Abstract
Aim
Both hyperglycemia and hyperlipidemia increase oxidative stress, and contribute to the development of diabetic nephropathy (DN). We investigated effects of α-lipoic acid, a natural antioxidant and a cofactor in the multienzyme complexes, on the development of DN in diabetic apolipoprotein E-deficient mice.
Methods
Twelve-weeks-old male apoE−/− mice on C57BL/6J genetic background were made diabetic with injections of streptozotocin (STZ). STZ-treated diabetic apoE−/− mice and non-diabetic control were fed with a synthetic high fat (HF) diet with or without LA supplementation. Multiple parameters including plasma glucose, cholesterol, oxidative stress markers, cytokines, and kidney cortex gene expression, and glomerular morphology were evaluated.
Results
LA supplementation markedly protected the beta cells and reduced cholesterol levels, attenuated albuminuria and glomerular mesangial expansion in the diabetic mice. Reno-protection by LA was equally effective regardless of whether the dietary supplementation was started 4 weeks before, simultaneously with, or 4 weeks after the induction of diabetes by STZ. LA supplementation significantly improved DN and oxidative stress in the diabetic mice. Severity of albuminuria was positively correlated with level of thiobarbituric acid reactive substances (TBARs) in the kidney (r2=0.62, P<0.05). Diabetes significantly changed the kidney expression of Rage, Sod2, Tgfb1 and Ctgf, Pdp2, nephrin and Lias. LA supplementation corrected these changes except that it further suppressed the expression of the Lias gene coding for lipoic acid synthase.
Conclusions
Our data indicate that LA supplementation effectively attenuates the development and progression of DN through its antioxidant effect as well as enhancing glucose oxidation.
Keywords: lipoic acid, antioxidants, oxidative stress, diabetes, diabetic nephropathy, apolipoprotein E null mice, streptozotocin
1. Introduction
Diabetes mellitus (DM) is the leading cause of end-stage renal disease and the relative risk for cardiovascular diseases is 10-fold higher for type 1 diabetic patients with nephropathy compared to those without diabetic nephropathy (Tuomilehto, et al., 1998). Proteinuria, an indicator of underlying diabetic nephropathy, usually worsens with progression of diabetic kidney disease. Progression of kidney disease is characterized by declining glomerular filtration rate and kidney structural changes, including thickening of the basement membranes, mesangial sclerosis, and arteriolar hyalinosis.
Hyperglycemia is a major cause of increased glycation of protein and lipid that, in turn, enhance the generation of reactive oxygen species (ROS) (Lee, et al. 2010, Aljofan & Ding, 2010). Thus, diabetes is usually accompanied by both an increased production of ROS and impaired antioxidant defenses leading to increased oxidative stress that is considered important in the development and progression of diabetes and its complications (Baynes & Thorpe, 1999). Despite current recommendations for management of diabetic complications, including administration of angiotensin converting enzyme inhibitors (ACEIs) or angiotensin receptor antagonists (ARBs) to control proteinuria and attenuate progression of chronic kidney disease (CKD) to end stage kidney disease (ESRD) [Kidney Disease Outcome Quality Initiative (KDOQI) recommendations], prevalence of CKD secondary to diabetes continues to increase (United States Renal Data Systems [USRDS] 2009; World Health Organization [WHO]). Accordingly, there has been increasing interest in the protective function of dietary antioxidants as potential adjunctive therapy to prevent diabetic complication.
In diabetics with suboptimal glycemic control, hyperlipidemia may develop. In addition, even in subjects with optimal glycemic control, abnormal lipid profile that is potential atherogenic may exist. Indeed, hyperlipidemia is now considered an independent and major determinant of progression of renal disease in diabetes (Rosario & Prabhakar, 2006). In this regard, an association between high consumption of saturated fat and albuminuria in type 1 diabetic patients has been reported (Riley & Dwyer, 1998).
Alpha-lipoic acid (1, 2-dithiolane-3-pentanoic acid, LA) is a natural antioxidant and a cofactor for mitochondrial α-ketoacid dehydrogenases (Reed, 1998), and consequently may work as a regulator of glucose metabolism (Packer, et al., 1995). LA is synthesized in mitochondria and, as well, may be derived from food. It is noteworthy that plasma LA levels are decreased in diabetic patients (Kleemann, et al., 1989). Interestingly, accumulating evidence suggests that LA supplementation is beneficial in both types 1 and 2 diabetes and, for diabetic complications in patients and animals (Jacob, et al., 1999; Ziegler 2004; Melhem, et al., 2002; Yi & Maeda 2006). The present study examined the effect of dietary LA supplementation on diabetes-induced kidney damage in apoE−/− mice, an animal model previously demonstrated to have a series of pathological conditions including dyslipidemia and atherosclerosis. Hyperlipidemia per se, is associated with development of early renal lesions in apoE−/− mice (Wen, et al., 2002). Mice were rendered diabetic by intraperitoneal (IP) administration of low doses of STZ over 5 days, an established approach for inducing type 1 diabetes mellitus (Kromann et al., 1982). Given the above features, STZ-induced diabetic ApoE−/− mice fed high fat diet were used in our studies to accelerate DN and probe the role of dyslipidemia in DN development (Erdely, et al., 2009; Cui, et al., 2009; Kasiske et al. (1990)). We here show that dietary LA supplementation led to significant changes in biomarkers of oxidative stress and reduced kidney damage in diabetic apoE−/− mice. The effect was associated with the reduction of plasma levels of glucose, cholesterol, and IL-6, and changes in expression of nephrin, Rage, Sod2, Pdp2, Lias, Tgfb1 and Ctgf in the kidney. The reno-protective effect of LA supplementation was equally effective regardless of whether the supplement ation was given prior to, simultaneously or after the induction of diabetes by STZ.
2. Materials and Methods
2.1. Experimental design
All animal experiments were carried out under protocols approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill. One hundred twenty-five male apoE−/− mice on C57BL/6J genetic background (12 weeks-old) were randomly divided into 4 groups: diabetic and control groups, with or without LA supplementation. Mice were made diabetic with multiple intraperitoneal injections of STZ at a low dose (40 mg/kg body weight in 0.1M citrate buffer, pH 4.5, (Sigma, St. Louis) to avoid nephrotoxic effects, following the protocol by the Animal Models of Diabetic Complications Consortium. The control group received buffer alone. Mice with blood glucose levels higher than 300 mg/dl at 4 week after the STZ treatment were considered to be diabetic. All mice were maintained on regular chow until being switched to a synthetic high fat (HF) diet containing 21% (w/w, or 45% calories) fat, 0.05% cholesterol, 20% sucrose (Research Diets, Inc. New Brunswick, NJ), with or without 200 mg/kg body weight of racemic LA (Sigma, St. Louis, MO). In order to test whether dietary LA supplementation can attenuate STZ-induced diabetes and whether different timing of LA supplementation can produce different protective and/or therapeutic effect, three sets of experiments were carried out: pre-STZ group in which LA supplementation was started 4 weeks before STZ injection; co-STZ group in which the LA-supplemented diets were initiated simultaneously with the STZ injection; and post-STZ group to which the LA-supplemented diets were provided starting at 4 weeks after the initiation of the STZ injection. Approximately 10% of diabetic mice on HF diet without LA became lethargic and were euthanized. Remaining animals were sacrificed at 20 weeks after STZ treatment.
2.2. Biochemical analyses
Mice were fasted for 4 hours and blood samples were collected from the orbital sinus. Systolic pressure was measured in conscious mice using a computerized noninvasive tail cuff system (Visitech Systems, Cary. NC). At 3 and 5 months after the STZ injection, mice were individually placed in metabolic cages for three consecutive days to monitor their water and food consumption, urine volume, and body weight. Immediately after mice were euthanized by an overdose of tribromoethanol, the right kidneys were quick-frozen in liquid N2 and stored at −80°C. Tissues were rinsed with isotonic saline and homogenized in phosphate-buffered saline (0.1mol/L, pH 7.4). The protein was measured by the Bradford method (Bio-Rad, Hercules, CA) with bovine serum albumin as the standard.
Plasma glucose, cholesterol and triglyceride were measured using the kits from Wako (Richmond, VA). Plasma and tissue lipid peroxide content was determined using TBARS assay (Lapenna et al., 2001) with malondialdehyde as the standard. Erythrocyte GSH levels were determined by using an assay kit (Calbiochem, San Diego, CA). Urinary 8-isoprostane and albumin were determined using the EIA kit from Cayman (Ann Arbor, MI, USA) and from Exocel Inc. (Philadelphia, PA), respectively. Superoxide dismutase (SOD) activity in kidney cortex was measured by using superoxide dismutase assay kit for all three types of SOD (Cayman). Advanced glycation end-products (AGEs) was measured as Nε-(carboxymethyl) lysine (CML), which was determined in serum and kidney homogenates with ELISA (CycLex Co., Ltd.,Nagano, Japan). Serum TNF-α and IL-6 were measured using the Luminex-100 system (Luminex Corporation, Austin, Texas) and analyzed with Flowmetrix software (Luminex) according to the manufacturer’s protocols.
2.3. Histological evaluation
Paraffin sections (3 µm thick) were stained with H&E and Periodic acid Schiff (PAS). Mesangial matrix expansion (MME) was derived from assessment of 100 glomeruli of 5 diabetic and 5 non-diabetic mice per dietary group. PAS-positive regions in mesangial zones as well as the total glomerular tuft area were quantitatively measured using NIH Image J program 1.34 (http://rsb.info.nih.gov/ij). A ratio of PAS positive mesangial zone over total glomerular tuft area was calculated to evaluate the size of the MME. For transmission electron microscopy (TEM), sections were prepared in the UNC Pathology Core Facility and examined with LEO 910 electron microscope (LEO Electron Microscopy Inc. Thornwood, New York). Immunostaining of the insulin positive cells of the pancreatic tissues was determined as previously described (Yi & Maeda, 2006).
2.4. RT-PCR
Total RNA was extracted from kidney cortex using TRIzol reagent (Vitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Gene expressions were determined using real-time quantitative reverse transcription PCR (Applied Biosystems, Foster City, CA) with β-actin as the reference gene.
2.5. Statistical analysis
All the biochemical assays were carried out in duplicates. Statistical analysis was performed with JMP software (SAS Cary NC). Diabetes effect, LA effect and their interaction were analyzed using two-way ANOVA, and Tukey-Kramer HSD test was applied for post-hoc pair-wise comparisons. Time effect was analyzed by MANOVA for repeated measurement. When there were no statistical effects of the dietary groups, data from the three dietary groups were combined and analyzed jointly.
3. Results
3.1. Timing of LA administration
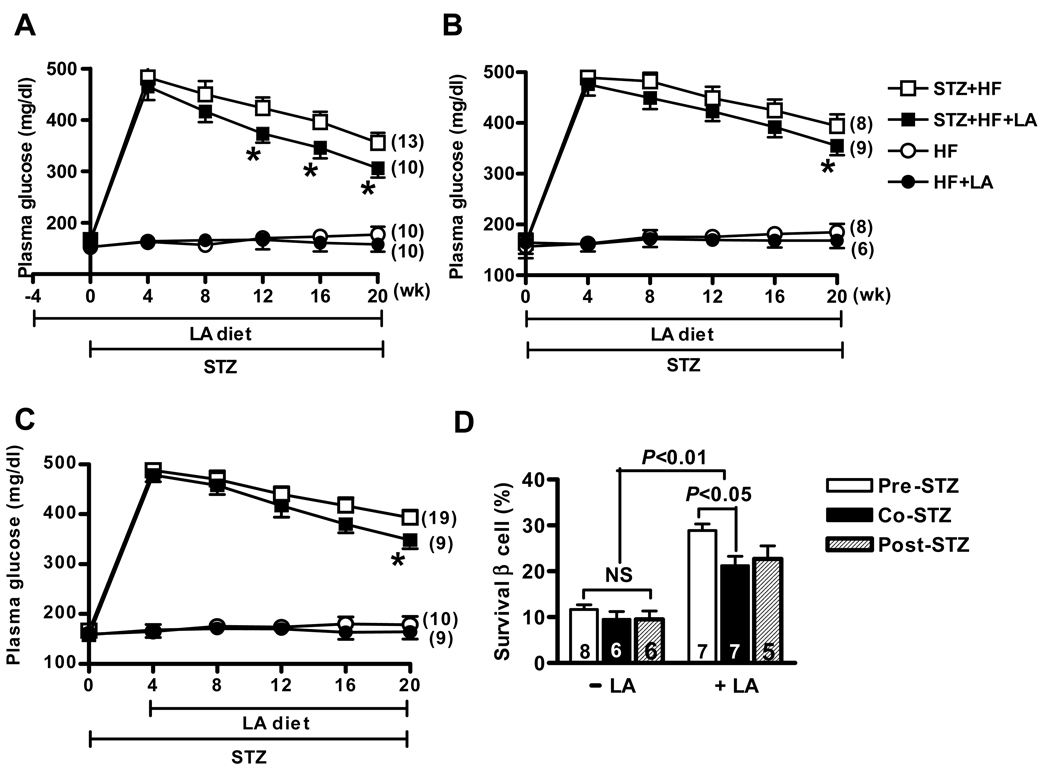
Plasma glucose levels in the initial stage of diabetes (at 4 weeks after STZ injection) were not significantly different among three groups, nor between diabetic mice with and without LA supplementation (P=0.74 for the three groups, P=0.06 for diabetic mice with LA versus without LA, by two-way ANOVA analysis). Plasma glucose levels in all diabetic mice gradually decreased during the study period, and LA supplementation accelerated this process (Figure 1). In pre-STZ group, plasma glucose values from the diabetic mice with LA were significantly lower than those in diabetic mice without LA, and the difference was noticeable as early as 12 weeks after initial STZ injection (Figure 1A, P<0.003 for overall LA effect by ANOVA ). In contrast, in the co-STZ and post-STZ groups, LA supplementation did not influence plasma glucose levels before 20 weeks (Figure 1B and 1C).
Figure 1.
Changes in plasma glucose levels of diabetic and non-diabetic mice in pre-STZ group (A), co-STZ group (B) and post-STZ group (C). Dietary lipoic acid (LA) was provided at 4 weeks before STZ treatment or at the same time with STZ or at 4 weeks after STZ, respectively. The number of animals in each group is in parentheses. *P<0.05 against mice without LA in the diabetic group (open squares). (D). Pancreatic β-cells survival rate in the STZ-induced diabetic mice with or without lipoic acid supplementation in three dietary groups. All results are expressed as mean ± SEM.
Morphometric quantification of the ratio of insulin-immunoreactive areas to total islet areas in diabetic fed LA showed that 28.9 ± 1.4% of islets were staining positive for insulin in the pre-STZ group, 21.4 ± 2.0 % in the co-STZ group and 22.6±2.8% in the post-STZ group (Figure 1D). Insulin reactive area in the diabetic mice without LA was not significantly lower than the mice with LA, and pre-STZ group had the highest survival β cells among the three groups. No pathological changes were observed in non-diabetic mice.
STZ-induced diabetic mice that did not receive LA lost weight, but the diabetic mice administered LA were able to partially maintain their body weight (P<0.01, Table 1). In addition, LA supplementation significantly slowed the HF diet-induced weight gain in the non-diabetic mice, although there was no significant difference of daily dietary intake in the non-diabetic mice with or without LA (Table 1).
Table 1.
Effects of STZ treatment and dietary LA supplementation on ApoE−/− mice.
| STZ | Control | P (ANOVA) | |||||
|---|---|---|---|---|---|---|---|
| −LA (n=31) |
+LA (n=29) |
−LA (n=28) |
+LA (n=25) |
STZ | LA | Interaction | |
| Food intake (g/day) |
6.7±0.3 | 6.4±0.3 | 5.4±0.2 | 4.8±0.2 | <0.01 | 0.16 | 0.85 |
| Urine volume (ml/day) |
9.5±1.0 | 6.8±0.9 | 1.9±0.1 | 1.6±0.1 | <0.01 | 0.08 | 0.17 |
| Body weight (BW, g) |
27.2±0.4 | 32.2±0.5 | 45.5±0.7 | 39.0±0.4 | <0.01 | 0.18 | 0.01 |
| Plasma | |||||||
| Triglyceride (mg/dl) |
99±6 | 83±4 | 77±5 | 68±6 | 0.16 | 0.25 | 0.5 |
| Cholesterol (mg/dl) |
708±43 | 493±18 | 443±10 | 454±14 | <0.01 | <0.05 | 0.02 |
| TBARS (nmol/ml) |
2.89±0.19 | 2.26±0.10 | 1.89±0.14 | 1.47±0.15 | <0.01 | <0.05 | 0.22 |
| AGE (ng/ml) | 1168±161 | 1057±87 | 421±55 | 385±53 | <0.05 | NS | 0.13 |
| IL-6 (pg/ml) | 439±23 | 236±19 | 441±41 | 339±35 | <0.001 | <0.01 | 0.001 |
| TNFα (pg/ml) | 64±7 | 63±7 | 56±6 | 56±6 | 0.23 | 0.91 | 0.95 |
| Erythrocyte GSH (nmol/ml) |
23.6±0.6 | 34.5±1.7 | 30.2±1.0 | 47.9±1.8 | <0.01 | <0.01 | 0.08 |
| Kidney weight (KW, g) |
0.26±0.04 | 0.25±0.03 | 0.31±0.02 | 0.25±0.01 | <0.05 | <0.01 | 0.01 |
| KW/BW (%) | 9.6±0.1 | 7.8±0.2 | 6.8±0.1 | 6.4±0.1 | <0.01 | <0.05 | 0.01 |
| Kidney TBARS (nm/mg Protein) |
3.24±0.17 | 2.29±0.18 | 2.30±0.08 | 2.11±0.13 | <0.05 | <0.01 | 0.03 |
| Urine Albumin (µg/day) |
77.6±6.3 | 45.7±8.0 | 17.6±3.2 | 15.2±2.8 | <0.01 | <0.05 | 0.02 |
| Urine 8- isoprostene (ng/mg Creat) |
254±12 | 186±11 | 79±5 | 60±5 | <0.01 | <0.01 | 0.08 |
Data shown are mean values ±SEM of mice, 5 months after the treatment with STZ or with the buffer (control).
The number of animals in each group is in parenthesis.
Although hyperglycemia and weight loss were attenuated in mice within the pre-STZ group, in association with more survival β-cells, data from mice within three dietary groups were not significantly affected by the timing of LA administration. Therefore, we analyzed these data together in the following sections.
3.2. Oxidative stress markers in plasma, urine and kidneys
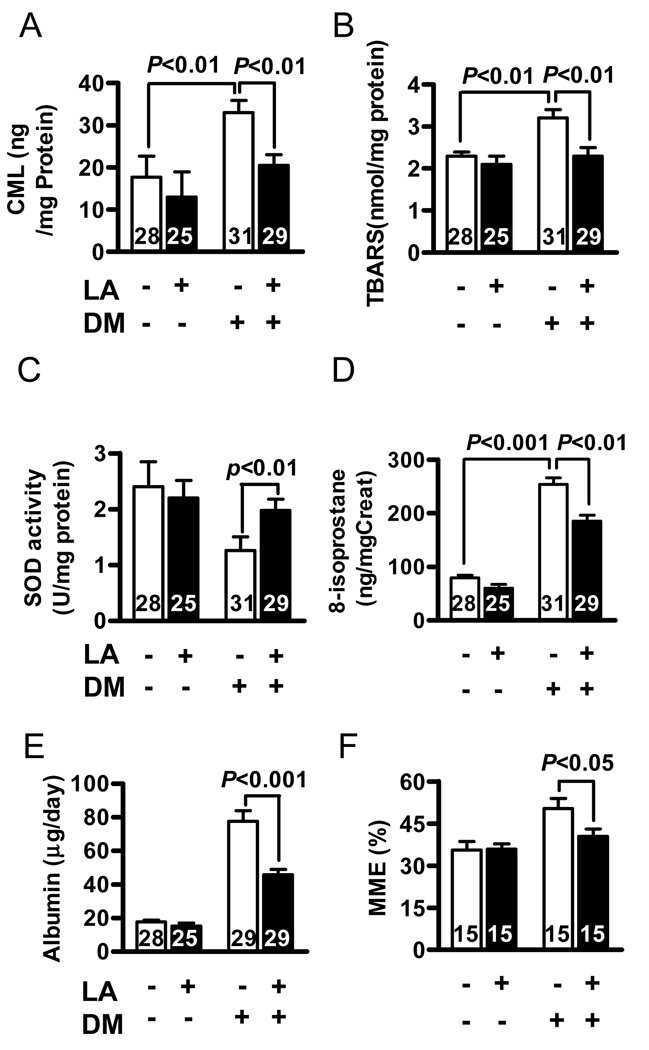
Diabetes significantly increased oxidative stress, as reflected by reduction in erythrocyte GSH and increases in plasma TBARS; LA supplementation ameliorated these changes (Table 1). Similarly, AGEs and TBARS in kidney cortex, as well as urinary 8-isoprostane were significantly increased by diabetes, but LA supplementation attenuated these increases (Figure 2A, 2B and 2D). Total SOD activity in kidney cortex was decreased in diabetic mouse, and treatment with dietary LA partially restored its activity (Figure 2C). Taken together, these data strongly suggested that dietary LA supplementation significantly suppressed oxidative stress in diabetes.
Figure 2.
Oxidative stress markers in the kidney cortex of apoE−/− mice with LA (black bars) and without LA (white bars) at 5 months after onset of diabetes. (A) kidney Nε-(carboxymethyl) lysine (CML) used for measurement of advanced glycation end products (B) kidney thiobarbituric acid reactive substances (TBARS). (C) kidney total superoxide dismutase activity (D). urinary 8-isoprostane. (E) daily urinary albumin excretion. (F) mesangial matrix fraction was quantified as the region of positive periodic acid Schiff staining, expressed as a function of total glomerular tuft area. The numbers inside bars indicate number of animals. Results are expressed as mean ± SEM.
3.3. Plasma cytokines
In order to evaluate changes in inflammatory mediators in the diabetic state and explore potential anti-inflammatory effects of LA, we monitored serum concentrations IL-6 and TNF-α in each mouse in the course of 5-month. IL-6 levels in the diabetic mice significantly increased at 3 months after STZ injection (data not shown) and increased 10-fold at 5 months post-STZ injection compared to the non-diabetic mice (Table 1). LA attenuated the increase of IL-6 in diabetic mice. In contrast, plasma TNF-α levels in diabetic and non-diabetic mice were not significantly different at either time point.
3.4. Renal damage
All diabetic mice suffered from polyuria. LA supplementation showed a trend towards improvement of this disorder, although the effect was not statistically significant (Table 1). Kidney weight over body weight (KW/BW) in the diabetic mice was 40% higher than that of non-diabetic mice (P<0.05). This change may partially reflect the reduced BW in diabetic mice. Dietary LA significantly decreased the KW/BW ratio in diabetic mice (Table 1). The daily urinary albumin excretion was markedly elevated in diabetic mice at 5 months after STZ treatment and LA significantly attenuated albuminuria (Figure 2E).
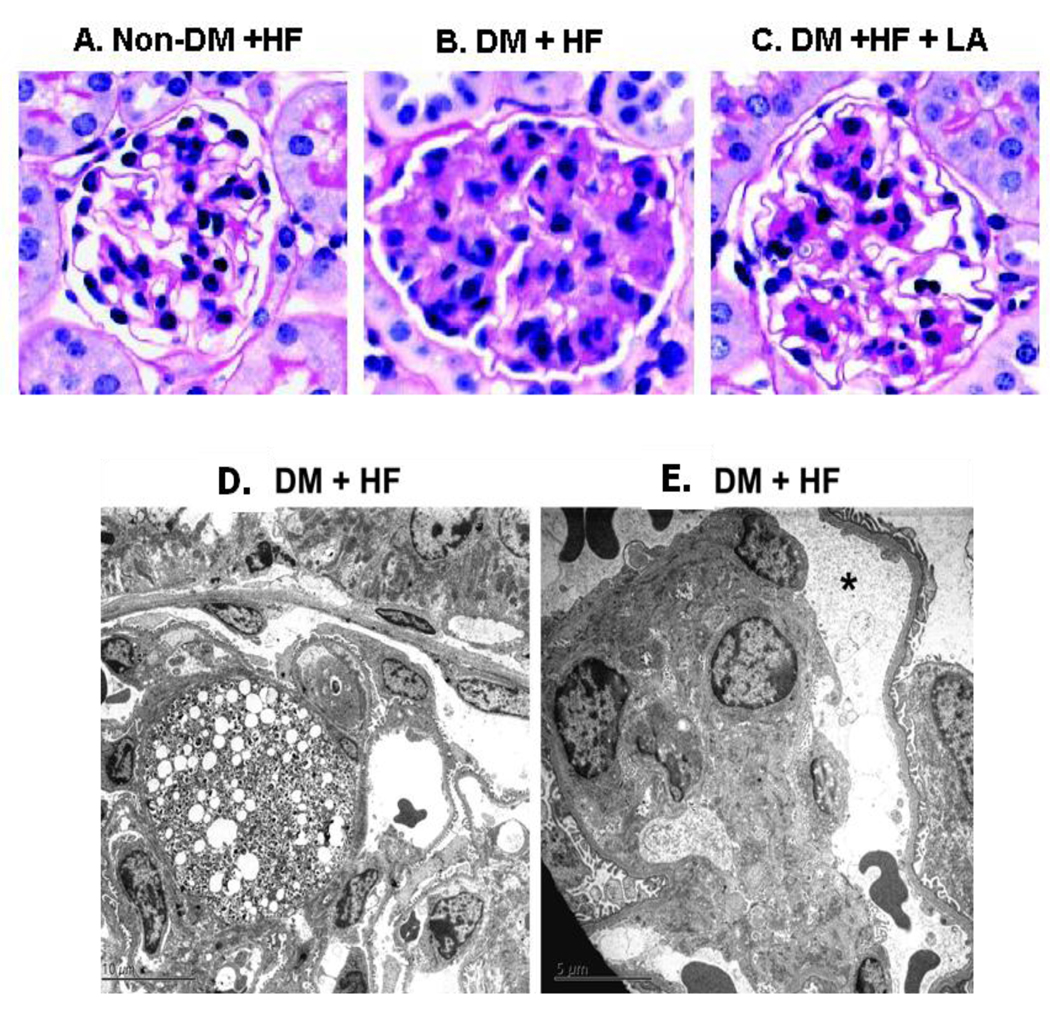
The gross appearance of kidneys in diabetic apoE−/− mice was unaltered. Light microscopy examinations revealed mesangium hypercellularity, nodular and diffuse MME, and rare foci of mesangiolysis in diabetic mice without LA supplementation (Figure 3B). In contrast, diabetic animals with LA had significantly reduced MME compared to diabetic mice without LA (Figure 2F, 3C). Electron microscopy showed that glomeruli from diabetic mice that did not receive dietary LA exhibited intracapillary lipid-laden mesangium and mesangial expansion (Figure 3D). Peripheral glomerular capillaries were aneurysmatically dilated and lost anchoring points to the mesangial cells (Figure 3E). No obvious alterations of podocyte foot processes were detected in diabetic mice, possibly due to a very early stage of diabetic nephropathy for our model (Gurley, et al., 2006). The tubulointerstitial compartment in diabetic animals either with or without LA supplementation showed only minimal non-specific changes. No significant morphological abnormalities were observed in the control glomeruli with or without dietary LA (Figure 3A).
Figure 3.
Mesangial expansion and ultrastructural changes in diabetic apoE−/− mice at 5 month after induction of diabetes. (A) non-diabetic mice without LA (B) diabetic mice without LA. (C) diabetic mice with LA. PAS staining. Original magnification X200.
(D) Segmentally expanded mesangial areas due to accumulation of lipid laden cells, presumably mesangial cells. (E) Aneurysmatic dilation of a peripheral glomerular capillary (asterisk), and anchoring points between peripheral capillary walls and the mesangium were lost.
3.5. Gene expression in the kidney
No obvious alterations of podocyte foot processes were detected in the diabetic mice, but the glomerular expression of nephrin was down-regulated in the diabetic mice and LA restored its expression (Table 2). In diabetic mice that did not receive LA, kidney expression of transforming growth factor beta 1(Tgfb1) and connective tissue growth factor (Ctgf) was increased at 20 weeks after injection of STZ (Table 2). Dietary LA significantly attenuated the up-regulation of the genes. In contrast, changes in the expression of the collagen I and collagen IV genes in diabetic animals were not significant. Rage (receptors for AGEs) was increased in diabetic mice while LA supplementation reduced it.
Table 2.
Kidney gene expression
| STZ | Control | P (ANOVA) | |||||
|---|---|---|---|---|---|---|---|
| −LA | +LA | −LA | +LA | STZ | LA | Interaction | |
| nephrin | 0.44±0.16 (5) |
0.92±0.17 (4) |
1.00±0.12 (12) |
0.98±0.18 (12) |
<0.05 | <0.05 | 0.65 |
| tgfβ1 | 1.65±0.12 (15) |
1.04±0.07 (13) |
1.00±0.19 (13) |
0.91±0.19 (12) |
<0.01 | <0.05 | 0.70 |
| ctgf | 1.55±0.13 (15) |
1.05±0.11 (13) |
1.00±0.16 (13) |
0.83±0.07 (12) |
<0.05 | <0.05 | 0.91 |
| Col1 | 1.22±0.16 (15) |
0.93±0.12 (15) |
1.00±0.19 (13) |
1.11±0.04 (12) |
NA | NA | 0.78 |
| Col4 | 1.15±0.10 (15) |
1.02±0.84 (15) |
1.00±0.12 (13) |
0.85±0.29 (12) |
NA | NA | 0.11 |
| RAGE | 2.47±0.48 (12) |
1.13±0.37 (12) |
1.00±0.24 (12) |
0.94±0.11 (12) |
<0.05 | <0.05 | 0.23 |
| SOD2 | 0.69±0.14 (12) |
0.98±0.07 (13) |
1.00±0.14 (12) |
0.92±0.09 (12) |
<0.01 | <0.01 | 0.24 |
| eNOS | 1.88±0.68 (15) |
1.10±0.65 (15) |
1.00±0.16 (12) |
0.87±0.12 (12) |
NA | NA | 0.31 |
| P22phox | 1.60±0.15 (12) |
1.02±0.17 (12) |
1.00±0.13 (12) |
0.88±0.11 (12) |
<0.05 | NA | 0.09 |
| p47phox | 1.92±0.15 (12) |
1.07±0.13 (12) |
1.00±0.27 (12) |
0.86±0.08 (12) |
<0.05 | <0.05 | 0.07 |
| GPX1 | 0.78±0.14 (15) |
1.26±0.46 (12) |
1.00±0.16 (12) |
1.21±0.26 (11) |
NA | NA | 0.93 |
| Lias | 0.27±0.03 (15) |
0.19±0.02 (13) |
1.00±0.14 (12) |
0.70±0.11 (12) |
<0.001 | <0.05 | 0.23 |
| Pdp2 | 0.27±0.06 (12) |
0.74±0.10 (12) |
1.00±0.12 (12) |
1.34±0.21 (12) |
<0.001 | <0.01 | 0.88 |
| Pdk4 | 0.79±0.21 (12) |
0.89±0.23 (12) |
1.00±0.30 (9) |
1.13±0.19 (9) |
NA | NA | 0.96 |
mRNA levels in kidney cortex are expressed as mean ± SE relative to the mean levels of the control without LA as 1.00. The numbers of animals are in the parentheses.
Among the genes related to oxidative stress in the kidneys, Sod2 expression in diabetic mice without LA was significantly lower than in non-diabetic mice, while p47phox and p22phox (a regulatory subunit and a membrane associated flavocytochrome subunit for NADPH oxidase) was increased. LA supplementation reversed these changes in diabetic mice. Expression of glutathione perioxidase-1 (Gpx-1) showed a non-significant downward trend in diabetic mice. Notably, the expression of genes for Lias and PDH phosphatase 2 (Pdp2) decreased markedly to about 30% normal levels in the diabetic kidney. Dietary LA significantly attenuated the down-regulation of Pdp2 expression, but further reduced the Lias gene expression (Table 2).
3.6. Blood pressure
Systolic blood pressure in diabetic mice with or without LA supplementation were not significantly different (105± 5 mmHg, n=8 vs. 114 ± 6 mmHg, n=7, P=0.08) at 5 month after STZ injection in pre-STZ and co-STZ groups.
3.7. Dislipidemia and nephropathy in diabetic apoE−/− mice
High plasma cholesterol levels were observed in some but not all diabetic apoE−/− mice at 5 months after the onset of diabetes. Livers from mice with abnormally high plasma lipids showed characteristic of steatohepatitis such as visible lipid droplets and inflammatory cells (data not shown).
Daily albumin excretion was positively correlated with plasma glucose levels in the diabetic apoE−/− mice that were not fed LA (R2=0.28, P<0.01, n=27) and to a lesser degrees in mice treated with LA (R2=0.12, P=0.08, n=22). Plasma and kidney TBARS were positively correlated with albumin excretion (R2=0.33, P<0.005, n=39 and R2=0.62, P<0.05, n=19, respectively) only in the diabetic apoE−/− mice treated with LA.
Discussion
The current findings support previous observations that LA supplementation attenuated hyperglycemia in diabetes and DN (Melhem, et al., 2002). We also report the novel observation that regardless of the timing of LA administration (that is, before, at the same time or one month after initiation of diabetes), LA supplementation significantly reduced hyperglycemia, especially in post-STZ group where hyperglycemia was confirmed before LA treatment. In addition, the observations of increased inflammatory cytokine and altered renal gene expression suggested that inflammation played a role in DN development. The data strongly implicate a therapeutic effect of LA on established diabetes.
The effects of LA on hyperglycemia and hyperglycemia-induced nephropathy are incompletely understood (Winiarska, et al. 2008). LA exerts hypoglycemic effects, but current evidence does not indicate that LA can function as an insulin secretagogue (Khamaisi, et al., 1997). LA may exert hypoglycemic effects via other mechanisms including increased insulin sensitivity in type 2 diabetes (Jacob et al., 1999) and direct binding site at the insulin receptor tyrosine kinase domain (Diesel, et al., 2007). Early administration of LA may predispose the β cells to increased antioxidant capacity, enhance their resistance to oxidative insults and consequently promote a better recovery after exposure to STZ, leading to accelerated drop of hyperglycemia. Pancreatic β cells express very low levels of antioxidant enzyme and are highly susceptible to free radical damage (Grankvist, Marklund & Talijedal, 1981). Over-expression of antioxidant enzymes in β cells enhances their resistance to oxidative stress and facilitates β cell recovery and even regeneration in animals (George, et al., 2002).
Our findings of elevated urinary 8-isoprostane, a reliable marker of in vivo lipid peroxidation (Morrow & Roberts, 1997), increased plasma and kidney TBARS, and decreased erythrocyte GSH clearly indicate enhanced oxidative stress in diabetic mice. The results are consistent with other investigations (Melhem, et al., 2002). Mitochondria are believed to be a major organelles that produce superoxide and are also a primary target for oxidative insult due to lack of protective histone and DNA repair ability (Shigenaga, et al., 1994). Accordingly, mitochondrial superoxide production has been proposed as a single unifying mechanism for diabetic complications (Brownlee, 2005). The active form of MnSOD distributes in mitochondrial matrix and it works as the first line of defense against superoxide and protects mitochondria from oxidative damage. In the current study, total SOD activity and Sod2 in diabetic kidney cortex was markedly reduced whereas LA supplementation restored SOD activity and Sod2 expression. These results suggest that LA reinforces antioxidant defenses such as SOD2 and other superoxide dismutase, in order to protect the kidney from damage by excessive superoxide production.
The observations of mesangiolysis and foamy glomerular mesangial cells in the diabetic kidney of apoE−/− mice suggest a role for hyperlipidemia in the pathogenesis of DN. However, hyperlipidemia alone cannot explain glomerular changes because kidneys of apoE−/− mice with higher plasma lipid levels can be completely normal (Bruneval et al., 2002). Similar fatty changes in mesangial cells and glomerular sclerosis were previously observed by us in apoE−/−mice that were also lacking eNOS (Knowles et al., 2000). Reduced NO bioavailability in diabetes is likely involved in the pathogenesis of mesangial changes and may explain why LA significantly attenuated these changes (Nakagawa, et al. 2008).
The role of LA in inhibition of AGE formation in vivo is inconclusive. We postulate that LA is a potential inhibitor of AGEs in two ways. First, LA improved glucose metabolism and decreased methylglyoxal. Methylglyoxal is primarily generated from glyceraldehyde-3-phosphate, an intermediate product in glycolysis pathway and has been considered the most important agent leading to AGEs formation (Shinohara, 1998). LA feeding increases Krebs cycle activity (Sudheesh, et al., 2009), leading to increased glyceraldehyde-3-phosphate is consumption in the TCA cycle and increased ATP generation. As a result, less glyceraldehyde-3-phosphate is available to form methyglyoxal. Our recently published paper showed that ApoE−/− mice with genetically reduced lipoic acid production had impaired PDC activity (Yi, et al. 2010). Secondly, LA prevents lipid peroxidation and reduces aldehyde production
We observed that the endogenous production of LA is markedly compromised in diabetes as judged by the reduction of Lias mRNA to less than 30% of normal levels. [Free LA in plasma is very low and plasma lipoic acid cannot be quantified without special equipment; instead, we examined mRNA level of Lias gene]. Although LA supplementation can improve general health of the diabetic animals, including prevention of severe weight loss, it failed to restore Lias gene expression, suggesting that glucose metabolism is responsible for this reduction. The capacity to execute oxidative respiration is reduced in diabetes, as a consequence of a decrease in PDC activity. Reduction in LA, an essential cofactor of PDC, could facilitate the switch from glucose oxidation to glycolysis in diabetes. Since the decrease of endogenous LA production coincidentally reduces cellular availability of LA for antioxidant defense, down-regulation of the Lias gene expression by high glucose may accelerate diabetic complications. In addition, activity of mammalian PDC is regulated by reversible phosphorylation-dephosphorylation of three specific serine residues of E1, catalyzed by specific pyruvate dehydrogenase kinases (PDKs) and phospho-pyruvate dehydrogenase phosphatases (PDPs). Our result showed that expression of Pdp2 in kidney was significantly down-regulated during diabetes, whereas LA supplementation partially restored Pdp2 expression. As a result, phosphorylation of PDC is increased, leading to increased activity of PDC.
LA is not only a naturally occurring antioxidant but also a cofactor in several mitochondrial enzymes that are involved in glucose oxidation and ATP generation. We could not ascribe all effects of LA supplementation (e.g. altered expression of Lias gene) to antioxidant property of LA. Thus, the beneficial effects of LA on diabetes and diabetic nephropathy were most likely derived from combined antioxidant and metabolic regulations including increase of glucose oxidation, rather than acting as an antioxidant alone.
In summary, our study showed that dietary LA supplementation not only exerts a better hypoglycemia function if given prior to onset of diabetes but also LA effectively treats established diabetes in mice. In addition, reno-protective effect of LA is equivalent in three dietary groups irrespective of the time when treatment is initiated. The protective action of LA is dependent on its function both as an antioxidant and as a regulator of glucose oxidation.
Supplementary Material
Acknowledgements
The authors thank Dr. Oliver Smithies for discussions and Mr. Lance Johnson, Mr. Benjamin Bleasdale and Ms. Sylvia Hiller for comments on the manuscript. This work was supported by grants HL087946 and HL42630 from the NIH and the UNC Research Council grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors have no conflict of interest.
References
- Aljofan M, Ding H. High glucose increases expression of cyclooxygenase-2, increases oxidative stress and decreases the generation of nitric oxide in mouse microvessel endothelial cells. J Cell Physiol. 2010;222(3):669–675. doi: 10.1002/jcp.21986. [DOI] [PubMed] [Google Scholar]
- Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Bruneval P, Bariety J, Belair MF, Mandet C, Heudes D, Nicoletti A. Mesangial expansion associated with glomerular endothelial cell activation and macrophage recruitment is developing in hyperlipidaemic apoE null mice. Nephrol Dial Transplant. 2002;17:2099–2107. doi: 10.1093/ndt/17.12.2099. [DOI] [PubMed] [Google Scholar]
- Cui J, Le G, Yang R, Shi Y. Lipoic acid attenuates high fat diet-induced chronic oxidative stress and immunosuppression in mice jejunum: a microarray analysis. Cell Immunol. 2009;260(1):44–50. doi: 10.1016/j.cellimm.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Diesel B, Kulhanek-Heinze S, Höltje M, Brandt B, Höltje HD, Vollmar AM, Kiemer AK. Alpha-lipoic acid as a directly binding activator of the insulin receptor: protection from hepatocyte apoptosis. Biochemistry. 2007;46(8):2146–2155. doi: 10.1021/bi602547m. [DOI] [PubMed] [Google Scholar]
- Erdelyi I, Levenkova N, Lin EY, Pinto JT, Lipkin M, Quimby FW, Holt PR. Western-style diets induce oxidative stress and dysregulate immune responses in the colon in a mouse model of sporadic colon cancer. J Nutr. 2009;139(11):2072–2078. doi: 10.3945/jn.108.104125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George M, Ayuso E, Casellas A, Costa C, Devedjian JC, Bosch F. Beta cell expression of IGF-I leads to recovery from type 1 diabetes. J Clin Invest. 2002;109:1153–1163. doi: 10.1172/JCI12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grankvist K, Marklund S, Taljedal IB. Superoxide dismutase is a prophylactic against alloxan diabetes. Nature. 1981;294:158–160. doi: 10.1038/294158a0. [DOI] [PubMed] [Google Scholar]
- Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM. Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol. 2006;290:F214–F222. doi: 10.1152/ajprenal.00204.2005. [DOI] [PubMed] [Google Scholar]
- Jacob S, Rett K, Henriksen EJ, Häring HU. Thioctic acid--effects on insulin sensitivity and glucose-metabolism. Biofactors. 1999;10(2–3):169–174. doi: 10.1002/biof.5520100212. [DOI] [PubMed] [Google Scholar]
- Kasiske BL, O'Donnell MP, Cowardin W, Keane WF. Lipids and the kidney. Hypertension. 1990;15:443–450. doi: 10.1161/01.hyp.15.5.443. [DOI] [PubMed] [Google Scholar]
- Khamaisi M, Potashnik R, Tirosh A, Demshchak E, Rudich A, Tritschler H, Wessel K, Bashan N. Lipoic acid reduces glycemia and increases muscle GLUT4 content in streptozotocin-diabetic rats. Metabolism. 1997;46(7):763–768. doi: 10.1016/s0026-0495(97)90120-7. [DOI] [PubMed] [Google Scholar]
- Kleemann A, Borbe HO, Ulrich H. Thioctsaure:a-liponsaure. In: Borbe HO, Ulrich H, editors. Thioctsaure: Neue biochemische, pharmakologische und klinische Erkenntnisse zur Thioctsaure. pmi Verlag: Frankfurt am Main; 1989. pp. 11–26. [Google Scholar]
- Knowles JW, Reddick RL, Jennette JC, Shesely EG, Smithies O, Maeda N. Enhanced atherosclerosis and kidney dysfunction in eNOS(−/−)Apoe(−/−) mice are ameliorated by enalapril treatment. J Clin Invest. 2000;105:451–458. doi: 10.1172/JCI8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromann H, Christy M, Lernmark A, Nedergaard M, Nerup J. The low dose streptozotocin murine model of type 1 (insulin-dependent) diabetes mellitus: studies in vivo and in vitro of the modulating effect of sex hormones. Diabetologia. 1982;22:194–198. doi: 10.1007/BF00283752. [DOI] [PubMed] [Google Scholar]
- Lapenna D, Ciofani G, Pierdomenico SD, Giamberardino MA, Cuccurullo F. Reaction conditions affecting the relationship between thiobarbituric acid reactivity and lipid peroxides in human plasma. Free Radic Biol Med. 2001;31:331–335. doi: 10.1016/s0891-5849(01)00584-6. [DOI] [PubMed] [Google Scholar]
- Lee SH, Heo SJ, Hwang JY, Han JS, Jeon YJ. Protective effects of enzymatic digest from Ecklonia cava against high glucose-induced oxidative stress in human umbilical vein endothelial cells. J Sci Food Agric. 2010;90(2):349–356. doi: 10.1002/jsfa.3833. [DOI] [PubMed] [Google Scholar]
- Melhem MF, Craven PA, Liachenko J, DeRubertis FR. Alpha-lipoic acid attenuates hyperglycemia and prevents glomerular mesangial matrix expansion in diabetes. J Am Soc Nephrol. 2002;13:108–116. doi: 10.1681/ASN.V131108. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Roberts LJ. The isoprostanes: unique bioactive products of lipid peroxidation. Prog Lipid Res. 1997;36:1–21. doi: 10.1016/s0163-7827(97)00001-5. [DOI] [PubMed] [Google Scholar]
- Nakagawa T. Uncoupling of VEGF with NO as a mechanism for diabetic nephropathy. Diabetes Res Clin Pract. 2008;13(82 Suppl 1):S67–S69. doi: 10.1016/j.diabres.2008.09.030. [DOI] [PubMed] [Google Scholar]
- Packer L, Witt EH, Tritschler HJ. alpha-Lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995;19(2):227–250. doi: 10.1016/0891-5849(95)00017-r. [DOI] [PubMed] [Google Scholar]
- Riley MD, Dwyer T. Microalbuminuria is positively associated with usual dietary saturated fat intake and negatively associated with usual dietary protein intake in people with insulin-dependent diabetes mellitus. Am J Clin Nutr. 1998;67(1):50–57. doi: 10.1093/ajcn/67.1.50. [DOI] [PubMed] [Google Scholar]
- Reed LJ. From lipoic acid to multi-enzyme complexes. Protein Sci. 1998;7:220–224. doi: 10.1002/pro.5560070125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario RF, Prabhakar S. Lipids and diabetic nephropathy. Curr Diab Rep. 2006;6(6):455–462. doi: 10.1007/s11892-006-0079-7. [DOI] [PubMed] [Google Scholar]
- Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci. 1994;91(23):10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Thornalley PJ, Giardino I, Beisswenger P, Thorpe SR, Onorato J, Brownlee M. Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J Clin Invest. 1998;101(5):1142–1147. doi: 10.1172/JCI119885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudheesh NP, Ajith TA, Janardhanan KK, Krishnan CV. Palladium α-lipoic acid complex formulation enhances activities of Krebs cycle dehydrogenases and respiratory complexes I–IV in the heart of aged rats. Food and Chemical Toxicology. 2009;47(8):2124–2128. doi: 10.1016/j.fct.2009.05.032. [DOI] [PubMed] [Google Scholar]
- Tuomilehto J, Borch-Johnsen K, Molarius A, Forsen T, Rastenyte D, Sarti C, Reunanen A. Incidence of cardiovascular disease in Type 1 (insulin-dependent) diabetic subjects with and without diabetic nephropathy in Finland. Diabetologia. 1998;41:784–790. doi: 10.1007/s001250050988. [DOI] [PubMed] [Google Scholar]
- Wen M, Segerer S, Dantas M, Brown PA, Hudkins KL, Goodpaster T, Kirk E, LeBoeuf RC, Alpers CE. Renal injury in apolipoprotein E-deficient mice. Lab Invest. 2002;82:999–1006. doi: 10.1097/01.lab.0000022222.03120.d4. [DOI] [PubMed] [Google Scholar]
- Winiarska K, Malinska D, Szymanski K, Dudziak M, Bryla J. Lipoic acid ameliorates oxidative stress and renal injury in alloxan diabetic rabbits. Biochimie. 2008;90(3):450–459. doi: 10.1016/j.biochi.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Yi X, Maeda N. alpha-Lipoic acid prevents the increase in atherosclerosis induced by diabetes in apolipoprotein E-deficient mice fed high-fat/low-cholesterol diet. Diabetes. 2006;55:2238–2244. doi: 10.2337/db06-0251. [DOI] [PubMed] [Google Scholar]
- Yi X, Xu L, Kim K, Kim HS, Maeda N. Genetic reduction of lipoic acid synthase expression modestly increases atherosclerosis in male, but not in female, apolipoprotein E-deficient mice. 2010 doi: 10.1016/j.atherosclerosis.2010.03.009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler D. Thioctic acid for patients with symptomatic diabetic polyneuropathy: a critical review. Treat Endocrinol. 2004;3:173–189. doi: 10.2165/00024677-200403030-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.