Abstract
Background
Recent evidence suggests that storage induced alterations of the red blood cell (RBC) are associated with adverse consequences in susceptible hosts. As RBCs have been shown to form Advanced Glycation Endproducts (AGEs) following increased oxidative stress and under pathologic conditions, we examined whether stored RBCs undergo modification with the specific AGE, N-(Carboxymethyl)lysine (Nε-CML) during standard blood banking conditions.
Study Design and Methods
Purified, fresh RBCs from volunteers were compared to stored RBCs (d 35–42 old) obtained from the Blood Bank. Nε-CML formation was quantified using a competitive enzyme-linked immunosorbent assay. The receptor for advanced glycation end-products (RAGE) was detected in human pulmonary microvascular endothelial cells by real-time PCR, western blotting, and flow cytometry. Intracellular reactive oxygen species (ROS) generation was measured by the use of 5-(and 6-)chloromethyl-2',7'-dichlorodihydrofluorescein diacetate, acetyl ester based assays.
Results
Stored RBCs showed increased surface Nε-CML formation when compared with fresh RBCs. Human Pulmonary Microvascular Endothelial Cells (HMVEC-L) showed detectable surface RAGE expression constitutively. When compared to fresh RBCs, stored RBCs triggered increased intracellular ROS generation in both Human Umbilical Vein Endothelial Cells (HUVEC) and Human Pulmonary Microvascular Endothelial Cells (HMVEC-L). RBC-induced endothelial ROS generation was attenuated in the presence of soluble RAGE (sRAGE) or RAGE blocking antibody.
Conclusion
The formation of the AGE Nε-CML on the surface of stored RBCs is one functional consequence of the storage lesion. AGE-RAGE interactions may be one mechanism by which transfused RBCs cause endothelial cell damage.
Keywords: AGE (advanced glycation endproducts), Nε-CML (N-Carboxymethyl-lysine), RAGE (receptor for advanced glycation endproducts)
Introduction
Oxidative damage is known to occur during red cell storage.1,2 In banked human red blood cells, there is a time-dependent decline in glutathione peroxidase activity that correlates with membrane protein and lipid oxidation.1,3,4 During the normal aging process and under pathologic conditions such as diabetes, erythrocytes form advanced glycation endproducts (AGEs), a heterogeneous group of chemically active compounds formed through nonezymatic reaction of aldose sugars with amino groups of proteins.5,6 AGEs are also formed through glucose-independent mechanisms. The myeloperoxidase system of phagocytes, phagocyte-derived oxidants, and lipid peroxidation increase the formation of the AGE, Nε-(carboxymethyl) lysine (Nε-CML).7–9 Given that oxidants increase AGE formation, it is plausible that AGE formation on the surface of erythrocytes occurs during RBC storage. 7,8
Diabetic erythrocytes interact with endothelial cells leading to increased oxidant stress, activation of NF-κB, and the mediators of this interaction are AGEs on the surface of the RBC that are capable of binding to the receptor for advanced glycation endproducts (RAGE) on endothelial cells. 10 Incubation of human umbilical vein endothelial cells (HUVEC) with diabetic RBCs increases endothelial cell hydrogen peroxide generation, whereas normal RBCs do not induce this effect.11 Furthermore, AGE signaling through RAGE induces endothelial cell ROS through both NADPH oxidase and the mitochondrial electron transport system.12
Thus, we hypothesized that erythrocytes undergo AGE modification during standard blood banking conditions. We further hypothesized that AGEs on stored erythrocytes engage RAGE on endothelial cells, increasing ROS generation.
Study Design and Methods
“Fresh” erythrocytes from healthy volunteers
Studies involving human subjects were approved by the University of Pennsylvania Institutional Review Board. Healthy volunteers between the ages of 18 and 65 years gave written informed consent. Volunteers were excluded for any of the following conditions: immunosuppressed state, diabetes mellitus, infectious processes, any serious comorbidity requiring hospitalization within the last year, pregnancy, anemia or a clotting disorder. Whole blood was obtained from healthy volunteers using citrate-phosphate-dextrose-adenine (Sigma-Aldrich, St. Louis MO) as an anticoagulant (9:1 ratio). Erythrocytes were purified from leukocytes and platelets as previously described using sephadex/microcellulose columns.13 Erythrocytes were combined with storage solution ( 150mM sodium chloride, 45 mM dextrose, 29 mM mannitol, 2 mM adenine, 3:1 ratio of erythrocytes to storage solution) and transferred to 100 ml plasticizer storage bags (Charter Medical Inc., Winston-Salem NC). The bags were heat sealed, stored at 4 °C, and these “fresh” RBCs were used within 24 hours.
Banked erythrocytes
Prestorage leukoreduced PRBC units (AS-1 or AS-3) were obtained from the Blood Bank at the Hospital of the University of Pennsylvania. When prestorage leukoreduced units were unavailable, leukoreduction was performed with Sepacell filters (Baxter Inc., Deerfield Il) or sephadex/microcellulose columns immediately prior to assay. For experiments where post-storage leukoreduction was performed, leukoreduction was performed within 24 hours of the assay and erythrocytes were washed three times in sterile PBS immediately prior to use. “Stored” RBCs refers to erythrocytes obtained from PRBC units stored between 35–42 d.
Detection of surface Nε- CML on erythrocytes
Erythrocytes (1×106), either freshly isolated or obtained from a 21d old PRBC unit, were washed 3x in sterile PBS and then resuspended in FACS buffer (2% FBS + PBS). Erythrocytes were labeled with anti-Nε-CML antibody (final concentration 10μg/ml, R&D Systems, Minneapolis MN) or IgG2b isotype control (final concentration 10μg/ml, Southern Biotech). Erythrocytes were subsequently washed 3x with FACs buffer prior to labeling with phycoerythrin (PE) conjugated secondary antibody (Sigma Aldrich, St Louis MO). Following 3 additional washes, FACS analysis was performed using a flow cytometer (FACsCaliber, Beckton Dickenson, Franklin Lakes NJ). Data analysis was performed using Flowjo software (v 7.2.5, Treestar Inc., Ashland OR).
Quantification of Nε- CML formation on erythrocytes using competitive ELISA
96-well plates (Nunc Maxisorp, Thermo-Fisher Scientific, Rochester NY) were coated with Nε-CML BSA [500 ng/mL] (MBL Int., Woburn MA) for 2 hours at 37°C. After blocking and washing (with 0.5% gelatin in PBS and 0.05% Tween-20 in PBS respectively), Nε-CML BSA standards or erythrocytes were added with mouse anti-Nε-CML antibody (75 ng/mL, R&D Systems, Minneapolis MN). Bound anti-Nε-CML antibody was detected using HRP conjugated donkey anti-mouse antibody (Jackson ImmunoResearch Laboratories, West Grove PA). After subsequent washes, substrate solution (BD Biosciences, San Jose CA) was added to the plate for 15 minutes. The reaction was stopped with stop solution (2N H2SO4). OD was measured at 450 nm.
Detection of RAGE transcript on endothelial cells
RNA was isolated from Pulmonary Microvascular Endothelial Cells (HMVEC-L) and Human Embryonic Kidney Cells (293) using a commercially available kit (RNeasy Minikit, Quiagen, Valencia, CA). Three micrograms of RNA from either cell type was reverse transcribed using 0.5 μg oligo(dT) (Promega, Madison, WI), 10 mmol/L deoxynucleotide triphosphates (Clontech, Palo Alto, CA), and 1 unit SuperScript III reverse transcriptase in 1x First-Strand Buffer and 10 mmol/L DTT (Clontech) for 60 minutes at 50°C. Equal amounts of cDNA from each cell type were isolated. Forward primer 5’-AGCCACTGGTGCTGAAGTGT-3’ and reverse primer 5’-GAATCTGGTAGACACGGACTC-3’ were designed to detect a 267 bp PCR product that identified human RAGE. Semi-quantitative analysis of gene expression was done using a Cepheid Smart Cycler (Sunnyvale, CA) following the manufacturer's protocol (Light Cycler RNA Amplification Kit SYBR Green I, Roche Applied Sciences, Indianapolis IN). cDNA concentrations from each pool were normalized using GAPDH as a control gene. RAGE expression in HMVEC-L and 293 cells (normalized to GAPDH) were determined. Each sample was run in triplicate.
Detection of RAGE protein on endothelial cells by immunoblotting
HMVEC-L or 293 cell lysates were prepared using cell lysis buffer (50 mmol/L Tris-HCl (pH 8), 150 mmol/L NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS and protease inhibitor cocktail (Mini Complete, Roche Applied Science, Indianopolis, IN). Cell lysate protein concentrations were assayed using a commercially available kit (Pierce BCA Protein assay, Thermo-Fisher Scientific, Rochester NY). 20 μg of protein was added to each lane of a 4% to 12% SDS-polyacrylamide gel (NuPage, Invitrogen, Carlsbad CA). The samples were then resolved with SDS page under reducing conditions and immunoblotting was performed as described above using mouse anti-human monoclonal (IgG2a) α-RAGE antibody (Abcam, Cambridge MA) and polyclonal horseradish peroxidase – conjugated secondary donkey anti-mouse antibody ( Jackson ImmunoResearch Laboratories, West Grove PA).
Detection of surface RAGE on endothelial Cells by flow cytometry
HMVEC-L at 1 × 106 cells were suspended in FACS buffer (2% FBS + PBS). Cells were labeled with mouse anti-human monoclonal (IgG2a) α-RAGE antibody (final concentration 10 μg/mL, Abcam, Cambridge MA) or mouse anti-human monoclonal (IgG1) α-PECAM antibody (final concentration 10 μg/mL) which was kindly provided by Dr. Peter Newman (the Blood Center of Wisconsin). The appropriate isotype controls were used (final concentration 10 μg/ml, Southern Biotech, Birmingham AL). The endothelial cells were then washed 3x with FACs buffer and labeled with PE conjugated secondary antibody (Sigma Aldrich, St Louis MO). FACS analysis and data analysis were performed as described above.
Measurement of endothelial cell intracellular ROS production: fluorimeter based assay
Endothelial cell intracellular reactive oxygen species (ROS) production was measured using 5-(and 6-)chloromethyl-2',7'-dichlorodihydrofluorescein diacetate, acetyl ester (DCF, Invitrogen, Carlsbad CA). Human Umblical Vein Endothelial Cells (HUVEC) or Human Pulmonary Microvascular Endothelial Cells (HMVEC-L) were grown to confluence on 96 well plates. The endothelial cells were incubated with 1x108 stored or freshly isolated erythrocytes × 4 hours at 37°C. Endothelial cells were subsequently washed five times using HBSS and subsequently loaded with DCF (10μM). The cells were washed once and then incubated in HBSS for 25 minutes at 37°C. Fluorescence intensity (excitation 495 nm, emission 530 nm) was measured with a spectroflourimeter (SPECTRAmax GEMINI XS, Molecular Devices, Sunnyvale CA). All samples were performed in triplicate and repeated at least 2 times. Results are reported as the mean ± standard error of the mean and data are presented as fold increase over baseline (EC loaded with DCF). Two-tailed Student’s t test was used to determine significance. P values less than or equal to 0.05 were considered to be significant.
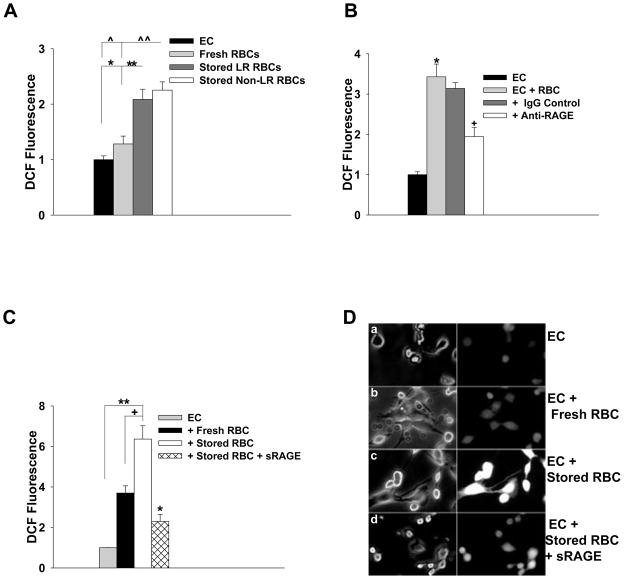
For the fluorimeter based assays using HUVEC (Figure 3A), stored LR RBCs represent RBCs obtained from a non-leukoreduced PRBC unit that was leukoreduced through a sephadex/microcellulose column and washed immediately prior to the assay. Stored Non-LR RBCs represent RBCs obtained from the same PRBC unit that were washed immediately prior to the assay. For experiments involving the presence of RAGE blocking antibody, immortalized HUVEC were preincubated with anti-human RAGE antibody (100 μg/mL R&D Systems, Minneapolis MN), or goat IgG control (100 μg/mL), for 2 hours at 37 °C. In order to accelerate the storage lesion the erythrocytes employed for these studies were stored plasma free and without additive solution for >48 hours (Figure 3B). Measurement of ROS generation with DCF was performed as described above.
Figure 3. Endothelial Cell ROS Generation Following Incubation with Stored Erythrocytes is attenuated by RAGE blockade.
(A) HUVEC were incubated with fresh or stored erythrocytes and ROS generation was measured as described in the methods. Incubation with fresh erythrocytes did not increase EC ROS generation (p=0.143), whereas incubation with stored erythrocytes increased EC ROS generation (*p=0.005 for LR, ^ p=0.002 for non-LR erythrocytes). When compared with fresh erythrocytes, both the LR and non-LR erythrocytes increased EC ROS generation (**p=0.026 for LR, ^^p =0.009 for non-LR erythrocytes). (B) Immortalized Human Umbilical Vein Endothelial Cells (IVECs) were incubated with erythrocytes, with or without RAGE blocking antibody or IgG control (100 ug/mL) as described in the methods. Endothelial cell ROS generation was increased over baseline following incubation with erythrocytes (*p=0.001). This effect was attenuated with RAGE blocking antibody (+p=0.004) but not in the presence of IgG control antibody (p=0.428). (C and D) Measurement of DCF fluorescence generated by HMVEC-L following incubation with fresh erythrocytes, stored erythrocytes or stored erythrocytes in the presence of soluble RAGE (sRAGE, 75 μg/mL). (C) Fresh erythrocytes did not increase ROS generation over baseline (p=0.100). Stimulation of endothelial cells with stored erythrocytes increased ROS generation when compared with ECs alone (**p=0.001) and ECs stimulated with fresh erythrocytes (+p=0.024). In the presence of sRAGE, EC ROS generation following stimulation with stored erythrocytes was significantly attenuated (*p=0.002). (D) Phase (left panel) and fluorescence (right panel) images of (subpanel a) EC with or without RBC + sRAGE as indicated in figure.
Measurement of endothelial cell intracellular ROS production: direct visualization
HMVEC-L were seeded in 60 x10mm plates and grown to confluence. The endothelial cells were subsequently washed once with HBSS and then incubated with 5x108 stored erythrocytes obtained from PRBC units that were leukoreduced on the day of the assay with a Sepacell filter (Baxter Inc, Deerfield, IL) or fresh erythrocytes for 4 hours at 37 °C. For select studies erythrocytes were added to the endothelial cells in the presence of sRAGE (75 μg/mL). The endothelial cells were subsequently washed 3-4 times using HBSS and loaded with DCF (10 μM) for 20 min at 37 °C. Images were acquired with an epifluorescence microscope (Nikon Diaphot TMD, Melville, NY). For each condition, the data was quantified over 3-4 fields using Metamorph Software (Molecular Devices, Downington PA). Imaging studies were repeated 2x.
Results
Stored Human RBC express the RAGE ligand, Nε-Carboxymethylysine (Nε-CML)
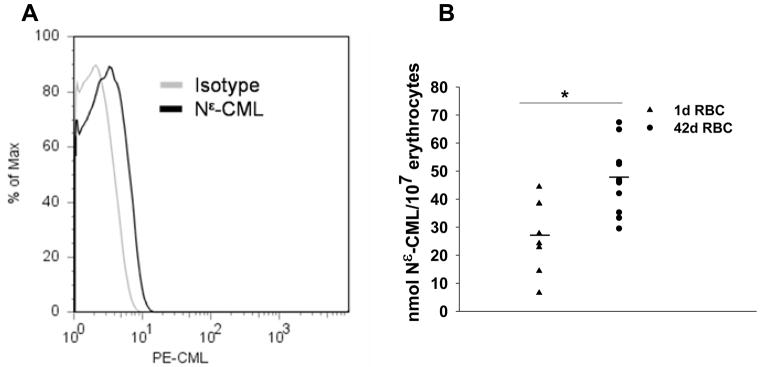
Because the AGE Nε-(Carboxymethyl)lysine (Nε-CML) has been implicated in RAGE-mediated endothelial dysfunction in vivo and vitro, we determined whether Nε-CML was detectable on the surface of stored erythrocytes. 14 Surface Nε-CML was detectable on stored red blood cells by flow cytometry using a monoclonal antibody that was used to develop a competitive ELISA to quantify Nε-CML formation on intact red cells (Figure 1A). This technique was reproducible, as 1 d RBCs from 3 individual donors or stored PRBC units assayed on 3 different days produced consistent results for each sample assayed (data not shown). Thus, similar to the findings of others, the competitive ELISA is a reliable and reproducible technique for quantifying Nε-CML content.15–17 The competitive ELISA appeared more sensitive in discriminating differences in surface Nε-CML content than flow cytometry, as we were able to show individual heterogeneity in concentrations of surface Nε-CML between fresh and stored erythrocytes with this method (Figure 1B) but not by flow cytometric detection (data not shown). By competitive ELISA, stored RBCs showed higher amounts of Nε-CML formation (Figure 1B, p=0.003).
Figure 1. Nε-CML expression on stored RBC.
(A) Nε-CML is detectable on erythrocytes from a 21 d stored PRBC unit. 3 independent studies were performed, data is representative of 1 experiment. (B) Nε-CML on fresh erythrocytes obtained from healthy donors (triangles) or on erythrocytes obtained from 42d old PRBCs units (circles). Erythrocytes from stored PRBC units showed increased Nε-CML content when compared with fresh erythrocytes (p=0.003). Two-tailed Student’s t test was used to determine significance using SigmaPlot 10 software (Systat Software Inc., Chicago, IL, USA).
Human Pulmonary Microvascular Endothelial Cells express the counter-receptor RAGE
Under basal conditions, HMVEC-L expressed RAGE transcripts when compared to human embryonic kidney cells (293 cells) which do not express RAGE (Figure 2A).23 As shown in Figure 3B, endothelial cells express a band of the appropriate size (55kD), compared with minimal expression by 293 cells. Although RAGE was not as highly expressed as the endothelial marker PECAM-1, HMVEC-L showed detectable surface RAGE expression (Figure 2C). Thus, pulmonary microvascular endothelial cells constitutively express the multiligand receptor RAGE.
Figure 2. RAGE Expression on Human Pulmonary Microvascular Endothelial Cells.
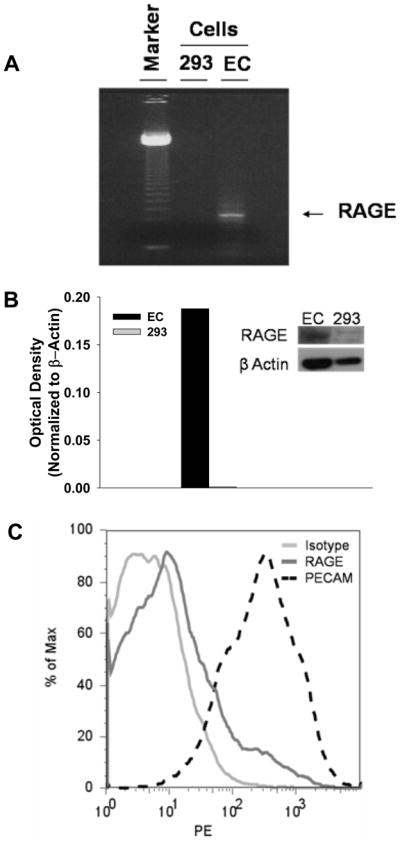
(A) RNA gel of RAGE mRNA in Human Pulmonary Microvascular Endothelial Cells (HMVEC-L) or Human Embryonic Kidney Cells (293) demonstrates increased RAGE transcripts in HMVEC-L (B) Western blot of extracts from HMVEC-L or 293 cells demonstrates increased RAGE expression in HMVEC-L. Densitometry is normalized to ß Actin. (C) FACS analysis demonstrates surface expression of RAGE on HMVEC-L, PECAM expression serves as a positive control. FACS analysis was repeated 3 times.
Stored Erythrocytes Increase Endothelial Cell ROS Generation that is attenuated by either RAGE blockade or soluble RAGE
We initially compared the effects of leukoreduction on red cell-induced endothelial ROS generation. Figure 3A shows that stored RBCs can trigger increased ROS generation in HUVEC compared to basal conditions (*p=0.005 for LR, ^ p=0.002 for non-LR erythrocytes). In contrast, incubation of HUVEC with fresh RBCs did not increase ROS generation over baseline (p=0.143). Stored red cells from the same PRBC unit bag, either leukoreduced or non-leukoreduced, were compared to one another. Both leukoreduced and nonleukoreduced stored RBCs increased endothelial ROS when compared with fresh RBCs (Fig. 3A. **p=0.026 for LR, ^^p =0.009 for non-LR erythrocytes), indicating that leukocyte removal from stored RBCs had no effect on endothelial cell ROS production. To determine whether red cell-induced endothelial ROS generation occurs through RAGE ligation, endothelial cells were incubated with erythrocytes that underwent accelerated storage in the presence or absence of RAGE blocking antibody. Endothelial ROS was significantly attenuated following incubation with RBCs in the presence of RAGE blocking antibody (Fig. 3B; +p=0.004), suggesting that stored RBCs induce endothelial cell ROS through RAGE ligation.
As mediators in stored PRBC have been implicated in transfusion related lung injury and ROS in the pathogenesis of acute lung injury, we examined endothelial ROS generation in a more relevant cell line, HMVEC-L.24,25 HMVEC-L showed minimal baseline fluoresence (Fig. 3D-a). Addition of fresh erythrocytes did not increase endothelial cell ROS (p=0.10, Figure 3C and 3D-b). Stimulation with stored erythrocytes from standard PRBC units led to a 6-fold and 2-fold increase in ROS generation when compared with unstimulated ECs (p=0.001, Figure 3C and 3D-c) and ECs stimulated with fresh erythrocytes (p=0.024, Figure 3C and 3D-c), respectively. Consistent with the findings using RAGE blocking antibody in HUVEC, ROS generation in HMVEC-L by RBCs was attenuated in the presence of sRAGE (*p = 0.002, Figure 3C and 3D-d). Thus, direct blockade of RAGE on endothelial cells or scavenging of RAGE ligands by sRAGE produced similar effects. Although AGE can ligate receptors such as CD36 or other scavenging receptors, we are unaware of published work that show that ligation of these receptors can trigger endothelial ROS generation. Collectively, our data show that AGE formation on stored RBCs can induce endothelial ROS generation through a RAGE-dependent mechanism.
Discussion
In this study, we show that erythrocytes have varying amounts of Nε-CML-modified proteins on their surface at baseline and that Nε-CML modifications increase during storage. Furthermore, we demonstrate that stored RBCs induce ROS generation in pulmonary endothelial cells through ligation of RAGE. These findings indicate that the formation of biologically active ligands on the erythrocyte capable of directly engaging cellular receptors, such as RAGE, may be one functional consequence of the erythrocyte storage lesion. We propose that transfusion of stored erythrocytes may sustain inflammation in susceptible hosts through activation of effector cells.
Nε-CML is one of the most abundant AGE structures detected in human subjects and is a well characterized mediator of EC activation.14,18,26 One previous study has reported increased AGE formation on PRBCs over time. However, this study examined total protein lysates from PRBC units.18 Our findings expand upon those in the previous study as we demonstrate a specific AGE, Nε-CML, present on the surface of intact erythrocytes, where it would need to be located to engage cellular receptors.
One limitation of our study is that Nε-CML levels in “fresh” and “stored” erythrocytes from the same donation were not studied. This may be relevant as we observed significant variation in baseline Nε-CML levels. Future studies will require serial determination of erythrocyte Nε-CML formation during storage, as our observation of substantial donor heterogeneity suggests that donor factors (i.e. blood glucose levels, age, smoking status), in addition to storage conditions, may contribute to alterations in erythrocyte Nε-CML levels. Relevant to this observation is the fact that Nε-CML is detectable in the serum of healthy individuals and is elevated with certain disease states. 16,27,28
Another question of future interest is which molecules on the surface of the RBC are modified by Nε-CML. Consistent with previous reports of Nε-CML modification of Band 3, we were able to detect Nε-CML on Band 3 from stored erythrocyte lysates.29,30 However, in preliminary studies, we did not demonstrate consistent differences in the amount of Nε-CML modification of Band 3 between fresh and stored erythrocytes (data not shown). One plausible explanation is that differences in Nε-CML seen with storage are not due to modification of solely Band 3, but are due to modification of other erythrocyte membrane proteins and/or lipids. We also speculate that Nε-CML modified Band 3 may be preferentially distributed to the microparticle fraction during storage- as storage dependent reductions in erythrocyte Band 3, and increases in Band 3 and glycated proteins such as hemoglobin have been identified in microparticles.31-33 Another explanation for our inability to detect differences in Nε-CML modified Band 3 with storage is that packed red blood cell units and fresh RBCs both contain an equal distribution of young and “senescent” erythrocytes. As “senescent” erythrocytes have increased Nε-CML modification of Band 3 compared to young erythrocytes, examination of Band 3 Nε-CML levels in fresh and stored erythrocytes while adjusting for erythrocyte age would be required.29 Thus, studies examining storage-mediated Nε-CML modification, with respect to erythrocyte age in addition to storage time are currently an area of investigation. Future studies, using proteomic approaches may be the most useful in answering which erythrocyte membrane proteins appear to be the most important with regard to Nε-CML modification.
RAGE is a relatively promiscuous receptor as it binds to a wide range of advanced glycation products in addition to Nε-CML, and other ligands such as high mobility group box (HMGB)-1, S100/calgranulins, and amyloid β fibrils.34–37 The exact identities of all RAGE ligands on the surface of RBCs are not known. Although RAGE is known to be present on other types of endothelial cells such as HUVEC, there is some controversy regarding the expression of RAGE on lung capillary endothelium as some studies have failed to demonstrate RAGE on the capillary endothelium while others have demonstrated its presence both in vitro and in vivo.19–22 This controversy may be due to technical issues, as we have observed that RAGE is cleaved by proteases and commonly used tissue culture agents, such as trypsin. Thus, some studies using immunohistochemistry may have had RAGE cleaved during tissue processing, as it has previously been shown that RAGE is cleaved by metalloproteinases.38 For these reasons, we sought to verify RAGE expression in our endothelial cells using several techniques. Our findings show human pulmonary microvascular endothelial cells express both RAGE transcript and protein constitutively. As high mobility group box 1 (HMGB1) and other RAGE ligands have been shown to upregulate expression of RAGE, our findings of RAGE expression in lung endothelium may have implications for transfusion related lung injury during sepsis and hemorrhagic shock as elevated levels of HMGB1 have been demonstrated during these states.39–44
Previous studies have shown that endothelial cells exposed to Nε-CML-modified ovalbumin showed both an increase in VCAM expression and NF-κB activation.14 Furthermore, infusion of Nε-CML -BSA increased VCAM expression in the lungs of mice.14 These effects were attenuated by RAGE blockade suggesting that Nε-CML -RAGE interactions can sustain inflammatory responses in the lung. Furthermore, transfusion of AGE-RBCs may have detrimental consequences in the microcirculation of susceptible hosts as AGEs have been shown to inhibit eNOS activity in HUVEC and dysregulation of NO bioavailability and activity have recently been implicated in the RBC storage lesion.45–47 Thus, it is plausible that transfusion of AGE or Nε-CML modified erythrocytes, found in stored blood, may perpetuate inflammatory responses and augment lung injury in susceptible hosts.
In summary, we provide evidence that the RAGE ligand, Nε-CML, is present on stored RBCs. We provide further evidence that stored RBCs can induce ROS in pulmonary endothelial cells through interaction with RAGE. Future studies determining the function of endothelial cell RAGE in the pathogenesis or perpetuation of lung injury following RBC transfusion are required.
Acknowledgments
The authors would like to thank Shirley Arrington, MT (ASCP) (Blood Bank, Hospital of the University of Pennsylvania) for providing the PRBC units and Dr. Jing Sun for her technical assistance.
Funding Support: NIH grants HL091644 and HL098362 (NSM), NIH 1-P01-HL079063-05 (SMA), and HL086884 (JSL).
Footnotes
Conflict of interest disclosure: All authors have no conflict of interest to declare.
References
- 1.Dumaswala UJ, Wilson MJ, Wu YL, Wykle J, Zhuo L, Douglass LM, Daleke DL. Glutathione loading prevents free radical injury in red blood cells after storage. Free Radic Res. 2000;33(5):517–29. doi: 10.1080/10715760000301061. [DOI] [PubMed] [Google Scholar]
- 2.Tinmouth A, Fergusson D, Yee IC, Hebert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46(11):2014–27. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 3.Dumaswala UJ, Zhuo L, Jacobsen DW, Jain SK, Sukalski KA. Protein and lipid oxidation of banked human erythrocytes: role of glutathione. Free Radic Biol Med. 1999;27(9–10):1041–9. doi: 10.1016/s0891-5849(99)00149-5. [DOI] [PubMed] [Google Scholar]
- 4.Dumaswala UJ, Zhuo L, Mahajan S, Nair PN, Shertzer HG, Dibello P, Jacobsen DW. Glutathione protects chemokine-scavenging and antioxidative defense functions in human RBCs. Am J Physiol Cell Physiol. 2001;280(4):C867–73. doi: 10.1152/ajpcell.2001.280.4.C867. [DOI] [PubMed] [Google Scholar]
- 5.Miller JA, Gravallese E, Bunn HF. Nonenzymatic glycosylation of erythrocyte membrane proteins. Relevance to diabetes. J Clin Invest. 1980;65(4):896–901. doi: 10.1172/JCI109743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vlassara H, Valinsky J, Brownlee M, Cerami C, Nishimoto S, Cerami A. Advanced glycosylation endproducts on erythrocyte cell surface induce receptor-mediated phagocytosis by macrophages. A model for turnover of aging cells. J Exp Med. 1987;166(2):539–49. doi: 10.1084/jem.166.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson MM, Heinecke JW. Production of N(epsilon)-(carboxymethyl)lysine is impaired in mice deficient in NADPH oxidase: a role for phagocyte-derived oxidants in the formation of advanced glycation end products during inflammation. Diabetes. 2003;52(8):2137–43. doi: 10.2337/diabetes.52.8.2137. [DOI] [PubMed] [Google Scholar]
- 8.Anderson MM, Requena JR, Crowley JR, Thorpe SR, Heinecke JW. The myeloperoxidase system of human phagocytes generates Nepsilon-(carboxymethyl)lysine on proteins: a mechanism for producing advanced glycation end products at sites of inflammation. J Clin Invest. 1999;104(1):103–13. doi: 10.1172/JCI3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu M-X, Requena JR, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR. The Advanced Glycation End Product, N[IMAGE]-(Carboxymethyl)lysine, Is a Product of both Lipid Peroxidation and Glycoxidation Reactions. J Biol Chem. 1996;271(17):9982–6. doi: 10.1074/jbc.271.17.9982. [DOI] [PubMed] [Google Scholar]
- 10.Wautier JL, Wautier MP, Schmidt AM, Anderson GM, Hori O, Zoukourian C, Capron L, Chappey O, Yan SD, Brett J, et al. Advanced glycation end products (AGEs) on the surface of diabetic erythrocytes bind to the vessel wall via a specific receptor inducing oxidant stress in the vasculature: a link between surface-associated AGEs and diabetic complications. Proc Natl Acad Sci U S A. 1994;91(16):7742–6. doi: 10.1073/pnas.91.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280(5):E685–94. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- 12.Basta G, Lazzerini G, Del Turco S, Ratto GM, Schmidt AM, De Caterina R. At least 2 distinct pathways generating reactive oxygen species mediate vascular cell adhesion molecule-1 induction by advanced glycation end products. Arterioscler Thromb Vasc Biol. 2005;25(7):1401–7. doi: 10.1161/01.ATV.0000167522.48370.5e. [DOI] [PubMed] [Google Scholar]
- 13.Mangalmurti NS, Xiong Z, Hulver M, Ranganathan M, Liu XH, Oriss T, Fitzpatrick M, Rubin M, Triulzi D, Choi A, Lee JS. Loss of red cell chemokine scavenging promotes transfusion-related lung inflammation. Blood. 2009;113(5):1158–66. doi: 10.1182/blood-2008-07-166264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Du Yan S, Hofmann M, Yan SF, Pischetsrieder M, Stern D, Schmidt AM. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem. 1999;274(44):31740–9. doi: 10.1074/jbc.274.44.31740. [DOI] [PubMed] [Google Scholar]
- 15.Koito W, Araki T, Horiuchi S, Nagai R. Conventional antibody against Nepsilon-(carboxymethyl)lysine (CML) shows cross-reaction to Nepsilon-(carboxyethyl)lysine (CEL): immunochemical quantification of CML with a specific antibody. J Biochem. 2004;136(6):831–7. doi: 10.1093/jb/mvh193. [DOI] [PubMed] [Google Scholar]
- 16.Semba RD, Bandinelli S, Sun K, Guralnik JM, Ferrucci L. Plasma carboxymethyl-lysine, an advanced glycation end product, and all-cause and cardiovascular disease mortality in older community-dwelling adults. J Am Geriatr Soc. 2009;57(10):1874–80. doi: 10.1111/j.1532-5415.2009.02438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Frischmann M, Kientsch-Engel R, Steinmann K, Stopper H, Niwa T, Pischetsrieder M. Two immunochemical assays to measure advanced glycation end-products in serum from dialysis patients. Clin Chem Lab Med. 2005;43(5):503–11. doi: 10.1515/CCLM.2005.089. [DOI] [PubMed] [Google Scholar]
- 18.Lysenko L, Mierzchala M, Gamian A, Durek G, Kubler A, Kozlowski R, Sliwinski M. The effect of packed red blood cell storage on arachidonic acid and advanced glycation end-product formation. Arch Immunol Ther Exp (Warsz) 2006;54(5):357–62. doi: 10.1007/s00005-006-0042-y. [DOI] [PubMed] [Google Scholar]
- 19.Durr E, Yu J, Krasinska KM, Carver LA, Yates JR, Testa JE, Oh P, Schnitzer JE. Direct proteomic mapping of the lung microvascular endothelial cell surface in vivo and in cell culture. Nat Biotechnol. 2004;22(8):985–92. doi: 10.1038/nbt993. [DOI] [PubMed] [Google Scholar]
- 20.Fehrenbach H, Kasper M, Tschernig T, Shearman MS, Schuh D, Muller M. Receptor for advanced glycation endproducts (RAGE) exhibits highly differential cellular and subcellular localisation in rat and human lung. Cell Mol Biol (Noisy-le-grand) 1998;44(7):1147–57. [PubMed] [Google Scholar]
- 21.Morbini P, Villa C, Campo I, Zorzetto M, Inghilleri S, Luisetti M. The receptor for advanced glycation end products and its ligands: a new inflammatory pathway in lung disease? Mod Pathol. 2006;19(11):1437–45. doi: 10.1038/modpathol.3800661. [DOI] [PubMed] [Google Scholar]
- 22.van Zoelen MA, Schouten M, de Vos AF, Florquin S, Meijers JC, Nawroth PP, Bierhaus A, van der Poll T. The receptor for advanced glycation end products impairs host defense in pneumococcal pneumonia. J Immunol. 2009;182(7):4349–56. doi: 10.4049/jimmunol.0801199. [DOI] [PubMed] [Google Scholar]
- 23.Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, Elliston K, Stern D, Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267(21):14998–5004. [PubMed] [Google Scholar]
- 24.Chabot F, Mitchell JA, Gutteridge JM, Evans TW. Reactive oxygen species in acute lung injury. Eur Respir J. 1998;11(3):745–57. [PubMed] [Google Scholar]
- 25.Silliman CC, Voelkel NF, Allard JD, Elzi DJ, Tuder RM, Johnson JL, Ambruso DR. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J Clin Invest. 1998;101(7):1458–67. doi: 10.1172/JCI1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jono T, Nagai R, Lin X, Ahmed N, Thornalley PJ, Takeya M, Horiuchi S. Nepsilon-(Carboxymethyl)lysine and 3-DG-imidazolone are major AGE structures in protein modification by 3-deoxyglucosone. J Biochem (Tokyo) 2004;136(3):351–8. doi: 10.1093/jb/mvh124. [DOI] [PubMed] [Google Scholar]
- 27.Semba RD, Fink JC, Sun K, Bandinelli S, Guralnik JM, Ferrucci L. Carboxymethyl-lysine, an advanced glycation end product, and decline of renal function in older community-dwelling adults. Eur J Nutr. 2009;48(1):38–44. doi: 10.1007/s00394-008-0757-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semba RD, Fink JC, Sun K, Windham BG, Ferrucci L. Serum Carboxymethyl-Lysine, a Dominant Advanced Glycation End Product, Is Associated With Chronic Kidney Disease: The Baltimore Longitudinal Study of Aging. J Ren Nutr. 2009 doi: 10.1053/j.jrn.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ando K, Beppu M, Kikugawa K, Nagai R, Horiuchi S. Membrane proteins of human erythrocytes are modified by advanced glycation end products during aging in the circulation. Biochem Biophys Res Commun. 1999;258(1):123–7. doi: 10.1006/bbrc.1999.0606. [DOI] [PubMed] [Google Scholar]
- 30.Grossin N, Wautier MP, Picot J, Stern DM, Wautier JL. Differential effect of plasma or erythrocyte AGE-ligands of RAGE on expression of transcripts for receptor isoforms. Diabetes Metab. 2009;35(5):410–7. doi: 10.1016/j.diabet.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Bosman GJ, Lasonder E, Groenen-Dopp YA, Willekens FL, Werre JM, Novotny VM. Comparative proteomics of erythrocyte aging in vivo and in vitro. J Proteomics. 2009 doi: 10.1016/j.jprot.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Bosman GJ, Lasonder E, Luten M, Roerdinkholder-Stoelwinder B, Novotny VM, Bos H, De Grip WJ. The proteome of red cell membranes and vesicles during storage in blood bank conditions. Transfusion. 2008;48(5):827–35. doi: 10.1111/j.1537-2995.2007.01630.x. [DOI] [PubMed] [Google Scholar]
- 33.Bosman GJ, Werre JM, Willekens FL, Novotny VM. Erythrocyte ageing in vivo and in vitro: structural aspects and implications for transfusion. Transfus Med. 2008;18(6):335–47. doi: 10.1111/j.1365-3148.2008.00892.x. [DOI] [PubMed] [Google Scholar]
- 34.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97(7):889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 35.Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995;270(43):25752–61. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108(7):949–55. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382(6593):685–91. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 38.Raucci A, Cugusi S, Antonelli A, Barabino SM, Monti L, Bierhaus A, Reiss K, Saftig P, Bianchi ME. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10) Faseb J. 2008;22(10):3716–27. doi: 10.1096/fj.08-109033. [DOI] [PubMed] [Google Scholar]
- 39.Bopp C, Bierhaus A, Hofer S, Bouchon A, Nawroth PP, Martin E, Weigand MA. Bench-to-bedside review: The inflammation-perpetuating pattern-recognition receptor RAGE as a therapeutic target in sepsis. Crit Care. 2008;12(1):201. doi: 10.1186/cc6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, Yang H, Czura CJ, Sama AE, Tracey KJ. HMGB1 as a late mediator of lethal systemic inflammation. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1768–73. doi: 10.1164/ajrccm.164.10.2106117. [DOI] [PubMed] [Google Scholar]
- 41.Yang H, Tracey KJ. High mobility group box 1 (HMGB1) Crit Care Med. 2005;33(12 Suppl):S472–4. doi: 10.1097/01.ccm.0000187005.81616.a9. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Xiang M, Yuan Y, Xiao G, Zhang J, Jiang Y, Vodovotz Y, Billiar TR, Wilson MA, Fan J. Hemorrhagic shock augments lung endothelial cell activation: role of temporal alterations of TLR4 and TLR2. Am J Physiol Regul Integr Comp Physiol. 2009;297(6):R1670–80. doi: 10.1152/ajpregu.00445.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan J, Li Y, Levy RM, Fan JJ, Hackam DJ, Vodovotz Y, Yang H, Tracey KJ, Billiar TR, Wilson MA. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J Immunol. 2007;178(10):6573–80. doi: 10.4049/jimmunol.178.10.6573. [DOI] [PubMed] [Google Scholar]
- 44.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201(7):1135–43. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, McMahon TJ. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci U S A. 2007;104(43):17063–8. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gladwin MT, Kim-Shapiro DB. Storage lesion in banked blood due to hemolysis-dependent disruption of nitric oxide homeostasis. Curr Opin Hematol. 2009;16(6):515–23. doi: 10.1097/MOH.0b013e32833157f4. [DOI] [PubMed] [Google Scholar]
- 47.Xu B, Chibber R, Ruggiero D, Kohner E, Ritter J, Ferro A. Impairment of vascular endothelial nitric oxide synthase activity by advanced glycation end products. Faseb J. 2003;17(10):1289–91. doi: 10.1096/fj.02-0490fje. [DOI] [PubMed] [Google Scholar]