Abstract
Acoustic communication is the major component of social behavior in anuran amphibians (frogs and toads) and has served as a neuroethological model for the nervous system’s processing of social signals related to mate choice decisions. The male’s advertisement or mating call is its most conspicuous social signal, and the nervous system’s analysis of the call is a progressive process. As processing proceeds through neural systems, response properties become more specific to the signal and, in addition, neural activity gradually shifts from representing sensory (auditory periphery and brainstem) to sensorimotor (diencephalon) to motor (forebrain) components of a behavioral response.
A comparative analysis of many anuran species shows that the first stage in biasing responses toward conspecific signals over heterospecific signals, and toward particular features of conspecific signals, lies in the tuning of the peripheral auditory system. Biases in processing signals are apparent through the brainstem auditory system, where additional feature detection neurons are added by the time processing reaches the level of the midbrain. Recent work using immediate early gene expression as a marker of neural activity suggests that by the level of the midbrain and forebrain, the differential neural representation of conspecific and heterospecific signals involves both changes in mean activity levels across multiple subnuclei, and in the functional correlations among acoustically active areas. Our data show that in frogs the auditory midbrain appears to play an important role in controlling behavioral responses to acoustic social signals by acting as a regulatory gateway between the stimulus analysis of the brainstem and the behavioral and physiological control centers of the forebrain. We predict that this will hold true for other vertebrate groups such as birds and fish that produce acoustic social signals, and perhaps also in fish where electroreception or vibratory sensing through the lateral line systems plays a role in social signaling, as in all these cases ascending sensory information converges onto midbrain nuclei which relay information to higher brain centers.
Introduction: Acoustic communication in anuran amphibians
Social behavior emerges from neural processing that transforms sensory representations of social signals into the expression of a behavioral response. The acoustic communication system of anuran amphibians (frogs and toads) has served as a valuable neuroethological model for this process[1–4]. Anurans are relatively uniform in the basic communication strategy employed for reproductive social interactions. Almost universally, males aggregate at breeding sites where they produce an advertisement, or mating, call that serves to attract females. This acoustic communication system plays an important role in the evolutionary processes of speciation and sexual selection: the conspecific call and the female’s preferential response to it can restrict genetic exchange to members of the same species, and female preferences for calls among conspecifics can lead to the further evolutionary elaboration of calls[5,6] (for perspectives on other taxa, see [7,8]). Males also respond to male advertisement calls, usually by counter-calling, and like females they respond preferentially to conspecific calls over heterospecific ones.
A major focus of neuroethological work on anuran acoustic communication has been on interspecific variation in vocal signals and the relationship of that signal variation to interspecific variation in auditory processing. This work is driven by the question of how species-specific signals are encoded by the nervous system in a manner that guides receivers to respond preferentially to conspecific over heterospecific signals. Work by many labs suggests that the analysis of advertisement calls leading to response decisions is a progressive process from the periphery to the forebrain. Recent work has shown that the process is more complex than a simple processing hierarchy, moving from less to more complex neural responses, although that certainly is an important component. Changes also occur in the way neural activity patterns in different brain regions are correlated with each other; that is, changes in functional connectivity are a critical component underlying decision making. Figure 1 outlines the various processes that take place as acoustic social signals are processed by the frog brain, processes which we cover in this review.
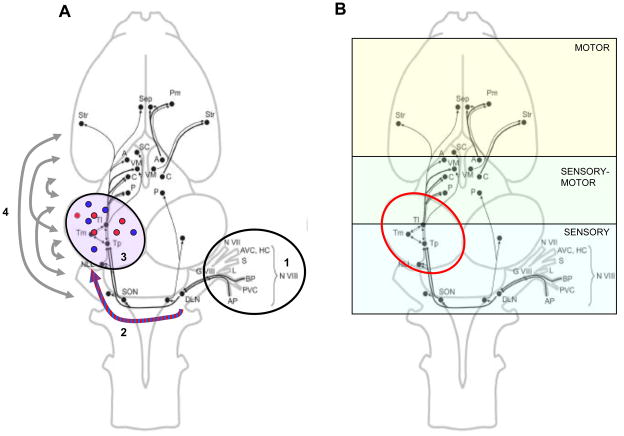
Figure 1.
Aspects of social signal processing in the nervous system of anuran amphibians, superimposed on schematic dorsal view of a frog brain showing the basic ascending auditory pathways (not all connections are shown). Rostral is at the top, caudal at the bottom. List of abbreviations: Components of the VIIIth cranial nerve complex: G VIII vestibulocochlear nerve ganglion; N VIII vestibulocochlear nerve; AP amphibian papilla; BP basilar papilla; AVC anterior vertical canal; PVC posterior vertical canal; HC horizontal canal; S sacculus; L lagena. Lower brainstem auditory areas: CN caudal nucleus; DLN dorsal lateral nucleus; SON superior olivary nucleus; NLL nucleus of the lateral lemniscus. Midbrain torus semicircularis subdivisions: Tl laminar nucleus; Tm magnocellular nucleus; Tp principal nucleus. Diencephalon: A anterior thalamic nucleus; C central thalamic nucleus; P posterior thalamic nucleus; VM ventromedial thalamic nucleus; SC suprachiasmatic nucleus. Telencephalon: Pm medial pallium; Sep septal complex; Str striato-pallidal complex. Illustration of frog brain and connections from[11].
A. Processes involved in the analysis of conspecific social signals as information ascends from the ear though the brain. 1. Neural responses in the ear generated by two auditory end organs, the amphibian and basilar papillae, are biased toward the spectral features of the conspecific call. 2. As information ascends from the lower brainstem to the midbrain, responses properties of cells become more specialized for coding elemental spectral and temporal features of the conspecific call. 3. In the midbrain, many neurons act as specialized feature detectors for either spectral or temporal call characteristics, which in turn relay this information to forebrain areas regulating motor and physiological responses to the call. However, the call is best represented there by activity distributed in multiple midbrain neurons across its subnuclei, that is, by a process of population coding. 4. In addition to changes in the firing rates of neurons within brain nuclei, behavioral salient signals like conspecific social signals induce changes in the correlated patterns of activity locally and across brain divisions, that is, a change in the functional connectivity of brain areas.
B. Coding of acoustic social signals at different levels of the frog nervous system. In the brainstem (medulla and midbrain), neural activity is driven by sensory features of the signal. Activity in thalamic nuclei is correlated with sensory features and movement during the stimulus period. Telencephalic activity in most areas is more strongly correlated with movement than with stimulus features. The torus semicricularis of the midbrain (circled in red) is a key gateway for transferring sensory information from lower levels to the motor and endocrine control areas of the forebrain and its properties are important for generating sex differences in responding as well as changes in responding with physiological state.
Species differences exist at the level of the ear in differences in band-pass characteristics and tuning that generally match the frequency composition of important components of the advertisement call. Robert R. Capranica articulated the behavioral relevance of species specializations in peripheral tuning in his classic papers in the 1960’s, where he proposed the “match filter hypothesis” [9,10]. Capranica’s idea was that tuning of the ear generally matches the spectral frequency peaks of a species’ call, thus biasing the organism’s sensory system to detecting their species’ call among the noise and irrelevant heterospecific signals that threaten to obscure it. An individual frog could use the match between call and tuning to determine whether a signal was in fact that of a conspecific particularly when there are multiple frequency peaks in the call. In that case, behavioral responses depend on the activation of those sensitive portions of the ear simultaneously or in some characteristic temporal sequence (depending on the frequency structure of the call).
Capranica noted that the central nervous system would need to extract information from the ear and further process it. In fact, the frog central auditory system does gradually construct neurons with feature-detector characteristics that respond to particular frequency combinations or temporal characteristics that typify the species advertisement call as processing proceeds sequentially from caudal to rostral auditory nuclei in the brain. This species-typical tuning bias is carried through lower brainstem auditory nuclei. Additional frequency specializations may occur, and responses to temporal features of the call begin to appear in this part of the brain. It is not until the midbrain, however, that a significant integration of auditory information into complex feature detectors first occurs.
The midbrain auditory center as an analyzer of social signals
The anuran auditory midbrain is the torus semicircularis, a homolog of the inferior colliculus[11]. It is composed of several subnuclei, the most significant of which is the laminar nucleus. Laminar nucleus neurons provide a significant portion of the torus’ output to both forebrain and brainstem areas, and are rich in receptors for a variety of hormones and neuromodulators. Walkowiak and Luksch [12] and others [11,13–15] proposed the torus to be a sensory-motor interface, that is, a key neural area that links sensory input to behavioral responses.
Throughout the anuran torus, neurons show complex response properties with special sensitivity to behavioral important call features[11,16–18]. Some cells respond to combinations of tones characteristic of calls. Others are temporally tuned to features such as pulse or amplitude modulation rate that are important for call recognition. Edwards et al. [19,20] (see also [21]) reported a remarkable example of this specialization: toral neurons that “count” pulses and are extremely sensitive to the interpulse interval. These neurons respond only after a certain number of pulses have occurred and only if the interpulse intervals are consistent during that counting phase. Such specialized neurons provide a mechanism for the torus to ascertain critical temporal information needed to identify species-specific advertisement calls in those species that employ amplitude modulated trills. Rose and Brenowitz[22], in fact, found that male Hyla regilla use interpulse interval information of the kind important for these toral cells to discriminate advertisement from aggressive calls of neighboring males and mount the appropriate behavioral response.
The spectral and temporal tuning characteristics of toral neurons show clear links between midbrain auditory processing and behavioral expression. However, the accumulated electrophysiological studies show that the individual midbrain cells each code only a portion of the information necessary for true call representation[16,17]. Spectrally tuned cells, for example, may respond best to particular combinations of tones, but none is so selective that it only responds to a conspecific call. Moreover, spectrally tuned cells do not have a particularly strong bias toward temporal properties. Similarly, the temporally tuned neurons have broad spectral bandpass characteristics, so have no preference for the frequency characteristics of the signals carrying the temporal information. If frogs attend to both the spectral composition and the temporal patterns that define their calls, it must be with at least two populations of neurons, as no individual midbrain cells are capable of doing both and in fact none of either type capture more than one or two call features. Therefore, if information necessary to recognize conspecific calls reliably is present at the level of the midbrain, it must be encoded in the population response across some area of the torus. Population coding has been invoked to explain the representation of complex stimuli in many mammalian sensory systems[23–29], often in regards to cortical processing. In the mammalian auditory system, population coding in the cortex and midbrain have provided insights into processing amplitude, location, and spectro-temporal features of complex signals.
Investigations of anuran population coding and call processing have not reached the level of sophistication represented by these studies. However, recent work using immediate early gene (IEG) expression[30,31] as a marker of stimulus-evoked neural activity has suggested that population coding is an important component of call representation in the frog auditory midbrain. Using IEGs to denote neural responses does have its limitations[32], but it allows one to overcome some significant technical limitations encountered in traditional electrophysiological studies in non-mammalian vertebrates such as amphibians, reptiles and fish. Single cell recording in such small animals generally provide a limited sample of neurons in any one individual, constraining the statistical approaches one might use to extract population codes. Moreover, their very small brains and the general lack of stereotaxic methods and maps make it difficult to mark multiple recording sites in a single individual with enough precision to reliably delineate subarea locations. IEGs allow precise anatomical measurements of activity (gene expression) levels across a brain area and between multiple nuclei throughout the central nervous system. Hoke et al.[31] used this approach to assess IEG expression (using the immediate early gene egr-1 (also called ZENK, zif268, NGFI-A, and krox-24) in the torus of the túngara frog (Physalaemus pustulosus) in response to a variety of conspecific, heterospecific, and biologically neutral acoustic stimuli. A simple analysis of mean expression levels showed that IEG expression was higher in response to conspecific calls in most toral subnuclei, with the laminar toral nucleus showing the clearest difference to behaviorally salient conspecific calls compared to other stimuli. These results in themselves are not surprising given the sensory bias toward conspecific calls apparent even in the auditory periphery. What did emerge, however, from a discriminant function analysis was that information in the activity levels across all four toral subnuclei responding to calls was the best predictor of stimulus type, correctly identifying different types of calls 85% of the time. Even more interesting, the results were not explainable by any simple acoustic features. Rather, the activity pattern across the torus appears to be an emergent property related to overall characteristics and behaviorally salience of complex acoustic signals.
Functional network changes as codes for social signals
Population coding is a process whereby responses across different neurons (or regions) represent information about a signal. Signals may also differentially change the relationship among brain regions in their responses; that is, how tightly activity in one brain area correlates with activity in other areas. The activity correlations among brain regions define their ‘functional connectivity’, that is, how activity in one brain area predicts activity in another, a process that can be the result of direct connections, indirect connections, or common inputs. Such correlated activity patterns emerge rapidly and transiently and are tied to a particular context or input. Functional connectivity has emerged as an important concept explaining the emergence of cortical patterns of activity underlying higher cognitive processes examined in human imaging studies[33–36]. Although rarely used this way, IEG studies can form the basis of functional connectivity studies[37] by providing a record of neural activation during a stimulus. They can be valuable when functional imaging is not feasible, as is the case for many small animals and non-mammals. IEG mapping in túngara frogs has revealed that shifts in functional connectivity accompany changes in activity levels when frogs are hearing conspecific signals. Hoke et al.[38] compared IEG patterns in túngara frogs hearing a conspecific advertisement call (the “whine”), a similar but ecologically meaningless heterospecific call, a conspecific aggressive “mew” call, and a silent control. A correlation analysis showed that hearing the conspecific advertisement call induced much great coupling of activity (in the sense of correlated patterns of IEG expression) across the brain, linking more closely activity levels in the brainstem, midbrain, and forebrain, than did hearing of the other signals (Figure 2). An investigation[39] of functional networks showed that different patterns of functional connectivity within the hypothalamus were elicited by hearing conspecific calls, heterospecific calls, and silence. Correlation changes were apparent even among hypothalamic nuclei that did not show statistically significant mean activity differences to different signals.
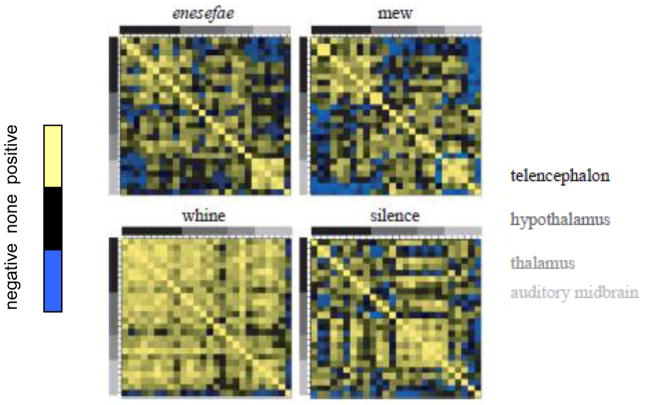
Figure 2.
Matrix indicating patterns of correlated activity, measured by level of egr-1 expression, across regions of the telencephalon, hypothalamus, thalamus, and midbrain (coded by gray scale) when Physalaemus pustulosus frogs heard a heterospecific Physalaemus enesefae advertisement call (upper left), a conspecific aggressive call (the mew, upper right), a conspecific advertisement calls (the whine, lower left), or silence. Positive correlations are in yellow, negative in blue, and no significant correlation is indicated by black. When hearing a conspecific advertisement call, neural activation within and across brain regions becomes significantly more correlated indicating a change in functional connectivity. From[38].
The correlation analyses made possible by IEG studies show that the behavioral relevance of a signal is represented by interactions among both closely and distantly related brain areas as much as it is represented by changes in mean activity within any one area. What these changes in functional connectivity actually mean for perception or behavior is still unresolved and is very difficult to test experimentally. Nevertheless, these results and those related to population coding provide a new way to consider the relationship between brain and behavior. This different view depends less on the idea that there are discrete brain “centers” for particular behaviors activated by stimuli, as opposed to considering behavior as emerging from patterns of neural activity tying sets of neurons or brain areas together in different ways depending on the context or meaning of stimuli.
Sex differences in responding to signals
All of the work on frogs reviewed above treats the behavioral neuroscience of social communication as a species-specific problem, that is, how does the typical individual of that species recognize its social signals? But of course, there are considerable differences among individuals within a species in terms of what a particular social signals means to them and how they respond to those signals. Sex differences are a clear example of this. Males and females respond very differently to male signals. For the acoustic communication behavior of frogs, evidence points to the midbrain auditory center, the torus semicircularis, as a playing a key role in mediating the transformation of signal perception into a sexually differentiated response.
One of the most common features of animal social communication is that, as a general rule, females respond to a more restricted range of potential male signals than do males to either female signals or male signals. This sex difference is predicted by the different costs to the sexes in responding inappropriately to sexual solicitation signals (higher in females than males) compared to the costs of not responding to a potential sexual signal (higher in males than females) [40,41]. In the case of túngara frogs, males and females differ in terms of how they respond to calls: males most often respond vocally, and much less often by moving toward the call, whereas females move toward the call and do not vocalize. In addition, the sexes differ in their selectivity: males vocalize in response to a wide range of audible signals whereas females are much more choosy when responding phonotactically [42–45]. In principle, the neural correlates of sex differences could be found in sensory areas, in brain areas serving as sensorimotor links, or in motor areas (or any combination). By identifying the processing levels at which these stages take place, one can then ascertain where male and female neural responses differ.
This task can be accomplished through a covariance approach that determines whether IEG activation in different areas of the brain correlate best with either conspecific signal presentation, or with movement that occurred when a signal was present, or varied with both signal presentation and movement[38]. Within the brainstem auditory nuclei up to the midbrain, IEG expression predicts that signal reception is on average higher for conspecific calls than other sounds or silence, but does not covary with the individual’s movement during the stimulus period. This would be expected based on neurophysiological studies of frog auditory processing and based on the fact that the areas assessed are standard auditory nuclei increasingly dominated by specializations to detect conspecific calls at progressively more rostral levels. In the diencephalic targets of the midbrain, however, IEG activity covaries with both stimulus type and with movement. Multiple forebrain areas also have increased IEG expression to conspecific calls[39,46–48], but a covariate analysis reveals that telencephalic limbic and basal ganglia areas are either correlated to movement alone or exhibited a movement by stimulus interaction. These results indicate that ascending pathways in the frog brain mediate a transition from primarily sensory, to sensorimotor, to motor processing as the information makes it way from brainstem, to thalamus, to telencephalon.
Where, then, do sex differences in activation arise? Expression patterns in lower brainstem areas do not differ in males and females (Figure 3). However, the laminar nucleus of the torus does show a sex difference related directly to the sex difference in the range of signals generating a response[49] (Figure 3). In males, midbrain IEG expression to conspecific Physalaemus pustulosus calls and to heterospecific P. petersi calls are not significantly different, which is consistent with the male’s behavior—males will vocalize in response to both. Female toral IEG expression is significantly greater in response to the P. pustulosus call than to the P. petersi call, and females make phonotaxis responses to the former, but not the latter; again, the analyses of brain and behavior are congruent. These results suggest that the midbrain is a key component of the neural mechanisms generating sex differences in responding to social signals, and it acts by regulating the range of signals passing from the auditory system to the forebrain. Examining the functional connectivity between midbrain and forebrain also implicates the torus as a gateway contributing to the sex differences in behavioral responses to signals[47]. In females, there is a significant linear correlation between laminar nucleus IEG expression and forebrain expression; in males, the correlation is not significant. Assuming that the correlation patterns indicate something about the transfer of information from midbrain to forebrain, one could interpret this result as showing much tighter control of the information flow in females.
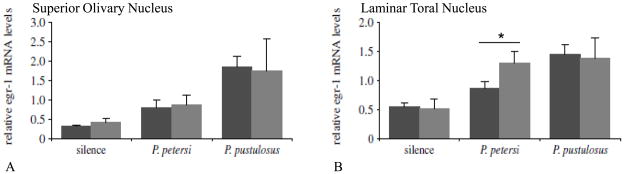
Figure 3.
Relative expression of egr-1 in the superior olivary nucleus (A) and laminar nucleus of the torus semicircularis (B) in male (gray bars) and female (black bars) túngara frogs hearing a conspecific Physalaemus pustulosus advertisement call, a heterospecific Physalaemus petersi advertisement call, or silence. The results show that the superior olivary nucleus, a lower brainstem auditory center, responds best to the conspecific call in both sexes and there is no sex difference. In the midbrain, however, male neural response is equally strong to the conspecific and heterospecific calls, whereas female responses are significantly lower to the heterospecific call. This matches the behavioral response: males will call in response to either call, whereas females express phonotaxis only to the conspecific call. Modified from[49].
Somewhat surprisingly, the patterns of correlation among forebrain nuclei do not differ in males and females despite sex differences in IEG expression in several forebrain areas[47]. This suggests that once activated, forebrain areas work together similarly in males and females, again indicating that the midbrain link between auditory processing in the brainstem, and forebrain operations underlying behavioral responses, is the key to generating sex differences in behavior. It is important to note that these results do not reveal the reason for the more major sex difference in what behavior males (vocal response) and females (phonotaxis) most predictably express in response to the same social signal (the male’s advertisement call). Anatomical sex dimorphisms in descending vocal control pathways have been identified[50,51], and such anatomical differences could play a role. Furthermore, neurophysiological or within-area circuits may exist at any level of processing, something that IEG studies can not identify. Nevertheless, the idea that some type of a gateway regulates the passage of information from sensory to motor areas, and that differences in that choke point reveal themselves in sex and possibly other differences in behavior has been advanced before. Kimchi et al.[52] suggested something similar for sex differences in response to olfactory social signals.
Neural correlates of the túungara frog’s intrasexual differences in responding to conspecific signals also point to the laminar nucleus of the torus as a key regulatory gateway. Female frogs (like many female vertebrates) vary in their receptivity toward male advertisement signals depending on their hormonal state[53,54]. Female túngara frogs also vary in the range of signals to which they will respond: when estrogen is high, females are more receptive and more permissive in their responses[54]. Toral activity levels, whether measured electrophysiologically or by IEG expression, vary in a way that predicts the behavioral pattern[55–57]. Neural responses in the torus are high when estrogen levels and female behavioral responses are high, and decrease significantly after females release their eggs and their receptivity drops. Thus the auditory midbrain might serve to control intrasexual differences in social responding as well as intersexual differences, in both cases by regulating the passage of sensory information to effector regions of the forebrain.
The results of IEG studies on sex differences and changes with hormonal state help to distinguish from among three possibilities for the neural systems variation underlying behavioral differences (Figure 4). Rather than arising from differences in basic sensory processing, or in the basic organization of forebrain motor or physiological control areas, the most important mechanism may be employing a strategically located gateway that regulates the flow of information from sensory to motor areas.
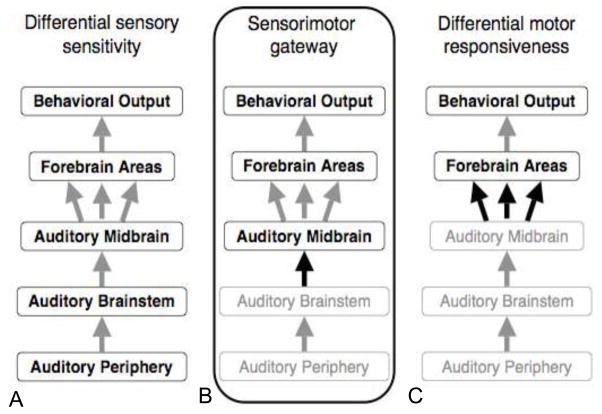
Figure 4.
Three possible mechanisms of could account for differences in the responses to male social signals between sexes, among individuals with in a sex, or within an individual depending on physiological state or external conditions. A. Sensory systems may be different, thereby biasing responses at higher levels. B. A neural locus acting as a ‘gateway’ controls access to behavioral control centers so that information transfer to higher behavioral or physiological response areas is different. C. Both the sensory processing and the transfer of information is constant, but the behavioral control centers have different thresholds, filter functions, or other characteristics leading to the behavioral differences. Immediate early gene results in túngara frogs suggest that the most likely possible mechanism is B, with the gateway being the midbrain auditory center relaying information about calls to forebrain areas controlling motor and endocrine functions. Figure courtesy of K. Hoke.
Anuran communication as a model for behavioral neuroscience
Acoustic communication in anuran amphibians may be a specialized trait in that vertebrate group, but the principles of neural processing underlying it can be applied to the behavioral neuroscience of social communication more generally. As in any other social behavioral context, signals produced by conspecifics must be received, represented in the central nervous system, and transformed into a behavioral response. The anuran brain shows a pattern in which sensory biases starting in the periphery are honed into feature detectors representing elemental aspects of species-typical signals as the information is gradually transmitted through the sensory pathway. These feature detectors are not sufficient to explain either the overall preference for conspecific over heterospecific signals, or the variation in responding in different physiological or environmental contexts. Instead, more complete information about a social signal is contained in a population code as well as in the pattern of functional connectivity that emerges depending on salience and context. It should be noted that not all behavioral responses need depend on a full analysis of information emerging from processes at all levels of the brain. Some responses may occur based solely on processing at lower sensory levels, much as happens for simple motor reflexes. Some male frogs can respond vocally to conspecific calls so rapidly that it is unlikely that the forebrain is involved[58]. It is very likely that this complex, multidimensional mode of representation is important in many aspects of social behavior, and the work in amphibians suggests that it is not a process restricted to higher areas of the mammalian cortex.
In anurans, signal analysis culminates at the level of the midbrain rather than in a sensory cortex (in fact, based on its functional anatomy of the telencephalon, a functional equivalent of primary sensory cortex may be lacking in amphibians[11]). Nevertheless, the idea that processing proceeds to sensorimotor representation, then to motor representation in forebrain centers is an idea that can be applied to many functional systems. Also important is the idea that a gateway regulating the transfer of sensory information to effector centers. Our data show that in amphibians the midbrain acts as the gateway, as it is located in a strategic position between the purely sensory areas of the lower brainstem and the motor and endocrine regulatory areas of the forebrain. We believe that the same may hold true for other vertebrates such as birds and sound-producing fish that use acoustic social signals. The auditory midbrain in fish, for example, occupies such a position anatomically[59], has neurons that code features of conspecific vocal signals[60–62], and it contains steroid hormone receptors that may regulate auditory processing there[63]. The processing of social signals using other sensory systems, such as the electrosensory system that, like the auditory system, depend on a midbrain relay, might similarly employ the midbrain as a gateway. The location of the gateway is likely different depending on the sensory system being used and the behavior being mediated. One might not expect the midbrain to act as the sensory gate for olfactory or visual signaling, for example, because these sensory systems do not pass through an obligatory midbrain relay en route to the forebrain. But if the amphibian acoustic communication paradigm can be generalized to communication in other modalities, it would predict a gatekeeper function at some critical node between sensory and motor systems. Finally, this concept implies that both the perceptual analysis features and the functional circuits defining different male and female behaviors are present in both sexes; what generates the sex difference in behavior is the way in which the perceptual process is connected to those functional circuits. Testing the limits of this idea would be important for understanding the behavioral neuroscience of social behavior and the true nature of sex differences.
Acknowledgments
We wish to thank the National Science Foundation and the National Institute of Mental Health for their support of many aspects of our research. We also thank Dr. Russell D. Fernald for comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Walter Wilczynski, Neuroscience Institute and Center for Behavioral Neuroscience, Georgia State University.
Michael J. Ryan, Section of Integrative Biology, University of Texas at Austin
References and Recommended Reading
*Of special interest
**Of outstanding interest
- 1.Fritzsch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W, editors. The Evoution of the Amphibian Auditory System. New York: Wiley; 1988. [Google Scholar]
- 2.Narins PN, Feng AS, Fay RR, Popper AN, editors. Hearing and Sound Communication in Amphibians. New York: Springer-Verlag; 2007. [Google Scholar]
- 3.Ryan MJ, editor. Anuran Communication. Washington DC: Smithsonian Institution Press; 2001. [Google Scholar]
- *4.Wells KD. The social behavior of anuran amphibians. Animal Behaviour. 1977;25:666–693. Well’s paper is an excellent, comprehensive review of the literature on anuran acoustic communication up to its publication date. It is widely considered a starting point for background information and general overview of anuran acoustic communication across species, including male signaling and both male and female responses. [Google Scholar]
- *5.Ryan MJ, Bernal XE, Rand AS. Female mate choice and the potential for ornament evolution in túngara frogs. Physalaemus pustulosus Current Zoology. 2010;56:343–357. Ryan et al. review experimental studies of female mate choice in túngara frogs to examine an important general issue in evolutionary animal behavior, the evolution elaborate male signals. The authors show that a variety of additions to the conspecific mate recognition signal, both naturally occurring and artificial, enhance the attractiveness of the call to females. The authors argue that latent female preferences for a variety of signal elaborations or innovations can drive the evolution of male signals. [Google Scholar]
- **6.Ryan MJ, Rand AS. Species recognition and sexual selection as a unitary problem in animal communication. Evolution. 1993;47:647–657. doi: 10.1111/j.1558-5646.1993.tb02118.x. Ryan and Rand investigate the response of female túngara frogs to a variety of conspecific and heterospecific male calls as a way to investigate the relationship between conspecific recognition and mate choice preferences for variation in conspecific signals. The authors find that females may prefer heterospecific calls if they fall along preference continua that define female behavior toward variation in conspecific signals. They conclude that both conspecific recognition and intraspecific preferences are aspects of the same female preference functions. [DOI] [PubMed] [Google Scholar]
- 7.Byers BE, Kroodsma DE. Female mate choice and songbird song repertoires. Animal Behaviour. 2009;77:13–22. [Google Scholar]
- 8.Seehausen O, Terai Y, Magalhaes IS, Carleton KL, Mrosso HDJ, Miyagi R, van der Sluijs I, Schneider MV, Maan ME, Tachida H, et al. Speciation through sensory drive in cichlid fish. Nature. 2008;455:620–U623. doi: 10.1038/nature07285. [DOI] [PubMed] [Google Scholar]
- 9.Capranica RR. Vocal response of bullfrog to natural and synthetic mating calls. Journal of the Acoustical Society of America. 1966;40:1131–1139. [Google Scholar]
- **10.Frishkopf LS, Capranica RR, Goldstein MHJ. Neural coding in the bullfrog’s auditory system-- a teleological approach. Proceedings IEEE. 1968;56:969–980. Work by Capranica and colleagues in this classic paper helped define the field of neuroethology. The experiments here show that the distribution tuning among peripheral auditory fibers matches in its areas of peak sensitivities the spectral peaks in the male advertisement call. Moreover, the relationship between the spectral frequency distribution in the call and the neurophysiological characteristics of peripheral auditory cells can explain important aspects of male behavioral responses to the call. [Google Scholar]
- 11.Wilczynski W, Endepols H. Central auditory pathways in anuran amphibians: the anatomical basis of hearing and sound communication. In: Narins PN, Feng AS, Fay RR, Popper AN, editors. Hearing and Sound Communication in Amphibians. Springer-Verlag; 2007. pp. 221–249. [Google Scholar]
- 12.Walkowiak W, Luksch H. Sensory-motor interfacing in acoustic behavior of anurans. American Zoologist. 1994;34:685–695. [Google Scholar]
- 13.Endepols H, Feng AS, Gerhardt HC, Schul J, Walkowiak W. Roles of the auditory midbrain and thalamus in selective phonotaxis in female gray treefrogs (Hyla versicolor) Behavioural Brain Research. 2003;145:63–77. doi: 10.1016/s0166-4328(03)00098-6. [DOI] [PubMed] [Google Scholar]
- 14.Endepols H, Walkowiak W. Integration of ascending and descending inputs in the auditory midbrain of anurans. Journal of Comparative Physiology a-Sensory Neural and Behavioral Physiology. 2000;186:1119–1133. doi: 10.1007/s003590000159. [DOI] [PubMed] [Google Scholar]
- 15.Strake J, Luksch H, Walkowiak W. Audio-motor interface in anurans. European Journal of Morphology. 1994;32:122–126. [PubMed] [Google Scholar]
- 16.Rose GJ, Gooler DM. Function of the amphibian central auditory system. In: Narins PN, Feng AS, Fay RR, Popper AN, editors. Hearing and Sound Communication in Amphibians. Springer-Verlag; 2007. pp. 250–290. [Google Scholar]
- 17.Feng AS, Hall JC, Gooler DM. Neural basis of sound pattern recognition in anurans. Progress in Neurobiology. 1990;34:313–329. doi: 10.1016/0301-0082(90)90008-5. [DOI] [PubMed] [Google Scholar]
- 18.Gooler DM, Feng AS. Temporal coding in the frog auditory midbrain - the influence of duration and rise-fall time on the processing of complex amplitude-modulated stimuli. Journal of Neurophysiology. 1992;67:1–22. doi: 10.1152/jn.1992.67.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Edwards CJ, Leary CJ, Rose GJ. Counting on inhibition and rate-dependent excitation in the auditory system. Journal of Neuroscience. 2007;27:13384–13392. doi: 10.1523/JNEUROSCI.2816-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards CJ, Leary CJ, Rose GJ. Mechanisms of long-interval selectivity in midbrain auditory neurons: Roles of excitation, inhibition, and plasticity. Journal of Neurophysiology. 2008;100:3407–3416. doi: 10.1152/jn.90921.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *21.Leary CJ, Edwards CJ, Rose GJ. Midbrain auditory neurons integrate excitation and inhibition to generate duration selectivity: An in vivo whole-cell patch study in anurans. Journal of Neuroscience. 2008;28:5481–5493. doi: 10.1523/JNEUROSCI.5041-07.2008. This paper is a conceptually and technically sophisticated analysis of the neurophysiological mechanisms by which frog midbrain neurons are converted into feature detectors for temporal characteristics of conspecific calls. The authors show how a combination of inhibition and time- and activity-dependent changes in excitation strength can allow neurons to code for signal duration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rose GJ, Brenowitz EA. Pacific treefrogs use temporal integration to differentiate advertisement from encounter calls. Animal Behaviour. 2002;63:1183–1190. [Google Scholar]
- 23.Covey E. Neural population coding and auditory temporal pattern analysis. Physiology & Behavior. 2000;69:211–220. doi: 10.1016/s0031-9384(00)00203-1. [DOI] [PubMed] [Google Scholar]
- 24.Dean I, Harper NS, McAlpine D. Neural population coding of sound level adapts to stimulus statistics. Nature Neuroscience. 2005;8:1684–1689. doi: 10.1038/nn1541. [DOI] [PubMed] [Google Scholar]
- 25.Miller LM, Recanzone GH. Populations of auditory cortical neurons can accurately encode acoustic space across stimulus intensity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:5931–5935. doi: 10.1073/pnas.0901023106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panzeri S, Pola G, Petersen RS. Coding of sensory signals by neuronal populations: The role of correlated activity. Neuroscientist. 2003;9:175–180. doi: 10.1177/1073858403009003010. [DOI] [PubMed] [Google Scholar]
- 27.Panzeri S, Pola G, Petroni F, Young MP, Petersen RS. A critical assessment of different measures of the information carried by correlated neuronal firing. Biosystems. 2002;67:177–185. doi: 10.1016/s0303-2647(02)00075-8. [DOI] [PubMed] [Google Scholar]
- 28.Petersen RS, Panzeri S, Diamond ME. Population coding in somatosensory cortex. Current Opinion in Neurobiology. 2002;12:441–447. doi: 10.1016/s0959-4388(02)00338-0. [DOI] [PubMed] [Google Scholar]
- 29.Petersen RS, Panzeri S, Diamond ME. Population coding of stimulus location in rat somatosensory cortex. Neuron. 2001;32:503–514. doi: 10.1016/s0896-6273(01)00481-0. [DOI] [PubMed] [Google Scholar]
- *30.Burmeister SS, Mangiamele LA, Lebonville CL. Acoustic modulation of immediate early gene expression in the auditory midbrain of female túngara frogs. Brain Research. 2008;1190:105–114. doi: 10.1016/j.brainres.2007.11.008. Burmeister et al. provide important base-line information on the use of immediate early genes egr-1 and fos on amphibians, focusing on acoustically evoked expression in the anuran midbrain. They outline the time course of expression as well as note that different IEGs provide different expression profiles in the midbrain subnuclei to the same acoustic stimulus, providing a caution that the use of different IEGs may lead to different functional interpretations. [DOI] [PubMed] [Google Scholar]
- 31.Hoke KL, Burmeister SS, Fernald RD, Rand AS, Ryan MJ, Wilczynski W. Functional mapping of the auditory midbrain during mate call reception. Journal of Neuroscience. 2004;24:11264–11272. doi: 10.1523/JNEUROSCI.2079-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **32.Clayton DF. The genomic action potential. Neurobiology of Learning and Memory. 2000;74:185–216. doi: 10.1006/nlme.2000.3967. Clayton’s review of immediate early genes as a tool for analyzing patterns of neural activation is an important reference work for such studies. Clayton clearly explains the benefits and the limitations of this technique. [DOI] [PubMed] [Google Scholar]
- *33.Damoiseaux JS, Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Structure & Function. 2009;213:525–533. doi: 10.1007/s00429-009-0208-6. The authors review eight human brain imaging studies that provide information on both functional connectivity (indicated by correlations in fMRI-indicated activation strength) and anatomical connections. They find that there is a strong correlation between functional connectivity and the strength of direct anatomical connections. However, there are several cases in which significant functional connectivity can be seen in the absence of direct anatomical connections between structures. These results indicate that while anatomy does predict functional connectivity to some extent, functional connectivity studies can reveal additional important relationships among brain areas. [DOI] [PubMed] [Google Scholar]
- 34.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honey CJ, Kotter R, Breakspear M, Sporns O. Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10240–10245. doi: 10.1073/pnas.0701519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiltz CA, Bremer QZ, Landry CF, Kelley AE. Food-associated cues alter forebrain functional connectivity as assessed with immediate early gene and proenkephalin expression. Bmc Biology. 2007;5:1–20. doi: 10.1186/1741-7007-5-16. This study by Schlitz et al. is one of the few to apply the ideas of functional connectivity to data generated by immediate early gene expression patterns. The authors show here that patterns of correlated expression between cortico-striatal networks and the basolateral amygdala emerge in motivated states related to food reward. The work is an interesting example showing that functional connectivity can emerge in systems related to natural rewards and is not limited to processes associated with higher cognitive cortical function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **38.Hoke KL, Ryan MJ, Wilczynski W. Integration of sensory and motor processing underlying social behaviour in túngara frogs. Proceedings of the Royal Society B-Biological Sciences. 2007;274:641–649. doi: 10.1098/rspb.2006.0038. The authors compare patterns of correlated expression of immediate early gene expression within and across broad areas of the túngara frog brain in individuals exposed to conspecific advertisement calls vs. a variety of control stimuli. The results show that there is a progressive transformation of IEG activation from sensory-driven in the brainstem, to sensorimotor related in the thalamus, to motor driven in the telencephalon as animals hear biologically salient signals. Furthermore, hearing such signals induces large scale patterns of functional correlation stretching across the brain. In addition to providing these functional insights, the study outlines the functional connectivity approach to analyzing IEG data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoke KL, Ryan MJ, Wilczynski W. Social cues shift functional connectivity in the hypothalamus. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10712–10717. doi: 10.1073/pnas.0502361102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Searcy WA, Brenowitz EA. Sexual differences in species recognition of avian song. Nature. 1988;332:152–154. [Google Scholar]
- 41.Wiley RH. Signal detection and animal communication. Advanced Studies in Behavior. 2006;36:217–247. [Google Scholar]
- 42.Baugh AT, Ryan MJ. Mate choice in response to dynamic presentation of male advertisement signals in túngara frogs. Animal Behaviour. 2010;79:145–152. [Google Scholar]
- 43.Bernal XE, Akre KL, Baugh AT, Rand AS, Ryan MJ. Female and male behavioral response to advertisement calls of graded complexity in túngara frogs, Physalaemus pustulosus. Behavioral Ecology and Sociobiology. 2009;63:1269–1279. [Google Scholar]
- 44.Bernal XE, Rand AS, Ryan MJ. Sex differences in response to nonconspecific advertisement calls: receiver permissiveness in male and female túngara frogs. Animal Behaviour. 2007;73:955–964. [Google Scholar]
- 45.Bernal XE, Rand AS, Ryan MJ. Sexual differences in the behavioral response of túngara frogs, Physalaemus pustulosus, to cues associated with increased predation risk. Ethology. 2007;113:755–763. [Google Scholar]
- 46.Hoke KL, Ryan MJ, Wilczynski W. Functional coupling between substantia nigra and basal ganglia homologues in amphibians. Behavioral Neuroscience. 2007;121:1393–1399. doi: 10.1037/0735-7044.121.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoke KL, Ryan MJ, Wilczynski W. Sexually dimorphic sensory gating drives behavioral differences in túngara frogs. Journal of Experimental Biology. 2010 doi: 10.1242/jeb.043992. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mangiamele LA, Burmeister SS. Acoustically-evoked immediate-early gene expression in the pallium of the túngara from. Brain Behavior and Evolution. 2008;72:239–250. doi: 10.1159/000171481. [DOI] [PubMed] [Google Scholar]
- **49.Hoke KL, Ryan MJ, Wilczynski W. Candidate neural locus for sex differences in reproductive decisions. Biology Letters. 2008;4:518–521. doi: 10.1098/rsbl.2008.0192. Hoke et al. use patterns of immediate early gene expression in male and female tungara frogs to conspecific and heterospecific calls to determine where neural differences related to behavioral differences are located. They find that males and females respond with a similar pattern in the superior olivary nucleus, a lower brainstem auditory nucleus, with conspecific calls inducing more IEG expression than heterospecific calls. In the midbrain torus semicircularis, however, the male and female expression is different: males respond equally to conspecific and heterospecific calls while females respond more selectively with a greater response to the conspecific calls. These difference mirror the sexual dimorphism in responses to these stimuli: males respond vocally to both, whereas females only respond to the conspecific call. The results thus suggest that sexually monomorphic responses to social signals in the lower auditory system are transformed in to sexually dimorphic responses in the midbrain center that relays auditory information to the forebrain areas controlling motor and endocrine responses to social signals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Emerson SB, Boyd SK. Mating vocalizations of female frogs: Control and evolutionary mechanisms. Brain Behavior and Evolution. 1999;53:187–197. doi: 10.1159/000006594. [DOI] [PubMed] [Google Scholar]
- 51.Zornik E, Yamaguchi A. Sexually differentiated central pattern generators in Xenopus laevis. Trends in Neurosciences. 2008;31:296–302. doi: 10.1016/j.tins.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **52.Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448:1009–1014. doi: 10.1038/nature06089. Kimchi et al. investigate the social behavioral responses to pheromones in mice deficient for Trpc2, an ion channel specifically in vomeronasal organ neurons that is important for the transduction of chemical signals there. They find that male mice lacking the channel gene are impaired responding to social odors and that female mice lacking the gene show both a reduction in female behavior and an increase in male-typical behavior when presented with such odors. The authors provide evidence for two very important points in behavioral neuroscience, first that the circuitry for both male and female social behavior exists in both sexes, and second that a sensory ‘switch’ responsible for relaying sensory information about social signals may be responsible for which circuit, and hence which behavior, is expressed in each sex. [DOI] [PubMed] [Google Scholar]
- 53.Lea J, Halliday T, Dyson M. Reproductive stage and history affect the phonotactic preferences of female midwife toads, Alytes muletensis. Animal Behaviour. 2000;60:423–427. doi: 10.1006/anbe.2000.1482. [DOI] [PubMed] [Google Scholar]
- 54.Lynch KS, Crews D, Ryan MJ, Wilczynski W. Hormonal state influences aspects of female mate choice in the túngara Frog (Physalaemus pustulosus) Hormones and Behavior. 2006;49:450–457. doi: 10.1016/j.yhbeh.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *55.Lynch KS, Wilczynski W. Reproductive hormones modify reception of species-typical communication signals in a female anuran. Brain Behavior and Evolution. 2008;71:143–150. doi: 10.1159/000111460. This study uses immediate early genes to show that the midbrain auditory center (torus semicircularis) changes its response in females to conspecific male calls depending on the female’s reproductive state. When estrogen levels are high, and females are both more receptive to calls and less choosy in terms of which calls she will behavior toward as a indicating an acceptable mate, IEG responses to conspecific calls are high; when estrogen levels are low, and females are less receptive to calls and more choosy when they do respond behaviorally, IEG responses to the same stimulus is low. The results thus show that at midbrain levels sensory responses are not static, but are instead sensitive to the hormonal state of the recipient. Furthermore, the results indicate the midbrain as a key regulator of behavioral responses to social signals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miranda JA, Wilczynski W. Female reproductive state influences the auditory midbrain response. Journal of Comparative Physiology a-Neuroethology Sensory Neural and Behavioral Physiology. 2009;195:341–349. doi: 10.1007/s00359-008-0410-7. [DOI] [PubMed] [Google Scholar]
- 57.Goense JBM, Feng AS. Seasonal changes in frequency tuning and temporal processing in single neurons in the frog auditory midbrain. Journal of Neurobiology. 2005;65:22–36. doi: 10.1002/neu.20172. [DOI] [PubMed] [Google Scholar]
- 58.Ryan MJ. Synchronized calling in a treefrog (Smilisca sila) Brain Behavior and Evolution. 1986;29:196–206. doi: 10.1159/000118681. [DOI] [PubMed] [Google Scholar]
- 59.Bass AH, McKibben JR. Neural mechanisms and behaviors for acoustic communication in teleost fish. Progress in Neurobiology. 2003;69:1–26. doi: 10.1016/s0301-0082(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 60.Bodner DA, Bass AH. Midbrain combinatorial code for temporal and spectral information in concurrent acoustic signals. Journal of Neurophysiology. 1999;81:552–563. doi: 10.1152/jn.1999.81.2.552. [DOI] [PubMed] [Google Scholar]
- 61.Bodner DA, Bass AH. Temporal coding of concurrent acoustic signals in auditory midbrain. Journal of Neuroscience. 1997;17:7553–7564. doi: 10.1523/JNEUROSCI.17-19-07553.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maruska KP, Tricas TC. Encoding properties of auditory neurons in the brain of a soniferous damselfish: response to simple tones and complex conspecific signals. Journal of Comparative Physiology a-Neuroethology Sensory Neural and Behavioral Physiology. 2009;195:1071–1088. doi: 10.1007/s00359-009-0480-1. [DOI] [PubMed] [Google Scholar]
- 63.Forlano PM, Deitcher DL, Bass ARH. Distribution of estrogen receptor alpha mRNA in the brain and inner ear of a vocal fish with comparisons to sites of aromatase expression. Journal of Comparative Neurology. 2005;483:91–113. doi: 10.1002/cne.20397. [DOI] [PubMed] [Google Scholar]