changes were found in adult waist circumference in prior studies in which visceral adiposity levels changed by 25% (19) and 48% (20) as a result of an exercise intervention.
Without high quality measurement, it is unknown whether different attempts to change eating and activity behaviors impact children’s fat distribution differently. Evidence from adults suggests that increasing physical activity may be a critical component to abdominal fat loss (21). Recent studies in children document negative cross-sectional associations between more objectively-measured (e.g., accelerometry, not self-reported) physical activity and child whole-body and trunk fat (22, 23). After adjusting for child whole body fat, we found that more physically active overweight children had lower visceral fat, but not lower subcutaneous abdominal fat (24). A recent review found that increasing physical activity, even without dietary change, reduces children’s whole body fat, but concluded that evidence on specific impacts of increasing physical activity on children’s visceral fat is limited (25).
Prior efficacious family-based pediatric overweight treatment programs have used behavioral change strategies to help children and parents make dietary and physical activity changes to achieve better weight management (3, 26). However, these trials have failed to examine the impact on visceral fat accumulation and to isolate whether dietary change alone can reduce visceral fat. In the present study it was hypothesized that overweight children targeted to improve dietary intake and physical activity would lose more visceral fat than children targeted to only improve dietary intake within the context of a family-based pediatric overweight treatment program.
METHODS
Design, Setting, and Participants
This pilot, randomized, parallel group trial was conducted in outpatient clinical settings. Participants were recruited from mass mailings sent to households in the surrounding communities. Eligible children were above the 85th percentile for BMI for sex and age but below a BMI that is 125% above the median BMI for a child of the same sex and age, were not engaged in any active treatment or taking medications that would significantly impact their weight or growth, were able to engage in at least moderate intensity physical activity, had at least one parent with a BMI > 25, and had a parent or caregiver interested and available in participating with the child.
The sample size sought for this pilot trial was determined by study resources and the expected difference in visceral fat change using on a 30 minute/day difference in physical activity and an expected resulting 45 cm3 difference at post-treatment in visceral fat by condition. This latter estimate was based on our prior cross-sectional study on physical activity and visceral fat in overweight children (24). Hypothesizing an effect size difference by condition of 1.0 (Cohen’s d), 14 children per condition would provide sufficient statistical power (>80%) at p<.10. This study was approved by the IRB at Cincinnati Children’s Hospital and Regional Medical Center and Seattle Children’s Hospital. Parents provided consent and children provided assent to participate.
Randomization
Stratified block randomization was used to determine treatment condition assignment. Children were stratified by gender and level of overweight (low = percent overweight <60%; high = percent overweight ≥ 60%) and then assigned to either condition within blocks that varied randomly in size from 2 to 6. Randomization was conducted blind to other information about the child/family. Treatment was conducted in three cohorts starting in July 2006, April 2007, and September 2007, with both treatment conditions conducted during each cohort.
Measurements
All measurements were performed prior to treatment beginning (pre-treatment) and immediately following treatment cessation (post-treatment, which was 15 weeks after pre-treatment). Assessors were trained research staff who were not interventionists, but were not blind to treatment assignment (with the exception of the DXA scan & MRI technologists and processors, who were blind to treatment assignment and measurement timepoint). Interventionists were doctoral or masters degree level professionals trained to deliver the intervention by the first author. Anthropometrics obtained for the child and participating parent were height, weight, and waist circumference. Child and parent BMI were calculated as kg/m2. Child percent overweight was calculated as the percentage the child’s actual BMI was above the median BMI for age and gender [((actual BMI – median BMI for age and gender)/median BMI for age and gender) * 100] (27). BMI z-score was determined using the LMS method, with Box-Cox transformation, and the Centers for Disease Control and Prevention National Center for Health Statistics 2000 growth curves (28). There is no consensus regarding the most appropriate whole body composition metric for evaluating change in pediatric obesity treatment (27, 29), so the most common metrics of change are reported. Estimates of children’s whole-body fat, percent body fat, and total lean mass were obtained by whole-body dual energy x-ray absorptiometry (DXA) scans. Estimates of children’s total abdominal, subcutaneous abdominal, and visceral fat volumes were obtained using magnetic resonance imaging (MRI). Details of the DXA and MRI procedures are described elsewhere (24). DXA demonstrates good reliability and validity for the measurement of body fat (30). In a similar child population and with the same type of DXA scan, Wosje and colleagues found low measurement error across duplicate scans of 1.29% for fat mass (31). DXA validity, as assessed most commonly by animal scan and subsequent carcass chemical analysis, generally find standard errors of estimate <1.0 kg (32, 33). Reproducibility estimates for MRI assessments of abdominal fat components are acceptable, with coefficients of variation mostly <10% (34), and although less often examined, validity based on volume estimates of known phantoms appears acceptable (35). In this study, a random selection of the MRI images from 15 participants (randomly and blindly selected at pre- or post-treatment) was processed by an independent rater. The single-measure intraclass correlation (absolute agreement criterion) between raters was .996, with a very small mean difference of 2.8 cm3 (95% CI −2.05, 7.68).
Children and parents’ physical activity and dietary intake were also evaluated at pre- and post-treatment. Physical activity was assessed by accelerometry, with acquisition epoch set at 1 minute (Actigraph model 71256, Fort Walton, FL). The accelerometer was worn on a belt and positioned above the right hip. Children and parents were instructed to wear the accelerometer for 7 days (including 2 weekend days) during all waking hours, with re-wearing (rewearing needed for n=4 children & n=5 parents at pre-treatment and n=7 children & n=3 parents at post-treatment) required if worn for less than 6 valid days (a valid day was defined as a day in which there were 10+ hours in which there was no 30-min blocks of consecutive 0 activity counts). At pre-treatment, children wore the accelerometer on 6.7 days (SD = 0.5) for a total of 80.7 hours (SD = 12.5), and parents wore it on 6.5 days (SD = 0.9) for a total of 88.6 hours (SD = 17.6). At post-treatment, children wore the accelerometer on 6.7 days (SD = 0.7) for a total of 69.9 hours (SD = 24.8), and parents wore it on 6.8 days (SD = 0.6) for a total of 88.3 hours (SD = 13.2). Minute-based activity counts were converted to per day estimates of moderate (either 3–6.9 metabolic equivalents or METs or 4–6.9 METs) and vigorous (7+ METs) intensity physical activity. For children, age-based thresholds (rounded down to whole years) for moderate and vigorous physical activity were used (36), as in recent large epidemiologic studies with youth and accelerometry (37, 38). For participating parents, Freedson thresholds for moderate (≥ 1952 and <5724 activity counts) and vigorous (≥ 5725) activity were used (39).
Children and parents were instructed on how to complete 3-day food logs, with the parent assisting children in remembering and recording foods/beverages and amounts. Upon return, logs were evaluated for completeness (i.e., full descriptions of foods/beverages, amounts for each food/beverage) and follow-up queries were made with the parent (for n=1 parent, one day from the parent’s last treatment week’s food monitoring book was used to get a 3-day intake estimate). Food log information was entered into Nutrition Data Systems (NDS) software for analysis and averages across the 3 days were calculated.
At post-treatment, parents reported on the helpfulness of treatment in getting their child to eat more healthfully and be more physically active, by rating each component separately (family, child group, parent group sessions) from 1=not at all helpful to 5=very helpful. Parents also rated their satisfaction (1=not at all satisfied to 5=very much satisfied) with treatment.
Treatment conditions
The delivery format of both treatment conditions was modeled after family-based behavioral pediatric weight management treatment with demonstrated efficacy (2, 3). Interventionists had training in clinical psychology or pediatrics and specific training prior to the intervention. Weekly supervision sessions were provided to interventionists by the first author. The child and participating parent were seen at the clinical site weekly for 14 weeks, with each weekly visit including a 20–30 minute parent-child session with an interventionist, and 40–45 minute separate parent and child group sessions. In these parent-child and group sessions, children and parents were instructed in the use of the behavioral skills of monitoring, goal setting and contingency management, and environmental control (40). At the beginning of each visit, both the child and parent were weighed. The family session included discussion of the weekly weight change and links to specific behaviors that may have contributed to that change. Children and parents were encouraged to complete daily food logs. In both conditions, children and parents were instructed in the use of a modified Stoplight Eating Plan (41), in which foods were categorized into green (‘go’ foods), yellow (‘caution’ foods), or red foods (‘stop’ foods), based on fat and sugar content. Children and parents in both conditions worked toward meeting 3 specific eating/weight goals introduced in week 2 and continuing through the end of treatment: 1) reducing calories to 1000–1200/day for children and 1200–1400/day for parents on at least 5 days/week, 2) reducing red foods to ≤ 15 servings/week, and 3) reducing weight (0.3kg/week).
The STANDARD and ADDED conditions differed only in the emphasis on physical activity. In the STANDARD condition, children and parents were encouraged to get at least 60 minutes of physical activity on most days of the week, and were simply asked to check off in their daily logs if they obtained this level of physical activity each day. Family and group interventionists provided little or no other additional information or attention to discussion of physical activity. In contrast, children and parents in the ADDED condition were instructed to increase physical activity up to at least 90 minutes of moderate-to-vigorous physical activity per day on at least 6 days each week. Using pre-treatment estimates of physical activity as a starting point, starting in week 3 of treatment, goals for physical activity increased gradually up to this 90 min/day goal. To help families in the ADDED condition meet this recommendation, children and parents were each 1) provided with pedometers (that measured steps and amount of time spent stepping), 2) asked to set weekly and long-term physical activity goals and contingent rewards based on physical activity goal attainment, 3) instructed in ways to increase both lifestyle and structured physical activity. Visits occurred in outpatient clinic offices at Cincinnati Children’s Hospital and the University of Washington Child Health Institute.
Statistical analysis
The primary outcome was change in various aspects of child body composition, with secondary outcomes being change in parent body anthropometrics and child and parent physical activity and dietary behaviors. Analysis of covariance (ANCOVAs) were conducted to explore post-treatment differences by condition, with pre-treatment values of the respective measure included as a covariate. Child sex (a randomization stratification variable) was also included as a covariate in analyses of child outcomes. Given the pilot nature of the study, p ≤ .10 was used to signify statistical significance and no adjustments were made for multiple tests. Effect sizes were estimated by Cohen’s d. All analyses were intent-to-treat, with baseline values carried forward for missing post-treatment values.
RESULTS
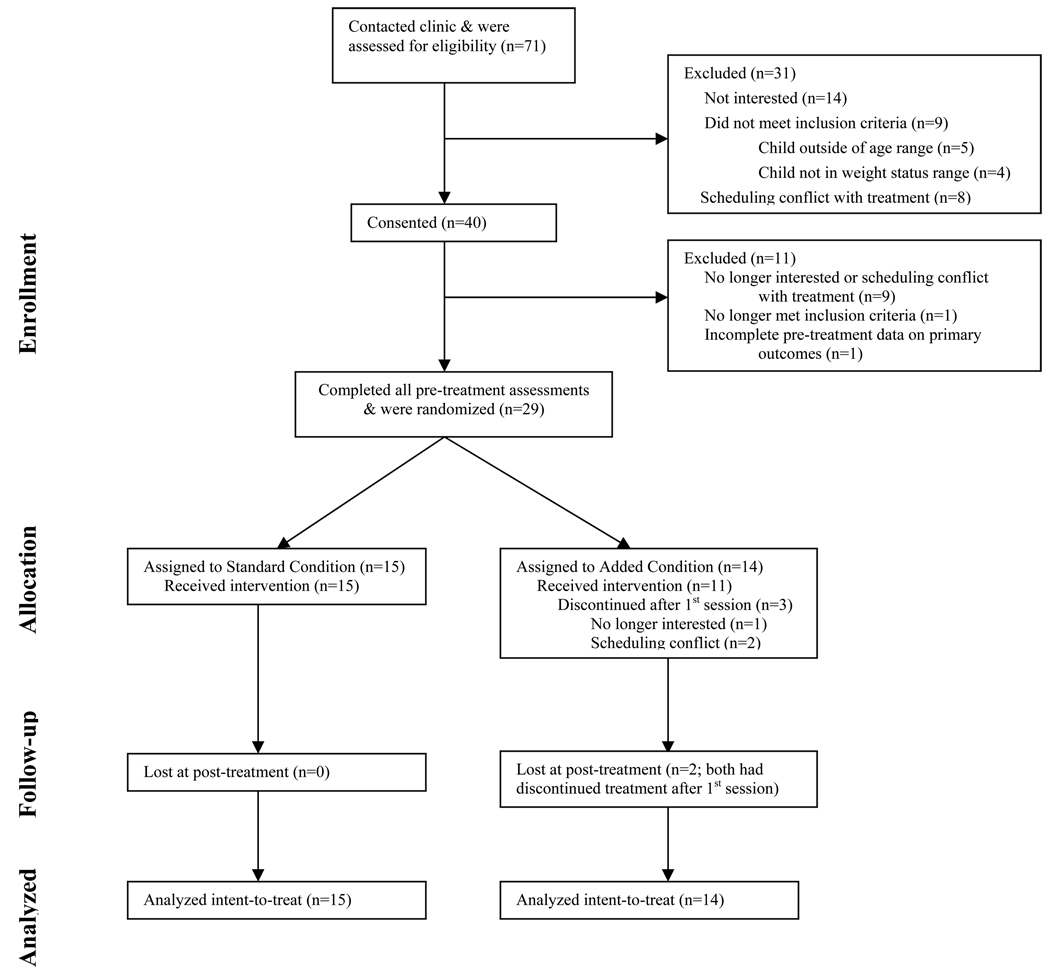
Twenty-nine children, aged 7–11 years old, were randomly assigned to one of two treatment conditions (see Figure 1 for participant flow). Demographic data by treatment condition are presented in Table 1. Child pre-treatment body composition and behavioral data are presented in Table 2. Parent pre-treatment body composition and behavioral data are presented in Table 3. Parent/child dyads attended on average 90.6% (STANDARD) or 88.0% (ADDED) or approximately 12 of the 14 weekly visits. At post-treatment, parents reported that treatment was similarly helpful in getting their child to eat more healthfully in both the STANDARD (mean=4.2; SD=0.6) and ADDED conditions (mean=4.5, SD=0.5). In contrast and expectedly given differences in approach between conditions, the parents in the STANDARD condition reported treatment was significantly less helpful for increasing their children’s physical activity (mean satisfaction=2.7, SD=1.3) than in the ADDED condition (mean satisfaction=4.3, SD=0.7) (p<.004). Participating parents and children reported no adverse events.
Figure 1.
Participant flow.
Table 1.
Baseline demographic characteristics by condition
| STANDARD condition (n=15) |
ADDED condition (n=14) |
|
|---|---|---|
| Mean child age ± SD, y | 10.1 (1.1) | 9.8 (1.2) |
| Child sex | 7 boys / 8 girls | 6 boys / 8 girls |
| Child ethnicity/race, n (%) | ||
| - Caucasian, non-Hispanic | 11 (73.3%) | 8 (57.1%) |
| - African-American | 2 (13.3%) | 2 (14.3%) |
| - Caucasian, Hispanic | 2 (13.3%) | 2 (14.3%) |
| - Multi-racial | 0 (0%) | 1 (7.1%) |
| - Refused | 0 (0%) | 1 (7.1%) |
| Participating parent | 13 mothers / 2 fathers | 12 mothers / 2 fathers |
| Participating parent martial status, n (%) | ||
| - Married | 13 (86.7%) | 9 (64.3%) |
| - Divorced or separated | 2 (13.3%) | 3 (21.4%) |
| - Never married | 0 (0%) | 2 (14.3%) |
| Annual household income, median US dollars |
$80,000–89,000 | $60–69,000 |
Table 2.
Child pre-treatment anthropometric and DXA-derived and MRI-derived body composition and physical activity and diet by condition [Mean (Standard Deviation)]
| STANDARD condition (n=15) |
ADDED condition (n=14) |
|
|---|---|---|
| Height, cm | 147.8 (8.9) | 146.8 (10.8) |
| Weight, kg | 60.1 (13.3) | 56.1 (16.3) |
| BMI, kg/m2 | 27.2 (4.0) | 25.6 (4.2) |
| BMI z-score | 2.12 (0.37) | 1.99 (0.41) |
| Percent overweight, % | 61.3 (21.8) | 53.3 (23.2) |
| Whole-body fat, kg | 23.4 (7.3) | 20.9 (7.0) |
| Percent body fat, % | 38.1 (5.2) | 36.7 (4.8) |
| Total body lean, kg | 36.4 (7.9) | 34.6 (10.0) |
| Waist circumference, cm | 90.2 (10.5) | 87.4 (11.2) |
| Total abdominal fat, cm3 | 1372.7 (418.9) | 1107.2 (469.8) |
| Visceral fat, cm3 | 206.3 (77.0) | 188.2 (134.5) |
| Subcutaneous abdominal fat, cm3 | 1166.4 (416.3) | 919.0 (397.4) |
| Moderate intensity PA (3–6.9 METs), mins/d | 100.5 (43.8) | 120.4 (50.6) |
| Moderate intensity PA (4–6.9 METs), mins/d | 41.1 (23.6) | 49.0 (23.6) |
| Vigorous intensity PA (7+ METs), mins/d | 8.6 (7.9) | 10.2 (6.1) |
| Total MVPA (3+ METs), mins/d | 109.1 (48.7) | 130.6 (53.7) |
| Total MVPA (4+ METs), mins/d | 49.6 (30.1) | 59.2 (27.8) |
| Caloric intake, kcal | 1945.1 (301.2) | 1939.7 (414.0) |
| Fat intake, % kcal | 34.3 (4.7) | 32.3 (7.8) |
| Carbohydrate intake, % kcal | 50.2 (5.7) | 54.1 (7.7) |
| Protein intake, % kcal | 16.5 (2.4) | 15.8 (2.6) |
Note. BMI=body mass index; PA=physical activity; MVPA=moderate-to-vigorous physical activity; n=1 child in the ADDED condition did not have adequate physical activity data; n=3 children in the ADDED condition did not have adequate dietary data.
Table 3.
Participating parent pre-treatment anthropometric and physical activity and diet by condition [Mean (Standard Deviation)]
| STANDARD condition (n=15) |
ADDED condition (n=14) |
|
|---|---|---|
| Weight, kg | 99.1 (22.3) | 91.2 (19.3) |
| BMI, kg/m2 | 35.8 (7.9) | 32.4 (5.2) |
| Waist circumference, cm | 109.8 (18.4) | 104.4 (13.8) |
| Moderate intensity PA, mins/d | 37.0 (26.5) | 28.6 (20.8) |
| Vigorous intensity PA, mins/d | 1.6 (3.2) | 2.7 (6.0) |
| Total MVPA, mins/d | 38.6 (29.3) | 31.3 (22.8) |
| Caloric intake, kcal | 2073.6 (401.0) | 1970.5 (488.5) |
| Fat intake, % kcal | 34.3 (5.6) | 36.4 (5.4) |
| Carbohydrate intake, % kcal | 48.8 (4.8) | 45.8 (8.5) |
| Protein intake, % kcal | 16.9 (3.6) | 18.3 (4.5) |
Note. BMI=body mass index; PA=physical activity; MVPA=moderate-to-vigorous physical activity; n=13 in the ADDED condition, since one parent did not have adequate pre-treatment physical activity data; n=11 in the ADDED condition, since three parents did not have adequate pre-treatment dietary data.
Primary outcomes
Child BMI, BMI z-score, and percent overweight were lower in both the STANDARD and ADDED condition at post-treatment than pre-treatment. These overall child weight status changes were similar across treatment conditions, with no differences by condition at post-treatment. Similarly, there were no post-treatment condition differences in more specific child whole body measures, including whole-body fat, percent body fat, or total body lean mass (Table 4).
Table 4.
Post-treatment child and participating parent anthropometrics and child DXA- and MRI derived fat and lean body mass by condition and condition difference statistics
| STANDARD condition (n=15) |
ADDED condition (n=14) |
Difference (ADDED – STANDARD) |
P value | Effect size estimate |
|
|---|---|---|---|---|---|
| Adjusted mean (SE) | Mean (95% CI) | Cohen’s d | |||
| Child | |||||
| Height, cm | 148.8 (0.3) | 149.1 (0.3) | 0.26 (−0.7 – 1.2) | .571 | .22 |
| Weight, kg | 56.1 (0.9) | 55.3 (0.9) | −0.8 (−3.4 – 1.8) | .526 | .25 |
| BMI, kg/m2 | 25.0 (0.4) | 24.4 (0.5) | −0.5 (−1.9 – 0.8) | .422 | .31 |
| BMI z-score | 1.80 (0.06) | 1.78 (0.06) | −0.02 (−0.19 – 0.16) | .864 | .07 |
| Percent overweight, % | 47.2 (2.7) | 44.1 (2.8) | −3.1 (−11.2 – 4.9) | .430 | .31 |
| Whole-body fat, kg | 20.5 (0.7) | 19.7 (0.7) | −0.8 (−2.8 – 1.2) | .432 | .31 |
| Percent body fat, % | 35.7 (0.6) | 35.1 (0.6) | −0.6 (−2.5 – 1.2) | .479 | .28 |
| Total body lean, kg | 35.0 (0.4) | 35.1 (0.4) | 0.09 (−1.0 – 1.1) | .867 | .07 |
| Waist circumference, cm | 85.0 (1.3) | 82.4 (1.3) | −2.6 (−6.4 – 1.2) | .164 | .55 |
| Total abdominal fat, cm3 | 1150.1 (61.7) | 1001.2 (64.0) | −148.9 (−336.2 – 38.3) | .114 | .63 |
| Visceral fat, cm3 | 178.2 (16.2) | 137.8 (16.7) | −40.5 (−88.4 – 7.5) | .095 | .67 |
| Subcutaneous abdominal fat, cm3 |
959.7 (50.7) | 878.5 (52.5) | −81.2 (−235.0 – 72.7) | .288 | .42 |
| Participating parent | |||||
| Weight, kg | 90.1 (1.1) | 90.2 (1.1) | 0.1 (−3.2 – 3.4) | .931 | .03 |
| BMI, kg/m2 | 32.3 (0.4) | 32.4 (0.4) | 0.2 (−1.0 – 1.4) | .787 | .10 |
| Waist circumference, cm | 101.9 (1.5) | 102.9 (1.6) | 1.1 (−3.5 – 5.6) | .638 | .08 |
Note. Based on intent-to-treat (with values for n=2 missing in the ADDED condition being baseline carried forward); child outcomes analyses were adjusted for child sex and corresponding pre-treatment values; parent outcome analyses were adjusted for corresponding pre-treatment values; SE=standard error; CI=confidence interval; BMI=body mass index.
There was some evidence of larger differences between treatment conditions in measures of child abdominal fat at post-treatment. At post-treatment, children in the ADDED condition tended to have lower total abdominal fat and specifically lower visceral fat deposition (p=.095, d=.67), measured by MRI, compared with children in the STANDARD condition. The differences between conditions at post-treatment were smaller for total abdominal and subcutaneous abdominal fat. Similarly, the differences between treatment conditions for children’s waist circumference at post-treatment were in the expected direction (ADDED>STANDARD), although the size of this difference was somewhat smaller than that observed for MRI-derived metrics of total abdominal and visceral fat (Table 4).
Secondary outcomes
The condition difference in children’s moderate-to-vigorous physical activity (MVPA) at post-treatment was in the expected direction, with the children in the ADDED condition tending to have more MVPA than children provided STANDARD treatment at post-treatment. The effect size was greater using the 4+ METs threshold (d=.68, p=.096) than using the 3+ METs threshold (d=.50, p=.218). Condition differences at post-treatment for separate measures of moderate intensity or vigorous intensity physical activity were also both in the expected direction (i.e., children in the ADDED condition higher at post-treatment). However, the Pearson product moment correlation between child pre- to post-treatment change in physical activity and change in visceral fat over this time period was weak regardless of which MET cut-off was used (r=.016, p=.938 for 3+ METs; r=-.045, p=.823 for 4+ METs; completers only n=27). The average child in the ADDED condition met the physical activity goal on 5.3 weeks (SD=3.3; median = 6.0) out of the possible 11 weeks in which physical activity goals were established. There were no condition differences at post-treatment for any child dietary intake measures, with children on average in both conditions reducing overall caloric intake and percent calories from fat and increasing the percentage of calories from carbohydrate and protein (Table 5).
Table 5.
Post-treatment child and participating parent physical activity and diet by condition and condition difference statistics
| STANDARD condition |
ADDED condition |
Difference (ADDED – STANDARD) |
P value |
Effect size estimate |
|
|---|---|---|---|---|---|
| Adjusted mean (SE) | Mean (95% CI) | Cohen’s d |
|||
| Child | |||||
| Moderate intensity PA (3–6.9 METs), mins/d | 100.9 (7.9) | 114.4 (8.5) | 13.5 (−10.7 – 37.8) | .260 | .45 |
| Moderate intensity PA (4–6.9 METs), mins/d | 38.8 (4.7) | 51.1 (5.1) | 12.3 (−2.2 – 26.8) | .094 | .69 |
| Vigorous intensity PA (7+ METs), mins/d | 8.7 (2.3) | 12.8 (2.5) | 4.1 (−2.9 – 11.1) | .239 | .47 |
| Total MVPA (3+ METs), mins/d | 110.0 (8.9) | 126.8 (9.6) | 16.8 (−10.6 – 44.1) | .218 | .50 |
| Total MVPA (4+ METs), mins/d | 47.6 (6.3) | 63.8 (6.8) | 16.2 (−3.1 – 35.5) | .096 | .68 |
| Caloric intake, kcal | 1388.1 (80.2) | 1455.0 (93.7) | 66.9 (−189.5 – 323.3) | .594 | .22 |
| Fat intake, % kcal | 27.1 (2.0) | 26.8 (2.3) | −0.2 (−6.6 – 6.2) | .943 | .03 |
| Carbohydrate intake, % kcal | 55.2 (2.1) | 56.3 (2.4) | 1.1 (−5.7 – 7.9) | .742 | .14 |
| Protein intake, % kcal | 18.8 (0.9) | 19.1 (1.1) | 0.3 (−2.6 – 3.2) | .847 | .08 |
| Parent | |||||
| Moderate intensity PA, mins/d | 26.8 (5.7) | 38.1 (6.1) | 11.4 (−5.9 – 28.7) | .189 | .53 |
| Vigorous intensity PA, mins/d | 2.9 (1.2) | 4.8 (1.3) | 1.9 (−1.7 – 5.5) | .283 | .43 |
| Total MVPA, mins/d | 29.3 (6.5) | 43.2 (6.9) | 13.9 (−5.7 – 33.5) | .157 | .57 |
| Caloric intake, kcal | 1319.8 (84.7) | 1300.2 (99.0) | −19.6 (−289.5 – 250.4) | .882 | .06 |
| Fat intake, % kcal | 28.6 (2.3) | 28.7 (2.7) | 0.05 (−7.3 – 7.4) | .990 | <.01 |
| Carbohydrate intake, % kcal | 52.7 (2.5) | 50.5 (3.0) | −2.2 (−10.4 – 5.9) | .577 | .23 |
| Protein intake, % kcal | 19.1 (0.9) | 22.5 (1.0) | 3.4 (0.6 – 6.2) | .021 | 1.0 |
Note. SE=standard error; CI=confidence interval; PA=physical activity; MVPA=moderate-to-vigorous physical activity; for physical activity data n=15 for STANDARD condition and n=13 for ADDED condition; for dietary data, n=15 for STANDARD condition and n=11 for ADDED condition.
Participating parents’ BMI and waist circumference did not substantively differ by condition at post-treatment (Table 4). The condition difference in parents’ physical activity at post-treatment was not statistically significant, although in the expected direction. There were no condition differences at post-treatment for all but one of the parent dietary intake values. The percentage calories from protein was unexpectedly higher among parents in the ADDED versus STANDARD condition (p=.021, d=1.0) (Table 5).
DISCUSSION
This pilot randomized controlled trial found preliminary evidence that targeting greater physical activity along with dietary change could better help decrease abdominal fat relative to targeting only dietary change among overweight children engaged in family-based behavioral weight control treatment. With all children and parents targeted for dietary change, the differential effects of less versus more emphasis on changing physical activity on children’s abdominal fat were most evident in MRI-derived measures. Findings highlight the potential stronger influence of physical activity on visceral fat versus subcutaneous abdominal fat reduction among overweight children within the context of simultaneous dietary change. However, given the small sample size and resulting low statistical power, and the greater absolute magnitude of post-treatment differences in subcutaneous versus visceral abdominal fat between the ADDED and STANDARD conditions by treatment end, the approach targeting increases in physical activity appears to be impacting overweight children’s overall abdominal fat. In contrast to condition differences in abdominal fat measures, smaller or minimal differences between children or parents in the ADDED versus STANDARD condition were observed the overall weight status (e.g., BMI, z-BMI) and whole-body fat measures.
Evidence continues to emerge regarding the benefits of greater physical activity and physical fitness on various aspects of children’s health, including better insulin sensitivity (42), blood pressure (43), lung function (44), and vascular function (particularly for vigorous physical activity) (45). More recent research that employed accelerometers to measure children’s physical activity finds that not just overall quantity, but higher physical activity intensity and bouts may be important for obesity prevention and lower central adiposity (46, 47). Physical activity also appears to reduce cardiovascular health risk independent of its impact on overall weight status or whole body fat (48, 49). Finding in the present study are also consistent with other findings regarding the incremental health benefit of more physical activity above and beyond the benefits achieved by dietary modification alone among children (50). In adults increasing exercise intensity may add to or interact with exercise volume to more dramatically reduce visceral fat (51). More research is needed in overweight children regarding the impact on visceral fat of modifying physical activity intensity, volume, and other aspects of physical activity.
The present study demonstrated the ability of focused behavioral strategies to increase physical activity and the impact of establishing a higher physical activity recommendation (90 minutes versus 60 minutes as recommended for general children health; (52)) among overweight youth in weight management treatment. After adjusting for pre-treatment physical activity, children in the ADDED condition nonetheless had on average more than 15 more minutes per day of physical activity at post-treatment. The average child in the present trial was similarly active to the average child in a sample of U.S. 9–15 year olds (not selected based on weight status) measured with similar accelerometers and the same thresholds for determining moderate and vigorous physical activity (3.0+ METs) (37). It is noteworthy that the estimates of MVPA were markedly lower when the higher 4.0+ METs threshold was used, with children having approximately 50% less physical activity on average. The average child in the ADDED condition was self-reporting meeting physical activity goals only slightly more than ½ the treatment weeks in which the goal was established, with considerable variability (range 0–11 weeks met goal; 3 weeks was the 25th percentile and 7 weeks was the 7th percentile) in goal attainment. Individual children’s change in physical activity, defined in this study as the change from the 1 week period of accelerometry measured before treatment and at post-treatment, was only very weakly associated with their pre- to post-treatment change in visceral fat. Objective assessment of physical activity throughout intervention would likely better determine whether physical activity was the primary mechanism of visceral fat change.
The pattern by condition for the parents’ physical activity was similar to that of their children. The overall discrepancy in physical activity at post-treatment between parents in the ADDED versus STANDARD conditions was similar to the condition differences for the children. There were no MRI data among parents to test whether condition differences existed in parents’ visceral versus subcutaneous abdominal fat.
Children and parents had the expected decreases in caloric and specifically dietary fat intake, based on the prescribed intervention eating plan. Given the eating plan did not differ by condition, the lack of differences in all child and most parent dietary intake outcomes between conditions was expected. The difference between the parents in the ADDED versus STANDARD conditions in protein intake at post-treatment was unexpected and may be related to possible insufficient number of days that parents recorded dietary intake.
As found in the present study, waist circumference may not be a sufficient proxy for visceral fat or change in visceral fat, at least among already overweight children (53–55). The use of MRI herein allowed for distinct estimates of change in subcutaneous abdominal and visceral fat and demonstrated where changes in the abdominal fat depot occurred. The present study is among the first to directly measure change in different abdominal fat depots in children provided comprehensive weight management treatment (56). Simple waist circumference measures appeared somewhat sensitive to differential change in total abdominal fat in the ADDED versus STANDARD conditions, but this may be due to the sensitivity of this measure to the larger subcutaneous fat depot (18). Waist circumference measurement does not allow for estimating the potential differential effect on visceral versus subcutaneous abdominal fat. There may also be gender-based differences in the utility of waist circumference to estimate visceral fat (57). More evidence is needed to determine how weight management in general and different approaches to weight management among overweight children impacts abdominal fat and the methods needed to evaluate such changes.
There were many strengths of this pilot study, including the high quality measurement of whole body and abdominal fat among children, the more objective measure of physical activity through accelerometry, and the high treatment session attendance. The clinical significance of the observed child abdominal fat changes is not totally clear. In adults, there is evidence that distribution of visceral versus subcutaneous abdominal fat depots may confer specific health risks (i.e., cardiovascular disease risk versus insulin sensitivity) (58). Such evidence in children is more limited and equivocal (13, 59–61). Limitations to this trial included the small sample size (observed power of only .52 for p<.10), the relatively short length of treatment with no assessments of physical activity throughout treatment or any outcomes beyond post-treatment, and the predominantly White middle-class sample (although representative of the geographic areas in which the trial took place). In addition, using accelerometers and common child-based thresholds for activity intensity, the average child before treatment was already meeting the 90 min/day physical activity goal of the trial. The threshold used may be capturing some light intensity, rather than moderate-to-vigorous intensity physical activity. Applying significantly more stringent thresholds for activity intensity (62) yielded much smaller absolute minutes of physical activity (e.g., 19.6 minutes/day at pre-treatment across conditions (SD=13.0)), although the direction and magnitude of condition differences at post-treatment were similar. The small sample size precludes an adequate evaluation of factors that might interact with treatment to impact abdominal fat outcomes. However, given the interest in individual factors (e.g., child age, sex, ethnicity) that impact fat distribution (57), post-treatment differences for visceral fat by condition are presented separately for boys and girls in Table 6. These data should be viewed cautiously given the small sample sizes and large confidence intervals. The dietary assessment used in this study may have been sufficient for group comparisons, but likely included under-reporting (e.g., younger children not being able to recall well and parents not being able to assist if not having observed the child, like at school lunch) and bias (e.g., children and parents knowing it is socially desirable to report less intake at post-treatment) (63). Future impacts of child or other factors (e.g., parental predispositions for abdominal fat distribution) on visceral fat outcomes should be explored.
Table 6.
Post-treatment visceral fat volume (cm3) estimates separately for girls and boys by condition
| Mean (SE) | 95% CI | ||
|---|---|---|---|
| STANDARD | Girls (n=8) | 176.7 (22.5) | 130.4 – 223.1 |
| Boys (n=7) | 179.0 (23.8) | 129.9 – 228.0 | |
| ADDED | Girls (n=8) | 155.6 (22.2) | 109.8 – 201.4 |
| Boys (n=6) | 115.2 (25.6) | 62.4 – 168.0 |
Note. SE=standard error; CI=confidence interval.
Future trials in pediatric overweight treatment should also continue to explore the specific impacts on body fat distribution resulting from different dietary and physical activity change approaches and evaluate these changes more completely throughout treatment (e.g., in the present study, physical activity was not assessed throughout treatment) and their impact on other health indicators (e.g., insulin resistance). Further testing in child overweight treatment could also explore the benefit of matching children with specific health risks with different intervention approaches. This pilot demonstrates it is feasible to increase physical activity more in pediatric weight management treatment and that this increase may have significant health benefits beyond those conferred by whole body weight or fat changes alone resulting from dietary intervention. Data from the present study will also help to estimate the sample size needed to conduct a larger randomized clinical trial to further elucidate the specific effects of physical activity within the context of pediatric weight management.
In summary, this study provides some preliminary evidence of an incremental benefit of interventions that target increased physical activity and dietary change on reducing abdominal fat, particularly visceral fat, among overweight children in a family-based weight management treatment. It is noteworthy that condition-based visceral fat differences were observed in the absence of whole body composition (e.g., BMI) or fat measure differences between conditions. Increasing physical activity is commonly a target in obesity-related interventions, but it is important to document its potential specific effects, perhaps particularly when dietary intervention is challenging.
ACKNOWLEDGMENTS
This project was supported by NIH/NIDDK K23 60476, with additional support from USPHS M01 RR 08084 to Cincinnati Children’s Hospital and Regional Medical Center and CTSA Grant Number 1 UL1 RR025014-01 to the University of Washington and Seattle Children’s Hospital from the National Center for Research Resources (NCRR). Manuscript content is solely the responsibility of the authors and does not necessarily represent the official views of NCRR or NIH.
The authors would like to thank the interventionists and the children and parents who participated in this trial.
Footnotes
This research was conducted at the Child Health Institute of the University of Washington (6200 NE 74th Street, Seattle, WA, USA) and Cincinnati Children’s Hospital Medical Center (3333 Burnet Ave, Cincinnati, OH, USA)
Conflicts of interest: None declared
Clinical Trial Registration: ClinicalTrials.gov identifier: NCT00359957
REFERENCES
- 1.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008;299:2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 2.Epstein LH, Paluch RA, Roemmich JN, Beecher MD. Family-based obesity treatment, then and now: twenty-five years of pediatric obesity treatment. Health Psychol. 2007;26:381–391. doi: 10.1037/0278-6133.26.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilfley DE, Tibbs TL, Van Buren DJ, et al. Lifestyle interventions in the treatment of childhood overweight: a meta-analytic review of randomized controlled trials. Health Psychol. 2007;26:521–532. doi: 10.1037/0278-6133.26.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savoye M, Shaw M, Dziura J, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA. 2007;297:2697–2704. doi: 10.1001/jama.297.24.2697. [DOI] [PubMed] [Google Scholar]
- 5.Faith MS, Saelens BE, Wilfley DE, Allison DB. Behavioral treatment of childhood and adolescent obesity: Current status, challenges, and future directions. In: Thompson JK, Smolak L, editors. Body image, eating disorders, and obesity in children and adolescents: Theory, assessment, treatment, and prevention. Washington, DC: American Psychological Association; 2001. pp. 313–340. [Google Scholar]
- 6.Brochu M, Tchernof A, Dionne IJ, et al. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrionol Metab. 2001;86:1020–1025. doi: 10.1210/jcem.86.3.7365. [DOI] [PubMed] [Google Scholar]
- 7.Depres J-P. The insulin-resistance-dyslipidemic syndrome of visceral adiposity: effect on patients’ risk. Obes Res. 1998;6:8S–17S. doi: 10.1002/j.1550-8528.1998.tb00683.x. [DOI] [PubMed] [Google Scholar]
- 8.Lemieux S, Prud'homme D, Nadeau A, et al. Seven-year change in body fat and visceral adipose tissue in women: associations with indexes of plasma glucose-insulin homeostasis. Diabetes Care. 1996;19:983–991. doi: 10.2337/diacare.19.9.983. [DOI] [PubMed] [Google Scholar]
- 9.Mathieu P, Poirier P, Pibarot P, Lemieux I, Despres JP. Visceral obesity: the link among inflammation, hypertension, and cardiovascular disease. Hypertension. 2009;53:577–584. doi: 10.1161/HYPERTENSIONAHA.108.110320. [DOI] [PubMed] [Google Scholar]
- 10.Lee CM, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol. 2008;61:646–653. doi: 10.1016/j.jclinepi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Caprio S, Hyman LD, McCarthy S, et al. Fat distribution and cardiovascular risk factors in obese adolescent girls: importance of the intraabdominal fat depot. Am J Clin Nutr. 1996;64:12–17. doi: 10.1093/ajcn/64.1.12. [DOI] [PubMed] [Google Scholar]
- 12.Daniels SR, Morrison JA, Sprecher DL, Khoury P, Kimball TR. Association of body fat distribution and cardiovascular risk factors in children and adolescents. Circulation. 1999;99:541–545. doi: 10.1161/01.cir.99.4.541. [DOI] [PubMed] [Google Scholar]
- 13.Owens S, Gutin B, Ferguson M, et al. Visceral adipose tissue and cardiovascular risk factors in obese children. J Pediatr. 1998;133:41–45. doi: 10.1016/s0022-3476(98)70175-1. [DOI] [PubMed] [Google Scholar]
- 14.Goran MI, Bergman RN, Gower BA. Influence of total vs visceral fat on insulin action and secretion in African American and white children. Obes Res. 2001;9:423–431. doi: 10.1038/oby.2001.56. [DOI] [PubMed] [Google Scholar]
- 15.Gower BA, Nagy TR, Goran MI. Visceral fat, insulin sensitivity, and lipids in prepubertal children. Diabetes. 1999;48:1515–1521. doi: 10.2337/diabetes.48.8.1515. [DOI] [PubMed] [Google Scholar]
- 16.Krebs NF, Himes JH, Jacobson D, et al. Assessment of child and adolescent overweight and obesity. Peds. 2007;120 Suppl 4:S193–S228. doi: 10.1542/peds.2007-2329D. [DOI] [PubMed] [Google Scholar]
- 17.Moreno LA, Pineda I, Rodriguez G, et al. Waist circumference for the screening of the metabolic syndrome in children. Acta Paediatr. 2002;91:1307–1312. doi: 10.1080/08035250216112. [DOI] [PubMed] [Google Scholar]
- 18.Benfield LL, Fox KR, Peter DM, et al. Magnetic resonance imaging of abdominal adiposity in a large cohort of British children. Int J Obes. 2008;32:91–99. doi: 10.1038/sj.ijo.0803780. [DOI] [PubMed] [Google Scholar]
- 19.Thomas EL, Brynes AE, McCarthy J, et al. Preferential loss of visceral fat following aerobic exercise, measured by magnetic resonance imaging. Lipids. 2000;35:769–776. doi: 10.1007/s11745-000-0584-0. [DOI] [PubMed] [Google Scholar]
- 20.Mourier A, Gautier JF, De Kerviler E, et al. Mobilization of visceral adipose tissue related to the improvement in insulin sensitivity in response to physical training in NIDDM. Effects of branched-chain amino acid supplements. Diabetes Care. 1997;20:385–391. doi: 10.2337/diacare.20.3.385. [DOI] [PubMed] [Google Scholar]
- 21.Kay SJ, Fiatarone Singh MA. The influence of physical activity on abdominal fat: a systematic review of the literature. Obes Rev. 2006;7:183–200. doi: 10.1111/j.1467-789X.2006.00250.x. [DOI] [PubMed] [Google Scholar]
- 22.Dencker M, Thorsson O, Karlsson MK, et al. Daily physical activity related to body fat in children aged 8–11 years. J Peds. 2006;149:38–42. doi: 10.1016/j.jpeds.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Ness AR, Leary SD, Mattocks C, et al. Objectively measured physical activity and fat mass in a large cohort of children. PLoS medicine. 2007;4:e97. doi: 10.1371/journal.pmed.0040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saelens BE, Seeley RJ, van Schaick K, Donnelly LF, O'Brien KJ. Visceral abdominal fat is correlated with whole-body fat and physical activity among 8-y-old children at risk of obesity. Am J Clin Nutr. 2007;85:46–53. doi: 10.1093/ajcn/85.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atlantis E, Barnes EH, Singh MA. Efficacy of exercise for treating overweight in children and adolescents: a systematic review. Int J Obes (Lond) 2006;30:1027–1040. doi: 10.1038/sj.ijo.0803286. [DOI] [PubMed] [Google Scholar]
- 26.Whitlock EP, O'Connor EA, Williams SB, Beil TL, Lutz KW. Effectiveness of weight management programs in children and adolescents. Evidence Report/Technology Assessment No. 170 (Prepared by the Oregon Evidence-based Practice Center under Contract No. 290-0200024) Rockville, MD: Agency for Healthcare Research and Quality; 2008. Sep, No. AHRQ Publication No. 08-E014. [Google Scholar]
- 27.Paluch RA, Epstein LH, Roemmich JN. Comparison of methods to evaluate changes in relative body mass index in pediatric weight control. Am J Hum Biol. 2007;19:487–494. doi: 10.1002/ajhb.20608. [DOI] [PubMed] [Google Scholar]
- 28.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States advance data from vital and health statistics; no. 314. Hyattsville, MD: National Center for Health Statistics; 2000. [PubMed] [Google Scholar]
- 29.Berkey CS, Colditz GA. Adiposity in adolescents: change in actual BMI works better than change in BMI z score for longitudinal studies. Annals of Epidemiol. 2007;17:44–50. doi: 10.1016/j.annepidem.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Lohman TG, Chen Z. Dual-energy x-ray absorptiometry. In: Heymsfield SB, Lohman TG, Wang Z, Going SB, editors. Human body composition 2nd edition. Champaign, IL: Human Kinetics; 2005. pp. 63–77. [Google Scholar]
- 31.Wosje KS, Knipstein BL, Kalkwarf HJ. Measurement error of DXA: interpretation of fat and lean mass changes in obese and non-obese children. J Clin Densitom. 2006;9:335–340. doi: 10.1016/j.jocd.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Ellis KJ, Shypailo RJ, Pratt J, Pond WG. Accuracy of dual-energy x-ray absorptiometry for body-composition measurements in children. Am J Clin Nutr. 1994;60:660–665. doi: 10.1093/ajcn/60.5.660. [DOI] [PubMed] [Google Scholar]
- 33.Koo WW, Hammami M, Hockman EM. Use of fan beam dual energy x-ray absorptiometry to measure body composition of piglets. J Nutr. 2002;132:1380–1383. doi: 10.1093/jn/132.6.1380. [DOI] [PubMed] [Google Scholar]
- 34.Ross R, Janssen I. Computed tomography and magnetic resonance imaging. In: Heymsfield SB, Lohman TG, Wang Z, Going SB, editors. Human body composition: second edition. Champaign, IL: Human Kinetics; 2005. pp. 89–108. [Google Scholar]
- 35.Donnelly LF, O'Brien KJ, Dardzinski BJ, et al. Using a phantom to compare MR techniques for determining the ratio of intraabdominal to subcutaneous adipose tissue. Am J Roentgenol. 2003;180:993–998. doi: 10.2214/ajr.180.4.1800993. [DOI] [PubMed] [Google Scholar]
- 36.Trost SG, Pate RR, Sallis JF, et al. Age and gender differences in objectively measured physical activity in youth. Med Sci Sports Exerc. 2002;34:350–355. doi: 10.1097/00005768-200202000-00025. [DOI] [PubMed] [Google Scholar]
- 37.Nader PR, Bradley RH, Houts RM, McRitchie SL, O'Brien M. Moderate-to-vigorous physical activity from ages 9 to 15 years. JAMA. 2008;300:295–305. doi: 10.1001/jama.300.3.295. [DOI] [PubMed] [Google Scholar]
- 38.Troiano RP, Berrigan D, Dodd KW, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 39.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30:777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 40.Spear BA, Barlow SE, Ervin C, et al. Recommendations for treatment of child and adolescent overweight and obesity. Peds. 2007;120:S254–S288. doi: 10.1542/peds.2007-2329F. [DOI] [PubMed] [Google Scholar]
- 41.Epstein LH, Squires S. The stoplight diet for children. Boston, MA: Little, Brown and Co.; 1988. [Google Scholar]
- 42.Jago R, Wedderkopp N, Kristensen PL, et al. Six-year change in youth physical activity and effect on fasting insulin and HOMA-IR. Am J Prev Med. 2008;35:554–560. doi: 10.1016/j.amepre.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Mark AE, Janssen I. Dose-response relation between physical activity and blood pressure in youth. Med Sci Sports Exerc. 2008;40:1007–1012. doi: 10.1249/MSS.0b013e318169032d. [DOI] [PubMed] [Google Scholar]
- 44.Berntsen S, Wisloff T, Nafstad P, Nystad W. Lung function increases with increasing level of physical activity in school children. Pediatric exercise science. 2008;20:402–410. doi: 10.1123/pes.20.4.402. [DOI] [PubMed] [Google Scholar]
- 45.Hopkins ND, Stratton G, Tinken TM, et al. Relationships between measures of fitness, physical activity, body composition and vascular function in children. Atherosclerosis. 2009;204:244–249. doi: 10.1016/j.atherosclerosis.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Mark AE, Janssen I. Influence of bouts of physical activity on overweight in youth. Am J Prev Med. 2009;36:416–421. doi: 10.1016/j.amepre.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 47.Moliner-Urdiales D, Ruiz JR, Ortega FB, et al. Association of objectively assessed physical activity with total and central body fat in Spanish adolescents; The HELENA Study. Int J Obes. 2009 doi: 10.1038/ijo.2009.139. [DOI] [PubMed] [Google Scholar]
- 48.Wong SL, Katzmarzyk P, Nichaman MZ, et al. Cardiorespiratory fitness is associated with lower abdominal fat independent of body mass index. Med Sci Sports Exerc. 2004;36:286–291. doi: 10.1249/01.MSS.0000113665.40775.35. [DOI] [PubMed] [Google Scholar]
- 49.Woo KS, Chook P, Yu CW, et al. Effects of diet and exercise on obesity-related vascular dysfunction in children. Circulation. 2004;109:1981–1986. doi: 10.1161/01.CIR.0000126599.47470.BE. [DOI] [PubMed] [Google Scholar]
- 50.Epstein LH, Goldfield GS. Physical activity in the treatment of childhood overweight and obesity: Current evidence and research issues. Med Sci Sports Exerc. 1999;31:S553–S559. doi: 10.1097/00005768-199911001-00011. [DOI] [PubMed] [Google Scholar]
- 51.Irving BA, Davis CK, Brock DW, et al. Effect of exercise training intensity on abdominal visceral fat and body composition. Med Sci Sports Exerc. 2008 doi: 10.1249/MSS.0b013e3181801d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: U.S. Department of Health and Human Services; 2008. [Google Scholar]
- 53.van der Kooy K, Leenen R, Seidell JC, et al. Waist-hip ratio is a poor predictor of changes in visceral fat. Am J Clin Nutr. 1993;57:327–333. doi: 10.1093/ajcn/57.3.327. [DOI] [PubMed] [Google Scholar]
- 54.Fox KR, Peters DM, Sharpe P, Bell M. Assessment of abdominal fat development in young adolescents using magnetic resonance imaging. Int J Obes Relat Metab Disord. 2000;24:1653–1659. doi: 10.1038/sj.ijo.0801464. [DOI] [PubMed] [Google Scholar]
- 55.Owens S, Litaker M, Allison J, et al. Prediction of visceral adipose tissue from simple anthropometric measurements in youths with obesity. Obes Res. 1999;7:16–22. doi: 10.1002/j.1550-8528.1999.tb00386.x. [DOI] [PubMed] [Google Scholar]
- 56.Torigoe K, Numata O, Matsunaga M, et al. Effect of weight loss on body fat distribution in obese children. Acta Paediatr Jpn. 1997;39:28–33. doi: 10.1111/j.1442-200x.1997.tb03551.x. [DOI] [PubMed] [Google Scholar]
- 57.Lee S, Kuk JL, Hannon TS, Arslanian SA. Race and gender differences in the relationships between anthropometrics and abdominal fat in youth. Obesity. 2008;16:1066–1071. doi: 10.1038/oby.2008.13. [DOI] [PubMed] [Google Scholar]
- 58.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endo Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 59.Goran MI, Gower BA. Relation between visceral fat and disease risk in children and adolescents. Am J Clin Nutr. 1999;70:149S–156S. doi: 10.1093/ajcn/70.1.149s. [DOI] [PubMed] [Google Scholar]
- 60.Maffeis C, Manfredi R, Trombetta M, et al. Insulin sensitivity is correlated with subcutaneous but not visceral body fat in overweight and obese prepubertal children. J Clin Endocrinol Metab. 2008;93:2122–2128. doi: 10.1210/jc.2007-2089. [DOI] [PubMed] [Google Scholar]
- 61.Owens S, Gutin B, Barbeau P, et al. Visceral adipose tissue and markers of the insulin resistance syndrome in obese black and white teenagers. Obes Res. 2000;8:287–293. doi: 10.1038/oby.2000.34. [DOI] [PubMed] [Google Scholar]
- 62.Puyua MR, Adolph AL, Vohra FA, Butte NF. Validation and calibration of physical activity monitors in children. Obes Res. 2002;10:150–157. doi: 10.1038/oby.2002.24. [DOI] [PubMed] [Google Scholar]
- 63.Livingstone MB, Robson PJ. Measurement of dietary intake in children. The Proceedings of the Nutrition Society. 2000;59:279–293. doi: 10.1017/s0029665100000318. [DOI] [PubMed] [Google Scholar]