Abstract
Background
Lubricin and hyaluronic acid lubricate articular cartilage and prevent wear. Because lubricin loss occurs following ACL injury, intra-articular lubricin injections may reduce cartilage damage in the ACL deficient knee.
Purpose
To determine if lubricin and/or hyaluronic acid supplementation will reduce cartilage damage in the ACL deficient knee.
Study Design
Controlled laboratory study
Methods
36 male rats, 3 months old, underwent unilateral ACL transection. They were randomized to four treatments: 1) saline (PBS), 2) hyaluronic acid (HA), 3) purified human lubricin (LUB), and 4) LUB and HA (LUB+HA). Intra-articular injections were given twice weekly for four weeks starting one week after surgery. Knees were harvested one week following final injection. Radiographs of each limb and synovial fluid lavages were obtained at harvest. Histology was performed to assess cartilage damage using Safranin O/Fast green staining. Radiographs were scored for the severity of joint degeneration using the modified Kellgren-Lawrence scale. Synovial fluid levels of sulfated glycosaminoglycan, collagen II breakdown, IL-1β, TNF-α and lubricin were measured using ELISA.
Results
Treatment with LUB or LUB+HA significantly decreased radiographic and histologic scores of cartilage damage (p=0.039, p=0.015, respectively) when compared to the PBS and HA conditions. There was no evidence of an effect of HA nor was the LUB effect HA dependent suggesting that the addition of HA did not further reduce damage. The synovial fluid of knees treated with LUB had significantly more lubricin in the synovial fluid at euthanasia, though there were no differences in the other cartilage metabolism biomarkers.
Conclusions
Supplemental intra-articular LUB reduced cartilage damage in the ACL transected rat knee 6 weeks after injury, while treatment with HA did not.
Clinical Relevance
Although longer-term studies are needed, intra-articular supplementation (tribosupplementation) with lubricin following ACL injury may protect the articular cartilage in the ACL injured knee.
Keywords: Cartilage, ACL injury, arthritis, lubricin, hyaluronic acid
Introduction
Anterior cruciate ligament (ACL) injury is associated with a high prevalence of post-traumatic osteoarthritis (OA), the rate of which appears not to be reduced following the current gold standard of treatment, surgical ACL reconstruction.38,42 In humans, cartilage loss associated with OA occurs over the course of years, a process stimulated by cytokines and degradative enzymes produced by the synovium and chondrocytes.2,40,43,48 The role of mechanical stress in perpetuating the progression of OA is not completely understood, but altered joint mechanics and subsequent cartilage wear are thought to contribute to the onset of OA in these patients.13,51 It remains unknown whether the development of OA after ACL injury is initiated due to inflammation, cartilage impact, abnormal contact biomechanics, or a combination thereof.
In healthy synovial joints, the articular surfaces serve as an extremely low friction bearing. The integrity of this bearing is protected by multiple lubrication methods, which depend on synovial fluid composition as well as cartilage matrix structure.8,10,22,29–31,34–37,58 Inadequate cartilage surface lubrication due to the acute inflammatory destruction of lubricin has been proposed as one likely mechanism linking acute joint injury to subsequent OA. This hypothesis is supported by previous studies that have shown that synovial fluid lubricin levels are decreased in patients with recent ACL injury,11 and that this decrease is associated with short term increases in cytokine levels and long term increases in cartilage damage following ACL transection in experimental models.12,56 An unanswered question is whether preserving or supplementing the joint lubricating mechanisms following ACL injury will slow or halt the progression of post-traumatic OA in these patients.
Intra-articular injections of hyaluronic acid (HA) and, more recently, lubricin (LUB) have been proposed as two possible means to prevent OA progression by enhancing the lubricating ability of synovial fluid after injury.53 HA contributes to the viscosity of synovial fluid so that it can act as a cushion and stabilizer for hydrodynamic lubrication,26,31,39 and thus could provide chondroprotection by preventing wear. In vitro, HA has also been reported to modulate the expression of inflammatory cytokines,41 and stimulate chondrocytes to synthesize matrix proteins and proteoglycans, which could slow disease progression,6,17 but animal models are not in agreement as to whether HA delays,1,49 accelerates,15–16 or has no effect on cartilage damage.52 Despite the limited evidence for disease-modifying effects in vivo, several clinical studies have been performed to determine whether the use of intra-articular HA reduces pain and increases joint motion in the shoulder,5 hip,46 and ankle,59 and in the knee after meniscectomy and ACL reconstruction.19–20 Intra-articular injections of HA are currently approved for the treatment of pain associated with knee OA since analgesia is maintained for up to several months following injection.6,17 However, it is unknown if any of the reported clinical effects of HA in reducing pain symptoms correlate with long-term chondroprotection.
LUB is a mucinous glycoprotein that is highly glycosylated with O-linked Gal(β1-3)-GalNAc-NeuAc in its central mucin domain.45,55 This protein is configured to form a discontinuous nanofilm that exerts repulsive forces, and is the basis for its anti-adhesive and lubricating properties when bound to the cartilage surface.45 Flannery et al. have reported short-term chondroprotective effects using the rat meniscectomy model following treatment with recombinant lubricin.14 Jones et al.28 also found that in cartilage explants with an intact articular surface, injurious compression resulted in elevated surface friction accompanied by increased chondrocyte lubricin expression, suggesting a possible compensatory response.
Given that LUB and HA are both thought to contribute to the lubrication of the articular surface in normal synovial joints, the objective of this study was to determine whether supplementing the synovial fluid of an ACL-deficient knee with LUB and/or HA would reduce cartilage damage six weeks following injury in the rat model. The hypotheses were: 1) that intra-articular supplementation of lubricin will reduce cartilage damage following ACL transection when compared to those treated with a sham injection; 2) that intra-articular supplementation of HA will reduce cartilage damage when compared to the sham treated animals; and 3) that intra-articular supplementation of lubricin plus HA will further reduce cartilage damage when compared to sham treated animals or those treated with HA or LUB in isolation. Cartilage damage was assessed using radiographic and histological analyses, and served as the primary outcome measures. In addition, biomarkers of articular cartilage metabolism in synovial fluid were also evaluated after 6 weeks. The synovial fluid biomarkers included indicators of cartilage degradation (sGAG, CTX-II), joint inflammation (IL-1b, TNF-a), and lubricin.
Methods
Experimental Design
After the study received IACUC approval, thirty-six male Lewis rats, three months of age, underwent unilateral surgical transection of the ACL of the right knee joint. The rat ACLT model has been previously characterized12,18 and implemented to assess cartilage damage following traumatic knee injury.12,18 These animals were randomized following surgery to one of four intra-articular injection treatment groups: 1) 40 μL phosphate-buffered saline (Sham); 2) 40 μL 3.33 mg/mL high molecular weight hyaluronic acid (HA) (Healon, Advanced Medical Optics, Upsala, Sweden); 3) 40 μL 200 μg/mL purified human lubricin (LUB); or 4) 40 μL 200 μg/mL LUB with 3.33 mg/mL HA (LUB+HA). There were nine animals in each treatment group. Within all treatment groups, the injections of the ACL transected knee were initiated one week after surgery, and performed twice weekly for four weeks. Both knees from each animal were harvested one week after the series of injections were completed. The total time from surgery to harvest was six weeks. After euthanasia with carbon dioxide gas, serum samples and synovial fluid lavages were obtained from both knees of each animal. Anteroposterior and lateral radiographs were taken of each knee using a high resolution (14-bit) radiography system (MX-20; Faxitron, Wheeling, IL). The images were obtained at 28kV, with adjustments in kV as needed, to obtain adequate penetration and image quality. Coronal sections from each knee were then obtained for histologic analysis of cartilage degeneration.
ACL Transection procedure
Animals were sedated prior to surgery with an intraperitoneal injection of ketamine (75 mg/kg) and metomidine (0.5mg/kg). Subcutaneous buprenorphine (0.03 mg/kg) was given immediately following surgery and twice daily for three days to provide post-operative analgesia. After the animal was anesthetized, the operative knee was shaved and prepared using an iodine solution and alcohol. The limb was sterilely draped with the animal supine. A 1 cm midline incision was made over the anterior knee and the skin was mobilized to expose the patellar tendon. A medial parapatellar incision was made to open the joint capsule. To expose the notch, the patella was subluxed laterally, rather than everted, to avoid additional damage to the articular cartilage. With the knee in flexion, the ACL was carefully sectioned with the tip of a scalpel. Complete ACL transection was confirmed by manually testing the joint for anterior laxity. The peripatellar capsular incision was then closed using 4-0 Vicryl sutures. The skin was closed with staples. Post-operatively, animals were allowed to bear weight as tolerated.
Intra-articular Injections
Prior to performing the intra-articular injections, anesthesia was induced in a chamber with 5% isoflurane. Once anesthetized, the animal was placed supine with its nose set in a cone and dosed with 2% isoflurane to maintain anesthesia. The knee was shaved and prepped to provide a sterile field. Intra-articular injections were then performed through the patellar tendon with the knee in flexion using a 3/10 cc, 29 gauge pediatric insulin syringe. Intra-articular placement of the injection was confirmed by the lack of resistance to flow with injection and by feeling the distention of the knee joint capsule medial to the patellar tendon.
Purified Lubricin Preparation
Human synovial fluid lubricin was purified from synovial fluid samples that were obtained at the time of total knee arthroplasty as previously described.25 The synovial fluids samples for making the purified human lubricin were obtained under an Institutional Review Board approved protocol. Following joint aspiration, samples were centrifuged at 10,000g at 4°C for 1 hour to remove cellular debris. The processed samples were pooled and stored at −20°C. To obtain purified lubricin, the human synovial fluid was filtered through 0.22 μm sterile filter units (Nalgene, Rochester, NY) at 4°C over two days. The retentate was scraped off the filter membranes and resuspended with 50 mM NaAc buffer, pH 5.5, containing proteolytic inhibitors, to the original synovial fluid volume. Digestion of hyaluronic acid was then carried out with Streptomyces hyaluronidase (Sigma, St. Louis, MO) 1U/ml of resuspended synovial fluid at 37°C for 18 h. The digest was loaded onto diethylaminoethyl (DEAE) agarose, washed with 50 mM NaAc, and eluted with 1 M NaCl. Eluate was concentrated and dialyzed against polyethylene glycol in 25 mM PBS at pH 7.4, containing 0.5 mM CaCl2. The DEAE-bound eluate was fractionated on a peanut agglutinin (PNA)-agarose affinity column with a settled bed volume of 25 ml, equilibrated at room temperature with 25 mM PBS (pH 7.4). Pre-purified lubricin was maximally eluted in the presence of a step-wise gradient of 0.15 M α-lactose in 25 mM PBS (pH 7.4). After dialysis against PBS, contaminating fibronectin was removed by a monoclonal antibody directed against human fibronectin (Zymed Laboratories, San Francisco, CA) immobilized on Actigel ALD agarose (Sterogene Bioseparations, Arcadia, CA).25 Final purity of the lubricin with this method has been found to be greater than 95% by comparing the ELISA recovery using a lubricin-specific Ab and 280 nm absorbance.21
Harvest/Specimen Collection
Following euthanasia, blood samples were obtained by intracardiac aspiration. The samples were centrifuged at 10,000g at 4°C for 20 minutes to remove cellular debris. The aliquots of serum were then frozen at −80°C. Following harvest, each hindlimb was disarticulated, the skin removed, and 100 μL of isotonic saline was injected intra-articularly into the knee joint. The knee was manually cycled through flexion and extension ten times to distribute the fluid throughout the joint. The fluid was then aspirated from the joint and frozen at −80°C.
Radiographic Scoring
Radiographs were scored using a modification of the Kellgren-Lawrence scoring system.32 The Kellgren-Lawrence score for knee OA, as originally described, assigns a score from 0–4 based on severity of disease in human subjects. In this study, the scoring was performed on rat knees. Although there are some anatomic differences between rats and humans, the images were scored relative to control radiographs taken of the non-operative contralateral limb. Radiographs were scored by an orthopaedic surgeon who specializes in adult joint reconstruction with over thirty years of clinical experience treating advanced OA and a third year orthopaedic surgery resident. Both examiners were blinded to the animal number and treatment group when scoring. The respective scores of both examiners for each animal were averaged for analysis.
Quantification of sGAG, CTXII, IL-1β, TNF-α, lubricin
sGAG concentrations, a marker of proteoglycan turnover, in the SF lavages of the ACLT joints were measured using Alcian Blue dye binding assay (Alpco Diagnostics, Windham, NH, USA).4 The assay is based on the formation of an insoluble blue colored complex between sGAG and Alcian blue, which can be quantified by absorbance at 590 nm. The sGAG concentrations were determined using serially diluted chondroitin-6 sulfate as a standard. SF lavage CTXII levels, a marker of collagen II breakdown, were measured using serum preclinical Cartilaps ELISA (Immunodiagnostic Systems, Fountain Hills, AZ, USA). The presence of inflammatory cytokines (TNF-α and IL-1β) in the SF lavages was quantified using commercially available ELISA kits (Invitrogen, Carlsbad, CA, USA). The reported minimum detection limits for TNF-α and IL-1β assays are 0.3 and 0.1 pg/ml, respectively.
The lubricin concentrations in SF lavages were quantified with a sandwich enzyme-linked immunosorbent assay (ELISA) using peanut agglutinin (PNA) (EY Laboratories, San Mateo, CA) and monoclonal antibody 9G3 as previously decribed.12 This antibody is specific for the lubricin mucin domain, which is highly conserved across both humans and rats. High-binding 96-well plates (Costar; Cole-Parmer, Vernon Hills, IL) were coated overnight with PNA in 50 mM sodium bicarbonate buffer, pH 9.5, at a final concentration of 10 μg/mL. After 24 hours, serial dilutions of purified human lubricin and diluted lavaged SF samples were incubated on the PNA-coated plates for 60 minutes at room temperature. PBS plus 0.1% Tween 20 was then used to wash the plates. Monoclonal antibody 9G3 was added at 1:10,000 dilution and incubated for 60 minutes at room temperature, followed by a second washing with PBS plus 0.1% Tween 20. Peroxidase-conjugated goat anti-mouse IgG (1:1,000 dilution; Invitrogen, Carlsbad, CA) was added to the plate for 60 minutes, followed by washing with PBS plus 0.1% Tween 20 and then with PBS alone. Last, tetramethylbenzidine reagent (Pierce, Rockford, IL) was added, and the absorbance was measured at 450 nm.
Normalization to urea
Because lavages with PBS were required to obtain the synovial fluid samples from the rat knees, the concentrations of the biomarkers were adjusted using the dilution factor established by comparing the concentrations of urea in both the serum and synovial fluids.33 Urea enters the joint by passive diffusion and is therefore in equilibrium between the serum and synovial fluid. It is not produced or broken down within the joint, and so it has been used to correct for the dilutional effects of a lavage on joint effusions of variable volume when direct aspiration of synovial fluid is not possible.33 The concentrations of urea in the synovial fluid lavages and serum were measured using a commercially available kit (Bioassay systems, Hayward, CA, USA).
Histology
The joints were processed for histology by removing the soft tissues from the femur and tibia, while leaving the knee joint capsule intact to facilitate fixation. The specimens were immersed in 10% formalin for a minimum of 72 hours, and then decalcified in Richman-Gelfand-Hill solution. After removing the proximal femur and distal tibia, the knee joints were embedded in a single block of Paraplast X-tra (Fisher, Santa Clara, CA) using a Tissue-Tek VIP 1000 tissue processor (Model#4617, Miles, Elkhart, IN). The blocks were trimmed to produce coronal sections for the assessment of articular cartilage integrity in the weight bearing regions of the tibial plateau using a rotary microtome (Model#2030, Reichart-Jung, Austria). The samples were sectioned 6 microns thick, mounted on slides, and stained with Safranin-O/fast green.47 The slides were viewed and photographed under light microscopy at 40X. OARSI scores 44 were assigned by four experienced independent examiners who were blinded to the treatment group. After independent scoring, the slides and scores were reviewed by the group, who were still blinded, in an effort to obtain consensus and a representative score. The scores for the tibial medial and lateral plateau were averaged for each specimen for statistical analysis.
Statistics
Statistical analyses were performed using two-way analyses of variance with factors representing the main effects of lubricin and hyaluronic acid and their interaction. Fisher’s LSD was used to perform pairwise comparison among treatment conditions (Sham, HA, LUB, LUB+HA). Primary and secondary outcome measures were the average medial and lateral compartment OARSI score for the tibial surface for each specimen, the modified Kellgren-Lawrence score, and the synovial fluid lavage concentrations of sGAG, CTXII, IL-1β, TNF-α, and lubricin in synovial fluid lavages at the time of harvest. All biomarker comparisons were performed on the urea-adjusted concentrations. Because the Kellgren-Lawrence and OARSI scores represent ordinal outcomes, the two-way analyses of variance for these outcomes were performed nonparametrically based on a rank transformation prior to analysis. Based on this sample size, this study was 80% powered to detect an effect size of 0.96 for all outcome measures. All statistical analyses were performed using SAS statistical software Version 9.0 (SAS Institute, Cary, NC).
Results
Gross Observations
A return to equal weight-bearing on the hindlimbs was observed within one week of surgery in all animals. No animals were lost following surgery or post-operative treatment injections. Following harvest, it was noted that all ACL-transected knees had a positive manual anterior drawer on examination.
Light Microscopy
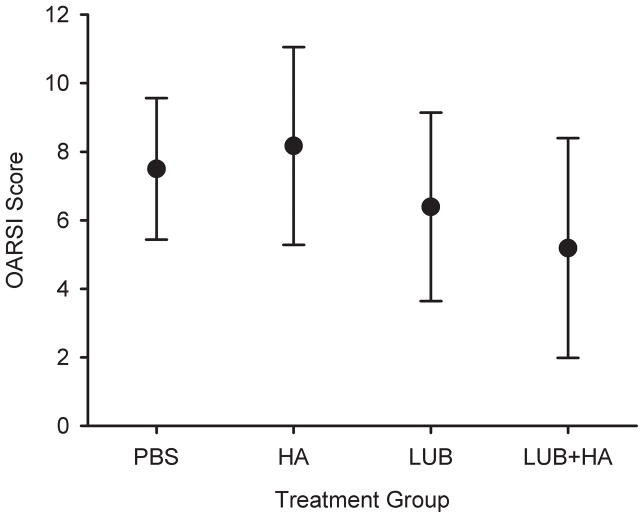
The mean OARSI scores were greatest in the saline- and HA-treated groups, and lower in the LUB- and LUB+HA-treated groups (Figure 1). Relative to PBS, HA treatment resulted in a 9% higher mean OARSI score, while LUB and LUB+HA reduced the mean OARSI score by 15% and 32%, respectively. All groups showed some evidence of cartilage damage that included surface fibrillation, hyper- and hypo-cellularity, and loss of proteoglycan staining (Figure 2). The joints treated with LUB had significantly lower OARSI histology scores than saline- or HA-treated knees (p=.015). There was no evidence that the addition of HA produced changes in OARSI scores (p=.51), nor did HA significantly influence the effect of LUB (p=.37).
Figure 1.
Mean OARSI scores for the PBS, HA, LUB, and LUB+HA treatment groups. The LUB and LUB+HA were significantly less than those of the PBS and HA groups (p<0.05). The error bars represent ± 1 standard deviation.
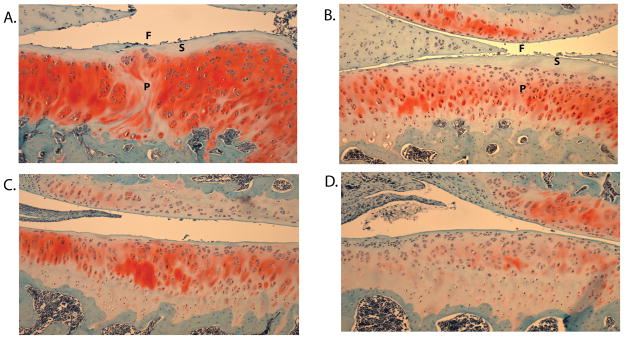
Figure 2.
Images taken of the samples with the median OARSI score for each treatment group: A) PBS, B) HA, C) LUB, and D) LUB+HA. Note the loss of superficial zone cells (S), proteoglycan (P) and increased surface fibrillation (F) in the PBS- and HA-treated sections.
Radiographs
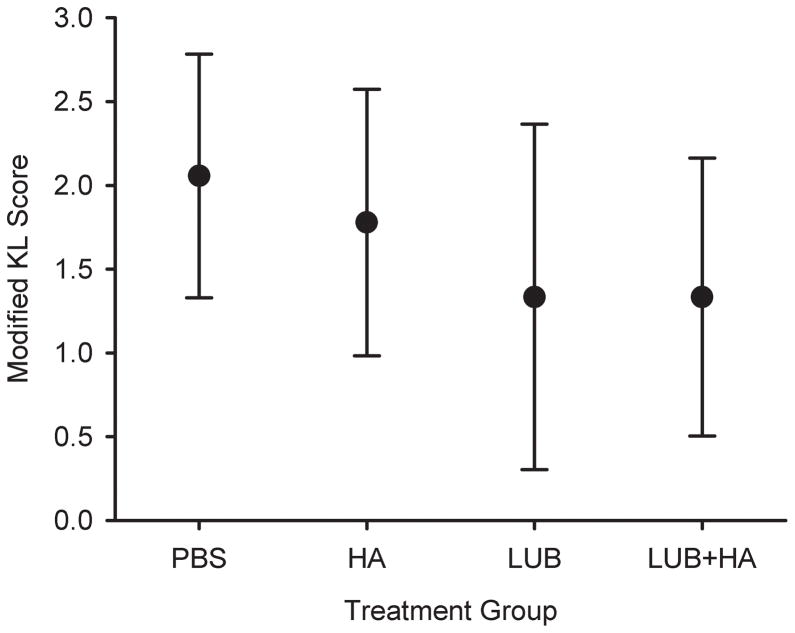
The modified Kellgren-Lawrence scores were highest in the saline- and HA-treated joints, and were significantly lower in the LUB- and LUB+HA-treated joints (p=0.039; Figure 3 & 4). Relative to PBS, HA treatment resulted in a 14% lower modified Kellgren-Lawrence score, while LUB and LUB+HA each reduced the mean score by 38% relative to PBS. There was no evidence that the LUB effect was HA dependent (p=.71).
Figure 3.
Mean modified Kellgren-Lawrence scores for the PBS, HA, LUB, and LUB+HA treatment groups. The LUB and LUB+HA were significantly greater than those of the PBS and HA groups (p<0.05). The error bars represent ± 1 standard deviation.
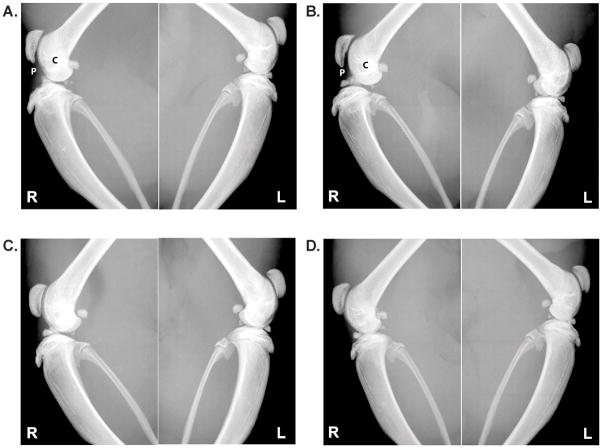
Figure 4.
Lateral images taken of the right (R, treated) and contralateral control left knees (L, ACL intact) with the median modified Kellgren_Lawrence (KL) score for each treatment group: A) PBS, B) HA, C) LUB, and D) LUB+HA. In the PBS- and HA-treated groups, patellar spurring (P) and contour irregularity (C) are prominent.
Synovial fluid analyses
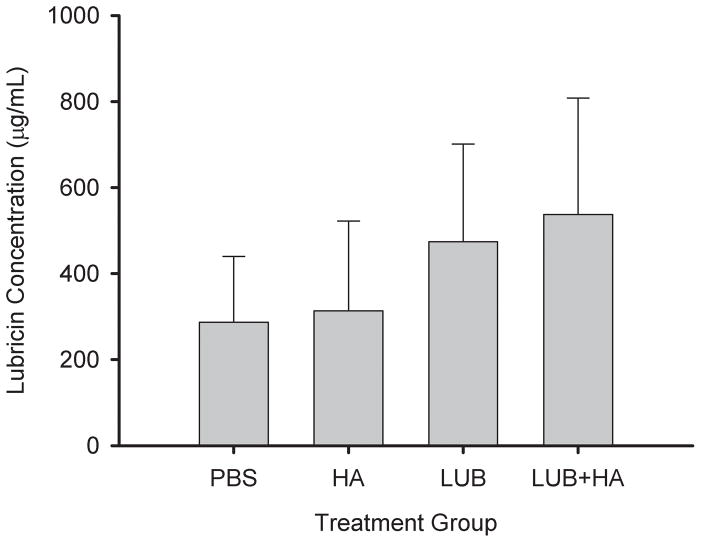
No statistically significant differences were observed in urea-adjusted synovial lavage concentrations of sGAG, CTX-II, IL-1β, and TNF-α at the time of harvest (Table 1). sGAG levels were observed to be lowest in the HA-treated group but overall were similar in all groups. CTX-II levels were lowest in the group treated with lubricin alone while PBS- and HA-treated subjects had similar elevated levels of CTX-II. LUB+HA-treated subjects showed intermediate levels of CTX-II. IL-1β was lowest in the HA-treated group. LUB- and PBS-treated samples had the highest levels of IL-1β. LUB+HA-treated joints showed intermediate levels of IL-1β. TNF-α levels were elevated in PBS-treated specimens, and HA-, LUB-, and LUB+HA-treated specimens had similar lower levels of TNF-α. Lubricin levels were significantly higher in the LUB and LUB+HA treatment groups at time of joint harvest compared to the PBS- and HA-treated groups (p=.008).
Table 1.
Synovial fluid lavage biomarkers (dilution adjusted by comparing the urea concentration in serum and synovial fluid) of cartilage metabolism. All values provided as the mean±standard deviation. No significant interaction between LUB and HA was observed for any of these biomarkers (significance not shown).
| Biomarker | PBS | HA | LUB | LUB+HA | p-value main effect LUB | p-value main effect HA |
|---|---|---|---|---|---|---|
| sGAG (pg/ml) | 890±1405.7 | 253±128.9 | 439±393.1 | 361±161.6 | p=.49 | p=.16 |
| CTX-II (ng/ml) | 4577±6079.8 | 5044±5382.7 | 1654±1202.2 | 3228±2307.0 | p=.11 | p=.48 |
| TNF-α (pg/ml) | 744±348.3 | 409±330.4 | 472±358.4 | 665±625.6 | P=.96 | p=.15 |
| IL-1β (pg/ml) | 760±116.5 | 448±416.7 | 465±216.4 | 623±254.0 | p=.64 | p=.08 |
Discussion
The results of our study confirm our first hypothesis that intra-articular supplementation with lubricin following ACL injury reduces articular cartilage damage as indicated by radiography and histology. However, we were unable to accept our second and third hypotheses since intra-articular supplementation with HA alone did not have an effect, and the addition of HA combined with lubricin did not further reduce articular cartilage damage compared to lubricin alone. Statistically significant differences in synovial fluid levels of inflammatory mediators and cartilage breakdown products were not seen, possibly due to the multifactorial processes by which post-traumatic cartilage degeneration occurs, the possible lack of a difference at the six week time point, the limited sample size, our ability to reproducibly perform the lavages in such a small knee, or other limitations of the rat model. Given that significant differences were present in the histologic and radiologic scores of cartilage damage, and that the lubricin concentrations in the synovial fluid of the lubricin treated animals remained elevated 1 week after the final injection, intra-articular supplementation with lubricin appears to be a promising approach to minimize the impact of post-traumatic OA in the ACL-deficient knee. However, more study is needed to understand the effects of lubricin and HA treatment on the metabolism of articular cartilage over the long term.
ACL transection has been used previously to induce OA in the rat knee.12,18 Using the modified OARSI scale, Hayami et al demonstrate that histological cartilage damage could be detected within 2 weeks of surgery, which would continued to progress over time.18 Visibly roughened articular surfaces, cartilage defects, and osteophyte formation were present 6 weeks post-surgery the interval selected for this study. Hayami concluded that the rat ACL transection model shared many characteristics (i.e. progressive cartilage damage, subchondral bone sclerosis and osteophyte formation) seen in the human and other animal models of OA.12,18 As with most animal OA models, arthrosis appears to be accelerated in the ACL-deficient rat knee when compared to the human. Although this can be perceived as a limitation, the rat provides a validated model that is small, easy to handle, relatively inexpensive, and that responds to both injury and treatment in a timely manner. It is ideal to provide proof of concept and to then design large animal and clinical studies to translate a novel chondroprotective therapy to clinical practice.
The concentration of lubricin for the injections used in our study, 200 μg/mL, was based upon the level of lubricin measured in normal synovial fluid.24,27,45,54–55 Of note, purified human lubricin was used for injection, rather than native rat lubricin, which may introduce the possibility of immunoreactivity and increased cartilage damage. However, our finding of decreased OA progression in the lubricin supplemented joints suggested that this was not the case. Also, the lubricin molecule is highly conserved across species further minimizing this concern. The concentration of HA and molecular weight of HA for the injection protocol in this study was also based on physiologic concentrations. HA is normally found in high concentrations, 2–4 mg/mL, with an average molecular weight of 1 × 106 Da.3,9,27
Following acute knee injury, synovial fluid levels of lubricin are decreased, likely as a consequence of inflammatory degradation and the subsequent loss of lubricating ability.11,23 It has been shown that patients with Camptodactyly Arthropathy Coxa Vara Pericarditis (CACP) syndrome, who lack the functional gene for lubricin expression, produce synovial fluid which lacks lubricating ability, and hence develop severe precocious arthritis.26 It has also been shown that the lack of the lubricin gene in a mouse model results in increased joint friction and accelerated arthritis progression;45 that increased joint friction results from brief proteolytic degradation of the mucin layer at the surface of intact joints;57 and that in late arthritis after ACL transection, low levels of lubricin are associated with increased whole joint friction.57 These findings lead to the question of whether supplementing the lubricating components of synovial fluid may delay or prevent the progression of post-traumatic OA in the ACL injured patient, the objective of the present study.
Our findings that intra-articular lubricin supplementation reduce radiographic and histologic measures of post-traumatic arthritis are consistent with the data published by Flannery et al., who showed delayed OA progression after intra-articular supplementation using recombinant lubricin.14 In their study, a recombinant form of lubricin, containing a shortened central mucin domain, was injection intra-articularly in the rat knee. OA was induced by sectioning the medial meniscus and medial collateral ligament. The rats received recombinant lubricin injections either 1 or 3 times per week for four weeks after surgery, which were matched with saline injection control animals. The outcome measures in their study showed significant reductions in cartilage degeneration scores, total joint scores, widths of severe lesions, and significant cartilage degeneration widths. Their study did not include radiographic or synovial fluid analyses nor did it use a full length natural lubricin control.14
In this study, the K-L score was selected to evaluate radiographic evidence of cartilage damage.32 Because the radiographs were taken post mortem, weightbearing radiographs were not obtained so joint space narrowing was not included in the K-L assessment. The modified numerical score corresponded to the following descriptors of OA: none, doubtful, minimal, moderate, and severe. When the assessments were being performed, the evaluators were blinded to the animal and treatment groups. The average of the two independent scores (an expert and a novice) were intentionally used to add variability. Despite this, there were significant differences between treatments. Poor reliability between examiners would only undermine the analysis if we did not find differences between groups.
Although HA is thought to contribute to the viscosity of synovial fluid,27,31,39 there has been recent debate about its ability to act as a surface lubricant.50 A recent study evaluating the ability of HA to interact with idealized bearing surfaces has shown that it does not effectively coat the articulating surfaces, as would be required for boundary lubrication.7 In addition, synovial fluid treated with hyaluronidase does not lose its lubricating ability, while synovial fluid treated with proteases, which would remove lubricin, is an ineffective lubricant.22 While HA may not be an effective boundary lubricant, it has been shown that lubricin alters the mechanical properties of HA-containing solutions: HA and lubricin in solution together to lower viscosity and alter diffusion compared to HA-only solutions.27 Based on the results of the current study, the combination of HA plus lubricin does not appear to be synergistic in preventing cartilage damage, though it should be noted that this may be due to the limited sample size which was only 80% powered to detect a 35% between the mean values.
Although significant differences in inflammatory markers (IL-1β, TNF-α) and cartilage breakdown products (CTX-II, sGAG) were not seen in this study, existing data suggest that both HA and lubricin may act as signaling molecules in addition to possessing mechanical lubricating properties. Of interest in this study, HA injection had the greatest effect in lowering IL1- β, while both lubricin- and HA-treated animals had lower levels of TNF- α. The cytokines IL-1β and TNF-α are of importance in cartilage degradation in OA. IL-1β has been found to be spontaneously released by OA cartilage,2 and both IL-β and TNF-α perpetuate cartilage matrix degradation by stimulating the release of stromelysin (MMP-3), collagenases (MMP-1/8/13), gelatinases (MMP-2/9), and by their own action.40,43 It should be noted that the cartilage metabolism biomarker data from this study must be treated with caution due to the relatively small sample size. For these four biomarkers we were only 80% powered to detect a shift of one standard deviation between treatments. Furthermore, lavages were only obtained after euthanasia so we do not know what the concentrations of each marker were during the active course of treatment. Further investigations will need to address the metabolic effects of lubricin and HA treatment on chondrocyte and synoviocyte metabolism, including their effects on inflammation and enzyme expression.
The progression of OA following injury is multifactorial, and a model in which OA progression occurs more slowly, for example after meniscal injury instead of ACL transection, will be studied in the future to further explore the metabolic effects of tribosupplementation. In addition, interval and longer term follow up periods will be included to determine if the observed reduction in OA progression persists after treatment. The delay in OA progression following lubricin treatment shown in this study did not correlate with significant alterations in inflammatory marker expression, but it is also possible that ongoing mechanical damage, which may have been partially prevented by supplementing the lubricating ability of synovial fluid, was the main contributor to OA progression. Further investigations will focus on the effects of supplemental lubricin and HA on the metabolism and mechanical properties of articular cartilage.
What is known about the subject:Lubricin and HA are lubricants of articular cartilage. Although HA is frequently used to treat the arthritic knee, its ability to reduce cartilage damage has not been demonstrated. Lubricin is down regulated following ACL injury, which in turn places the articular cartilage at greater risk for wear.
What this study adds to existing knowledge:This study demonstrates that lubricin provides chondral protection in the ACL injured knee while HA does not in this experimental model.
Figure 5.
Mean lubricin concentrations in the synovial fluid lavages at the time of joint harvest as a function of treatment group. The concentration was adjusted by comparing the urea concentration in serum and synovial fluid to obtain the dilution factors. The error bars represent ± 1 standard deviation.
Acknowledgments
This study was supported by grants from the National Institutes of Health (R01-AR049199; R01-AR050180; R21-AR055937; P20-RR024484 and R41-AR057276-01) and the RIH Orthopaedic Foundation. It should be noted that one of the authors (GDJ) owns patents related to recombinant lubricin.
References
- 1.Abatangelo G, Botti P, Del Bue M, et al. Intraarticular sodium hyaluronate injections in the Pond-Nuki experimental model of osteoarthritis in dogs. I. Biochemical results. Clin Orthop Relat Res. 1989:278–285. [PubMed] [Google Scholar]
- 2.Abramson SB, Attur M, Amin AR, Clancy R. Nitric oxide and inflammatory mediators in the perpetuation of osteoarthritis. Curr Rheumatol Rep. 2001;3:535–541. doi: 10.1007/s11926-001-0069-3. [DOI] [PubMed] [Google Scholar]
- 3.Balazs EA, Watson D, Duff IF, Roseman S. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritis human fluids. Arthritis Rheum. 1967;10:357–376. doi: 10.1002/art.1780100407. [DOI] [PubMed] [Google Scholar]
- 4.Bjornsson S. Simultaneous preparation and quantitation of proteoglycans by precipitation with alcian blue. Anal Biochem. 1993;210:282–291. doi: 10.1006/abio.1993.1197. [DOI] [PubMed] [Google Scholar]
- 5.Blaine T, Moskowitz R, Udell J, et al. Treatment of persistent shoulder pain with sodium hyaluronate: a randomized, controlled trial. A multicenter study. J Bone Joint Surg Am. 2008;90:970–979. doi: 10.2106/JBJS.F.01116. [DOI] [PubMed] [Google Scholar]
- 6.Brandt KD, Smith GN, Jr, Simon LS. Intraarticular injection of hyaluronan as treatment for knee osteoarthritis: what is the evidence? Arthritis Rheum. 2000;43:1192–1203. doi: 10.1002/1529-0131(200006)43:6<1192::AID-ANR2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 7.Chang DP, Abu-Lail NI, Coles JM, Guilak F, Jay GD, Zauscher S. Friction force microscopy of lubricin and hyaluronic acid between hydrophobic and hydrophilic surfaces. Soft Matter. 2009 doi: 10.1039/b907155e. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charnley J. The lubrication of animal joints. Symposium on Biomechanics Institution of Mechanical Engineers. 1959:12–19. [Google Scholar]
- 9.Dahl LB, Dahl IM, Engstrom-Laurent A, Granath K. Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Ann Rheum Dis. 1985;44:817–822. doi: 10.1136/ard.44.12.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowson D, Jin Z-M. Micro-elastohydrodynamic lubrication of synovial joints. Engin Med. 1986;15:63–65. doi: 10.1243/emed_jour_1986_015_019_02. [DOI] [PubMed] [Google Scholar]
- 11.Elsaid KA, Fleming BC, Oksendahl HL, et al. Decreased lubricin concentrations and markers of joint inflammation in synovial fluids from patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58:1707–1715. doi: 10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elsaid KA, Machan JT, Waller K, Fleming BC, Jay GD. The impact of anterior cruciate ligament injury on lubricin metabolism and the effect of inhibiting tumor necrosis factor alpha on chondroprotection in an animal model. Arthritis Rheum. 2009;60:2997–3006. doi: 10.1002/art.24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felson DT. The epidemiology of knee osteoarthritis: results from the Framingham Osteoarthritis Study. Semin Arthritis Rheum. 1990;20:42–50. doi: 10.1016/0049-0172(90)90046-i. [DOI] [PubMed] [Google Scholar]
- 14.Flannery CR, Zollner R, Corcoran C, et al. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. 2009;60:840–847. doi: 10.1002/art.24304. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh P, Read R, Armstrong S, Wilson D, Marshall R, McNair P. The effects of intraarticular administration of hyaluronan in a model of early osteoarthritis in sheep. I. Gait analysis and radiological and morphological studies. Semin Arthritis Rheum. 1993;22:18–30. doi: 10.1016/s0049-0172(10)80016-2. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh P, Read R, Numata Y, Smith S, Armstrong S, Wilson D. The effects of intraarticular administration of hyaluronan in a model of early osteoarthritis in sheep. II. Cartilage composition and proteoglycan metabolism. Semin Arthritis Rheum. 1993;22:31–42. doi: 10.1016/s0049-0172(10)80017-4. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg VM, Buckwalter JA. Hyaluronans in the treatment of osteoarthritis of the knee: evidence for disease-modifying activity. Osteoarthritis Cartilage. 2005;13:216–224. doi: 10.1016/j.joca.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Hayami T, Pickarski M, Zhuo Y, Wesolowski GA, Rodan GA, Duong le T. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone. 2006;38:234–243. doi: 10.1016/j.bone.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Huang MH, Yang RC, Chou PH. Preliminary effects of hyaluronic acid on early rehabilitation of patients with isolated anterior cruciate ligament reconstruction. Clin J Sport Med. 2007;17:242–250. doi: 10.1097/JSM.0b013e31812570fa. [DOI] [PubMed] [Google Scholar]
- 20.Huskin JP, Vandekerckhove B, Delince P, et al. Multicentre, prospective, open study to evaluate the safety and efficacy of hylan G-F 20 in knee osteoarthritis subjects presenting with pain following arthroscopic meniscectomy. Knee Surg Sports Traumatol Arthrosc. 2008;16:747–752. doi: 10.1007/s00167-008-0556-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jay GD, Britt DE, Cha CJ. Lubricin is a product of megakaryocyte stimulating factor gene expression by human synovial fibroblasts. J Rheumatol. 2000;27:594–600. [PubMed] [Google Scholar]
- 22.Jay GD, Cha CJ. The effect of phospholipase digestion upon the boundary lubricating ability of synovial fluid. J Rheumatol. 1999;26:2454–2457. [PubMed] [Google Scholar]
- 23.Jay GD, Elsaid KA, Zack J, et al. Lubricating ability of aspirated synovial fluid from emergency department patients with knee joint synovitis. J Rheumatol. 2004;31:557–564. [PubMed] [Google Scholar]
- 24.Jay GD, Haberstroh K, Cha CJ. Comparison of the boundary-lubricating ability of bovine synovial fluid, lubricin, and Healon. J Biomed Mater Res. 1998;40:414–418. doi: 10.1002/(sici)1097-4636(19980605)40:3<414::aid-jbm11>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 25.Jay GD, Harris DA, Cha CJ. Boundary lubrication by lubricin is mediated by O-linked beta(1-3)Gal-GalNAc oligosaccharides. Glycoconjugate Journal. 2001;18:807–815. doi: 10.1023/a:1021159619373. [DOI] [PubMed] [Google Scholar]
- 26.Jay GD, Torres JR, Rhee DK, et al. Association between friction and wear in diarthrodial joints lacking lubricin. Arthritis Rheum. 2007;56:3662–3669. doi: 10.1002/art.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jay GD, Torres JR, Warman ML, Laderer MC, Breuer KS. The role of lubricin in the mechanical behavior of synovial fluid. Proc Nat Acad Sci. 2007;104:6194–6199. doi: 10.1073/pnas.0608558104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones AR, Chen S, Chai DH, et al. Modulation of lubricin biosynthesis and tissue surface properties following cartilage mechanical injury. Arthritis Rheum. 2009;60:133–142. doi: 10.1002/art.24143. [DOI] [PubMed] [Google Scholar]
- 29.Jones ES. Joint Lubrication. Lancet. 1934;1:1426–1427. [Google Scholar]
- 30.Jones ES. Joint Lubrication. Lancet. 1936;1:1043–1044. [Google Scholar]
- 31.Kawano T, Miura H, Mawatari T, et al. Mechanical effects of the intraarticular administration of high molecular weight hyaluronic acid plus phospholipid on synovial joint lubrication and prevention of articular cartilage degeneration in experimental osteoarthritis. Arthritis Rheum. 2003;48:1923–1929. doi: 10.1002/art.11172. [DOI] [PubMed] [Google Scholar]
- 32.Kellgren JK, Lawrence JS. Radiological assessment of osteoarthritis. Ann Rheum Dis. 1957;15:494–501. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraus VB, Huebner JL, Fink C, et al. Urea as a passive transport marker for arthritis biomarker studies. Arthritis Rheum. 2002;46:420–427. doi: 10.1002/art.10124. [DOI] [PubMed] [Google Scholar]
- 34.Krishnan R, Kopacz M, Ateshian GA. Experimental verification of the role of interstitial fluid pressurization in cartilage lubrication. J Orthop Res. 2004;22:565–570. doi: 10.1016/j.orthres.2003.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krishnan R, Park S, Eckstein F, Ateshian GA. Inhomogeneous cartilage properties enhance superficial interstitial fluid support and frictional properties, but do not provide a homogeneous state of stress. J Biomech Engin. 2003;125:569–577. doi: 10.1115/1.1610018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar P, Oka M, Toguchida J, et al. Role of uppermost superficial surface layer of articular cartilage in the lubrication mechanism of joints. J Anat. 2001;199:241–250. doi: 10.1046/j.1469-7580.2001.19930241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linn FC. Lubrication of Animal Joints II: The Mechanism. J Biomech. 1968;1:193–205. doi: 10.1016/0021-9290(68)90004-3. [DOI] [PubMed] [Google Scholar]
- 38.Louboutin H, Debarge R, Richou J, et al. Osteoarthritis in patients with anterior cruciate ligament rupture: a review of risk factors. Knee. 2009;16:239–244. doi: 10.1016/j.knee.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Mabuchi K, Tsukamoto Y, Obara T, Yamaguchi T. The effect of additive hyaluronic acid on animal joints with experimentally reduced lubricating ability. J Biomed Mater Res. 1994;28:865–870. doi: 10.1002/jbm.820280805. [DOI] [PubMed] [Google Scholar]
- 40.Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Ther. 2003;5:54–67. doi: 10.1186/ar623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noble PW, Lake FR, Henson PM, Riches DW. Hyaluronate activation of CD44 induces insulin-like growth factor-1 expression by a tumor necrosis factor-alpha-dependent mechanism in murine macrophages. J Clin Invest. 1993;91:2368–2377. doi: 10.1172/JCI116469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sports Med. 2009;37:1434–1443. doi: 10.1177/0363546509338827. [DOI] [PubMed] [Google Scholar]
- 43.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237–1247. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 44.Pritzker KP, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13–39. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 45.Rhee DK, Marcelino J, Baker M, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115:622–631. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richette P, Ravaud P, Conrozier T, et al. Effect of hyaluronic acid in symptomatic hip osteoarthritis: a multicenter, randomized, placebo-controlled trial. Arthritis Rheum. 2009;60:824–830. doi: 10.1002/art.24301. [DOI] [PubMed] [Google Scholar]
- 47.Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971;53:69–82. [PubMed] [Google Scholar]
- 48.Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 2001;3:107–113. doi: 10.1186/ar148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schiavinato A, Lini E, Guidolin D, et al. Intraarticular sodium hyaluronate injections in the Pond-Nuki experimental model of osteoarthritis in dogs. II. Morphological findings. Clin Orthop Relat Res. 1989:286–299. [PubMed] [Google Scholar]
- 50.Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 2007;56:882–891. doi: 10.1002/art.22446. [DOI] [PubMed] [Google Scholar]
- 51.Sharma L, Kapoor D, Issa S. Epidemiology of osteoarthritis: an update. Curr Opin Rheumatol. 2006;18:147–156. doi: 10.1097/01.bor.0000209426.84775.f8. [DOI] [PubMed] [Google Scholar]
- 52.Smith GN, Jr, Myers SL, Brandt KD, Mickler EA. Effect of intraarticular hyaluronan injection in experimental canine osteoarthritis. Arthritis Rheum. 1998;41:976–985. doi: 10.1002/1529-0131(199806)41:6<976::AID-ART4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 53.Strauss EJ, Hart JA, Miller MD, Altman RD, Rosen JE. Hyaluronic acid viscosupplementation and osteoarthritis: current uses and future directions. Am J Sports Med. 2009;37:1636–1644. doi: 10.1177/0363546508326984. [DOI] [PubMed] [Google Scholar]
- 54.Swann DA, Radin EL. The molecular basis of articular lubrication. I. Purification and properties of a lubricating fraction from bovine synovial fluid. J Biol Chem. 1972;247:8069–8073. [PubMed] [Google Scholar]
- 55.Swann DA, Silver FH, Slayter HS, Stafford W, Shore E. The molecular structure and lubricating activity of lubricin isolated from bovine and human synovial fluids. Biochem J. 1985;225:195–201. doi: 10.1042/bj2250195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teeple E, Elsaid KA, Fleming BC, et al. Coefficients of friction and cartilage damage in the guinea pig knee. J Orthop Res. 2008;26:231–237. doi: 10.1002/jor.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teeple E, Fleming BC, Mechrefe AP, Crisco JJ, Brady MF, Jay GD. Frictional Properties of Hartley Guinea Pig Knees With and Without Proteolytic Disruption of the Articular Surfaces. Osteoarthritis Cartilage. 2007;15:309–315. doi: 10.1016/j.joca.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Unsworth A, Dowson D, Wright V. The Frictional Behavior of Synovial Joints – Part 1: Natural Joints. J Lubrication Technol. 1975:369–376. [Google Scholar]
- 59.Witteveen AG, Giannini S, Guido G, et al. A prospective multi-centre, open study of the safety and efficacy of hylan G-F 20 (Synvisc) in patients with symptomatic ankle (talo-crural) osteoarthritis. Foot Ankle Surg. 2008;14:145–152. doi: 10.1016/j.fas.2008.01.001. [DOI] [PubMed] [Google Scholar]