Abstract
ADHD is typically characterized as a disorder of inattention and hyperactivity/impulsivity but there is increasing evidence of deficits in motivation. Using PET we showed decreased function in the brain dopamine reward pathway in adults with ADHD, which we hypothesized could underlie the motivation deficits in this disorder. To evaluate this hypothesis we performed secondary analyses to assess the correlation between the PET measures of dopamine D2/D3 receptor and dopamine transporter availability (obtained with [11C]raclopride and [11C]cocaine, respectively) in the dopamine reward pathway (midbrain and nucleus accumbens), and a surrogate measures of trait motivation (assessed using the Achievement scale on the Multidimensional Personality Questionnaire or MPQ) in 45 ADHD participants and 41 controls. The Achievement scale was lower in ADHD participants than in controls (11±5 vs 14±3, p<0.001) and was significantly correlated with D2/D3 receptors (accumbens: r=0.39, p<0.008; midbrain: r=0.41, p<0.005) and transporters (accumbens: r=0.35, p < 0.02) in ADHD participants, but not in controls. ADHD participants also had lower values in the Constraint factor and higher values in the Negative Emotionality factor of the MPQ but did not differ in the Positive Emotionality factor - and none of these were correlated with the dopamine measures. In ADHD participants scores in the Achievement scale were also negatively correlated with symptoms of inattention (CAARS A, E and SWAN-I). These findings provide evidence that disruption of the dopamine reward pathway is associated with motivation deficits in ADHD adults, which may contribute to attention deficits and supports the use of therapeutic interventions to enhance motivation in ADHD.
Keywords: psychiatric disorder, brain imaging, PET, attention, catecholamines, personality
Attention Deficit Hyperactivity Disorder (ADHD) is the most recognized and treated psychiatric disorder of childhood1 and it is increasingly recognized and treated in adults2. ADHD is characterized by symptoms of inattention and/or hyperactivity/impulsivity that produce impairment across cognitive, behavioral and interpersonal domains of function1. However, the hypothesis that there is a dysfunction in reward and motivation was proposed over 2 decades ago3 and there is increasing evidence that this plays a role in ADHD3,4. For example, children with ADHD require stronger incentives to modify their behavior than those without ADHD5; they also show a failure to delay gratification, have impaired responses to partial schedules of reinforcement, and preference for small immediate rewards over larger delayed rewards6,7.
The mesoaccumbens dopamine (DA) pathway, which projects from the ventral tegmental area (VTA) in midbrain to the nucleus accumbens (NAcc) in the ventral striatum, is critically involved in reward and motivation8, and has been hypothesized to underlie the reward and motivational deficits observed in ADHD6,9. Indeed, using positron emission tomography (PET) we showed lower than normal availability of DA D2/D3 receptors (measured with [11C]raclopride) and of DA transporters (measured with [11C]cocaine) in the midbrain and in NAcc in drug naïve ADHD participants compared to non-ADHD control subjects10. Here we report on secondary analyses that were performed on a subset of subjects in whom we had collected personality measures, including trait measures of motivation, to test the hypothesis that disruption of the DA reward pathway is associated with the motivation deficit in ADHD.
For this purpose we measured the correlations between our PET measures of D2/D3 receptors and DA transporters (DAT) in the DA reward pathway (midbrain and NAcc) and questionnaire measures of trait motivation in drug-naïve ADHD participants and group-matched controls. DA D2/D3 receptor availability was measured with [11C]raclopride11 and DAT availability was measured with [11C]cocaine12. The Multidimensional Personality Questionnaire (MPQ)13, obtained in 45 ADHD participants (never medicated) and 41 controls, was used to obtain scores on the Achievement scale, which was used as a surrogate trait measure of motivation. The Achievement scale of the MPQ, evaluates a motivational disposition that comprises social dominance, enthusiasm, energy, assertiveness, ambitiousness, and achievement striving. We hypothesized that the deficits in the DA reward pathway in ADHD participants would be associated with lower scores in the Achievement scale (surrogate trait measure of motivation), and that lower scores in this surrogate measure of motivation would predict more severe ADHD symptoms.
Methods
Participants
ADHD participants were recruited from the ADHD programs at Duke University, Mount Sinai Medical Center and UC Irvine, and controls were recruited from advertisements in local newspapers at Brookhaven National Laboratory. The study included 45 never medicated ADHD participants (23 males; 32 ±8 years of age; 16 ±2 years of education; BMI 26 ±5) and 41 healthy controls (28 males, 31 ±5 years of age; 15 ±2 years of education; BMI 25 ±3) from an imaging study that measured group differences in DA D2/D3 receptor and DAT availability10. Details on subject inclusion and exclusion criteria have been described10. Briefly, inclusion criteria for ADHD participants were: DSM-IV diagnostic criteria for ADHD, presence of at least 6 of 9 inattention symptoms (ascertained with semi-structured psychiatric interviews), evidence that some symptoms of ADHD started during childhood (before age seven) and a Clinical Global Impressions Severity scale (CGI-severity)14 rating of 4 or greater. Exclusion criteria were past or present history of substance abuse (other than nicotine; 3 ADHD participants and 1 control were active smokers) or a positive urine drug screen during assessment, prior or current treatment with psychotropic medications (including stimulants), psychiatric co-morbidities (axis I or II diagnosis other than ADHD), neurological disease, medical conditions that may alter cerebral function (i.e., cardiovascular, endocrinological, oncological or autoimmune diseases) or head trauma with loss of consciousness (greater than 30 minutes). Controls met the same exclusion criteria but not the inclusion criteria for diagnosis of ADHD and were excluded if they described symptoms of inattention or hyperactivity that interfered with their everyday activities.
Clinical Scales
In addition to the categorical assessment of symptom severity by interview and questionnaires, we assessed the underlying traits of the two DSM-IV domains of attention and activity/reflectivity using the Strengths and Weaknesses of ADHD-symptoms and Normal-behavior (SWAN). The SWAN uses a 7-point scale (-3 to +3) to represent the full range of these dimensions in the population, with average behavior as a reference point (zero)15. The SWAN scores traits that are below average and represent severity of psychopathology on a positive scale (from 1 to 3), and scores traits above average on a negative scale (from -1 to -3). The SWAN was completed in 37 of the controls and 39 of the ADHD participants. We also obtained standard assessments of symptom severity with the Conners Adult ADHD Rating Scale (CAARS) long version16. The CAARS provides self-assessment of severity of ADHD symptoms on a 4-point scale (Not at All = 0, Just a Little = 1, Pretty Much = 2, and Very Much = 3). Eight subscales are provided: A. Inattention/Memory problems, B. Hyperactivity/Restlessness, C. Impulsivity/Emotional lability, D. Problems with self-concept, E. DSM-IV Inattentive symptoms, F. DSM-IV Hyperactive-impulsive symptoms, G. DSM-IV Symptom total. The CAARS was completed in 36 of the controls and 43 of the ADHD participants. The scores on these clinical scales are shown in Table 1.
Table 1.
Scores on the clinical scales in Controls and in ADHD participants and number of subjects for whom measures were obtained for each group. Measures correspond to means and standard deviations.
| Controls (n=36) | ADHD (n=43) | Motivation ADHD | ||
|---|---|---|---|---|
| CAARS | ||||
| A Inattention | 5 ±4 | 25 ±6 | -0.43, p<0.005 | |
| B Hyperactivity | 7 ±4 | 22 ±7 | NS | |
| C Impulsivity | 5 ±3 | 20 ±7 | NS | |
| D Self-concept | 3 ±2 | 9 ±4 | NS | |
| E DSM Inattentive | 3 ±3 | 20 ±4 | -0.43, p<0.005 | |
| F DSM Hyperactive | 3 ±3 | 15 ±5 | ||
| G Total symptoms | 6 ±5 | 35 ±6 | ||
| (n=37) | (n=39) | |||
| SWAN | ||||
| Inattention | -1.5 ±1 | 1.5 ±1 | 0.44, p<0.005 | |
| Hyperactivity | -1.2 ±1 | 0.4 ±1 |
Personality measures
The Multidimensional Personality Questionnaire (MPQ)13,17, which has 276 true-false items that score 3 broad factors (Positive Emotionality, Negative Emotionality, and Constraint) and 11 primary trait dimensions, was administered. The trait dimension of Achievement (hard working, driven, tenacious, perfectionistic, enthusiastic) was used as a surrogate trait measure of motivation. The Achievement scale consists of items such as “works hard, drives self, enjoys working hard, welcomes difficult and demanding tasks, persists where others give up, is ambitious, puts work and accomplishments before many other things, sets high standards, is a perfectionist” versus “does not like to work harder than is strictly necessary, avoids very demanding projects, sees no point in persisting when success seems unlikely, is not terribly ambitious or a perfectionist”. The MPQ Achievement scale has been shown to correlate with leadership role occupancy among a large sample (N=238) of identical male twins18, consistent with prior meta-analyses studies19. The activation and sustainment of achievement motivation has been conceptualized to be accomplished by central representations of delayed rewards20. To assess if the correlations with the DA measures were specific for the surrogate trait measure of motivation, we also inspected correlations with the broad trait measures of Positive Emotionality (combination of scores on well-being, social potency, achievement), Negative Emotionality (combination of scores for stress reaction, alienation, and aggression) and Constraint (combination of scores for self-control, harm avoidance and traditionalism).
PET Scans
We used a Siemens HR+ tomograph (resolution 4.5×4.5×4.5 mm full width half-maximum). Dynamic scans were started immediately after injection of 4-10 mCi of [11C]raclopride (specific activity 0.5-1.5 Ci/μM at end of bombardment) and after injection of 4-8 mCi of [11C]cocaine (specific activity > 0. 53 Ci/μmol at end of bombardment) and were obtained for a total of 60 minutes as described (12). Arterial blood was obtained to measure the concentration of unchanged [11C]raclopride and [11C]cocaine in plasma.
Image analysis and statistics
We manually obtained regions of interest (ROI) in ventral striatum (in the location of the NAcc), midbrain and cerebellum11. The carbon-11 concentration in each ROI was used to generate time activity curves for [11C]cocaine and for [11C]raclopride as previously described11,12. The time activity curves for tissue concentration and unchanged tracer in plasma were used to calculate the distribution volumes (DV) using a graphical analysis technique for reversible systems21 to estimate the equilibrium ratio of tissue concentration to plasma concentration in NAcc, midbrain and cerebellar regions. The ratios of DV in the accumbens and midbrain regions to that in cerebellum correspond to (Bmax/Kd) +1 and were used as measures of D2/D3 receptor and DAT availability. Pearson product moment correlations were used to assess the association between our trait measure of motivation and D2/D3 receptor and DAT availability in the midbrain and NAcc regions. These correlations were calculated first for all participants and then separately for each group of participants (i.e., for the ADHD and Control group separately). We also measured the correlation between the Achievement scale (surrogate trait measure of motivation) and the symptom ratings of inattention and hyperactivity in the ADHD participants using the CAARS and the dimensions of attention and activity/reflectivity from the SWAN. Significance for the a priori hypothesis (i.e., association of DA measures with surrogate trait measures of motivation, and association of surrogate trait measures of motivation with ADHD symptoms) was set at p < 0.05. The significance level for the correlations of the DA measures and scores on the 3 broad traits of the MPQ (Positive Emotionality, Negative Emotionality and Constraint factors) was set at p < 0.008 (i.e., Bonferroni correction for 3 personality and 2 DA measures).
Results
Personality measures
The scores on the Achievement scale (surrogate trait measure of motivation) was significantly lower in ADHD participants than in controls (11±5 vs 15±3; t = 3.5, p <0.001). Compared to control participants, the ADHD participants also showed lower scores for Constraint (t= 5.2, p < 0.0001) and a trend for higher scores on Negative Emotionality (t = 2.1, p < 0.05), but the 2 groups did not differ on scores of Positive Emotionality (Table 2).
Table 2.
Scores on the Achievement Scale (surrogate trait measure of motivation) and on the Positive and Negative Emotionality and Constraint factors from the Multidimensional Personality Questionnaire (MPQ) in controls and ADHD participants. Comparisons correspond to independent t tests (two tail). Measures correspond to mean and standard deviations.
| Controls (n=41) | ADHD (n=45) | p | |
|---|---|---|---|
| Achievement Scale | 15 ±3 | 11 ±5 | 0.0003 |
| Positive Emotionality | 52 ±10 | 48 ±14 | 0.13 |
| Negative Emotionality | 12 ±9 | 16 ±11 | 0.04 |
| Constraint | 52 ±10 | 42 ±12 | 0.0001 |
Dopamine measures
Compared to controls, the ADHD participants had significantly lower measures of D2/D3 receptor and of DAT availability in NAcc and midbrain regions (averaged for left and right regions) (see Table 3).
Table 3.
Measures of DA D2/D3 receptor availability (Bmax/Kd) in ADHD participants and controls in the NAcc and midbrain regions and correlations with the scores on the Achievement Scale (surrogate trait measure of motivation) for analysis done with all subjects (ALL) and for analysis done with only ADHD participants (ADHD). The correlations with controls were not significant. Measures correspond to mean and standard deviation.
| D2/D3 receptors | Controls (n=41) | ADHD (n=45) | Correlations with Achievement Scale |
|---|---|---|---|
| NAcc region* | 2.65 ±0.27 | 2.53 ±0.22 | ALL r=0.27, p<0.01; ADHD: r=0.39, p<0.008 |
| Midbrain** | 0.28 ±0.12 | 0.20 ±0.17 | ALL r=0.36, p<0.0007; ADHD r=0.41, p<0.005 |
| DAT | |||
| NAcc region* | 0.60 ±0.14 | 0.55 ±0.10 | ALL r=0.20, p<0.07; ADHD r=0.35, p<0.02 |
| Midbrain | 0.14 ±0.09 | 0.10 ±0.09 | ALL r=0.18, NS; ADHD R=0.17, NS |
Comparisons between groups correspond to independent samples t tests (two tail)
p < 0.05;
p < 0.01
Correlation Between DA and Personality Measures
The correlation analysis between the Achievement scale (surrogate trait measure of motivation) and DA measures in NAcc (averaged left and right ROI) was significant for D2/D3 receptors (r=0.27, p<0.01) but not for DAT (r=0.20, p<0.07) when all subjects were included. Separate group analyses showed the correlation was significant for ADHD participants for both D2/D3 receptors (r=0.39, p<0.008) and DAT (r=0.35, p < 0.02), but not for either in the control participants (see Figure 1).
Figure 1.
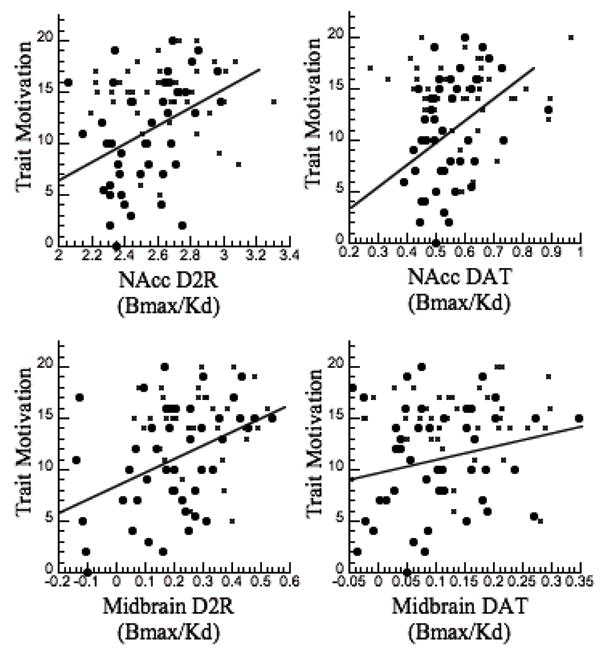
Scattegram showing the regression between the measures of DA D2/D3 receptor and of DAT availability in the NAcc and in the midbrain regions and Trait Motivations (MPQ Achievement scale, which was used as surrogate trait measure of motivation) in ADHD participants (circles) and in controls (x).
The correlation analysis between the Achievement scale and the DA measures in midbrain (averaged left and right ROI) when all subjects were included was significant for D2/D3 receptors (r=0.36, p<0.0007) but not DAT (r=0.18, NS). Separate group analyses showed the correlation was significant for ADHD participants for D2/D3 receptor (r=0.41, p<0.005) but not DAT; in controls neither correlation was significant (Figure 1).
The correlations with the other personality measures when all subjects were included revealed a significant correlations between Positive Emotionality and D2/D3 receptor in midbrain (r=0.34, p<0.002) and separate group analysis showed that this correlation was significant in ADHD participants (r=0.41, p<0.005) but not in controls. The correlations with Negative Emotionality and Constraint factors were not significant.
Correlation of Achievement (Surrogate Trait Measures of Motivation) and Clinical Symptoms
To assess if our surrogate trait measure of motivation contributed to symptoms in ADHD participants we also measured the correlation between the scores in the Achievement scale and ADHD symptoms, which was significant for the CAARS A (r=-0.43, p<0.005), CAARS E (r=-0.43, p<0.005) and the SWAN Attention dimension (r=-0.44, p < 0.005) (Figure 2); the lower the scores in the Achievement scale the greater the inattention. In viewing the regression slopes in Figure 2, note that the positive SWAN score in attention reflects greater symptoms whereas the negative values reflect the opposite of the symptom. The correlation between scores in the Achievement scale and ratings of hyperactivity were not significant. Correlations between the other personality measures and symptoms of inattention or hyperactivity were not significant.
Figure 2.
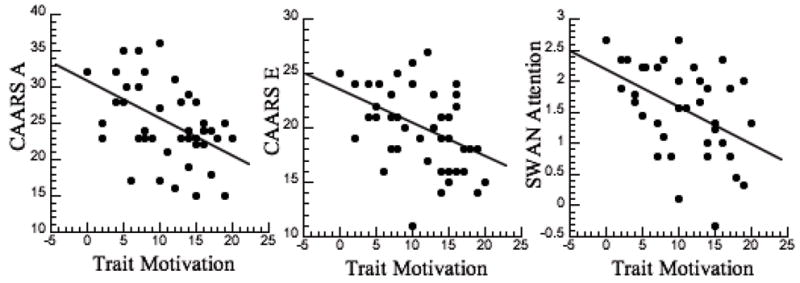
Scattegram for the regression between Trait Motivations (MPQ Achievement scale, which was used as surrogate trait measure of motivation) and the measures of inattention (CAARS A, CAARS E and SWAN-I) in the ADHD participants.
Discussion
This study provides evidence of the predicted association between synaptic DA markers in regions of the mesoaccumbens DA pathway (NAcc and midbrain) and the trait of motivation in adults with ADHD. These are key brain regions for reward22, and the observed decreased availability of D2/D3 receptors and DAT could explain the decreased motivation in these patients.
The correlation between our surrogate measure of motivation (Achievement scale) and symptoms of inattention also suggest that impaired motivation may contribute to severity of symptoms of inattention in ADHD. These findings are consistent with the clinical recognition that attentional deficits in individuals with ADHD are most evident in tasks that are boring, repetitive and considered uninteresting (i.e., tasks or assignments for which intrinsic motivation is low)23. However, the correlational approach in our study does not allow us to assess which of these dimensions is more primary; the motivation deficit produces inattention as opposed to the attention deficit resulting in decreased motivation. Alternatively these two dimensions could have common neurobiological substrates (DA reward pathway) as well as unique features (i.e., noradrenergic prefrontal pathways for inattention).
The observation of a deficit in the DA reward pathway is further evidence that deficits in motivation may be contributing to impairment in ADHD adults6. This finding is also consistent with a recent fMRI study that reported decreased activation of the ventral striatum (the location of the NAcc) in adults with ADHD when compared to controls for both immediate and delayed rewards24.
We also showed a significant positive correlation between Positive Emotionality and D2/D3 receptor availability in midbrain in ADHD participants. Since the Achievement scale is part of the Positive Emotionality factor this correlation is likely to reflect the association between D2/D3 receptor availability and this surrogate trait measure of motivation.
Clinical Implications of Findings
Our findings may have clinical relevance. They support the use of interventions to enhance the saliency of school and work tasks to improve motivation and performance. Indeed both motivational intervention and contingency management have been shown to improve performance in ADHD patients25. For example, the use of intrinsically interesting activities (perhaps in areas where the individual shows talent and has successes) to reinforce mundane but necessary behaviors offers a therapeutic strategy to overcome a motivation deficit. Also methylphenidate, which is one of the most frequent pharmacological interventions for ADHD, has been shown to increase motivation and interest in a cognitive task in proportion to the drug-induced DA increases in striatum26.
Decreased activity of the reward system in individuals with ADHD is likely to translate into problems in engaging in activities that are not inherently rewarding or reinforcing (and therefore may be described as less interesting or less motivating). By extrapolation to children, our observations in adults with ADHD could explain the reports of some parents that their child with ADHD can spend hours playing video games, but cannot focus attention on tasks at school, and the reports of children with ADHD that schoolwork is “boring”, which is commonly used to explain their lack of effort. The strong correlation between low scores in the surrogate trait measure of motivation and symptoms of inattention observed in this study suggests the need to consider the possibility of including “motivation or interest deficit” as part of the core pathology of ADHD.
Personality Measures and ADHD
Here we report lower scores on the Achievement scale (surrogate trait measure of motivation) in ADHD participants than in controls that was negatively associated with symptoms of inattention in ADHD. This is consistent with studies in children with ADHD in whom temperament measures of effortful control were linked to core symptoms of ADHD27. Specifically, Martel and Nigg (2006) described two overall dimensions of temperament, effort control and reactive control, and suggested that inattention symptoms might be considered as extreme of the former (effort control) and hyperactive/impulsive symptoms as the extreme of the latter (reactive control)28. In our study hyperactivity did not correlate with any of the personality measures. The difference between our findings and those in children with ADHD could reflect either the low occurrence of symptoms of hyperactivity in our adult ADHD participants, or indicate that the association of personality measures with ADHD symptoms is different in adults than in children with ADHD.
We also found decreases in scores on the Constraint factor of the MPQ in ADHD participants. This factor is a combination of scores for self-control, harm avoidance and traditionalism, but the main difference was due to the lower scores on the self-control subscale in ADHD participants than in controls (data not shown). This is consistent with prior studies documenting poor self-control as one of the main behavioral characteristics that distinguishes adults with ADHD from controls29. Moreover, impaired inhibition is considered a core symptom of ADHD30.
Using the Achievement scale we had previously documented in healthy controls an association with asymmetry in striatal measures of D2/D3 receptor availability such that greater scores on trait measures of motivation were associated with higher left relative to right receptor availability31. The current study did not corroborate this finding (data not shown), which may reflect the sensitivity of laterality measures to the position of the head in the field of view of the scanner.
Limitations
[11C]Raclopride measures are influenced by extracellular DA, so decreased binding could reflect low D2/D3 receptor levels or increased DA release32. However, the latter is unlikely since we had previously reported that DA release in a subgroup of the ADHD participants reported in this study was lower than in controls33.
The relatively low affinity of [11C]raclopride and [11C]cocaine for their targets and the relatively poor spatial resolution of PET decreases the signal to error in measures done in small brain regions such as NAcc, or in regions with lower relative concentration of D2/D2 receptors or of DAT such as midbrain. Future studies using radiotracers with higher affinity and PET instruments with higher resolution will enable more accurate assessments.
We used the MPQ Achievement scale as a surrogate trait measure of motivation, which in and of itself is a complex construct. However, we had previously shown that the Achievement scale correlated with the dorsolateral prefrontal cortical response to monetary reward, which was in turn correlated with reinforcement-driven reaction time in cocaine addicted individuals34. In the current study it would have been useful to have also collected measures of objective task motivation. Such a measure would have enabled us to assess the relationship between sustained performance on a challenging task and the self-reported measure of motivation in ADHD and in control participants. In future studies we plan to include reaction time measures in response to tasks of varying difficulty and reward expectation, which primates studies have shown reflect behavioral markers of state motivation35.
Our findings show a significant correlation between DA measures in NAcc and midbrain and the MPQ Achievement scale, which we interpret to suggest an association between these two measures. However, correlations do not necessarily connote causality and further studies are required to address this. Nonetheless, our previous findings in healthy controls, in whom we showed that increases in striatal DA induced by methylphenidate were also associated with increases in the motivation to perform a cognitive task, provides evidence that DA is involved in motivation23. Further studies are also required to assess the directionality of the association between inattention and motivation deficit (i.e., poor motivation resulting in inattention vs. inattention resulting in poor motivation).
In conclusion, these findings show that reductions in DA D2/D3 receptor and DAT availability in the DA reward pathway of ADHD participants are associated with low scores in the MPQ Achievement scale (surrogate trait measures of motivation). Moreover, the correlation between scores in the MPQ Achievement scale and symptoms of inattention in the ADHD participants suggests that deficits in motivation contribute to inattention in ADHD. Our findings and those from other studies reporting motivation deficits in ADHD strongly suggest that ADHD is a disorder not only of attention-deficit and hyperactivity/impulsivity but also of motivation-deficit, that appears to reflect an hypofunctional DA reward pathway.
Acknowledgments
This research was carried out at Brookhaven National Laboratory (BNL) and was supported in part by the Intramural Research Program of the National Institutes of Health (NIH), the National Institute of Mental Health (MH66961-02) and infrastructure support from the Department of Energy. We are grateful to the following BNL employees: Donald Warner for PET operations, David Schlyer and Michael Schueller for cyclotron operations, Pauline Carter, Millard Jayne and Barbara Hubbard for nursing care, Payton King for plasma analysis and Lisa Muench, Youwen Xu and Colleen Shea for radiotracer preparation, Karen Appelskog for protocol coordination; to the following Duke employees: Joseph English and Allan Chrisman, for subject recruitment and evaluation; to the following NIH employee: Linda Thomas for editorial assistance. We also thank the individuals who volunteered for these studies.
References
- 1.National Institutes of Health Consensus Development Conference Statement: diagnosis and treatment of attention-deficit/hyperactivity disorder (ADHD) J Am Acad Child Adolesc Psychiatry. 2000;39:182–193. doi: 10.1097/00004583-200002000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Dopheide JA, Pliszka SR. Attention-deficit-hyperactivity disorder: an update. Pharmacotherapy. 2009;29:656–679. doi: 10.1592/phco.29.6.656. [DOI] [PubMed] [Google Scholar]
- 3.Haenlein M, Caul WF. Attention deficit disorder with hyperactivity: a specific hypothesis of reward dysfunction. J Am Acad Child Adolesc Psychiatry. 1987;26:356–362. doi: 10.1097/00004583-198705000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Johansen EB, Killeen PR, Russell VA, Tripp G, Wickens JR, Tannock R, et al. Origins of altered reinforcement effects in ADHD. Behav Brain Funct. 2009;5:7. doi: 10.1186/1744-9081-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kollins SH, Lane SD, Shapiro SK. The experimental analysis of childhood psychopathology: A matching analysis of the behavior of children diagnosed with Attention Deficit Hyperactivity Disorder. Psychological Record. 1997;47:25–44. [Google Scholar]
- 6.Sonuga-Barke EJ. The dual pathway model of AD/HD: an elaboration of neuro-developmental characteristics. Neurosci Biobehav Rev. 2003;27:593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Tripp G, Wickens JR. Research review: dopamine transfer deficit: a neurobiological theory of altered reinforcement mechanisms in ADHD. J Child Psychol Psychiatry. 2008;49:691–704. doi: 10.1111/j.1469-7610.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- 8.Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- 9.Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol Psychiatry. 2005;57:1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, et al. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009;302:1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volkow ND, Fowler JS, Wang GJ, Dewey SL, Schlyer D, MacGregor R, et al. Reproducibility of repeated measures of carbon-11-raclopride binding in the human brain. J Nucl Med. 1993;34:609–613. [PubMed] [Google Scholar]
- 12.Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, Macgregor RR, et al. Mapping cocaine binding sites in human and baboon brain in vivo. Synapse. 1989;4:371–377. doi: 10.1002/syn.890040412. [DOI] [PubMed] [Google Scholar]
- 13.Tellegen A, Waller NG. Exploring personality through test construction: Development of the Multidimensional Personality Questionnaire. In: Boyle GJ, Matthews G, Saklofske DH, editors. Handbook of personality theory and testing: Vol. II. Personality measurement and assessment. Greenwich, CT: JAI Press; 2008. [Google Scholar]
- 14.Guy W. Clinical Global Impression (CGI) Scale. In: Rush AJ, First MB, Blacker D, editors. Handbook of Psychiatric Measures. Washington DC: American Psychiatric Publishing; 2000. [Google Scholar]
- 15.Young DJ, Levy F, Martin NC, Hay DA. Attention Deficit Hyperactivity Disorder: A Rasch Analysis of the SWAN Rating Scale. Child Psychiatry Hum Dev. 2009;40(4):543–549. doi: 10.1007/s10578-009-0143-z. [DOI] [PubMed] [Google Scholar]
- 16.Conners CK. Rating scales in attention-deficit/hyperactivity disorder: use in assessment and treatment monitoring. J Clin Psychiatry. 1998;59(Suppl 7):24–30. [PubMed] [Google Scholar]
- 17.Krueger RF, Caspi A, Moffitt TE, Silva PA, McGee R. Personality traits are differentially linked to mental disorders: a multitrait-multidiagnosis study of an adolescent birth cohort. J Abnorm Psychol. 1996;105:299–312. doi: 10.1037//0021-843x.105.3.299. [DOI] [PubMed] [Google Scholar]
- 18.Arvey RD, Rotundo M, Johnson W, Zhang Z, McGue M. The determinant of leadership role occupancy: Genetic and personality factors. Leadership Quaterly. 2006;17:1–20. [Google Scholar]
- 19.Judge TA, Bono JE, Ilies R, Gerhardt MW. Personality and leadership: a qualitative and quantitative review. J Appl Psychol. 2002;87:765–780. doi: 10.1037/0021-9010.87.4.765. [DOI] [PubMed] [Google Scholar]
- 20.Depue RA, Collins PF. Neurobiology of the structure of personality: dopamine, facilitation of incentive motivation, and extraversion. Behav Brain Sci. 1999;22:491–517. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- 21.Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- 22.Wise RA, Rompre PP. Brain dopamine and reward. Ann Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 23.Barkley RA. Attention Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. New York, NY: The Guilford Press; 1990. [Google Scholar]
- 24.Plichta MM, Vasic N, Wolf RC, Lesch KP, Brummer D, Jacob C, et al. Neural hyporesponsiveness and hyperresponsiveness during immediate and delayed reward processing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009;65:7–14. doi: 10.1016/j.biopsych.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Barkley RA. Adolescents with attention-deficit/hyperactivity disorder: an overview of empirically based treatments. J Psychiatr Pract. 2004;10:39–56. doi: 10.1097/00131746-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Volkow ND, Wang GJ, Fowler JS, Telang F, Maynard L, Logan J, et al. Evidence that methylphenidate enhances the saliency of a mathematical task by increasing dopamine in the human brain. Am J Psychiatry. 2004;161:1173–1180. doi: 10.1176/appi.ajp.161.7.1173. [DOI] [PubMed] [Google Scholar]
- 27.Martel MM, Nigg JT. Child ADHD and personality/temperament traits of reactive and effortful control, resiliency, and emotionality. J Child Psychol Psychiatry. 2006;47:1175–83. doi: 10.1111/j.1469-7610.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- 28.Nigg JT. Temperament and developmental psychopathology. J Child Psychology Psychiatry. 2006;47:395–422. doi: 10.1111/j.1469-7610.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- 29.De Quiros GB, Kinsbourne M. Adult ADHD. Analysis of self-ratings on a behavior questionnaire. Ann N Y Acad Sci. 2001;931:140–147. [PubMed] [Google Scholar]
- 30.Lampe K, Konrad K, Kroener S, Fast K, Kunert HJ, Herpertz SC. Neuropsychological and behavioural disinhibition in adult ADHD compared to borderline personality disorder. Psychol Med. 2007;37:1717–1729. doi: 10.1017/S0033291707000517. [DOI] [PubMed] [Google Scholar]
- 31.Tomer R, Goldstein RZ, Wang GJ, Wong C, Volkow ND. Incentive motivation is associated with striatal dopamine asymmetry. Biol Psychol. 2008;77:98–101. doi: 10.1016/j.biopsycho.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gjedde A, Wong DF, Rosa-Neto P, Cumming P. Mapping neuroreceptors at work: on the definition and interpretation of binding potentials after 20 years of progress. Int Rev Neurobiol. 2005;63:1–20. doi: 10.1016/S0074-7742(05)63001-2. [DOI] [PubMed] [Google Scholar]
- 33.Volkow ND, Wang GJ, Newcorn J, Telang F, Solanto MV, Fowler JS, et al. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64:932–940. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, et al. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am J Psychiatry. 2007;164:43–51. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satoh T, Nakai S, Sato T, Kimura M. Correlated coding of motivation and outcome of decision by dopamine neurons. J Neurosci. 2003;23:9913–9923. doi: 10.1523/JNEUROSCI.23-30-09913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


