Abstract
The mechano-sensitive responses of the heart and brain were examined in the chick embryo during Hamburger and Hamilton stages 10–12. During these early stages of development, cells in these structures are organized into epithelia. Isolated hearts and brains were compressed by controlled amounts of surface tension (ST) at the surface of the sample, and microindentation was used to measure tissue stiffness following several hours of culture. The response of both organs was qualitatively similar, as they stiffened under reduced loading. With increased loading, however, the brain softened while heart stiffness was similar to controls. In the brain, changes in nuclear shape and morphology correlated with these responses, as nuclei became more elliptical with decreased loading and rounder with increased loading. Exposure to the myosin inhibitor blebbistatin indicated that these changes in stiffness and nuclear shape are likely caused by altered cytoskeletal contraction. Computational modeling suggests that this behavior tends to return peak tissue stress back toward the levels it has in the intact heart and brain. These results suggest that developing cardiac and neural epithelia respond similarly to changes in applied loads by altering contractility in ways that tend to restore the original mechanical stress state. Hence, this study supports the view that stress-based mechanical feedback plays a role in regulating epithelial development.
1 Introduction
Epithelia (cell sheets) play a central role in embryonic development, as they elongate, shorten, fold, and invaginate to create structures such as the brain, spinal cord, eyes, blood vessels, the heart, lungs, and the gut40. These morphogenetic processes involve mechanical forces, and, prior to the 1980s, considerable effort was devoted to uncovering the physical mechanisms of development. More recent studies, however, have focused chiefly on genetic and molecular regulation. Although significant advances have been made in these areas, the links between the mechanistic and regulatory aspects of epithelial morphogenesis remain poorly understood.
Recent work has shown that cells are highly sensitive to their local mechanical environment. Substrate stiffness can direct cell differentiation11, motility37, and growth rate39, while mechanical stress and strain affects cell proliferation47, cell sorting27, cytoskeletal remodeling16, and patterns of growth10,32,47. The specific response depends on cell type, differentiation state, and loading characteristics6. In mature organisms, mechanical perturbations often elicit an adaptive response that tends to restore a homeostatic or optimal state consistent with evolutionary law21,25.
In the embryo, researchers have hypothesized that active responses to mechanical perturbations play a role in driving and regulating morphogenesis43. Experimental data accumulated by Beloussov and co-workers seem to support this view3,26, and we have found that the looping heart tube stiffens via cytoskeletal contraction when normal loads applied by the overlying splanchnopleuric membrane are removed33,36. Our data also suggest that this response represents a morphogenetic adaptation that restores normal looping.
Here, we study this contractile response in the heart under more controlled conditions. In addition, because the primitive myocardium is an epithelium, we wondered whether this behavior is a general characteristic of embryonic epithelia. Hence, we also examine the mechano-sensitive response of the neuroepithelium of the early brain tube. Although the present study does not address morphogenesis directly, understanding how embryonic tissues respond to mechanical loads is a crucial step in determining the physical mechanisms of development.
Some features of the brain make it more amenable to analysis than the heart. For example, the brain does not beat and its structure is simpler than that of the heart, as it consists of a single neuroepithelium rather than the three layers (endocardium, cardiac jelly, myocardium) of the heart wall. Also, neural precursor cells in the brain are more highly organized and aligned than myocardial cells in the heart tube. Although experiments were conducted on both the heart and brain, these characteristics foster a more in-depth study of the brain.
We subjected isolated brain and heart tubes from chick embryos to various compressive loads and used microindentation to measure tissue stiffness following several hours in culture. Despite differences in morphology and function, both organs stiffened on decreased loading, while only the brain softened on increased loading. Exposure to the myosin inhibitor blebbistatin indicated that this response is caused by changes in contractility. Further study of the brain revealed that, relative to controls, cell nuclei became more elongated in stiffer brains and more circular in softer brains. Computational modeling indicates that these adaptive changes in nuclear shape are consistent with changes in contractility and wall stress. The results indicate a significant role for tissue stress in regulating epithelial morphology and development.
2 Materials and Methods
Experimental Protocol
Fertilized white Leghorn chicken eggs (Sunrise Farms, Catskill, NY) were incubated for 36 hours to HH10− to 10+20. Embryos were removed from the egg using a filter paper carrier as previously described45. The brain or heart was then isolated from surrounding tissue using a fine glass needle and microscissors. Isolated tissues were transferred to semisolid 0.3% agar gels containing culture media (89% DMEM, 10% chick serum, 1% penicillin/streptomycin/neomycin) prepared in 24-well culture plates. Surface tension was varied by adding and removing liquid media in each well with a micropipette. Applied loads were tightly controlled for no surface tension (NST) samples (submerged in 0.7 mL liquid media) and high surface tension (HST) samples (excess liquid completely removed from the culture gel). A third moderate surface tension (MST) loading condition was slightly less controlled as just enough fluid was removed to match heart or brain diameter in the intact embryo. The purpose of this condition was to simulate the natural loading in the embryo as much as possible without accurately knowing the magnitude of these loads. Because of natural variability, applying the same precisely controlled force to each sample would not yield the same deformation.
Isolated brains were cultured dorsal side up for five hours prior to microindentation, whereas hearts were cultured on the left or right side for twelve hours through the process of c-looping. Samples were cultured in an environment maintained at 37 °C, 95% humidity, and 5% CO2. Imaging with optical coherence tomography verified that fluid volumes (and surface tension loads) remained relatively consistent during culture in all conditions. In some experiments, isolated tissues were cultured in 60 μM (−)-blebbistatin (Sigma, St. Louis, MO) in the dark to inhibit myosin-II based contractility. Contraction was enhanced in a group of HST brains using calyculin A (Sigma) mixed into the agar+media culture gel at a final concentration of 20 nM. Additional experiments were performed at intermediate time points during culture to test the reversibility of imposed loading and chemical perturbations. In a separate study, explant viability was verified for all culture conditions using 0.4% (w/v) Trypan Blue (Sigma).
Bright field images of isolated tissues were captured using a CCD camera (COHU, Model 4915, Coway, CA) attached to a dissecting microscope (Leica MZ8, Wetzlar, Germany). Morphology measurements were performed using commercially available image analysis software (Volocity; Improvision, Waltham, MA).
Optical Coherence Tomography (OCT)
OCT images were generated by detecting the time delay of backscattered light in a sample path versus that of a fixed reference path15 as previously described13. Briefly, our system, custom built in the laboratory of Dr. Andrew Rollins at Case Western Reserve University23, operates at a wavelength of 1310 nm with a laser output of 15 mW. Four images were acquired every 5 μm in a 2x2 mm scanning window and were later averaged together using a custom MATLAB (Mathworks) program. Alternatively, in isolated culture experiments, a Thorlabs (Newton, NJ) OCT system coupled to a Nikon FN1 microscope was used to more efficiently locate and image samples (isolated hearts were no larger than 1 mm3). Subsequent image analysis for both systems was performed using Volocity software, including image cropping, contrast optimization, and noise filtering. Three-dimensional volumes were automatically generated by cropping surrounding tissue and thresholding the inner cavity of the neural tube (see Fig. 3A′).
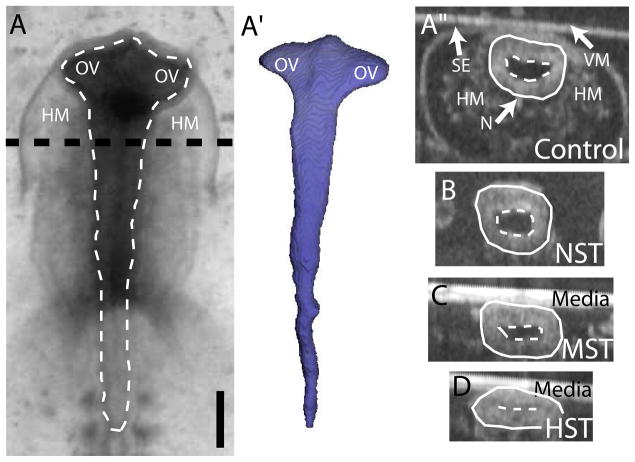
Figure 3. Morphology and loading of the brain tube.
(A) At HH10− the brain is a relatively cylindrical tube with evaginated optic vesicles (OVs) at the cranial end. (A′) 3-D reconstruction of the inner lumen (white outline in A) from optical coherence tomography (OCT) imaging. Transverse OCT cross sections show the prospective midbrain (dashed black line in A) in normal (A″) and altered loading conditions (B–D). (A″–D) For clarity, the basal (solid line) and apical (dashed line) sides of the brain tube have been delineated. After removing surrounding tissues (except the notochord, N), the brain is cultured under (B) no surface tension (NST), (C) moderate surface tension (MST), or (D) high surface tension (HST), depending on the amount of liquid media present during culture. HM = head mesenchyme; SE = surface ectoderm; VM = vitelline membrane. Scale bar: 250 μm.
Micro-indentation and Tissue Stiffness
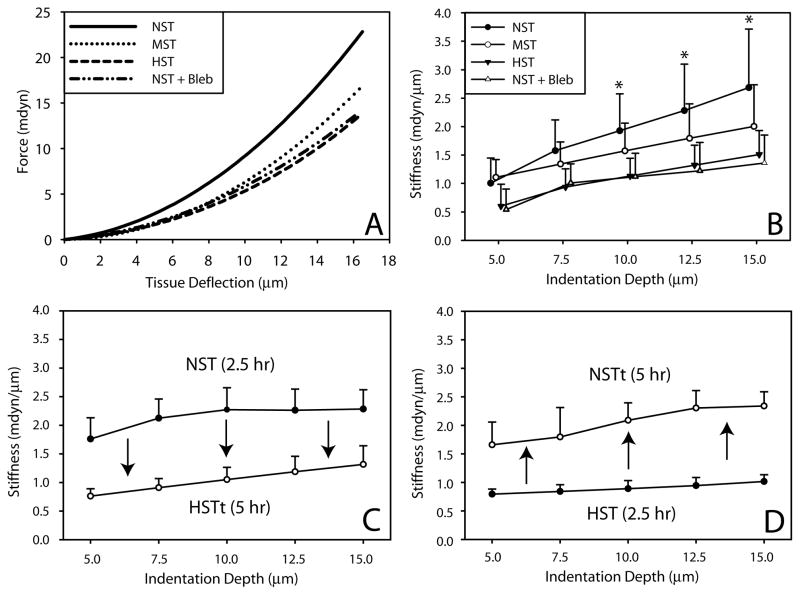
Stiffness was measured using a custom-built micro-indentation device49. Isolated tissues were supported on one side via suction from a micropipette, and a calibrated indenter (flexible glass cantilever beam) was periodically driven into the opposite side of the sample (0.1 Hz) by a piezoelectric motor. Optical measurements of beam deflection and tissue deformation were used to plot indentation force as a function of indentation depth (see Fig. 5A). Tangent stiffness was computed as the slope of this curve at a particular depth (see Fig. 5B). A linear fit was used to approximate the slope of the force-displacement curve at tissue indentation depths ≤ 5 μM, while a four-parameter exponential function was used for larger indentation depths as previously described49.
Figure 5. Stiffness of isolated brains cultured under different loading conditions.
Midbrain stiffness was measured on the dorsal side using a micro-indentation device. (A) Representative force-displacement curves for each of the sample groups are shown. Wall stiffness is the slope of this curve at a particular indentation depth. (B) Stiffness versus indentation depth. Brains cultured under no surface tension (NST, n = 13) for 5 hours were significantly stiffer than those cultured under high surface tension (HST, n = 7) or no surface tension + blebbistatin (NST + bleb, n = 5) at indentation depths ≥ 10 μm (*, p < 0.05). The stiffness of moderate surface tension (MST, n = 5) brains fell between NST and HST samples but differences were not statistically significant. (C,D) Effect of reversed loading conditions on brain stiffness. (C) Brains were isolated and cultured under NST for 2.5 hours, indented, transferred to HST, cultured for an additional 2.5 hours (HSTt), and then indented again. Stiffness significantly decreased at all indentation depths following this change in loading. (D) The opposite experiment (HST culture followed by NST culture) led to an opposite significant stiffening effect. Stiffness values were similar to those measured after 5 hours of culture in each respective culture condition (n ≥ 3 for each load reversal experimental set).
All tissues were indented at room temperature while fully submerged in phosphate buffered saline. Hence, surface tension had no effect on indentation testing. During this process morphology did not change noticeably. Isolated hearts were indented in 50 μM verapamil (L type calcium channel blocker - Sigma) to inhibit the heart beat. Previous studies have shown that this drug has little effect on diastolic myocardial stiffness36. Three indentations were recorded at each sampling location to verify a consistent response. Side studies confirmed measured stiffness was relatively consistent when indenting the same region but in slightly different locations.
Nuclear Geometry
Embryos were fixed in 3.7% formaldehyde overnight and washed in a series of increasing ethanol concentrations (70, 85, 95, and 100%) to dehydrate the tissues. Samples were next washed in xylene, transferred to paraffin, and 10 μm slices of the brain were cut using a microtome (Bausch and Lomb, Rochester, NY). Slices were re-hydrated (reverse ethanol concentrations above), washed in PBS, stained (DAPI, ProLong Gold, Invitrogen, Carlsbad CA), and imaged (Olympus IX70 microscope - 40x magnification). In another test of explant health, we found minimal regions of condensed DNA (indicative of cell death24) after 5 hours of culture.
Nuclear misalignment (the difference in angle between the major axis of the nucleus and the neuroepithelial wall) and circularity (minor/major axis of the best fit ellipse to the nucleus) were measured using ImageJ (http://rsbweb.nih.gov/ij/) and custom image processing routines implemented in Matlab (The Mathworks, Inc, Natick MA). Misalignment was not calculated in nuclei with a circularity ≥ 0.9. More than 80 nuclei were analyzed for each sample and at least four embryos were analyzed for each loading condition.
Statistics
All data are reported as mean +/− SD. Statistical analyses were performed using the statistics package in SigmaPlot (v11, Systat Software Inc.). Tissue morphology and stiffness as well as nuclear circularity and misalignment were compared across multiple sample groups using one-way ANOVA, with post hoc pairwise comparison made using the Bonferroni - Dunn Test (p < 0.05 for statistical significance). To compare samples indented before and after changes in loading or blebbistatin treatment, a two tailed, paired t-test was used.
3 Results
Isolated hearts stiffen under reduced loads
First, we consider the morphology, culturing, and mechanical testing of the isolated heart. The embryonic chick heart forms as a relatively simple tube at Hamburger-Hamilton stage 10 (HH10, approximately 33 hours)20. At this stage of development, an inner endocardium (one cell, approx. 10 μm thick) is separated from an outer myocardium (two cells, approx. 20 μm thick) by a viscous extracellular matrix dubbed the cardiac jelly (CJ) (Fig. 1A). Isolated hearts were studied over a time period of about 12 hours (HH10–HH12). These stages of development encompass the morphogenetic process of cardiac c-looping, as the initially straight heart deforms into a c-shaped tube29.
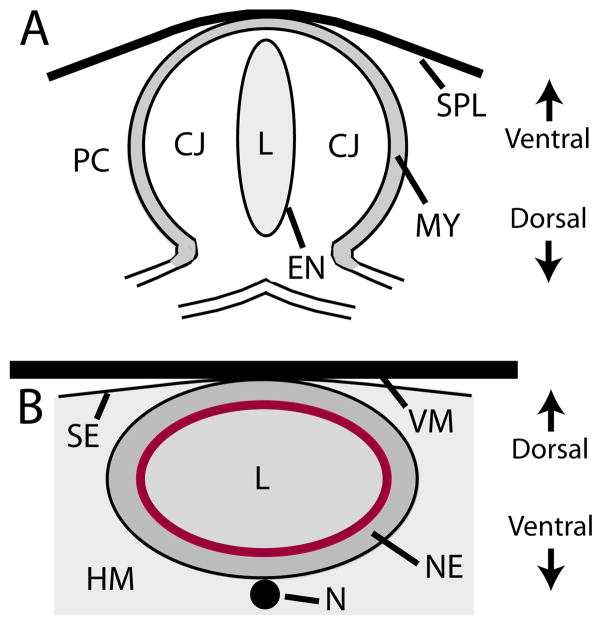
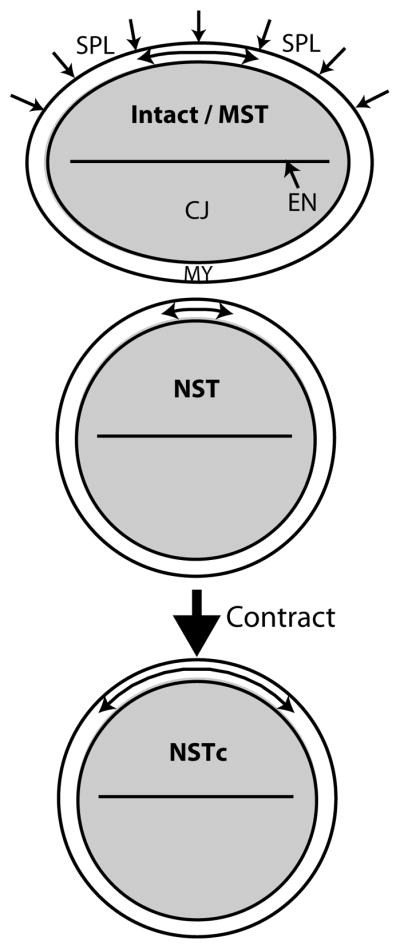
Figure 1. Cross-sectional schematics of heart and brain of early chick embryo.
(A) Primitive heart ventricle. The splanchnopleure (SPL) membrane compresses the ventral side of the myocardium (MY). The MY is separated from the endocardium (EN) by the acellular, viscous cardiac jelly (CJ). The EN surrounds the lumen (L) of the heart tube and the extracellular space outside the heart is the pericardial coelom (PC). (B) Midbrain. The neuroepithelium (NE) encloses the lumen (L), and the notochord (N) lies along the ventral side of the brain tube. The brain is constrained by the surrounding head mesenchyme (HM), surface ectoderm (SE), and vitelline membrane (VM). The red ring indicates the local concentration of actin microfilaments at the luminal (apical) side of the wall (see Fig. S1B).
Isolated HH10 hearts were cultured in fluid whose depth was adjusted to provide three types of loading (Fig. 2): no surface tension (NST), moderate surface tension (MST), and high surface tension (HST). The NST condition (fully submerged heart) effectively eliminated external loads from surrounding tissues45 (Fig. 2A). The MST case was used to approximate loads in the intact embryo, as liquid media was removed until the horizontal diameter of the isolated heart was similar to that of the intact heart (Fig. 2B). Finally, to culture explants under high surface tension (HST), liquid media was wicked away from the surface of the culture gel, greatly compressing the heart (Fig. 2C).
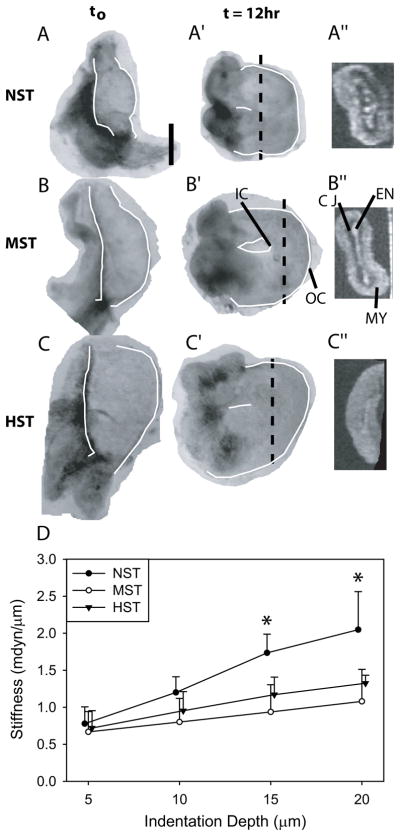
Figure 2. Isolated heart morphology and stiffness under altered loading conditions.
(A–C) Embryonic hearts were isolated at HH10− - 10+ and cultured under NST (no surface tension, n = 6), MST (moderate surface tension, n = 6), or HST (high surface tension, n = 5). (A′–C′) After 12 hours, the hearts were looped. (A″–C″) Less cardiac jelly (CJ) separated the endocardium (EN) and the myocardium (MY) as loads increased. Dashed lines in A′–C′ indicate OCT cross section locations in A″–C″. Heart tubes are outlined for clarity in bright field images; OC = outer curvature, IC = inner curvature. Scale bar: 250 μm. (D) Hearts were indented on the outer curvature after culture. Stiffness was similar at a 5 μm indentation depth, but diverged thereafter. NST hearts were significantly stiffer (*, p<0.05) than MST and HST hearts at indentation depths ≥15 μm. MST hearts were similar in stiffness to HST hearts at all indentation depths.
Cross sections obtained by optical coherence tomography (OCT) reveal that, as compressive loading increased, samples were flattened and less cardiac jelly separated the myocardium and the endocardium (Fig. 2A″–C″). In general, the internal lumen was closed after culture (Fig. 2A″–C″), but the isolated heart looped into a c-shaped tube in all culture conditions, with the dorsal mesocardium located at the inner curvature (Fig. 2A′–C′), further confirming the robust nature of this process in extraembryonic conditions4,38.
Microindentation tests were used to measure tissue stiffness, as hearts were indented on the outer curvature of the loop (OC, see Fig. 2B′). Past studies have found the outer layer of myocardium to be an order of magnitude stiffer than the inner layer of cardiac jelly49, suggesting that measured stiffness is primarily that of the myocardium. During these tests, verapamil (calcium channel blocker - 50 μM) was used to inhibit sarcomeric contraction, arresting the heart without changing the diastolic mechanical properties of the myocardium33,36. Note that verapamil affects sarcomeric contraction, but not non-sarcomeric (cytoskeletal) contraction.
Because force-displacement curves were nonlinear, the stiffness (slope) varied with indentation depth38. Consistent with our previous results for unloaded hearts in ovo33, hearts cultured under NST for 12 hours were significantly stiffer than hearts cultured under MST or HST at indentation depths ≥ 15 μm (Fig. 2D). Hearts cultured under MST were similar in stiffness to HST hearts (Fig. 2D). In summary, the stiffness of the heart increased significantly when compressive loads were removed, but the stiffness changed relatively little under loads greater than control. Previously, we showed that the increased stiffness in response to decreased loading is caused by cytoskeletal contraction38.
Isolated brains shorten, curl, and stiffen under reduced loads
The above experiments were repeated for the brain. Brains were removed from the embryo at HH10. At this stage, the brain is a tubular, pseudostratified neuroepithelium (one cell, approx. 40–50 μm thick) surrounded by the vitelline membrane, surface ectoderm, head mesenchyme, and notochord (Fig. 1B). Actin microfilaments are concentrated along the apical (inner) side of the tube (red outline in Fig. 1B, Fig. S1B). In addition, the optic vesicles (OVs) have evaginated laterally from the cranial end of the brain tube, although the brain ventricles cannot yet be easily identified (Fig. 3A,A′). In the intact embryo, the midbrain is compressed by the vitelline membrane and the head mesenchyme, yielding a slightly elliptic cross section (Fig. 3A″).
Under NST conditions, the lumen of the isolated brain opens further (Fig. 3B). As in the heart experiments, the MST state was obtained by removing liquid media until the diameter of the isolated brain was similar to that of the intact brain (Fig. 3C). Finally, removing most of the fluid gave HST, which compressed the brain until the lumen was no longer apparent (Fig. 3D). Isolated brains were cultured for 5 hours in each loading condition and resulting tissue lengths, stiffnesses, and nuclear shapes were measured.
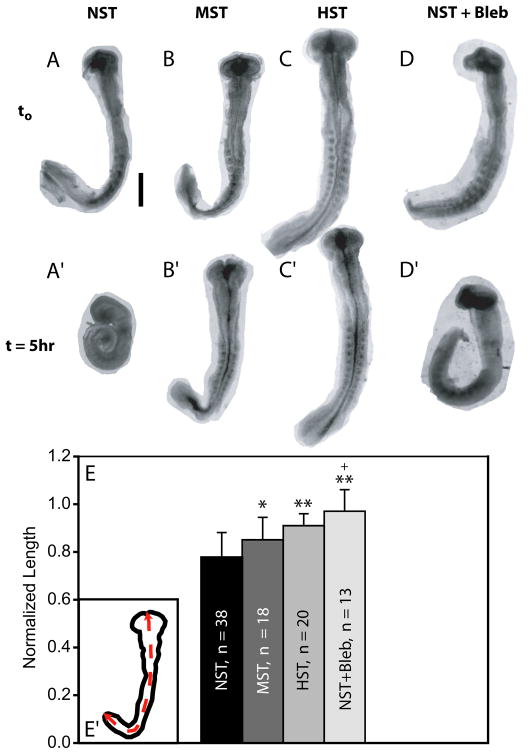
In the NST condition, the length of isolated brains decreased by approximately 25% during culture, and the brain tube curled with the relatively stiff notochord located at the inner curvature (similar to the dorsal mesocardium in the heart) (Fig. 4A,A′,E). MST and HST brains decreased in length but significantly less than the NST samples (MST, p<0.05; HST, p<0.001), and did not curl (in contrast to the heart) as these explants were pressed into the semi-solid media by the surface tension (Fig. 4B,B′,C,C′,E). While a significant difference in length change was not found between MST and HST conditions, in general, it appeared that shortening was inversely proportional to mechanical loading (Fig. 4E).
Figure 4. Isolated brain morphology under altered loading conditions.
Isolated brains were cultured for 5 hours under no surface tension (NST, n = 38), moderate surface tension (MST, n = 18), or high surface tension (HST, n = 20). Additional brains were cultured under NST in blebbistatin (60 μM, NST+bleb, n = 13) to inhibit cytoskeletal contraction. (A–D) HH10 brains at the beginning of the experiment. (A′–D′) Same brains after 5 hours of culture. (E) Ratio of brain tube length after culture to length before culture. Brains became shorter, with NST and MST brains shortening more than HST brains. Treating NST brains with bleb prevented the length decrease and curling observed in NST samples. (E′) All brains were measured along the midline. Statisitics: * = p < 0.05 and ** = p< 0.001 relative to the NST loading condition, and + = p < 0.05 relative to the MST loading condition. Scale bar: 500 μm.
Microindentation was used to measure tissue stiffness in the dorsal midbrain for each loading condition. In general, due in part to differences in global structure, isolated brains were stiffer than isolated hearts (Figs. 2D and 5B), a result consistent with past studies38,48. Explant stiffness increased as external loading decreased (Fig. 5B), a difference that was statistically significant for NST samples relative to HST brains for indentation depths ≥ 10 μm (Fig. 5B).
To explore the reversibility of these perturbations, we performed additional experiments in which loading was changed at an intermediate time point during culture. Brains were cultured for half the time (2.5 hours) with increased (HST) or decreased (NST) loading, indented, cultured the remaining 2.5 hours under the opposite loading and then indented again (Fig. 5C,D). At the halfway point, stiffness values were similar to those of brains cultured for the full five hours in each respective loading condition. Following transfer to the opposite loading condition (HSTt, NSTt), the stiffnesses of HSTt and NSTt brains decreased and increased, respectively. These results show that the responses to altered loading conditions are reversible.
Structural stiffness depends on both material properties and geometry. Because brain geometry varied greatly between sample groups (Fig. 3B–D), it was important to determine how geometry affected our results. To estimate the effects of geometry on apparent tissue stiffness, finite-element models for brains undergoing micro-indentation were created using COMSOL Multiphysics software (Fig. S2, Supplementary Methods). For the same material properties, the models showed that apparent stiffness was approximately 1.6 times higher in collapsed brains relative to those with an internal lumen (Fig. S2C). Hence, the flattening effect of high surface tension would increase the measured tissue stiffness, and it follows that for the same geometry, stiffness differences between HST versus NST and MST samples may in fact be even larger than our data indicate.
Changes in brain morphology and stiffness correlate with changes in cytoskeletal contractility
To test whether these changes in morphology and stiffness were caused by cytoskeletal contraction, we inhibited myosin II by culturing specimens in 60 μM blebbistatin (bleb) under NST conditions. The length of explants exposed to bleb decreased only slightly (<5%) (Fig. 4D,D′,E), and these explants curled less than those cultured under NST alone (Fig. 4A′,D′). Bleb-treated brains shortened significantly less than the MST (p<0.05) and NST (p<0.001) brains without bleb (Fig. 4E). Taken together, these data suggest that the embryonic brain actively shortens via cytoskeletal contraction when external loads are reduced.
In additional experiments, NST brains were cultured in bleb for 2.5 hours and then transferred to normal media. After bleb washout, curling resumed (Fig. S3A″). Also, when bleb was added to brains undergoing curling for 2.5 hours, curling ceased and the overall tissue size increased (Fig. S3B″). These results show that inhibiting contraction stops brain curling after it begins and that this effect is reversible.
Brain stiffness was measured to confirm the effects of bleb on contractility. Stiffness of HST brains was similar to the NST+bleb brains at all indentation depths, suggesting reduced contraction in HST explants (Fig. 5B). To further examine the effects of bleb exposure time, a subset of the NST explants (NSTs) were reincubated for an additional hour in 60 μM blebbistatin and reindented (Fig. S4). A statistically significant decrease in tissue stiffness was found at indentation depths ≥ 10 μm. Stiffness decreased to approximately the same value as tissues cultured for the full five hours in blebbistatin (dashed line in Fig. S4).
In summary, brain stiffness increased as externally applied loads decreased (Fig. 5B). This response was myosin-II dependent, as stiffness and length changes did not occur in blebbistatin-treated samples cultured under NST conditions (Figs. 4,5,S3,S4). The brain, therefore, contracts and stiffens when compressive loads are decreased, but relaxes and softens when loads are increased.
Brain nuclei change shape under changes in load
Lastly, we speculated that changes in contractility alter wall stresses, which in turn affect the shape of cell nuclei. Studying nuclear shape in the brain is easier than in the developing heart, because brain nuclei have more consistent patterns than those in the heart (Supplementary Information). In the normal brain tube, nuclei are generally elongated and aligned in the radial direction from HH10 to HH12 (Fig. S5). Hence, we analyzed changes in nuclear shape and orientation in the midbrain for each loading condition (Fig. 6). Nuclear misalignment was defined as the difference in angle between the major axis of the elliptically shaped nucleus and the normal to the neuroepithelial wall. Nuclear circularity (minor/major axis of the best-fit ellipse, 1 = perfect circle, 0 = straight line) was also calculated.
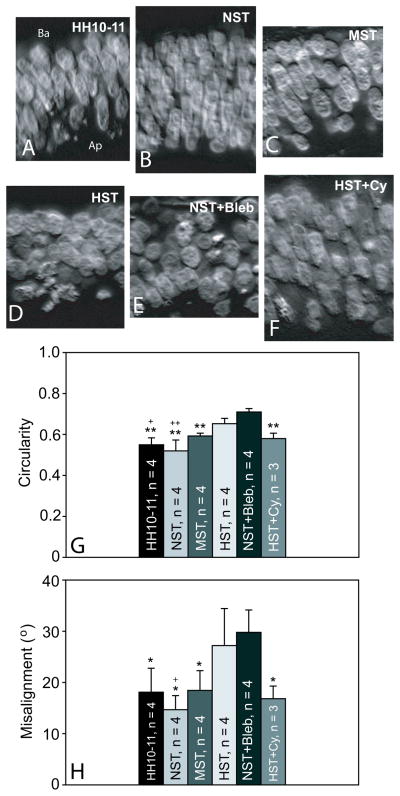
Figure 6. Nuclear shape and orientation in the brain under different loading conditions.
(A) Nuclei are elliptically shaped and radially aligned relative to the inner lumen in control embryos cultured from HH10 to 11. Ap: apical side of the neuroepithelial wall; Ba: basal side of the wall. (B,C) Culturing brains under reduced loading (NST) led to highly organized, more elliptical nuclei, while the morphology of MST (approximating normal loading conditions) samples mimicked HH10-11 controls. (D,E) Nuclei of highly loaded (HST) brains were more circular and randomly oriented than controls, and resembled the phenotype of blebbistatin-treated brains. (F) Stimulating contraction in HST brains with 20 nM calyculin (cy) prevented increases in nuclear circularity and misalignment. (G,H) Nuclear circularity and misalignment relative to the radial direction were quantified and compared between sample groups. As loading decreased, nuclei generally became more elliptical in shape and more aligned. The nuclei of loaded brains (MST and HST) were more circular and misaligned. Statisitics: * = p < 0.05 and ** = p < 0.001 relative to the NST+bleb loading condition; + = p < 0.05 and ++ = p <0.001 relative to the HST loading condition.
As in normal HH11 brains (Fig. S5B′), nuclei were radially elongated and relatively aligned in control brains cultured from HH10 to HH11 (Fig. 6A). This morphology was consistent across the neuroepithelial wall and did not vary in different regions of the midbrain (Fig. S6). In MST brains, nuclear morphology was similar to control (HH10-11) brains (Fig. 6C,G,H), while NST nuclei were more aligned and elliptical than controls, although differences were not statistically significant (Fig. 6B,G,H). Exposure to bleb resulted in rounder (p<0.001), more randomly oriented (p<0.05) nuclei (Fig. 6E,G,H), which were similar to nuclei of HST brains (Fig. 6D,G,H). As a corollary to the bleb experiments, we enhanced myosin contractility in HST samples by adding calyculin (cy - phosphatase inhibitor) to the culture gels at a final concentration of 20 nM. After the same 5 hour culture period, the nuclei of HST+cy brains were more elliptical and radially aligned than HST brains, and had a phenotype similar to the MST samples and the HH10-11 controls (Fig. 6F,G,H).
Taken together, our data suggest that as contractility increases, neuroepithelial nuclei become more elliptical in shape and more radially aligned. As contractility decreases, nuclei become rounder and more randomly oriented.
4 Discussion
The main finding of this study is that epithelia of the embryonic brain and heart respond similarly to altered mechanical loads. Our results indicate that these organs decrease in stiffness as compressive loads increase, and vice versa. Exposure to the myosin II inhibitor blebbistatin shows that these changes in stiffness are associated with changes in cytoskeletal contractility. In the brain, this response is also accompanied by changes in nuclear shape (heart nuclei were not studied). As discussed below, all of these effects are consistent with an adaptive response that tends to restore the original mechanical state.
Even though different types of cells can have widely disparate functions, studies have shown a remarkable similarity in how they respond to certain types of mechanical stimuli. For example, fibroblasts, smooth muscle cells, and endothelial cells all turn away from the direction of oscillatory stretch on a 2-D substrate5, and, for many cell types, the cytoskeleton rapidly fluidizes and then re-solidifies in response to transient stretch44. Of particular relevance here is the study of Mizutani et al. (2004)30, who found that fibroblasts become softer or stiffer in response to stretch or shortening, respectively. Like our results, these responses for single cells correlate with changes in actomyosin contractility. At the tissue level, the present results for the embryonic heart are consistent with previous findings, whereby removing a membrane (splanchnopleure) that normally compresses the looping heart elicits a contractile response that stiffens the myocardium33,36.
During early embryonic development, the heart and brain share some common features. Both are tubes composed primarily of epithelia. In the heart, the rate of growth depends on blood pressure7; in the brain, growth rate depends on cerebrospinal fluid pressure9,10. In addition, mechanically driven changes in tissue shape are crucial to both heart and brain morphogenesis14,19,41. Hence, it is no surprise that both types of epithelia (myocardium and neuroepithelium) exhibit similar mechano-sensitive behaviors.
In our experiments, both the isolated heart and brain contracted and stiffened when loads from surrounding tissues were removed (Figs. 2D and 5). Increased compressive loading induced cytoskeletal relaxation and softening of the brain (Fig. 5), but, in the heart, the stiffness of highly compressed (HST) samples was similar to that of control (MST) samples. The lack of softening in the heart is consistent with the finding that the myocardium of the heart tube contains relatively little myosin II-based cytoskeletal contractility (as opposed to sarcomeric contractility) under normal conditions38. In other words, the diastolic cytoskeleton is already in a relatively relaxed state. In addition, isolated brains were stiffer than isolated hearts in all culture conditions. While individual brain cells generally are softer than heart cells11, in the early embryo, the neuroepithelium (ectoderm) is much thicker than the myocardium (mesoderm), explaining in part the higher neuroepithelial indentation stiffness. Moreover, Krieg et al. (2008) found that early ectodermal cells are significantly stiffer (due to higher cortical tension) than mesodermal and endodermal cells27.
In the brain, these changes in contractility and stiffness are also associated with global changes in brain size and shape, as well as changes in the shape of cell nuclei. Longitudinal contraction causes the brain to shorten and curl (Figs. 4,S3). The curling, with the notochord along the inner curvature, is consistent with flexure that occurs during normal development17. (Similarly, like hearts in ovo, isolated hearts loop with the dorsal mesocardium at the inner curvature.) The enhanced magnitude of the bending in NST brains may be caused by contraction of the actin-rich notochord.31
During early development in ovo, neuroepithelial nuclei become increasingly elliptical and radially aligned (Fig. S5). In our experiments, nuclei became even more elliptical and aligned with the increased contraction induced by decreased loading (Fig. 6B). Decreasing contractility, whether through compressive loading (HST) or drug treatment (NST+bleb), produced circular nuclei with a less organized orientation (Fig. 6D,E,G,H). Since contraction affects wall stress, these results are consistent with studies showing that mechanical forces can alter nuclear shape18,28.
Forces deform the nucleus through cytoskeletal connections, causing conformational changes in DNA or chromatin structure, which can alter gene expression8,46. Progenitor cells are potentially more sensitive to changes in mechanical loads, as their nuclei are softer and more deformable than the nuclei of terminally differentiated cells34. Therefore, we speculate that changes in nuclear shape correlate with changes in wall stress. To illustrate the feasibility of this mechanism, we developed finite element models simulating compressive loading of the tubular heart and brain (Supplementary Information and Methods, Figs. S7–S10). The results and interpretation are summarized in Figs. 7 and 8.
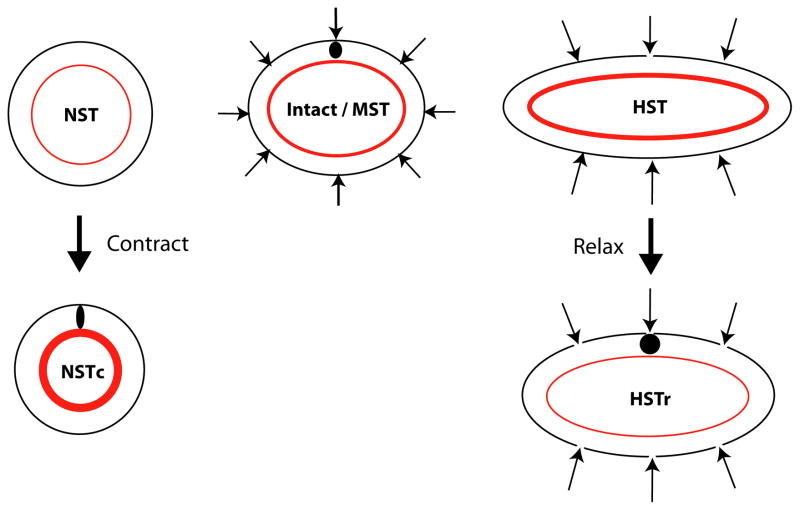
Figure 7. Response of heart to altered mechanical loads.
Schematic for active myocardial response to changes in compressive loading. MST: moderate surface tension (control). The myocardium (MY) is in a state of circumferential tension (shown by arrow in the MY). Tension is caused by loads applied by the cardiac jelly (CJ) as it swells. In ovo compression by the overlying splanchnopleure (SPL) membrane is simulated by the surface tension. The lumen surrounded by the endocardium (EN) is closed in isolated hearts (see Fig. 2). NST: no surface tension. Removing external loads decreases myocardial tension. NSTc: Active contraction increases tension in the MY, counteracting the initial loss in tension following compressive load removal. See Figs. S7,S8 for validation of this schematic by computational modeling.
Figure 8. Response of brain to altered mechanical loads.
Schematic for active response of the neuroepithelium to changes in loading. Line thickness of the apical actomyosin ring (red) reflects relative contractility causing these adaptive responses. MST: Moderate surface tension (control). Radial compressive loads are imposed by surrounding tissues in the intact embryo (see Fig. 1B). Nuclei are elliptical in shape and radially oriented (black circle). Actin ring is in a state of circumferential tension due to contraction. NST: Removing surrounding tissues decreases peak tension in actin ring. NSTc: Elevated contraction in the actomyosin ring increases to counter this decrease in tension. Circumferential compression increases in the remainder of the neuroepithelium, favoring radially elongated nuclei. HST: High compressive loading increases peak tension in ring. HSTr: The actomyosin ring relaxes to offset this increase in tension. This reduces circumferential tension across the remainder of the wall, favoring circular nuclei. See Figs. S9, S10 for validation of this schematic by computational modeling.
According to the model for the heart, reducing the normal compressive load decreases the myocardial tension caused by swelling and squeezing of cardiac jelly (Fig. 7 and S8, MST to NST). In response to this perturbation, actomyosin contraction restores myocardial tension (Figs. 7 and S8, NSTc). In a previous study, we found that a similar response to decreased loading can play a role in morphogenesis of the looping heart33,36.
The situation in the brain is a little more complicated. The main reason is that, since the neuroepithelial wall is relatively thick, compressing the brain causes bending stresses that are strongly inhomogeneous (Fig. S10). We assume that (1) contractile stress is generated predominately by the actomyosin-rich network concentrated at the apical (inner) side of the neuroepithelium (Fig. S1B); (2) apical actin is a key mechano-sensor in the brain tube; and (3) peak actin stress modulates the response of the entire actin network. The last assumption is consistent with recent work suggesting that local changes in tension can induce large-scale changes in contractility in neighboring cells6,12,35.
In the brain tube, increasing compressive loading (HST) increases the maximum tension in the apical actomyosin ring, while decreasing compression (NST) has the opposite effect (Fig. S10). In response, the inner ring contracts when loads are removed (NSTc) and relaxes when loads are increased (HSTr) (Figs. 8 and S10). These responses tend to reverse the direction of the original perturbation in peak actin stress (Fig. S10).
Furthermore, apical contraction causes circumferential compression throughout the remainder of the wall (Fig. S10, NST to NSTc). This change in stress favors elliptical, radially aligned nuclei as seen experimentally (Fig. 8, NSTc case; Fig. 6B). Conversely, apical relaxation has the opposite effect (Fig. S10, HST to HSTr), favoring the relatively circular nuclei observed after HST culture (Fig. 8, HSTr case; 6D).
The non-uniform stresses in the brain under all loading conditions (Figs. S9 and S10), however, does not seem consistent with the relatively uniform patterns in nuclear shape observed in the neuroepithelial wall (Fig. S6). One explanation for this discrepancy may be that our model is too simple, as it neglects, for example, radial contraction and the effects of 3-D geometry. Another possibility is that the nuclei intrinsically maintain a preferred shape that is modulated by a uniformly distributed molecular signal triggered by peak actin stress. Clearly more work is warranted to decipher how epithelial cell (and nuclear) shape is optimized with respect to changes in mechanical stress during development.
Together, our data suggest that embryonic epithelia respond to stress perturbations in ways that tend to restore the original (possibly peak) stress in the tissue, and that this adaptive response is brought about by alterations in cytoskeletal contractility. However, if our mechanically perturbed specimens were simply adapting ‘toward’ an original stress state, the nuclear shapes should begin to resemble those in control samples. In contrast, our results indicate that NST nuclei eventually become more elliptical than controls (MST), while HST nuclei become rounder than controls (Fig. 6). These data suggest that the response causes overshoots of the original nuclear shape and peak stress, in agreement with the hyper-restoration hypothesis proposed by Beloussov and co-workers2,3. The significance of the overshoot has been demonstrated by computational models, which show that if tissue stress returns to its original homeostatic value as in mature tissues, then morphogenesis ceases when homeostatic conditions are restored42. On the other hand, an overshoot in stress can induce further perturbations. In this way, a single perturbation can drive a relatively complete morphogenetic process1.
In conclusion, we have shown that changes in mechanical loading lead to changes in cytoskeletal contractility, which cause alterations in epithelial stiffness and nuclear shape in the developing embryo. We speculate that these changes are mediated by changes in tissue stress. Future studies are needed to determine the role of mechanical feedback in morphogenesis. Better understanding of how precursor cells adapt to changes in mechanical stress also could provide insight into the mechanisms of stress regulation and disease pathology in mature tissues21,22.
Supplementary Material
Acknowledgments
We thank Igor Efimov for access to his OCT system, as well as Anjul Davis at Thorlabs for access to the integrated microscope + OCT system that aided our isolated sample imaging. We also thank Dimitry Voronov and Dylan McCreedy for sectioning and staining advice, in addition to Shelly Sakiyama-Elbert for microscope access. Finally, we acknowledge Guy Genin for helpful insight into our stress analysis of the brain. This work was supported by NSF grant DMS-0540701 (LAT), NIH grant R01 GM075200 (LAT), and fellowships for BAF from NIH T90 DA022871 and the Mallinckrodt Institute of Radiology, Washington University.
References
- 1.Beloussov LV. The Dynamic Architecture of a Developing Organism: An Interdisciplinary Approach to the Development of Organisms. Kluwer Dordrecht; the Netherlands: 1998. [Google Scholar]
- 2.Beloussov LV. Mechanically based generative laws of morphogenesis. Phys Biol. 2008;5:15009. doi: 10.1088/1478-3975/5/1/015009. [DOI] [PubMed] [Google Scholar]
- 3.Beloussov LV, Grabovsky VI. Morphomechanics: goals, basic experiments and models. International Journal of Developmental Biology. 2006;50:81–92. doi: 10.1387/ijdb.052056lb. [DOI] [PubMed] [Google Scholar]
- 4.Butler JK. MS Thesis. University of Texas; 1952. An experimental analysis of cardiac loop formation in the chick. [Google Scholar]
- 5.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:H1209–1224. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- 6.Chowdhury F, Na S, Li D, Poh YC, Tanaka TS, Wang F, Wang N. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat Mater. 9:82–88. doi: 10.1038/nmat2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark EB, Hu N, Frommelt P, Vandekieft GK, Dummett JL, Tomanek RJ. Effect of Increased Pressure on Ventricular Growth in Stage 21 Chick Embryos. American Journal of Physiology. 1989;257:H55–H61. doi: 10.1152/ajpheart.1989.257.1.H55. [DOI] [PubMed] [Google Scholar]
- 8.Dahl KN, Ribeiro AJ, Lammerding J. Nuclear shape, mechanics, and mechanotransduction. Circ Res. 2008;102:1307–1318. doi: 10.1161/CIRCRESAHA.108.173989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desmond ME, Jacobson AG. Embryonic brain enlargement requires cerebrospinal fluid pressure. Developmental Biology. 1977;57:188–198. doi: 10.1016/0012-1606(77)90364-5. [DOI] [PubMed] [Google Scholar]
- 10.Desmond ME, Levitan ML, Haas AR. Internal luminal pressure during early chick embryonic brain growth: descriptive and empirical observations. Anat Rec A Discov Mol Cell Evol Biol. 2005;285:737–747. doi: 10.1002/ar.a.20211. [DOI] [PubMed] [Google Scholar]
- 11.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Gonzalez R, Zallen JA. Cell mechanics and feedback regulation of actomyosin networks. Sci Signal. 2009;2:pe78. doi: 10.1126/scisignal.2101pe78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filas BA, Efimov IR, Taber LA. Optical coherence tomography as a tool for measuring morphogenetic deformation of the looping heart. Anat Rec. 2007;290:1057–1068. doi: 10.1002/ar.20575. [DOI] [PubMed] [Google Scholar]
- 14.Filas BA, Knutsen AK, Bayly PV, Taber LA. A new method for measuring deformation of folding surfaces during morphogenesis. J Biomech Eng. 2008;130:061010. doi: 10.1115/1.2979866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujimoto JG. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nat Biotechnol. 2003;21:1361–1367. doi: 10.1038/nbt892. [DOI] [PubMed] [Google Scholar]
- 16.Galbraith CG, Skalak R, Chien S. Shear stress induces spatial reorganization of the endothelial cell cytoskeleton. Cell Motil Cytoskeleton. 1998;40:317–330. doi: 10.1002/(SICI)1097-0169(1998)40:4<317::AID-CM1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Goodrum GR, Jacobson AG. Cephalic flexure formation in the chick embryo. J Exp Zool. 1981;216:399–408. doi: 10.1002/jez.1402160308. [DOI] [PubMed] [Google Scholar]
- 18.Guilak F. Compression-induced changes in the shape and volume of the chondrocyte nucleus. J Biomech. 1995;28:1529–1541. doi: 10.1016/0021-9290(95)00100-x. [DOI] [PubMed] [Google Scholar]
- 19.Gutzman JH, Graeden EG, Lowery LA, Holley HS, Sive H. Formation of the zebrafish midbrain-hindbrain boundary constriction requires laminin-dependent basal constriction. Mech Dev. 2008;125:974–983. doi: 10.1016/j.mod.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Journal of Morphology. 1951;88:49–92. [PubMed] [Google Scholar]
- 21.Humphrey JD. Vascular adaptation and mechanical homeostasis at tissue, cellular, and sub-cellular levels. Cell Biochem Biophys. 2008;50:53–78. doi: 10.1007/s12013-007-9002-3. [DOI] [PubMed] [Google Scholar]
- 22.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10:63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkins MW, Rothenberg F, Roy D, Nikolski VP, Hu Z, Watanabe M, Wilson DL, Efimov IR, Rollins AM. 4D embryonic cardiography using gated optical coherence tomography. Optics Express. 2006;14:736–748. doi: 10.1364/opex.14.000736. [DOI] [PubMed] [Google Scholar]
- 24.Jurisicova A, Varmuza S, Casper RF. Programmed cell death and human embryo fragmentation. Mol Hum Reprod. 1996;2:93–98. doi: 10.1093/molehr/2.2.93. [DOI] [PubMed] [Google Scholar]
- 25.Kassab GS, Navia JA. Biomechanical considerations in the design of graft: the homeostasis hypothesis. Annu Rev Biomed Eng. 2006;8:499–535. doi: 10.1146/annurev.bioeng.8.010506.105023. [DOI] [PubMed] [Google Scholar]
- 26.Kornikova ES, Troshina TG, Kremnyov SV, Beloussov LV. Neuromesodermal patterns in artificially deformed embryonic explants: a role for mechanogeometry in tissue differentiation. Dev Dyn. 239:885–896. doi: 10.1002/dvdy.22238. [DOI] [PubMed] [Google Scholar]
- 27.Krieg M, Arboleda-Estudillo Y, Puech PH, Kafer J, Graner F, Muller DJ, Heisenberg CP. Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol. 2008;10:429–436. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- 28.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manner J. Cardiac looping in the chick embryo: a morphological review with special reference to terminological and biomechanical aspects of the looping process. Anatomical Record. 2000;259:248–262. doi: 10.1002/1097-0185(20000701)259:3<248::AID-AR30>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 30.Mizutani T, Haga H, Kawabata K. Cellular stiffness response to external deformation: tensional homeostasis in a single fibroblast. Cell Motil Cytoskeleton. 2004;59:242–248. doi: 10.1002/cm.20037. [DOI] [PubMed] [Google Scholar]
- 31.Munro EM, Odell GM. Polarized basolateral cell motility underlies invagination and convergent extension of the ascidian notochord. Development. 2002;129:13–24. doi: 10.1242/dev.129.1.13. [DOI] [PubMed] [Google Scholar]
- 32.Nelson CM, Jean RP, Tan JL, Liu WF, Sniadecki NJ, Spector AA, Chen CS. Emergent patterns of growth controlled by multicellular form and mechanics. Proc Natl Acad Sci U S A. 2005;102:11594–11599. doi: 10.1073/pnas.0502575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nerurkar NL, Ramasubramanian A, Taber LA. Morphogenetic adaptation of the looping embryonic heart to altered mechanical loads. Developmental Dynamics. 2006;235:1822–1829. doi: 10.1002/dvdy.20813. [DOI] [PubMed] [Google Scholar]
- 34.Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ, Discher DE. Physical plasticity of the nucleus in stem cell differentiation. Proc Natl Acad Sci U S A. 2007;104:15619–15624. doi: 10.1073/pnas.0702576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pouille PA, Ahmadi P, Brunet AC, Farge E. Mechanical signals trigger Myosin II redistribution and mesoderm invagination in Drosophila embryos. Sci Signal. 2009;2:ra16. doi: 10.1126/scisignal.2000098. [DOI] [PubMed] [Google Scholar]
- 36.Ramasubramanian A, Nerurkar NL, Achtien KH, Filas BA, Voronov DA, Taber LA. On Modeling Morphogenesis of the Looping Heart Following Mechanical Perturbations. Journal of Biomechanical Engineering. 2008;130:061018. doi: 10.1115/1.2978990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reinhart-King CA, Dembo M, Hammer DA. Cell-cell mechanical communication through compliant substrates. Biophys J. 2008;95:6044–6051. doi: 10.1529/biophysj.107.127662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Remond MC, Fee JA, Elson EL, Taber LA. Myosin-based contraction is not necessary for cardiac c-looping in the chick embryo. Anat Embryol (Berl) 2006;211:443–454. doi: 10.1007/s00429-006-0094-0. [DOI] [PubMed] [Google Scholar]
- 39.Saez A, Ghibaudo M, Buguin A, Silberzan P, Ladoux B. Rigidity-driven growth and migration of epithelial cells on microstructured anisotropic substrates. Proc Natl Acad Sci U S A. 2007;104:8281–8286. doi: 10.1073/pnas.0702259104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taber LA. Biomechanics of Growth, Remodeling, and Morphogenesis. Applied Mechanics Reviews. 1995;48:487–545. [Google Scholar]
- 41.Taber LA. Biophysical mechanisms of cardiac looping. International Journal of Developmental Biology. 2006;50:323–332. doi: 10.1387/ijdb.052045lt. [DOI] [PubMed] [Google Scholar]
- 42.Taber LA. Theoretical study of Beloussov’s hyper-restoration hypothesis for mechanical regulation of morphogenesis. Biomech Model Mechanobiol. 2008;7:427–441. doi: 10.1007/s10237-007-0106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taber LA. Towards a unified theory for morphomechanics. Philos Transact A Math Phys Eng Sci. 2009;367:3555–3583. doi: 10.1098/rsta.2009.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trepat X, Deng L, An SS, Navajas D, Tschumperlin DJ, Gerthoffer WT, Butler JP, Fredberg JJ. Universal physical responses to stretch in the living cell. Nature. 2007;447:592–595. doi: 10.1038/nature05824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voronov DA, Taber LA. Cardiac looping in experimental conditions: the effects of extraembryonic forces. Developmental Dynamics. 2002;224:413–421. doi: 10.1002/dvdy.10121. [DOI] [PubMed] [Google Scholar]
- 46.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 47.Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol. 2009;10:34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu G, Kemp PS, Hwu JA, Beagley AM, Bayly PV, Taber LA. Opening Angles and Material Properties of the Early Embryonic Chick Brain. J Biomech Eng-T Asme. 2010;132:071013. doi: 10.1115/1.4000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zamir EA, Srinivasan V, Perucchio R, Taber LA. Mechanical asymmetry in the embryonic chick heart during looping. Ann Biomed Eng. 2003;31:1327–1336. doi: 10.1114/1.1623487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.