Abstract
Osteonecrosis (ON) is a debilitating long-term complication of allogenic bone marrow transplantation (alloBMT) but may begin before alloBMT in some children because of their primary disease treatment. Therefore, to estimate the prevalence and associated risk factors for ON before alloBMT, we conducted a retrospective analysis of magnetic resonance (MR) studies of 118 children who underwent first alloBMT at our institution between December 2000 and September 2007. Of the 118 consecutive patients, 107 (90.7%) underwent prospective MR studies irrespective of symptoms (69 males; median age at alloBMT 12.9 years), and 11 underwent MR studies for symptoms. Amongst the 107 who had prospective imaging, 23 (21.5%) had ON; nearly 50% had at least 30% epiphyseal involvement. Knees were more frequently involved than were hips; severity of ON was greater in hips. ON prevalence before alloBMT was 23.72% when all 118 patients were included in the denominator. Risk factor analysis, limited to MR studies performed irrespective of symptoms, revealed female gender (P = 0.049) and age ≥10 years at the time of MR study (P = 0.03) as significant risk factors and primary diagnosis of lymphoid malignancies and aplastic anemia trended towards significance. ON prior to alloBMT is a common occurrence in children.
Keywords: Osteonecrosis, allogenic bone marrow transplantation, children, females, lymphoid malignancies, aplastic anemia
Introduction
Osteonecrosis (ON) is a potentially persistent and debilitating complication after allogenic bone marrow transplantation (alloBMT) 1-6.Incidences in pediatric populations range from 3.9% to 44.2%7-9. These lesions can progress and eventually lead to collapse of affected joints, 10-12 making joint replacement necessary while the individual is still a teenager or young adult. Joint replacement in teenagers and young adults is undesirable because these patients will typically require additional revisions and replacements as they age. 3,12,13
The prevalence of ON and its probable risk factors, such as acute or chronic graft versus host disease (GvHD) requiring steroids, increasing age, and a primary diagnosis of aplastic anemia or acute leukemia, have been documented in post-BMT settings in mixed populations 1,5,6.However, the prevalence and risk factors for developing ON before BMT remain undefined. Children who undergo BMT have primary diseases that are often associated with the occurrence of ON either as a direct result of disease pathology (e.g., in sickle-cell disease) 14,15 or secondary to the high-dose chemotherapy or steroids used for treatment (e.g., leukemias)16-18. Because these children are exposed to bone toxic factors even before undergoing BMT, it is possible that ON may develop before BMT in some cases.
Identifying the subset of children who will develop pretransplantation ON or who may be at risk of posttransplantation ON progression will help in early recognition of this debilitating condition, which may allow intervention in stages preceding collapse when joint-preserving procedures are possible 19. It will also allow suitable tailoring of medical management post–BMT and provide timely counseling of parents and patients. Early recognition will also help assess the true affect of BMT on the evolution of ON and allow development of interventions to ameliorate ON progression and maintain joint function. We therefore conducted a retrospective analysis of magnetic resonance (MR) images and medical records of 118 children who underwent first alloBMT at St. Jude Children’s Research Hospital between December 2000 and September 2007 to estimate the prevalence of ON and to identify risk factors for its development before alloBMT in this population.
Methods
Study population
We conducted a retrospective review of medical records and MR images of children who underwent first alloBMT for malignant or nonmalignant conditions at St. Jude Children’s Research Hospital between December 2000 and September 2007. Only patients for whom MR imaging results of hips, knees, or both before the first alloBMT were available were included in the reported cohort. This cohort was further divided into two groups: MR studies performed irrespective of symptoms (Group1) and MR studies performed for symptoms only (Group2). Since January 2002, patients undergoing evaluation for alloBMT at St. Jude Children’s Research Hospital undergo a baseline protocol-driven MR of hips and knees before alloBMT. Also included in group 1 were patients who had undergone MRs of hips, knees, or both as a part of their primary disease follow-up assessment irrespective of symptoms. Typically patients with acute lymphoid leukemia (ALL) undergo MR studies after every reinduction therapy. Patients with acute myeloid leukemia (AML) undergo prospective MR studies of the femur to assess marrow response to therapy, and patients with sickle-cell disease undergo MR studies because of the recognized increased risk of ON in these patients14,20. To avoid bias, risk factor analysis was restricted to Group 1 patients only. Institutional review board approval was obtained for this study, and data was managed according to the Health Insurance Portability and Accountability Act of 1996 (HIPAA).
Osteonecrosis
The presence of ON was determined by using MR imaging. As a standard of care at our institution, patients being evaluated for ON undergo coronal noncontrast T1-weighted and short tau inversion recovery (STIR) imaging of the hips and/or knees and sagittal FLASH 2-D imaging of the articular surfaces. Imaging studies were performed with either a Siemens 1.5 T Symphony or a Vision MR Unit (Siemens, Inc., Erlangen, Germany). Images were reviewed and interpreted by an experienced pediatric radiologist (SCK). As described previously, ON is defined as a geographic area of decreased signal on T1-weighted and increased signal on STIR 12,21 .ON locations were classified as epiphyseal, metaphyseal, or diaphyseal. Because the extent of epiphyseal involvement is an important predictor of joint outcome, the extent of epiphyseal involvement was categorized as ≥30% or <30% of the weight-bearing volume 12. Thus, hip joint involvement was coded according to the involvement of the capital femoral epiphysis, and knee joints were coded as involved if an ON lesion was present in either the distal femoral or the proximal tibial epiphysis. For patients who underwent multiple MR studies before transplantation, the examination demonstrating the most severe ON was the one used for analysis. The distribution of joint involvement is important for designing interventions to ameliorate ON. Therefore, the pattern of joint involvement was also studied.
Risk Factors
Risk factor analysis was done only for the patients who had MR studies irrespective of symptoms (i.e., Group1). The following risk factors were studied to determine their associations with ON: gender, race, age at MR imaging (i.e., <10 years or ≥10 years), age at primary diagnosis, primary diagnosis, and body mass index (BMI) at the time of the MR study. BMI at the time of MR study was calculated only for patients older than 2 years, per Centers for Disease Control and Prevention (CDC) criteria (www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html)22. Participants were categorized as healthy, underweight, overweight, or obese per CDC criteria22.
Malignant conditions were categorized as lymphoid [i.e., ALL, non-Hodgkin lymphoma (NHL), and Hodgkin disease] or non-lymphoid malignancies [i.e., acute myeloid leukemia/myelodysplastic syndrome (AML/MDS), chronic myeloid leukemia (CML), sarcoma, and neuroblastoma]. Also, because statistical power for eliciting the effect of individual treatment regimens was limited and individual cumulative doses of steroids received by each patient were not available, we categorized primary diagnoses on the basis of known contemporary treatment protocols to assess the potential effect of steroids. Diagnoses were grouped as follows: lymphoid malignancies and aplastic anemia or other conditions (i.e., AML/MDS, CML, sarcoma, sickle-cell disease, Duncan syndrome, hemophagocytic lymphohistiocytosis, and hyper–Ig M syndrome).
Statistical analyses
Descriptive statistics were used to describe the study population by gender, race, age at MR study, age at alloBMT, BMI at MR study, and primary diagnosis. To assess the internal validity of our cohort, t-tests and Chi-squared statistics were used to compare the characteristics of Group1 participants to those of all other alloBMT recipients treated during the same time.
ON prevalence rates were calculated for epiphyseal involvement of any of the four lower extremity joints as well as for hips and knees separately. The pattern of joint involvement was also examined by evaluating the number of lower extremity joints involved at the time point when the MR study showed the highest grade of ON.
Logistic regression models were used to examine the associations between potential risk factors and the development of ON in any of the four joints. Risk factors that were statistically significant in univariate analysis and those which were clinically significant were further examined in multiple-regression models. Data were analyzed by using SAS (Version 9.13; SAS Institute, Inc; Cary, NC). Statistical significance was set at an α-error level of 0.05.
Results
Demographic Characteristics of the Study Population
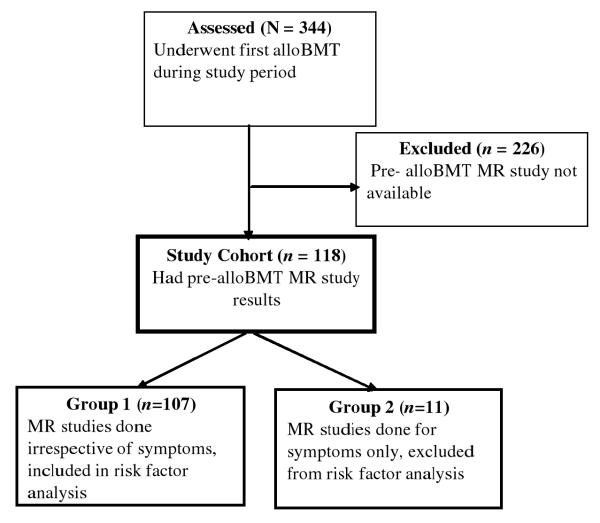
Of the 344 consecutive patients who underwent first alloBMT at St. Jude Children’s Research Hospital from December 2000 to September 2007, 226 were excluded from further analysis because they did not have a pre-alloBMT MR study (Figure 1).One hundred eighteen patients had pre-alloBMT MR studies. Among these patients, 107 (90.7%) had MR studies irrespective of symptoms (Group1), and 11(10.3%) patients had MR studies because of symptoms (Group2) (Figure1). All patients underwent treatment for their primary disease per institutional or cooperative group protocols. Demographic characteristics of Group1 are shown in Table 1, and demographic and clinical characteristics of Group 2 are in Table 2. The time from peak ON to alloBMT for the two groups is in Table 3.
Figure 1.
Study cohort eligibility.
Table1.
Demographic characteristics of patients who underwent MR studies irrespective of symptoms (Group 1)
| Characteristic | Number (N=107) |
Percentage (%) |
|---|---|---|
| Gender | ||
| Male | 69 | 63.89 |
| Female | 39 | 36.11 |
| Race | ||
| Other | 27 | 25.00 |
| Black | 24 | 22.22 |
| White | 57 | 52.78 |
| Diagnosis | ||
| Non-malignant | 18 | 16.67 |
| Malignant | 90 | 83.33 |
| BMI at MR1 (n=98) | ||
| Normal | 56 | 57.14 |
| Underweight | 6 | 6.12 |
| Overweight | 20 | 20.41 |
| Obese | 16 | 16.33 |
| Age at MR | ||
| <10 | 34 | 31.48 |
| ≥10 | 74 | 68.52 |
| Variable | Median | Range | Standard Deviation |
|---|---|---|---|
| Age at MR imaging (years) | 12.9 | 0.5-21.1 | 5.3 |
| Age at alloBMT (years) | 12.9 | 0.5-21.2 | 5.3 |
| Time from primary diagnosis to alloBMT (months) |
20.2 | 2.42-05.9 | 49.4 |
Data from patients younger than 2 years and those who did not have height and weight measured at MR date were not included.
Abbreviations: BMI, body mass index percentile; MR, magnetic resonance.
Table2.
Demographic and clinical characteristics of patients who underwent MR studies for symptoms (Group 2)
| Patient Demographics | Clinical Characteristics | MR Findings |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L. Hip | R. Hip | L Knee | R. Knee | ||||||||||
|
| |||||||||||||
| Patient No. |
Age at alloBMT (years) |
Sex | Race | Primary Diagnosis |
Time from Primary Diagnosis to alloBMT (months) |
Symptoms | Time from symptoms to alloBMT (months) |
L.PFEpi | R.PFEpi | L.DFEpi | L.PTEpi | R.DFEpi | R.PTEpi |
| 1 | 14 | F | W | ALL | 114.2 | Limping and ankle pain |
53.4 | 1 | 0 | NA | NA | NA | NA |
| 2 | 13 | F | W | ALL | 43.5 | Radicular pain in legs |
17.6 | 2 | 1 | 0 | 0 | 0 | 0 |
| 3 | 16 | M | W | NHL | 40.16 | Bilateral knee pain |
7.26 | NA | NA | NA | 0 | 0 | 0 |
| 4 | 15 | M | W | ALL | 14.6 | Pain | ≥3.5 referred study |
0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 19 | M | B | AA | 69.4 | Redness, swelling knees |
48.5 | NA | NA | 0 | 0 | 0 | 0 |
| 6 | 8 | M | W | ALL | 5.1 | Bilateral knee pain |
2.63 | 0 | 0 | 1 | 0 | 0 | 0 |
| 7 | 21 | M | W | ALL | 55.8 | Pain and swelling knees |
1.83 | 0 | 0 | 1 | 0 | 1 | 0 |
| 8 | 19 | M | W | ALL | 20.1 | Knee pain | 4 days | 0 | 0 | NA | NA | NA | NA |
| 9 | 6 | M | W | NB | 5.8 | Pain at multiple body sites |
138.2 | 0 | 0 | NA | NA | NA | NA |
| 10 | 8 | F | W | ALL | 138.3 | Proximal leg weakness |
17days | 2 | 2 | 2 | 2 | 2 | 2 |
| 11 | 13 | M | B | NHL | 8.8 | Bilateral knee swelling and severe pain in both legs |
2.3 | 0 | 0 | 0 | 0 | 1 | 0 |
Abbreviations: AA ,aplastic anemia; ALL, acute lymphoid leukemia; B, black; CML, chronic myeloid leukemia; DFEpi, distal femoral epiphysis; F ,female ;L, left; M, male; NB, neuroblastoma; NHL, non-Hodgkin lymphoma; PFEpi, proximal femoral epiphysis; PTEpi, proximal tibial epiphysis; R, Right; W, white.
0 = no ON
1 = <30% epiphyseal involvement
2 = ≥ 30% epiphyseal involvement
NA = results not available
Table 3.
Time from peak ON to alloBMT
| Patients | Location (n) | Median (months) |
Range (months) |
|---|---|---|---|
| Group 1 | Any joint (23) | 1.1 | 0.03-54.9 |
| (MRs irrespective of symptoms) |
Hips (12) | 0.8 | 0.03-32.3 |
| Knees (16) | 0.9 | 0.23-54.9 | |
| Group 2 | Any joint (5) | 2.3 | 0.93-17.9 |
| (MRs for symptoms) | Hips (2) | 9.4 | 0.93-17.9 |
| Knees (4) | 2.1 | 0.93-2.7 |
The median age of Group1 at the time of MR study was 12.9 years (range 0.5-21.1), and the median time from diagnosis to alloBMT was 20.2 months (range 2.4-205.9) (Table 1). Eighty nine patients (83.2%) had malignant diseases and 18 (16.8%) had nonmalignant diseases (Table 4, 5). Among the 107 patients examined prospectively, 76 (71.03%) had one, 9 (8.41%) had two, 9 (8.41%) had three, and 13 (12.15%) had more than three MR studies. Hip MR studies were available for 103 patients (96.26%), and knee MR studies were available for 84 patients (78.50%). Additionally, the distributions of gender, diagnoses, and BMI did not differ between Group 1and the remaining population, although the distribution of age at transplant and race did differ between these groups (Table 4).
Table 4.
Characteristics of patients who had pre-alloBMT prospective MR studies who were included in risk factor analysis i.e. (Group1) (N=107) vs. all others who underwent alloBMT during the study period {N=226+ 11(Group2)}
| Characteristics | Patients with prospective pre-BMT MR studies(Group1) [n(%)] |
All others who underwent alloBMT during the study period [n (%)] |
P value |
|---|---|---|---|
| Gender | |||
| Male | 69(31.94) | 147(68.06) | 0.66 |
| Female | 38(29.69) | 90(70.31) | |
| Race | |||
| Other | 27(42.86) | 36(57.14) | 0.03 |
| Black | 24(35.29) | 44(64.71) | |
| White | 56(26.29) | 157(73.71) | |
| Diagnosis | |||
| Non-malignant | 18(26.47) | 50(73.53) | 0.36 |
| Malignant | 89(32.25) | 187(67.75) | |
| BMI at alloBMT1 | |||
| Normal | 62(35.63) | 112(64.37) | 0.08 |
| Underweight | 5(27.78) | 13(72.22) | |
| Overweight | 14(26.92) | 38(73.08) | |
| Obese | 23(51.11) | 22(48.89) | |
| Age at Transplant | |||
| ≤2 | 3(5.88) | 48(94.12) | <0.001 |
| 2 – 10 | 28(22.40) | 97(77.60) | |
| ≥10 | 76(45.24) | 92(54.76) |
Data from patients younger than 2 and patients who did not have height/weight measured at MR date were not included.
Abbreviations: MR, magnetic resonance; BMI at MR, body mass index percentile at the time of MR study
Table 5.
Primary diagnoses of Group 1 (N = 107) and patients who had ON before alloBMT (n = 23)
| Primary Diagnosis | Group 1 [n (%)] |
Patients with ON [n (%)] |
|---|---|---|
| Malignant Conditions | 89 (83.17) | 19 (82.60) |
| Lymphoid Malignancies | ||
| ALL | 39 (36.45) | 10 (43.48) |
| NHL | 2 (1.87) | 0 (0) |
| Hodgkin Disease | 1 (0.93) | 1 (4.35) |
| Nonlymphoid Malignancies | ||
| AML/MDS | 33 (30.84) | 5 (21.74) |
| CML | 9 (8.41) | 2 (8.7) |
| Sarcoma | 5 (4.67) | 1 (4.35) |
| Nonmalignant Conditions | 18 (16.82) | 4 (17.4) |
| Sickle cell disease | 12 (11.21) | 2 (8.7) |
| Aplastic anemia | 3 (2.80) | 2 (8.70) |
| Duncan syndrome | 1 (0.93) | 0 (0) |
| Hemophagocytic lymphohistiocytosis |
1 (0.93) | 0 (0) |
| Hyper–Ig M Syndrome | 1 (0.93) | 0 (0) |
Abbreviations: ALL, acute lymphoid leukemia; AML/MDS, acute myeloid leukemia and myelodysplastic syndrome; CML, chronic myeloid leukemia; NHL, non-Hodgkin lymphoma.
Prevalence, severity, and extent of osteonecrosis
When all four joints were considered, prevalence of ON for Groups1 and 2 collectively was 28 of 118 (23.72 %).In those patients who had MR studies irrespective of symptoms, prevalence was 23 of 107 (21.5%) when all four joints were considered; 16 of 84 (23.5%) had knee involvement, and 12 of 103 (11.7%) had hip involvement (Table 6). Eight (66.7%) of 12 patients with hip ON had ≥30% of epiphyseal involvement, whereas only 3 (18.8%) of 16 patients with knee ON had ≥30% of epiphyseal involvement (Table 6).
Table 6.
Prevalence of osteonecrosis in Group1 (N = 107)
| Joint Involvement1 | Number (n) | Percentage (%) |
|---|---|---|
| Any joint | 84 | 78.50 |
| None | ||
| <30% epiphyseal | 12 | 11.21 |
| ≥30% epiphyseal | 11 | 10.28 |
| Hips | ||
| No data | 4 | 3.74 |
| None | 91 | 85.05 |
| <30% epiphyseal | 4 | 12.15 |
| ≥30% epiphyseal | 8 | 2.80 |
| Knees | ||
| No data | 23 | 21.50 |
| None | 68 | 63.55 |
| <30% epiphyseal | 13 | 12.15 |
| ≥30% epiphyseal | 3 | 2.80 |
Some patients did not undergo MR studies of both hips and both knees before alloBMT; therefore, the actual number of positive results in any joint might be higher than that presented.
Pattern of Joint Involvement
The pattern of joint involvement, in patients with ON in Group 1, is shown in Table 7. Of the 23 patients with ON, 12 (52.2%) had more than one joint involved (Table 7). The most frequent pattern of multiple joint involvement was that of bilateral knees.
Table7.
Pattern of joint involvement in patients with osteonecrosis (N = 23) in Group 1.
| Pattern | Frequency (n) |
Percentage (%) |
|---|---|---|
| Bilateral knees only | 6 | 26.1 |
| Unilateral knee only | 4 | 17.4 |
| Unilateral hip, knee result missing | 4 | 17.4 |
| Bilateral hips and bilateral knees or bilateral hips and unilateral knee |
3 | 13.0 |
| Unilateral hip and unilateral knee or unilateral hip only |
3 | 13.0 |
| Unilateral hip and bilateral knees or bilateral hips only |
2 | 8.7 |
| Bilateral knee, hip result missing | 1 | 4.3 |
Risk Factors
Age ≥10 years at MR imaging and female gender were associated with greater odds of developing ON (OR 4.50; 95%CI 1.16-17.17 and OR 2.70; 95%CI 1.00-7.27, respectively) (Table 8). There was also a trend towards significance for primary diagnosis of lymphoid malignancies or aplastic anemia (OR 2.72; CI, 0.99-7.48) after adjusting for age and gender. Other risk factors studied were not associated with an increased risk of developing ON (Table 8).
Table 8.
Risk-factor analysis before allo-BMT MR study results in ON-positive (n = 23) and ON-negative (n = 84) patients
| ON-positive [n (%)] |
ON-negative [n (%)]) |
Odds Ratio (95% CI) |
P value | |
|---|---|---|---|---|
| Gender | ||||
| Male | 10 (14.49) | 59 (85.51) | 0.02 | |
| Female | 13 (34.21) | 25 (65.79) | 3.07 (1.19, 7.92) | |
| Race | ||||
| Other | 3 (11.11) | 24 (88.89) | 0.28 | |
| Black | 5 (20.83) | 19 (79.17) | 2.11 (0.45, 9.95) | |
| White | 15 (26.79) | 41 (73.21) | 2.93 (0.77, 11.16) | |
| BMI at MR study 1 | ||||
| Normal | 9 (16.07) | 47 (83.93) | 0.65 | |
| Underweight | 2 (33.33) | 4 (66.67) | 2.61 (0.41, 16.46) | |
| Overweight | 5 (26.32) | 14 (73.68) | 1.87 (0.54, 6.48) | |
| Obese | 3 (18.75) | 13 (81.25) | 1.21 (0.28, 5.11) | |
| Age at MR study | ||||
| <10 | 3 (8.82) | 31 (91.18) | 0.04 | |
| ≥10 | 20 (27.40) | 53 (72.60) | 3.90 (1.07, 14.18) | |
| Age at Primary Diagnosis | ||||
| ≤2 | 2 (9.09) | 20 (90.91) | 0.27 | |
| 2 – 10 | 8 (21.62) | 29 (78.38) | 2.76 (0.53, 14.37) | |
| ≥10 | 13 (27.08) | 35 (72.92) | 3.71 (0.76, 18.15) | |
| Primary Diagnosis | ||||
| Others | 10 (16.13) | 52 (83.87) | 0.12 | |
| Lymphoid Malignancy & Aplastic Anemia |
13 (28.89) | 32 (71.11) | 2.11 (0.83, 5.38) |
| Multivariable analysis | ||
|---|---|---|
| Variable | Odds ratio (95%CI) | P value |
| Gender | ||
| Male (reference) | ||
| Female | 2.70 (1.00, 7.27) | 0.049 |
| Primary Diagnosis | ||
| Others (reference) | ||
| Lymphoid malignancies & Aplastic anemia |
2.72 (0.99, 7.48) | 0.053 |
| Age at MR study | ||
| ≥10 Years (reference) | ||
| ≥10 Years | 4.50 (1.16, 17.17) | 0.030 |
Data from patients younger than 2 and patients who did not have height/weight measured at MR date were not included.
Abbreviations: BMI, body mass index percentile; MR, magnetic resonance;
Discussion
One striking observation in our cohort is that approximately half the patients had at least 30% epiphyseal involvement, indicating the existence of significant joint morbidity before alloBMT. Although regression of small lesions has been reported 11,23, large lesions have consistently been associated with high rates of progression and joint collapse. For example, Karimova et al. found that lesions occupying 30% or more of the femoral head volume were associated with an increased risk of joint collapse or a need for arthroplasty in 48% of patients 12.
In our study, adolescent females emerged as a conspicuous risk group for developing ON. Studies in ALL and post alloBMT patients have identified treatment with intensive chemotherapy and age of 10 or more years as the major risk factors for the development of ON in children1,2,6,9,16,18. However, whether or not an association between gender and development of ON exists is unclear 1,5,16,18. Theoretically, an increased risk of ON in females may be related to earlier onset of puberty with earlier physeal closure 24,25. Mature bone may be more vulnerable to rises in intraosseous pressure than immature bone,26 leading to ischemia and ON. Because we did not assess pubertal stage, bone age, or sex hormone levels, we could not analyze the potential association between puberty and ON. Also, as suggested by Schulte et al.,5 sexual dimorphism in the morphogenesis potential of osteoblasts may need further exploration to clarify whether or not there exists an association between gender and the development of ON.
Our data indicate a potential association between primary diagnoses of lymphoid malignancies or aplastic anemia and the development of ON. These data are supported by earlier studies that indicate acute leukemias and aplastic anemia as risk factors for post-BMT ON 1. ON has recently been reported as a morbid skeletal complication among children being treated for ALL with incidences up to 38% 16,17. Park et al. reported ON in five children with aplastic anemia prior to any therapy27.
Although cumulative steroid dose information was not available for the children in our cohort, our trend toward significance among those with lymphoid malignancies or aplastic anemia indicates that steroids are a potential risk factor for ON. Previous literature indicates that ON among children with ALL is associated with higher doses of steroids and other chemotherapeutic agents16-18,28,29. Similarly, ON in patients with aplastic anemia is associated with antilymphocyte globulin followed by high-dose methylprednisolone 30. However, because information was not available on the cumulative doses of steroids in our cohort, we could not distinguish the effect of disease pathophysiology, if any, from treatment dose–related effects for these children. ON observed in our patients with other diagnoses such as sickle cell ds, CML, AML, sarcomas is well supported in literature 31-37 Reported risk factors for ON include steroids, vaso-oclusive episodes, chemotherapeutic agents such as methotrexate, asparaginase, and cyclophosphamide, and disease-related disturbances in coagulation mechanisms. A direct comparison between prevalence rates in this study and those of previous studies is difficult because of the smaller number of patients in individual groups and the diverse diagnostic criteria used. Furthermore, we found no significant relationship between ON and race or BMI, in contrast to reports indicating that white patients with ALL have a higher risk than do patients of other races18,38
Consistent with previous reports 1,2,16,17,27,39 , we found multi-articular involvement in our cohort. However, whereas previous studies using symptom-driven imaging to document ON indicated an increased frequency of hip involvement1,4,6,28,29, our study, using prospective imaging, indicated knee involvement to be more frequent than hip involvement 7,16. One possible reason for knee involvement to be under-diagnosed in symptomatic patients is that knee pain may be interpreted as referred pain from concurrent ipsilateral hip involvement.
The results of our study should not be interpreted without taking into account their potential limitations. First, this cohort was older than the overall pre-alloBMT population at St. Jude Children’s Research Hospital. Therefore, the prevalence calculated for our patients could be an overestimation, since older age has been consistently related to a higher incidence of ON. Second, we could not determine the effect of individual treatment regimens and disease pathophysiology on the development of ON. Therefore, because of the diversity of therapy for various diagnoses, we cannot generalize our findings to children undergoing alloBMT at other centers. Despite these limitations, our study provides insight into a skeletal toxicity that may develop before alloBMT and could possibly exacerbate during the transplantation period when the patient is subjected to additional bone-toxic therapy.
On the basis of this single-institution retrospective study of prospectively acquired MR studies in a modestly large pediatric population, we conclude that ON before alloBMT is a common occurrence in children. A high index of suspicion, and, if possible, prospective screening before alloBMT of adolescent females with lymphoid malignancies or aplastic anemia may help identify this debilitating disease at pre-collapse stages when joint preserving interventions are feasible in this young population. This complication also needs to be considered while developing preparatory and follow-up monitoring protocols for these patients and while counseling parents and children. Future studies are required to elucidate the physiologic basis of the effect of gender on the evolution of ON as well as to develop appropriate interventions to prevent or ameliorate ON.
Acknowledgements
This work was supported in part by grant number P30 CA-21765 from the National Institutes of Health, a Center of Excellence grant from the State of Tennessee, and the American Lebanese Syrian Associated Charities (ALSAC).
We also thank Vani J. Shanker and Cherise Guess for editing the manuscript, Sandra Gaither for manuscript preparation, and the physicians and nursing staff for providing outstanding clinical care to the patients in this study.
This work was supported in part by grant number P30 CA-21765 from the National Institutes of Health, a Center of Excellence grant from the State of Tennessee, and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Conflict of Interest: None
Reference List
- 1.Socie G, Selimi F, Sedel L, Frija J, Devergie A, Esperou BH, et al. Avascular necrosis of bone after allogeneic bone marrow transplantation: clinical findings, incidence and risk factors. Br. J Haematol. 1994;86:624–628. doi: 10.1111/j.1365-2141.1994.tb04795.x. [DOI] [PubMed] [Google Scholar]
- 2.Socie G, Cahn JY, Carmelo J, Vernant JP, Jouet JP, Ifrah N, et al. Avascular necrosis of bone after allogeneic bone marrow transplantation: analysis of risk factors for 4388 patients by the Societe Francaise de Greffe de Moelle (SFGM) Br. J Haematol. 1997;97:865–870. doi: 10.1046/j.1365-2141.1997.1262940.x. [DOI] [PubMed] [Google Scholar]
- 3.Zadegan F, Raould A, Bizot P, Nizard R, Sedel L. Osteonecrosis after allogeneic bone marrow transplantation. Clin. Orthop. Relat Res. 2008;466:287–293. doi: 10.1007/s11999-007-0044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattano L. The skeletal remains: porosis and necrosis of bone in the marrow transplantation setting. Pediatr. Transplant. 2003;7(Suppl 3):71–75. doi: 10.1034/j.1399-3046.7.s3.11.x. [DOI] [PubMed] [Google Scholar]
- 5.Schulte CM, Beelen DW. Avascular osteonecrosis after allogeneic hematopoietic stem-cell transplantation: diagnosis and gender matter. Transplantation. 2004;78:1055–1063. doi: 10.1097/01.tp.0000138026.40907.38. [DOI] [PubMed] [Google Scholar]
- 6.Enright H, Haake R, Weisdorf D. Avascular necrosis of bone: a common serious complication of allogeneic bone marrow transplantation. Am. J. Med. 1990;89:733–738. doi: 10.1016/0002-9343(90)90214-x. [DOI] [PubMed] [Google Scholar]
- 7.Kaste SC, Shidler TJ, Tong X, Srivastava DK, Rochester R, Hudson MM, et al. Bone mineral density and osteonecrosis in survivors of childhood allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;33:435–441. doi: 10.1038/sj.bmt.1704360. [DOI] [PubMed] [Google Scholar]
- 8.Balduzzi A, Gooley T, Anasetti C, Sanders JE, Martin PJ, Petersdorf EW, et al. Unrelated donor marrow transplantation in children. Blood. 1995;86:3247–3256. [PubMed] [Google Scholar]
- 9.Faraci M, Calevo MG, Lanino E, Caruso S, Messina C, Favr C, et al. Osteonecrosis after allogeneic stem cell transplantation in childhood. A case-control study in Italy. Haematologica. 2006;91:1096–1099. [PubMed] [Google Scholar]
- 10.Ohzono K, Saito M, Takaoka K, Ono K, Saito S, Nishina T, et al. Natural history of nontraumatic avascular necrosis of the femoral head. J Bone Joint Surg. Br. 1991;73:68–72. doi: 10.1302/0301-620X.73B1.1991778. [DOI] [PubMed] [Google Scholar]
- 11.Nam KW, Kim YL, Yoo JJ, Koo KH, Yoon KS, Kim HJ. Fate of untreated asymptomatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 2008;90:477–484. doi: 10.2106/JBJS.F.01582. [DOI] [PubMed] [Google Scholar]
- 12.Karimova EJ, Rai SN, Howard SC, Neel M, Britton L, Pui CH, et al. Femoral Head Osteonecrosis in Pediatric and Young Adult Patients With Leukemia or Lymphoma. J Clin Oncol. 2007;25:1525–1531. doi: 10.1200/JCO.2006.07.9947. [DOI] [PubMed] [Google Scholar]
- 13.Hungerford MW, Hungerford DS, Khanuja HS, Pietryak BP, Jones LC. Survivorship of femoral revision hip arthroplasty in patients with osteonecrosis. J Bone Joint Surg Am. 2006;88(Suppl 3):126–130. doi: 10.2106/JBJS.F.00777. [DOI] [PubMed] [Google Scholar]
- 14.Milner PF, Kraus AP, Sebes JI, Sleeper LA, Dukes KA, Embury SH, et al. Sickle cell disease as a cause of osteonecrosis of the femoral head. N Engl J Med. 1991;325:1476–1481. doi: 10.1056/NEJM199111213252104. [DOI] [PubMed] [Google Scholar]
- 15.Akinyoola AL, Adediran IA, Asaleye CM. Avascular necrosis of the femoral head in sickle cell disease in Nigeria: a retrospective study. Niger. Postgrad. Med J. 2007;14:217–220. [PubMed] [Google Scholar]
- 16.Niinimaki RA, Harila-Saari AH, Jartti AE, Seuri RM, Riikonen PV, Paakko EL, et al. High Body Mass Index Increases the Risk for Osteonecrosis in Children With Acute Lymphoblastic Leukemia. J Clin Oncol. 2007;25:1498–1504. doi: 10.1200/JCO.2006.06.2539. [DOI] [PubMed] [Google Scholar]
- 17.Ojala AE, Paakko E, Lanning FP, Lanning M. Osteonecrosis during the treatment of childhood acute lymphoblastic leukemia: a prospective MRI study. Med Pediatr. Oncol. 1999;32:11–17. doi: 10.1002/(sici)1096-911x(199901)32:1<11::aid-mpo4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 18.Mattano LA, Jr., Sather HN, Trigg ME, Nachman JB. Osteonecrosis as a Complication of Treating Acute Lymphoblastic Leukemia in Children: A Report From the Children’s Cancer Group. J Clin Oncol. 2000;18:3262–3272. doi: 10.1200/JCO.2000.18.18.3262. [DOI] [PubMed] [Google Scholar]
- 19.Marker DR, Seyler TM, McGrath MS, Delanois RE, Ulrich SD, Mont MA. Treatment of Early Stage Osteonecrosis of the Femoral Head. J Bone Joint Surg Am. 2008;90:175–187. doi: 10.2106/JBJS.H.00671. [DOI] [PubMed] [Google Scholar]
- 20.Hernigou P, Galacteros F, Bachir D, Goutallier D. Deformities of the hip in adults who have sickle-cell disease and had avascular necrosis in childhood. A natural history of fifty-two patients 1. J. Bone Joint Surg. Am. 1991;73:81–92. [PubMed] [Google Scholar]
- 21.Karimova EJ, Rai SN, Ingle D, Ralph AC, Deng X, Neel MD, et al. MRI of knee osteonecrosis in children with leukemia and lymphoma: Part 2, clinical and imaging patterns. AJR Am. J. Roentgenol. 2006;186:477–482. doi: 10.2214/AJR.04.1597. [DOI] [PubMed] [Google Scholar]
- 22.CDC Criteria, Centers for Disease Control and Prevention Available from: www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html.
- 23.Hungerford DS, Jones LC. Asymptomatic osteonecrosis: should it be treated? Clin Orthop. Relat Res. 2004:124–130. [PubMed] [Google Scholar]
- 24.Wheeler MD. Physical changes of puberty. Endocrinol. Metab Clin North Am. 1991;20:1–14. [PubMed] [Google Scholar]
- 25.Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: correspondence between hormonal and physical development. Child Dev. 2009;80:327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laroche M. Intraosseous circulation from physiology to disease. Joint Bone Spine. 2002;69:262–269. doi: 10.1016/s1297-319x(02)00391-3. [DOI] [PubMed] [Google Scholar]
- 27.Park J, Jun J, Kim Y, Lee J, Kim C, Hahn S. Osteonecrosis of the hip in patients with aplastic anemia. J Korean Med Sci. 2002;17:806–810. doi: 10.3346/jkms.2002.17.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arico M, Boccalatte MF, Silvestri D, Barisone E, Messina C, Chiesa R, et al. Osteonecrosis: An emerging complication of intensive chemotherapy for childhood acute lymphoblastic leukemia. Haematologica. 2003;88:747–753. [PubMed] [Google Scholar]
- 29.Kadan-Lottick NS, Dinu I, Wasilewski-Masker K, Kaste S, Meacham LR, Mahajan A, et al. Osteonecrosis in adult survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2008;26:3038–3045. doi: 10.1200/JCO.2007.14.9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marsh JC, Zomas A, Hows JM, Chapple M, Gordon-Smith EC. Avascular necrosis after treatment of aplastic anaemia with antilymphocyte globulin and high-dose methylprednisolone. Br J Haematol. 1993;84:731–735. doi: 10.1111/j.1365-2141.1993.tb03153.x. [DOI] [PubMed] [Google Scholar]
- 31.Shim K, MacKenzie MJ, Winquist E. Chemotherapy-associated osteonecrosis in cancer patients with solid tumours: a systematic review. Drug Saf. 2008;31:359–371. doi: 10.2165/00002018-200831050-00001. [DOI] [PubMed] [Google Scholar]
- 32.Niinimaki RA, Harila-Saari AH, Jartti AE, Seuri RM, Riikonen PV, Paakko EL, et al. Osteonecrosis in children treated for lymphoma or solid tumors. J Pediatr. Hematol. Oncol. 2008;30:798–802. doi: 10.1097/MPH.0b013e31818ab29d. [DOI] [PubMed] [Google Scholar]
- 33.Hanif I, Mahmoud H, Pui CH. Avascular femoral head necrosis in pediatric cancer patients. Med Pediatr. Oncol. 1993;21:655–660. doi: 10.1002/mpo.2950210909. [DOI] [PubMed] [Google Scholar]
- 34.Kozuch P, Talpaz M, Faderl S, O’Brien S, Freireich EJ, Kantarjian H. Avascular necrosis of the femoral head in chronic myeloid leukemia patients treated with interferon-alpha: a synergistic correlation? Cancer. 2000;89:1482–1489. doi: 10.1002/1097-0142(20001001)89:7<1482::aid-cncr10>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 35.Lackner H, Benesch M, Moser A, Smolle-Juttner F, Linhart W, Raith J, et al. Aseptic osteonecrosis in children and adolescents treated for hemato-oncologic diseases: a 13-year longitudinal observational study. J Pediatr. Hematol. Oncol. 2005;27:259–263. doi: 10.1097/01.mph.0000163215.37147.13. [DOI] [PubMed] [Google Scholar]
- 36.al-Bahar S, Pandita R, Hoffbrand AV. Bilateral osteonecrosis of the head of the femur complicating acute promyelocytic leukemia: a sequel to treatment of retinoic acid syndrome with dexamethasone. Acta Haematol. 1996;96:88–91. doi: 10.1159/000203722. [DOI] [PubMed] [Google Scholar]
- 37.Abhyankar D, Nair R, Menon H, Kapoor B, Advani S. Avascular necrosis of head of femur in a patient with acute promyelocytic leukemia. Leuk. Lymphoma. 2000;37:635–637. doi: 10.3109/10428190009058519. [DOI] [PubMed] [Google Scholar]
- 38.Relling MV, Yang W, Das S, Cook EH, Rosner GL, Neel M, et al. Pharmacogenetic risk factors for osteonecrosis of the hip among children with leukemia 1. J. Clin. Oncol. 2004;22:3930–3936. doi: 10.1200/JCO.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 39.Niinimaki RA, Harila-Saari AH, Jartti AE, Seuri RM, Riikonen PV, Paakko EL, et al. Osteonecrosis in children treated for lymphoma or solid tumors. J Pediatr. Hematol. Oncol. 2008;30:798–802. doi: 10.1097/MPH.0b013e31818ab29d. [DOI] [PubMed] [Google Scholar]