Abstract
Objectives
To examine the relations of race, sex, adiposity, adipokines and physical activity to telomere length in adolescents.
Study design
Leukocyte telomere length (T/S ratio) was assessed cross-sectionally in 667 adolescents (aged 14–18 years, 48% blacks, 51% girls) using a quantitative PCR method. Generalized Estimating Equations analyses were performed.
Results
Black adolescents had longer telomeres than white adolescents (age and sex adjusted T/S ratio ± SE: 1.32 ± 0.01 vs. 1.27 ± 0.01, p=0.014) and girls had longer telomeres than boys (age and race adjusted T/S ratio ± SE: 1.31 ± 0.01 vs. 1.27 ± 0.01, p=0.007). None of the adiposity or adipokine measures explained a significant proportion of the variance in telomere length. Vigorous physical activity was positively associated with telomere length (adjusted R2=0.019, p=0.009) and accounted for 1.9% of the total variance only in girls.
Conclusion
This study, conducted in a biracial adolescent cohort, demonstrated that: (1) race and sex differences in telomere length have already emerged during adolescence; (2) adiposity and adipokines are not associated with telomere length at this age; and (3) the anti-aging effect of vigorous physical activity may begin in youth especially in girls.
Keywords: Telomere length, race, sex, adiposity, adipokines, physical activity, adolescents
Telomeres, specialized chromatin structures located at the chromosomal ends, protect chromosome integrity and stability. Telomeres naturally shorten with every cell cycle, and cells with critically short telomeres undergo replicative senescence and apoptosis. Therefore, leukocyte telomere length (LTL) has emerged as a novel indicator of human aging, cardiovascular aging in particular. Despite no difference in LTL between white and black or male and female newborns,1 racial2–4 and sex differences5–7 have recently been identified in adulthood. At what stage of life race and sex differences emerge remains unclear.
LTL is not only genetically determined,8–10 but also shaped by environmental factors such as life stress, oxidative stress, inflammation and cigarette smoking.11–13 Obesity is characterized as a state of chronic inflammation and heightened oxidative stress. However, existing data on the relations between obesity and telomere length in adults have been mixed. Two studies conducted in pediatric populations have produced different outcomes. Zannolli et al14 found no difference in LTL between obese and normal weight white children, whereas Al-Attas et al15 showed that LTL was significantly shorter in obese Arab boys compared with lean boys.
Regular physical activity reduces the risks for obesity, type 2 diabetes, hypertension and cancer in adults. We have demonstrated that physical activity improves general and visceral adiposity, bone and fitness,16 and insulin resistance,17 in adolescents and overweight children. Recent studies show that physical activity is positively associated with LTL in adult populations suggesting an anti-aging property.18–21
In contrast to the considerable information available in adult literature, the knowledge about LTL in adolescent populations is limited. Therefore, the present study aimed to examine the relations of race, sex, adiposity, adipokines and physical activity to telomere length in a relatively large sample of healthy white and black adolescents.
Methods
Healthy adolescents aged 14–18 years including 348 whites and 319 blacks (324 boys) were previously recruited from local public high schools. Written informed consent was obtained from these 18 year olds. For 14–17 year olds, parental consent and subject assent were obtained. Race was determined by self-report of each subject, and by a parent if subject was under 18 years of age. The adolescents were apparently healthy, had no contraindications to any of the study procedures and were taking no medication that could influence the results. The Institutional Review Board at the Medical College of Georgia approved the study.22
Height and weight were measured using a wall-mounted stadiometer and a digital scale with subjects wearing light clothing and no shoes. Body mass index (BMI) was calculated (kg/m2). Waist circumference (cm) was measured twice at the center of the umbilicus over the shirt and the values were averaged. Percentage body fat (%BF) was assessed by dual-energy x-ray absorptiometry (DXA, QDR-4500W, Hologic Waltham, MA) as previously described.23 The intra-class correlation coefficient was 0.99 for repeat scans of the same subject on the same equipment (QDR-4500W). Visceral adipose tissue (VAT) and subcutaneous abdominal adipose tissue (SAAT) were determined with a 1.5-T magnetic resonance imaging system (General Electric Medical Systems, Milwaukee, WI). All images were analyzed by the same experienced observer. The intra-class correlation coefficients for separate-day repeat analyses of the same scan typically exceeded 0.99 for both VAT and SAAT.23 Serum leptin and adiponectin were measured by ELISA. The intra-assay and inter-assay coefficients of variation were 2% and 5% for leptin and 7.4% and 8.4% for adiponectin.
Free-living physical activities were assessed using CSA/MTI Actigraph monitors (model 7164; MTI Health Services, Fort Walton Beach, FL) as described previously.22 Subjects were instructed to wear the monitor for 7 days, remove it for sleep and any activity that may cause harm to either the monitor or another person (eg, during contact sports), and return the monitor 1 week later. Data from days 1 and day 7 were discarded because a full day of information was not available for those days. Daily movement counts were converted to average minutes per day spent in moderate physical activity (MPA) [3–6 metabolic equivalents (METs)] and vigorous physical activity (VPA) (>6 METs) by the software accompanying the device.
To assess pubertal development, subjects were provided privacy while reading a script and viewing pictures showing different stages of pubertal development. Boys self-determined gonadal and pubic hair development, and girls self-determined breast and pubic hair development on a scale of 1 to 5;22 88% of the adolescents were in Tanner stages 4 and 5.
Family socioeconomic status (SES) was calculated using the Hollingshead Four-Factor Index of Social Status, a weighted average of parental education and occupations.
Diet was measured by a trained dietitian with 7 non-consecutive recalls that covering the full 24 hours of the previous day with all recalls completed within 12 weeks.24 Youth who provided at least four recalls were included in the analyses. We have previously shown that energy intake (EI) and VPA were positively correlated and were negative predictors of %BF. In addition, EI was a negative predictor of VAT.24
Mean telomere length was determined from leukocyte DNA by a modified quantitative polymerase chain reaction (PCR)-based assay.25–26 The relative ratio of telomere repeat copy number (T) to single copy gene copy number (36B4 gene, encoding ribosomal phosphoprotein, located on chromosome 12, S) was determined using an 7500 Fast Real-time PCR System (Applied Biosystems, Foster City, CA). Samples were done in triplicate. Threshold values (Ct) were obtained by averaging the triplicates. Each 96-well plate contained a 5-point standard curve using the same control genomic DNA from 3 to 48 ng. Telomere PCRs and 36B4 PCRs were performed on separate plates, with the same sample well position. T/S ratio was calculated as: the amount of telomeric DNA (T) divided by the amount of single-copy control gene DNA (S). The intra-plate and inter-plate coefficients of variation for the T/S ratio were 5.6% and 6.8% respectively.
Statistical analyses
Prior to analysis, the distribution of all variables was checked. If the data were not normally distributed, either a natural log- or square root-transformation was applied. Because there were 50 siblings out of the 667 subjects, Generalized Estimating Equations (GEE) were used to examine differences in general characteristic variables as related to race and sex. GEE is a multiple regression technique that allows for non-independence of twin or family data yielding unbiased standard errors and p-values. GEE was further used to evaluate associations of race, sex, adiposity, adipokines and physical activity with LTL in a hierarchical model. First, we did analyses in which a base model of age, race and sex as well as their 2-way and 3-way interactions were determined. Separate models were then built to test the effects of adiposity and physical activity indices and their interactions with age, race and sex. A value of p < 0.05 was considered statistically significant. The statistical analyses were performed with STATA 8 (StataCorp, College Station, Texas).
Results
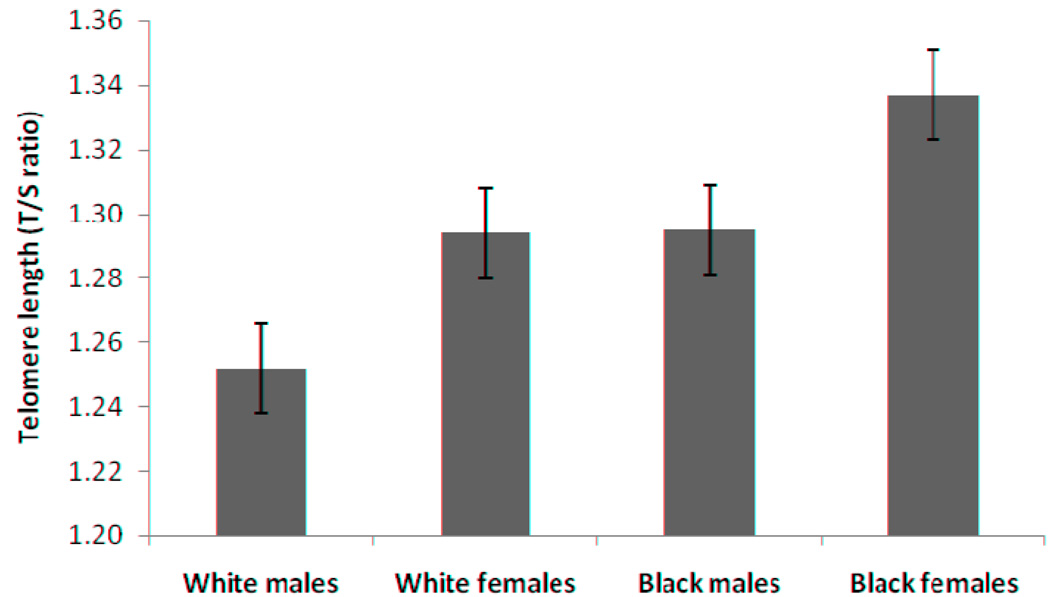
The participant characteristics are presented according to race and sex in Table I. Significant race and sex differences in LTL were identified (Table II), with black subjects having longer telomeres than white subjects (age and sex adjusted T/S ratio ± SE: 1.32 ± 0.01 vs. 1.27 ± 0.01, p=0.014) and girls having longer telomeres than boys (age and race adjusted T/S ratio ± SE: 1.31 ± 0.01 vs. 1.27 ± 0.01, p=0.007). The Figure shows age-adjusted LTL (mean±SE) by race and sex. The LTL (T/S ratio, mean ± SE) for white boys and girls are 1.25 ± 0.21, 1.30 ± 0.18 and for black boys and girls are 1.30 ± 0.24, 1.33 ± 0.20 respectively. The race and sex differences in LTL did not change after further adjustment of family SES, and pubertal status. Neither age nor two-way/three-way interactions among age, race and sex showed significant effects on LTL. Neither adiposity measures nor adipokines were associated with LTL. Moreover, no interactions between these variables and age, race or sex effects on LTL were identified.
Table 1.
General characteristics of study subjects
| White males | White females | Black males | Black females | P value |
||
|---|---|---|---|---|---|---|
| Ethnicity | Gender | |||||
| N | 169 | 179 | 155 | 164 | --- | --- |
| Age | 16.2±1.2 | 16.0±1.2 | 16.1±1.2 | 16.3±1.3 | NS | NS |
| BMI (kg/m2) | 22.2±4.3 | 21.8±4.1 | 23.4±5.1 | 24.7±6.1 | <0.001 | NS |
| Waist (cm) | 76.4±9.7 | 70.0±8.8 | 76.3±11.3 | 74.2±11.9 | 0.012 | <0.001 |
| % Body Fat | 18.6±8.0 | 29.3±7.0 | 17.3±9.4 | 29.7±8.5 | NS | <0.001 |
| VAT (cm3)* | 101.5±65.6 | 105.6±55.8 | 80.4±62.0 | 114.5±80.2 | NS | 0.001 |
| SAAT (cm3)* | 676.8±622.7 | 857.3±512.7 | 782.3±782.5 | 1323.0±970.0 | NS | <0.001 |
| Leptin (ng/mL)** |
5.5±8.1 | 14.8±12.4 | 7.2±10.0 | 20.7±14.8 | <0.001 | <0.001 |
| Adiponectin (ug/mL)*** |
7.6±3.8 | 10.8±5.7 | 7.0±5.0 | 7.8±4.3 | <0.001 | <0.001 |
| MPA (min/d)# |
46.4±25.8 | 30.4±16.7 | 47.8±27.6 | 31.7±21.5 | NS | <0.001 |
| VPA (min/d)# | 6.51±7.76 | 2.90±4.10 | 7.70±10.0 | 2.30±3.60 | NS | <0.001 |
| MVPA (min/d)# |
52.9±31.7 | 33.3±19.1 | 55.5±35.0 | 34.0±23.8 | NS | <0.001 |
| EI (kcal)## | 2316±572 | 1727±481 | 2101±579 | 1668±557 | 0.002 | <0.001 |
| Family SES### |
44.0±12.8 | 44.2±10.6 | 34.4±13.4 | 33.5±14.0 | <0.001 | NS |
Sample size for VAT and SAAT is 409;
Sample size for leptin is 617;
Sample size for adiponectin is 570;
Sample size for MPA, VPA and MVPA is 579;
Sample size for EI is 631;
Sample size for family SES is 618.
Table 2.
Results of the regression model fitting
| Models | Variables | Beta | SE | P |
|---|---|---|---|---|
| Base model | Age | −0.0122 | 0.0065 | 0.062 |
| Sex | 0.0438 | 0.0161 | 0.007 | |
| Race | 0.0405 | 0.0165 | 0.014 | |
| VPA model# | VPA | −0.0223 | 0.0197 | 0.257 |
| VPA×Age | 0.0007 | 0.0012 | 0.544 | |
| VPA×Sex | 0.0086 | 0.0035 | 0.013 | |
| VPA×Race | 0.00076 | 0.002559 | 0.765 |
Sample size for VPA is 579.
Figure.
Age adjusted telomere length by race and sex. Telomere length is plotted as T/S ratio. The values are mean ± SE. P value for race is 0.014; p value for sex is 0.007.
Physical activity was not associated with LTL in the entire cohort. However, an interaction between VPA and sex on LTL was identified (p=0.013, Table II). Stratification of the sample by sex showed that LTL was significantly and positively associated with the average minutes of VPA per day after adjustment for age and race in girls only (β=0.0079, SE=0.0030, p=0.009). Further adjustment for %BF, family SES, EI and pubertal status did not change the result. VPA accounted for 1.9% of the total variance in telomere length in girls.
Discussion
Black adolescents have longer telomeres than their white peers and girls have longer telomeres than boys. Further, VPA is a positive predictor of LTL in girls. Adiposity and adipokines were not related to LTL.
Few studies have investigated race or ethnic differences in LTL. Oduka et al 1 reported no difference in LTL in 168 white and black newborns. Hunt et al2 studied 2453 black and white adults from the Family Heart Study and the Bogalusa Heart Study. Black individuals had considerably longer telomeres and a steeper decline in LTL with age than their white counterparts. Roux et al4 assessed 981 white, black and Hispanic men and women aged 45–84 years participating in the Multi-Ethnic Study of Atherosclerosis. Blacks and Hispanics had shorter telomeres and showed greater differences in LTL associated with age than did the whites. Differences in age composition might have contributed to the differences between these two studies.4
It has been suggested that racial differences in telomere length may emerge and grow with age.4 It would be of great interest to know at what stage of life such differences occur. Our data show that blacks display longer telomeres than their white peers even as early as adolescence. The racial gap in telomere length is thought to be due to the factors that define leukocyte telomere dynamics during the formative years. Blacks display lower leukocyte and neutrophil counts than do whites,2, 27–28 therefore longer telomere length in blacks may arise from fewer replications of hematopoietic stem cells and progenitor cells. Blacks exhibit increased prevalence of risk factors for cardiovascular diseases. Possessing longer telomeres early in life could be a protective mechanism to compensate for higher rate of telomere shortening due to potentially deleterious conditions.2–3
No evidence for the effect of sex on LTL was shown at birth.1 However, several studies have shown that adult males generally have shorter telomeres than their female counterparts.5–6, 26, 29 In addition, women exhibit a significantly slower rate of age-dependent telomere attrition than men possibly due to the stimulating properties of estrogen on telomerase.7 A remarkable association between the levels of estrogen and telomerase activity has been shown under physiological conditions.30–31 Studies in vitro show that estrogen rapidly up-regulates the telomerase gene expression and activity.32–33 Here we provide the first evidence that the sex difference in LTL has already emerged during late pubertal adolescence.
Several large studies have shown that shorter telomeres are associated with obesity in adulthood. Valdes et al 13 reported that telomeres of white obese women were 240 base pairs shorter than those of lean women. In the Bogalusa Heart Study, an increase in BMI over ten years was associated with a decrease in telomere length.34 Nordfjall et al 29 found that associations between obesity variables and LTL exist only in women. The Sister Study showed that higher BMI and hip circumference were inversely associated with LTL.35 In addition, waist circumference, but not BMI, was inversely associated with LTL in the Nurses’ Health Study.36 The majority of associations identified so far have been in adult premenopausal women. By contrast, other studies of comparable size found no associations between BMI, adiposity measures and adipokines with LTL.7, 37–39
To the best of our knowledge, there have been only two studies conducted in pediatric populations. Zannolli et al14 studied 53 white children aged 3–15 years and found no difference in LTL between obese and normal weight children. On the other hand, Al-Attas et al15 examined 69 Arab boys and 79 Arab girls aged 5–12 years. LTL was significantly shorter in obese compared with lean boys. The contrasting results could be due to the different racial populations, small sample size and the use of indirect measures of adiposity. In the present study involving a substantial sample of white and black adolescents and more direct measures of adiposity, none of the adiposity measures or adipokines explained a significant proportion of the variance in LTL. It may take more years of exposure for the deleterious effects of obesity to be manifested in white and black populations than in Arab populations.
There is limited research exploring the relationship between physical activity and LTL, and the outcomes are inconsistent. Cherkas et al18 reported a significant, positive, dose-dependent relationship between self-reported physical activity and LTL in women from the UK Adult Twin Registry, providing the first evidence of a role of leisure time physical activity in modifying LTL. Ludlow et al19 showed an inverted ‘u’ relationship between reported physical activity and LTL such that a moderate level of activity was correlated with longer telomeres and light and heavy activity was correlated with shorter telomeres. Ponsot et al20 and LaRocca et al 21 reported that LTL can be maintained in elderly people engaged in regular MPA or VPA. On the other hand, the beneficial effect of exercise on LTL is much attenuated in elder Chinese men and women,40 and disappeared in 16 obese middle-aged women.41 The inconsistency may be due to different populations, different physical activity measurements used, as well as different duration and intensity of endurance training.
Our finding that girls who spent greater amounts of time engaging in VPA, as measured objectively with accelerometry, had longer telomeres parallels what Cherkas et al found in women. This relationship was independent of adiposity and social economic status. Moreover, we provide the first evidence that the beneficial effect of physical activity on LTL may begin in youth. These data suggest that to reap anti-aging benefits, adolescents especially girls should engage in more VPA. The reasons why the beneficial effects of vigorous activity on LTL were only seen in girls are not known. It is possible that estrogen has a direct role in the transcriptional up-regulation of human telomerase.32–33 Regular exercise increases bioavailability of endothelial nitric oxide and increases circulating endothelial progenitor cells.42 Both estrogen and nitric oxide are important mediators of signal transduction in a variety of tissues. Grasselli et al43 recently demonstrated the existence of a molecular circuitry of intracellular control of telomerase regulation, mediated by the association between estrogen receptor α (ERα) and the endothelial nitric oxide synthase (eNOS). eNOS acts as an essential cofactor of the ERα, and the eNOS/ERα complex activates human telomerase transcription. This model also assigns telomerase an important role in cardiovascular diseases. The greatest benefits in mortality risk occur with increasing physical activity and exercise capacity in the least fit and more sedentary44–45; thus even a small amount of VPA among typically inactive adolescent girls may have significant influence on their LTL.
The major strengths of the present study are: 1) our relatively large, healthy adolescent population including roughly equal numbers of white and black adolescents and boys and girls; 2) the narrow age range of our adolescent population, which minimizes the confounding effect of disease process and chronological age on telomere length; 3) the availability of sophisticated adiposity measures including % BF by DXA, VAT and SAAT by magnetic resonance imaging and two key adipokines, leptin and adiponectin; and 4) objective measurements of physical activity by accelerometer, which has been shown to be both valid and reliable for quantifying physical activity in a ‘real-life’ setting for children and young adolescents.46
They are several limitations in the present study. First, the cross-sectional nature of the study limits our findings to association, not causality. Longitudinal studies that evaluate the effects of race, sex, adiposity and physical activity on the rate of change in telomere length during adolescence are warranted. Second, VPA may have been underestimated by the accelerometer in boys because boys are more likely to play contact sports which would require them to remove their accelerometers; this would cause artificially deflated physical activities for boys. Third, our findings in white and black adolescents may not be generalizable to other populations such as Arab subjects.
Independent replication studies across different race and ethnic groups are warranted. Although physical activity has been shown to be associated with longer telomeres in adults, this is the first study to demonstrate that this association exists in female adolescents. Our findings underscore the importance of regular exercise to healthy aging for people of all ages including adolescents.
Acknowledgments
Supported in part by grants from National Heart, Lung, and Blood Institute (HL64157, HL85817 and HL77230).
Abbreviations
- BMI
Body mass index
- %BF
Percentage body fat
- DXA
Dual-energy X-ray absorptiometry
- EI
Energy intake
- eNOS
endothelial nitric oxide synthase
- ERα
estrogen receptor α
- GEE
Generalized Estimating Equations
- LTL
Leukocyte telomere length
- MPA
Moderate physical activity
- MVPA
Moderate + vigorous physical activity
- SAAT
Subcutaneous abdominal adipose tissue
- SES
Social economic status
- VAT
Visceral adipose tissue
- VPA
Vigorous physical activity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Okuda K, Bardeguez A, Gardner JP, Rodriguez P, Ganesh V, Kimura M, et al. Telomere length in the newborn. Pediatr Res. 2002 Sep;52:377–381. doi: 10.1203/00006450-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Hunt SC, Chen W, Gardner JP, Kimura M, Srinivasan SR, Eckfeldt JH, et al. Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell. 2008 Aug;7:451–458. doi: 10.1111/j.1474-9726.2008.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aviv A, Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, et al. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am J Epidemiol. 2009 Feb 1;169:323–329. doi: 10.1093/aje/kwn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roux AV, Ranjit N, Jenny NS, Shea S, Cushman M, Fitzpatrick A, et al. Race/ethnicity and telomere length in the Multi-Ethnic Study of Atherosclerosis. Aging Cell. 2009 Jun;8:251–257. doi: 10.1111/j.1474-9726.2009.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J, et al. Telomere length as an indicator of biological aging: The gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001 Feb;37:381–385. doi: 10.1161/01.hyp.37.2.381. [DOI] [PubMed] [Google Scholar]
- 6.Jeanclos E, Schork NJ, Kyvik KO, Kimura M, Skurnick JH, Aviv A. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension. 2000 Aug;36:195–200. doi: 10.1161/01.hyp.36.2.195. [DOI] [PubMed] [Google Scholar]
- 7.Bekaert S, De Meyer T, Rietzschel ER, De Buyzere ML, De Bacquer D, Langlois M, et al. Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell. 2007 Oct;6:639–647. doi: 10.1111/j.1474-9726.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 8.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994 Nov;55:876–882. [PMC free article] [PubMed] [Google Scholar]
- 9.Codd V, Mangino M, van der Harst P, Braund PS, Kaiser M, Beveridge AJ, et al. Common variants near TERC are associated with mean telomere length. Nat Genet. 2010 Mar;42:197–199. doi: 10.1038/ng.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy D, Neuhausen SL, Hunt SC, Kimura M, Hwang SJ, Chen W, et al. Genome-wide association identifies OBFC1 as a locus involved in human leukocyte telomere biology. Proc Natl Acad Sci U S A. 2010 May 18;107:9293–9298. doi: 10.1073/pnas.0911494107. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004 Dec 7;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006 Aug;5:325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 13.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005 Aug 20–26;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 14.Zannolli R, Mohn A, Buoni S, Pietrobelli A, Messina M, Chiarelli F, et al. Telomere length and obesity. Acta Paediatr. 2008 Jul;97:952–954. doi: 10.1111/j.1651-2227.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 15.Al-Attas O, Al-Daghri N, Bamakhramah A, Shaun Sabico S, McTernan P, Huang TT. Telomere length in relation to insulin resistance, inflammation and obesity among Arab youth. Acta Paediatr. 2010 Jun;99:896–899. doi: 10.1111/j.1651-2227.2010.01720.x. [DOI] [PubMed] [Google Scholar]
- 16.Barbeau P, Johnson MH, Howe CA, Allison J, Davis CL, Gutin B, et al. Ten months of exercise improves general and visceral adiposity, bone, and fitness in black girls. Obesity (Silver Spring) 2007 Aug;15:2077–2085. doi: 10.1038/oby.2007.247. [DOI] [PubMed] [Google Scholar]
- 17.Davis CLWJ, Boyle CA, Gutin B. Dose-response effect of aerobic exercise training on insulin resistance in overweight children. Obesity. 2007;15 Suppl.:A19. [Google Scholar]
- 18.Cherkas LF, Hunkin JL, Kato BS, Richards JB, Gardner JP, Surdulescu GL, et al. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med. 2008 Jan 28;168:154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- 19.Ludlow AT, Zimmerman JB, Witkowski S, Hearn JW, Hatfield BD, Roth SM. Relationship between physical activity level, telomere length, and telomerase activity. Med Sci Sports Exerc. 2008 Oct;40:1764–1771. doi: 10.1249/MSS.0b013e31817c92aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ponsot E, Lexell J, Kadi F. Skeletal muscle telomere length is not impaired in healthy physically active old women and men. Muscle Nerve. 2008 Apr;37:467–472. doi: 10.1002/mus.20964. [DOI] [PubMed] [Google Scholar]
- 21.Larocca TJ, Seals DR, Pierce GL. Leukocyte telomere length is preserved with aging in endurance exercise-trained adults and related to maximal aerobic capacity. Mech Ageing Dev. 2010 Feb;131:165–167. doi: 10.1016/j.mad.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutin B, Yin Z, Humphries MC, Barbeau P. Relations of moderate and vigorous physical activity to fitness and fatness in adolescents. Am J Clin Nutr. 2005 Apr;81:746–750. doi: 10.1093/ajcn/81.4.746. [DOI] [PubMed] [Google Scholar]
- 23.Gutin B, Johnson MH, Humphries MC, Hatfield-Laube JL, Kapuku GK, Allison JD, et al. Relationship of visceral adiposity to cardiovascular disease risk factors in black and white teens. Obesity (Silver Spring) 2007 Apr;15:1029–1035. doi: 10.1038/oby.2007.602. [DOI] [PubMed] [Google Scholar]
- 24.Stallmann-Jorgensen IS, Gutin B, Hatfield-Laube JL, Humphries MC, Johnson MH, Barbeau P. General and visceral adiposity in black and white adolescents and their relation with reported physical activity and diet. Int J Obes (Lond) 2007 Apr;31:622–629. doi: 10.1038/sj.ijo.0803587. [DOI] [PubMed] [Google Scholar]
- 25.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002 May 15;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGrath M, Wong JY, Michaud D, Hunter DJ, De Vivo I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2007 Apr;16:815–819. doi: 10.1158/1055-9965.EPI-06-0961. [DOI] [PubMed] [Google Scholar]
- 27.Phillips D, Rezvani K, Bain BJ. Exercise induced mobilisation of the marginated granulocyte pool in the investigation of ethnic neutropenia. J Clin Pathol. 2000 Jun;53:481–483. doi: 10.1136/jcp.53.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayr FB, Spiel AO, Leitner JM, Firbas C, Kliegel T, Jilma B. Ethnic differences in plasma levels of interleukin-8 (IL-8) and granulocyte colony stimulating factor (G-CSF) Transl Res. 2007 Jan;149:10–14. doi: 10.1016/j.trsl.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Nordfjall K, Eliasson M, Stegmayr B, Melander O, Nilsson P, Roos G. Telomere length is associated with obesity parameters but with a gender difference. Obesity (Silver Spring) 2008 Dec;16:2682–2689. doi: 10.1038/oby.2008.413. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka M, Kyo S, Takakura M, Kanaya T, Sagawa T, Yamashita K, et al. Expression of telomerase activity in human endometrium is localized to epithelial glandular cells and regulated in a menstrual phase-dependent manner correlated with cell proliferation. Am J Pathol. 1998 Dec;153:1985–1991. doi: 10.1016/S0002-9440(10)65712-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yokoyama Y, Takahashi Y, Morishita S, Hashimoto M, Niwa K, Tamaya T. Telomerase activity in the human endometrium throughout the menstrual cycle. Mol Hum Reprod. 1998 Feb;4:173–177. doi: 10.1093/molehr/4.2.173. [DOI] [PubMed] [Google Scholar]
- 32.Kyo S, Takakura M, Kanaya T, Zhuo W, Fujimoto K, Nishio Y, et al. Estrogen activates telomerase. Cancer Res. 1999 Dec 1;59:5917–5921. [PubMed] [Google Scholar]
- 33.Misiti S, Nanni S, Fontemaggi G, Cong YS, Wen J, Hirte HW, et al. Induction of hTERT expression and telomerase activity by estrogens in human ovary epithelium cells. Mol Cell Biol. 2000 Jun;20:3764–3771. doi: 10.1128/mcb.20.11.3764-3771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardner JP, Li S, Srinivasan SR, Chen W, Kimura M, Lu X, et al. Rise in insulin resistance is associated with escalated telomere attrition. Circulation. 2005 May 3;111:2171–2177. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
- 35.Kim S, Parks CG, DeRoo LA, Chen H, Taylor JA, Cawthon RM, et al. Obesity and weight gain in adulthood and telomere length. Cancer Epidemiol Biomarkers Prev. 2009 Mar;18:816–820. doi: 10.1158/1055-9965.EPI-08-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cassidy A, De Vivo I, Liu Y, Han J, Prescott J, Hunter DJ, et al. Associations between diet, lifestyle factors, and telomere length in women. Am J Clin Nutr. 2010 May;91:1273–1280. doi: 10.3945/ajcn.2009.28947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007 Jan 13;369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 38.Bischoff C, Petersen HC, Graakjaer J, Andersen-Ranberg K, Vaupel JW, Bohr VA, et al. No association between telomere length and survival among the elderly and oldest old. Epidemiology. 2006 Mar;17:190–194. doi: 10.1097/01.ede.0000199436.55248.10. [DOI] [PubMed] [Google Scholar]
- 39.Diaz VA, Mainous AG, Player MS, Everett CJ. Telomere length and adiposity in a racially diverse sample. Int J Obes (Lond) 2010 Feb;34:261–265. doi: 10.1038/ijo.2009.198. [DOI] [PubMed] [Google Scholar]
- 40.Woo J, Tang N, Leung J. No association between physical activity and telomere length in an elderly Chinese population 65 years and older. Arch Intern Med. 2008 Oct 27;168:2163–2164. doi: 10.1001/archinte.168.19.2163. [DOI] [PubMed] [Google Scholar]
- 41.Shin YA, Lee JH, Song W, Jun TW. Exercise training improves the antioxidant enzyme activity with no changes of telomere length. Mech Ageing Dev. 2008 May;129:254–260. doi: 10.1016/j.mad.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Laufs U, Werner N, Link A, Endres M, Wassmann S, Jurgens K, et al. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004 Jan 20;109:220–226. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 43.Grasselli A, Nanni S, Colussi C, Aiello A, Benvenuti V, Ragone G, et al. Estrogen receptor-alpha and endothelial nitric oxide synthase nuclear complex regulates transcription of human telomerase. Circ Res. 2008 Jul 3;103:34–42. doi: 10.1161/CIRCRESAHA.107.169037. [DOI] [PubMed] [Google Scholar]
- 44.Mandic S, Myers JN, Oliveira RB, Abella JP, Froelicher VF. Characterizing differences in mortality at the low end of the fitness spectrum. Med Sci Sports Exerc. 2009 Aug;41:1573–1579. doi: 10.1249/MSS.0b013e31819ca063. [DOI] [PubMed] [Google Scholar]
- 45.Leon AS, Connett J, Jacobs DR, Jr., Rauramaa R. Leisure-time physical activity levels and risk of coronary heart disease and death. The Multiple Risk Factor Intervention Trial. JAMA. 1987 Nov 6;258:2388–2395. [PubMed] [Google Scholar]
- 46.Trost SG, Ward DS, Moorehead SM, Watson PD, Riner W, Burke JR. Validity of the computer science and applications (CSA) activity monitor in children. Med Sci Sports Exerc. 1998 Apr;30:629–633. doi: 10.1097/00005768-199804000-00023. [DOI] [PubMed] [Google Scholar]