Abstract
Background
We have previously shown that heparin-binding EGF-like growth factor (HB-EGF) promotes angiogenesis and preserves mesenteric microvascular blood flow in several models of intestinal injury. The current study was designed to evaluate the effect of HB-EGF on pericytes, since these cells function to regulate capillary blood flow and new capillary growth.
Materials and Methods
C3H/10T1/2 mouse mesenchymal cells were differentiated into pericyte-like cells in vitro using transforming growth factor- β1 (TGF-β1). In addition, primary pericyte cultures were established from rat brain. The effect of HB-EGF on pericyte proliferation was assessed. In addition, cells were stressed by exposure to anoxia, and apoptosis determined. In vivo, we examined the effect of HB-EGF on pericytes in a model of intestinal I/R injury based on superior mesenteric artery occlusion (SMAO) in mice.
Results
Differentiated C3H/10T1/2 cells (pericyte-like cells) demonstrated morphologic characteristics of pericytes, and expressed pericyte specific markers. Addition of HB-EGF led to significant cell proliferation in differentiated pericyte-like cells, even under conditions of anoxic stress. Addition of the EGF receptor inhibitor AG 1478 led to complete inhibition of the proliferative effects of HB-EGF on pericyte-like cells. In addition, HB-EGF protected pericyte-like cells from anoxia-induced apoptosis. In addition, HB-EGF promoted cell proliferation in primary pericyte cultures. In vivo, administration of HB-EGF to mice subjected to intestinal I/R injury led to protection of pericytes from injury.
Conclusions
These results suggest that HB-EGF may function as a microcirculatory blood flow regulator, at least in part, via its effects on pericytes.
Keywords: heparin-binding EGF-like growth factor, pericytes, transforming growth factor- β1, microvasculature, intestine, ischemia/reperfusion injury
INTRODUCTION
Many intestinal disorders are manifested by deceased intestinal blood flow or failure of regulation of intestinal microcirculation. We have previously shown that heparin-binding EGF-like growth factor (HB-EGF) promotes angiogenesis (1) and preserves mesenteric microvascular blood flow in several models of intestinal injury including intestinal ischemia/reperfusion (I/R) injury (2), hemorrhagic shock and resuscitation (HS/R) (3) and experimental NEC (4). Furthermore, we have shown that HB-EGF acts as a vasodilator of isolated terminal mesenteric arterioles via upregulation of (NO) production in vascular EC and via increased expression of endothelin B (ETB) receptors in vascular smooth muscle cells (SMC) (5). Thus, it appears that HB-EGF has significant effects on the microvaculature.
Pericytes, also known as Rouget cells, are mural cells that are located in the capillaries and post-capillary venules of the microvasculature in intimate proximity to vascular endothelial cells (EC). Pericytes function as important regulators of microvascular blood flow and of angiogenesis (6). They have been shown to be multipotent, with the ability to differentiate into adipocytes, osteoblasts and phagocytes (6). In the brain, they demonstrate macrophage-like functions and clear up remnants of degenerated neurons (7, 8). They have also been shown to be specific precursors of vascular smooth muscle cells (SMC) (6). Immunocytochemical and biochemical studies provide evidence of contractile machinery within pericytes, indicating that they function in a manner analogous to the SMC of larger vessels (9, 10). Using ultrastructural morphometric techniques, pericytes have been found to have a contractile response to vasoactive agents and consequently have a compressive effect on EC membranes (11). Sodium nitroprusside has been shown to lead to relaxation of pre-contracted pericytes, suggesting that EC-derived nitric oxide (NO) may lead to relaxation of pericytes (12, 13).
Heparin-binding EGF-like growth factor (HB-EGF) was initially identified as a secreted product of cultured human macrophages (14) that is a member of the epidermal growth factor (EGF) family (15). HB-EGF is a 22-kDa glycoprotein which is produced as a membrane-anchored precursor (pro-HBEGF) that is processed to a soluble, secreted, mature form (sHBEGF). HB-EGF exerts its biological effects by binding to cell-surface EGF receptors (EGFR) (16, 17) and to the HB-EGF-specific receptor Nardilysin (Nrdc) (18, 19). HB-EGF is expressed in vivo in the vascular EC that are intimately associated with smooth muscle cells (SMC) or pericytes, suggesting an important role for HB-EGF in the recruitment of SMC/pericytes by vascular EC (20). EC have been shown to increase their expression of HB-EGF in response to tumor necrosis factor-α (TNF-α) (21). In addition, angiotensin II induces angiogenesis in the rat cornea via modulation of HB-EGF (22).
We have previously shown that HB-EGF induces capillary tube formation in vitro and angiogenesis in vivo (1). We and others have shown that HB-EGF does not have a direct mitogenic effect on EC (23). However, the effect of HB-EGF on pericytes, especially in the face of hypoxic injury, has not been studied in detail. Although the ability of HB-EGF to act as a mitogen for pericytes has been alluded to by Kirschi and D'Amore as unpublished observations (6), detailed studies of the effects of HB-EGF on pericytes have not been previously reported. The current study used C3H/10T1/2 mouse mesenchymal cells grown in the presence of transforming growth factor-β1 (TGF-β1), which causes differentiation of the mesenchymal cells towards a SMC/pericyte lineage (24–26), to study the effect of HB-EGF on pericytes under basal and injury conditions in vitro. Additionally, we used primary rat pericyte cultures to confirm the effect of HB-EGF on pericyte proliferation. Lastly, we confirmed our in vitro findings by examining the effect of HB-EGF on pericytes in vivo using a mouse model of intestinal I/R injury based on superior mesenteric artery occlusion (SMAO).
MATERIALS AND METHODS
C3H/10T1/2 Cell Culture and Differentiation
C3H/10T1/2 mouse mesenchymal cells (CCL226; ATCC, Rockville, MD) were grown in Dulbecco's modified Eagle's medium (DMEM; JRH Biosciences, Lenexa, KS) supplemented with 10% fetal calf serum (FCS; Hyclone Laboratories, Logan, UT), 233.6 μg/ml glutamine, 25 mM glucose (Sigma Chemical Company, St. Louis, MO), and 100 units/ml penicillin plus100 μg/ml streptomycin (GPS; Irvine Scientific, Santa Ana, CA). The cells were used under passage 10 and were maintained for 3 days at 80% confluence prior to use. C3H/10T1/2 cells were plated at 2 × 104 cells/well in 96-well plates or 2.5 × 105 cells/well in 6-well tissue culture dishes. Ninety minutes after plating, the culture medium was removed and replaced with serum-free medium. Recombinant human TGF-β1 (eBioscience, Inc. San Diego, CA) was added at 1 ng/ml in serum-free medium to promote cell differentiation into pericyte-like cells. The concentration of TGF-β1 was selected according to literature on the basis of dose response curves for stimulating C3H/10T1/2 differentiation into pericytes (25). Cells were differentiated with TGFβ1 for 48h prior to additional treatments.
Primary Pericyte Cultures
The animal procedure was approved by the Institutional Animal Care and Use Committee of the Research Institute at Nationwide Children's Hospital (Protocol 00203 AR). Rat cerebral pericytes were isolated according to established protocols (27–29). Pure cultures of rat cerebral pericytes were obtained by culturing of isolated brain cortex microvessel fragments under selective culture conditions.
Briefly, rat cerebral cortexes were obtained from 3-week-old male Sprague-Dawley (SD) rats. Meninges were carefully removed from forebrains and gray matter was minced into 1 mm pieces in ice-cold Dulbecco's modified Eagle's medium (DMEM). Homogenates were digested with collagenase type II (1 mg/ml; Worthington, Lakewood, NJ) and DNase I (37.5 μg/ml; Sigma, St. Louis, MO) in DMEM containing penicillin (100 units/ml) and streptomycin (100 μg/ml) at 37°C for 1.5 h with agitation. Neurons and glial ce lls were removed by centrifugation in 20% bovine serum albumin (BSA)-DMEM (1000 × g for 20 min). The microvessels obtained in pellets were further digested with collagenase type II (1 mg/ml) and DNase I (16.7 μg/ml) in DMEM at 37 °C for 45 min with agitation. Microvessel endothelial cell clusters were separated using 33% continuous Percoll (GE Healthcare, Sweden) gradient centrifugation (1000 × g for 10 min). Endothelial cell clusters were pipette and filtered through 70 um nylon mesh. Cell pellets were washed twice with DMEM (first 1000 × g for 8 min, then 700 × g for 5 min) and placed in uncoated culture flasks in DMEM supplemented with 10% FBS, L-glutamine (2 mM), glucose `(4.5 g/L), penicillin (100 units/ml) and streptomycin (100 μg/ml) at 37 °C with a humidified atmosphere of 5% CO2/95% air. Cells were allowed to adhere for 4–5 h and then nonadherent cells were removed. After 14 days in culture, rat pericytes overgrew brain endothelial cells and reached 80–90% confluency. Cells were used at passage 2–3 at which time there was ~95% purity as determined by NG2 and desmin double immunostaining.
Materials
Recombinant human HB-EGF corresponding to amino acids 74–148 of the mature HB-EGF precursor was produced using a Pichia pastoris expression system (Trillium Therapeutics, Toronto, Canada). The EGF receptor (EGFR) inhibitor AG1478 (4-(3-chloroanilino)-6,7-dimethoxyquinazoline) was from Calbiochem (San Diego, CA).
Detection of Pericyte Biomarkers by Immunocytochemistry
To evaluate the effect of TGFβ1 on C3H/10T1/2 cell differentiation, differentiated cells were immunostained for α-SMA using a 1:150 dilution of mouse anti-α-SMA (Upstate Biotechnology, New York), for desmin using a 1:50 dilution of mouse anti-desmin (Sigma, Saint Louis, MO) and for NG2 using a 1:100 dilution of rabbit anti-NG2 (Chemicon, Temecula, CA). The secondary antibodies, donkey anti-mouse IgG coupled to cy3, goat anti-mouse IgG coupled to cy2 and goat anti-rabbit IgG coupled to cy3 fluorochrome (Jackson Immunochemicals, West Grove, PA), were used at a 1:500 dilution. Rat primary pericytes were immunostained for desmin and for NG2 using the same primary antibodies, and secondary antibodies of goat anti-mouse IgG coupled to cy2 and goat anti-rabbit IgG coupled to cy3 fluorochrome at a 1:500 dilution. Antibodies were diluted in blocking solution consisting of 5% BSA/0.05% Tween-20 in PBS. Nuclei were identified by labeling with DAPI (500 ng/ml) (Sigma, Saint Louis, MO) for 5 min at RT. Sections were washed in 0.05% Tween-20 in PBS and permanently mounted with Gelvatol.
Trypan Blue Exclusion Assay for Assessment of Cell Viability
Differentiated C3H/10T1/2 cells were collected 24h, 48h, 72h or 96h after treatment with HB-EGF. When used, AG 1478 (500nM) was added to differentiated cells 30 min prior to the addition of HB-EGF. The trypan blue exclusion assay was used to determine total cell numbers and viable cell numbers by counting total and trypan blue-positive cells respectively using a hemocytometer. The percentage of cell survival was calculated as follows: percentage survival (%) = (mean viable cell number/mean total cell number) × 100%.
MTT Assay for Quantification of Cell Proliferation
The effect of HB-EGF on cell proliferation was quantified using the 3,(4,5-dimethyl-2-thiazolyl)-2,5-diphenylate-2H-tetrazolium bromide (MTT) assay. For the study of effect of HB-EGF on C3H/10T1/2 cells, 2 × 104 cells per well were seeded in 96-well plates and cells were differentiated with TGFβ1 for 48h. Differentiated as well as undifferentiated C3H/10T1/2 cells were assayed at 24, 48, 72 and 96h, in the presence or absence of HB-EGF, with or without the addition of the EGF receptor (EGFR) inhibitor AG1478. When used, AG1478 (500nM) was added to cells 30 min prior to addition of HB-EGF. For the study of effect of HB-EGF on rat primary pericytes, 2.5 × 104 cells per well were seeded in 96-well plates, cells were deprived of serum, and were assayed at 24, 48, 72 and 96h. After treatment, 10 μl MTT (5 mg/ml) reagent was added to each well (medium volume 100 μl) followed by incubation at 37°C for 4h. The MTT solution and culture medium were then discarded and 100 μl of dimethyl sulfoxide (DMSO) was added to dissolve the formazan crystals. Absorbance was measured at 570 nm using a microplate spectrophotometer SpectraMax M2 system (Molecular Devices, Sunnyvale, CA). Each treatment was completed in quintuplicate with each experiment repeated 3 times.
Flow Cytometry for Quantification of Cell Proliferation
Differentiated C3H/10T1/2 cells were treated with HB-EGF (10ng/ml). Cells were labeled with 5 (and 6) carboxyfluorescein diacetate succinimidyl ester (CFSE) for flow cytometry. Briefly, a freshly prepared 5mM stock solution of CFSE (Invitrogen, Carlsbad, CA) with DMSO was diluted in prewarmed PBS at a final concentration of 2 M. Cells were incubated with CFSE for 15 min at RT prior to resuspension in regular culture medium. Cells were cultured for 4d and then harvested and fixed with 2% paraformaldehyde. Cells were analyzed by division tracking flow cytometry on a BD LSR II flow cytometer (BD Biosciences) and analyzed using FlowJo software (TreeStar, Ashland, OR). Division was characterized by sequential halving of CFSE fluorescence, producing evenly spaced peaks on a logarithmic scale. Increased cell division was indicated by a left shift of mean fluorescent intensity of the peaks. Control cultures of unlabeled cells were analyzed for autofluorescence to determine background. At least 30,000 events were acquired for each sample.
Flow Cytometry for Quantification of Cellular Apoptosis
Apoptosis was determined by flow cytometry using an annexin V-FITC/PI Apoptosis Assay Kit (Invitrogen, Carlsbad, CA) according to the manufacturers' recommended protocol. Differentiated C3H/10T1/2 cells were stressed by anoxia (100% N) for 1h. Cells that were treated with HB-EGF (10ng/ml) received the growth factor for 1 h prior to anoxic stress. Twenty hours later, floating and attached cells were harvested, washed twice in PBS, and resuspended in 1× binding buffer at a cell density of 1×106 cells/ml. Cells were double stained in the dark for 15 min at RT with the fluorescein isothiocyanate (FITC)-labeled annexin V (5 μl) and PI (10 μl) prior to analysis by flow cytometry. Gates were set up using unstained and single stained negative controls. At least 30,000 events were acquired for each sample. Flow cytometry data were acquired on a BD LSR II flow cytometer (BD Biosciences) and analyzed using FlowJo software (TreeStar, Ashland, OR). Apoptosis was measured by plotting the events from a dual gate of a biparametric histogram using the Fl-1 channel (annexin-V) and Fl-2 channel (PI), respectively. The biparametric representation showed at least three distinct populations: (1) viable cells (annexin-V−/PI−), (2) early apoptotic cells (annexin-V+/PI−), and (3) late apoptotic cells (annexin-V+/PI+). The experiment was repeated 3 times.
TUNEL Assay
Apoptosis was also determined using the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay, using an Apop Tag Red in Situ Apoptosis Detection kit (Chemicon, Temecula, CA). Briefly, cells were grown in 6-well culture plates and differentiated for 48h. Cells were then stressed by anoxia (100% N) for 1h in the presence or absence of HB-EGF (10 ng/ml). Cells that were treated with HB-EGF received the growth factor for 1h prior to exposure to anoxia. After exposure to anoxia for 24h, cells were washed twice with ice-cold PBS and fixed with 2% paraformaldehyde in PBS for 20 min at RT. After washing twice with PBS, cells were treated with permeabilization solution (0.5% Triton X-100 in 0.1% sodium citrate) for 10 min on ice. Samples were then washed with PBS, equilibrated and incubated with TdT enzyme at 37°C for 1h, followed by incubation with anti-digoxigenin conjugate (rhodamine) in a humidified chamber for 30 min at RT in the dark. Cells were counterstained with DAPI and were washed twice with PBS, and then visualized using a Zeiss Axioskop fluorescent microscope (Carl Zeiss, New York, NY). Two random fields were counted per well in duplicate wells from three separate experiments.
Intestinal I/R Injury Animal Model
The animal procedure was approved by the Institutional Animal Care and Use Committee of the Nationwide Children's Hospital Research Institute (Protocol 00903 AR). Eight to ten week old male C57BL/6 mice (Charles River, Boston, MA) weighing 20–25g were fasted for 10–12 hours with access to water only. Mice were randomized to either sham surgery (sham), or superior mesenteric artery occlusion (SMAO) for 45 min followed by reperfusion for 8 h (I/R), or I/R plus HB-EGF treatment (I/R+HB-EGF). Animals were anesthetized with 3% isoflurane, and the abdominal skin was shaved and scrubbed with 70% ethanol. Anesthesia was maintained using 1% isoflurane and animal body temperature was kept at 37°C with the use of a heating pad.
A midline laparotomy was performed under a dissecting microscope. The superior mesenteric artery (SMA) as well as the collateral branches from the celiac artery were identified and occluded with non-traumatic vascular clamps. Intestinal ischemia was established by visualizing paleness of the small intestine wall and lack of pulsation in the mesentery. After 15 min of ischemia, mice in the I/R+ HB-EGF group received a 0.6mL intraluminal injection of HB-EGF (1200 μg/kg), diluted in 0.1% bovine serum albumin (BSA) with PBS, at the jejuno-ileal junction to ensure that the entire small bowel was filled with the HB-EGF solution. Animals in the I/R only group received 0.6mL of 0.1% bovine serum albumin (BSA) in PBS solution only. The vascular clamps were removed after 45 min of ischemia and the intestine was allowed to reperfuse for 8 h. In the sham-operated group, the SMA and collateral branches from the celiac axis were identified but were not clamped.
Pericyte Immunohistochemistry
Since no single pericyte marker can identify all pericytes (28), we performed double-staining using the well-established pericyte markers desmin and PDGFR-β. PDGFR-β has been shown to be an early marker of activated pericytes (29, 30). Paraffin sections of mouse ileum were used for the studies. Slides were deparaffinized, and antigen retrieval was performed with a pressure cooker using citrate buffer (10mM, pH 6.0). Sections were immunohistochemically stained for PDGFR-β using a 1:50 dilution of goat anti-PDGFR-β (Santa Cruz Biotechnology, Santa Cruz, CA) and for desmin using a 1:50 dilution of mouse anti-desmin (Sigma, Saint Louis, MO). The secondary antibodies, donkey anti-goat IgG coupled to Cy2 (Jackson Immunochemicals, West Grove, PA) and goat anti-mouse IgG coupled to Alexa (Invitrogen, Carlsbad, CA) were used at a 1:500 dilution. Antibodies were diluted in blocking solution of 5% donkey serum/0.05% Tween-20 in PBS. Nuclei were identified by labeling with DAPI (500 ng/ml) (Sigma, Saint Louis, MO) for 5 min at RT. Sections were washed with 0.05% Tween-20 in PBS, permanently mounted with Gelvatol, and visualized using a Zeiss Axioskop fluorescent microscope (Carl Zeiss, New York, NY).
Statistical Analyses
Experimental data were expressed as mean ± SD. Statistical differences between groups were compared using the Student's t-test or chi-square test. Differences were considered to be statistically significant if p<0.05.
RESULTS
Differentiated C3H/10T1/2 Cells Exhibit Characteristics of Pericytes
The C3H/10T1/2 cell line is a multipotent mouse embryonic cell line that differentiates into SMC/pericytes upon stimulation with TGF-β1 (26). To confirm C3H/10T1/2 cell differentiation into pericytes, we exposed the cells to TGF-β1 for 24–48h. Differentiated cells changed their shape from polygonal to spindle-shaped with long processes when viewed by phase contrast microscopy (Figure 1, A and B). In addition, differentiated cells are known to express a group of pericyte markers after 48h of differentiation (31). We therefore determined expression of pericyte markers including α-smooth muscle actin (α-SMA), desmin and neuron/glia-type 2 antigen (NG2). There was significantly increased expression of these markers in fully differentiated cells compared with undifferentiated cells (Figure 1, C–F) (NG2 expression not shown). We also noted the importance of TGF-β1 in maintenance of the differentiated state, since pericyte-like cells reversed their differentiated phenotype if TGF-β1was removed from the culture medium, as indicated by changing of cell shape from spindle-shaped back to polygonal, with decreased expression of α-SMA (data not shown).
Figure 1.
Morphologic and immunocytochemical analysis of differentiated 3H/10T1/2 cells. 3H/10T1/2 cells (2.5 × 105 cells/well) were cultured in 6-well plates for 90 min and then incubated in starvation medium for 48h with or without the addition of TGF-β1 (1ng/ml) for 48h. Shown are representative photomicrographs of: A, C, E) undifferentiated cells; B, D, F) differentiated cells; A,B) unstained cells, C,D) cells stained for α-SMA; E,F) cells stained for desmin. Panels A and B were visualized by phase contrast microscopy; panels C–F were visualized by fluorescent microscopy. 40× magnification. Note that differentiated cells are spindle-shaped with long processes when viewed by phase contrast microscopy, and have markedly increased expression of α-SMA and desmin. Results are representative of three separate experiments.
HB-EGF Stimulates Cell Proliferation of Differentiated C3H/10T1/2 Cells and Primary Rat Pericytes
Addition of HB-EGF to the culture medium of undifferentiated C3H/10T1/2 cells had no effect on cell proliferation (Figure 2A), however, addition of the growth factor to differentiated (pericyte-like) cells led to significantly increased cell proliferation (Figure 2, B–D). The proliferative effects of HB-EGF on pericyte-like cells was demonstrated using the MTT assay (Figure 2B), the trypan blue exclusion assay (Figure 2C), and by flow cytometry (Figure 2D). Using CFSE division tracking flow cytometry, increased cell division in HB-EGF stimulated cells was seen as a left shift of the mean fluorescent intensity of the peaks in the histograph from 8248 to 3133 (mean fluorescent intensity units on a log scale). In addition, the fluorescent intensity of HB-EGF-treated cells decreased significantly compared with non-HB-EGF-treated cells as seen in the dot plot graph. The decreased mean fluorescence of HB-EGF-treated cells represents fast cell division in these cells. Since the biological activity of HB-EGF is mediated through its interaction with EGF receptors (EGFR), we used AG1478, a potent and selective inhibitor of EGFR kinase, to determine whether HB-EGF-induced cell proliferation in pericyte-like cells was mediated via EGFR. We found that the proliferative effects of HB-EGF on pericyte-like cells was completely inhibited by EGFR inhibition in both the MTT (Figure 2B) and the trypan blue exclusion assay (Figure 2C). The proliferative effect of HB-EGF on pericyte-like cells was further investigated using primary rat pericyte cultures, confirming that HB-EGF promotes pericyte proliferation (Figure 2E).
Figure 2.
HB-EGF promotes cell proliferation in differentiated C3H/10T1/2 cells and in primary rat pericyte cultures. A) Cell proliferation as measured by the MTT assay in undifferentiated C3H/10T1/2 cells; B) cell proliferation as measured by the MTT assay in differentiated C3H/10T1/2 cells; C) cell proliferation as measured by the trypan blue assay in differentiated C3H/10T1/2 cells; D) cell proliferation as measured by CFSE flow cytometry in differentiated C3H/10T1/2 cells; and E) cell proliferation as measured by the MTT assay in primary rat pericyte cultures. Note that HB-EGF significantly increases cell proliferation of primary pericytes and of differentiated C3H/10T1/2 (pericyte-like) cells. In the latter, this effect is totally blocked by pretreatment of the cells with the EGFR inhibitor AG 1478. In A, B, and E, data at each time point were obtained in quintuplicate. All experiments were repeated 3 times with similar results. In panel D, differentiated C3H/10T1/2 cells were treated with HB-EGF (10ng/ml), labeled with 5 (and 6) carboxyfluorescein diacetate succinimidyl ester (CFSE) and examined by flow cytometry. Increased cell division is indicated by a left shift of the mean fluorescent intensity of the peaks. The decrease of mean fluorescence represents increased cell division in HB-EGF-treated cells compared with non-HB-EGF-treated cells (p<0.001; Student's t-test). This experiment was repeated three times with similar results. AG, AG1478. * p<0.05 and ** p<0.001 compared with control; Student's t-test.
HB-EGF Stimulates Cell Proliferation of Differentiated C3H/10T1/2 Cells under Conditions of Anoxic Stress
Given that HB-EGF has potent mitogenic effects on pericytes under basal conditions, that HB-EGF preserves microvascular blood flow under conditions of injury, and that pericytes are important regulators of microvascular blood flow, we next tested the effect of HB-EGF on differentiated C3H/10T1/2 cells under conditions of anoxic stress (100% N) for I h. Upon exposure to anoxia, pericyte-like cells exhibited a significant decrease in cell viability (Figure 3). However, addition of HB-EGF (10 ng/ml) prior to anoxia not only preserved cell viability, but actually promoted cell proliferation to levels higher than that of non-HB-EGF-treated cells grown under normoxic conditions.
Figure 3.
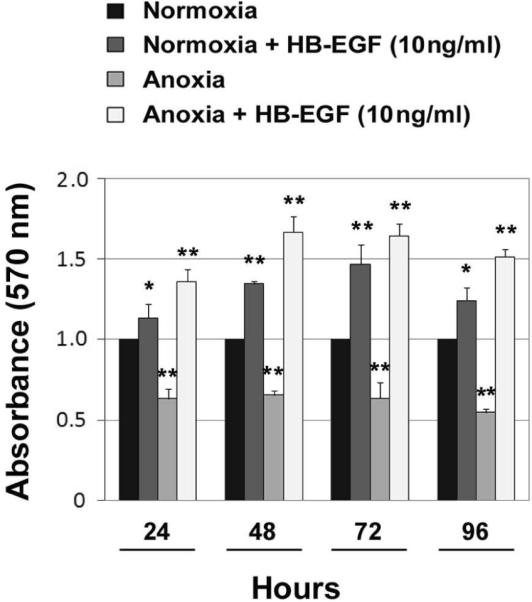
HB-EGF promotes cell proliferation in differentiated C3H/10T1/2 cells exposed to anoxic stress. Cell proliferation was measured using the MTT assay. HB-EGF (10 ng/ml) was added 1h prior to the initiation of anoxia and left in the culture until the cells were harvested. The proliferative effect of HB-EGF on pericyte-like cells persists in the presence of anoxic insult to the cells. * p<0.05 and ** p<0.001 compared with normoxia; Student's t-test.
HB-EGF Protects Differentiated C3H/10T1/2 Cells from Anoxia-induced Apoptosis
Previous studies have shown that mature HB-EGF acts as an anti-apoptotic factor in several different cell types. Based on this, we next examined the effect of HB-EGF on pericyte-like cells exposed to anoxia-induced apoptosis. Apoptosis was examined using both TUNEL assay (Figure 4, A–G) and flow cytometry (Figure 4H). Under conditions of anoxic stress (100% N for 1h), TUNEL-positive cells increased from 2% to 26% of the cell population 24h after exposure to anoxia (Figure 4G). However, addition of HB-EGF protected pericyte-like cells from anoxia-induced apoptosis, decreasing TUNEL positive cells from 26% to 8%. Apoptosis was further analyzed using two-dimensional flow cytometry with annexin V and propidium iodide dual staining (Figure 4H). Using flow cytometry, HB-EGF decreased apoptotic cells from 17.7% to 12.8% for early apoptotic activity and from 5.39% to 1.78% for late apoptotic activity.
Figure 4.
HB-EGF protects differentiated C3H/10T1/2 cells from anoxia-induced apoptosis. Apoptosis was determined by TUNEL staining (A–G) and by flow cytometry (H). A, C, E) differentiated cells without HB-EGF treatment; B, D, F) differentiated cells treated with HB-EGF (10 ng/ml). TUNEL positive cells are stained red and DAPI positive cell nuclei are stained in blue. G) quantification of apoptotic cells in panels A–F. All panels are representative of three separate experiments. * p<0.001 compared with control, ** p<0.05 compared with anoxia; Chi-square test. H) Apoptosis as determined by two-dimensional flow cytometry using annexin V and propidium iodide dual staining. Cells in the lower left quadrants [Annexin V-FITC(−)/PI(−)] represent viable cells. Cells in the lower right quadrants [Annexin V-FITC(+)/PI(−)] represent early apoptotic cells. Cells in the upper right quadrants [Annexin V-FITC(+)/PI(+)] represents late apoptotic/necrotic cells. HB-EGF protects pericyte-like cells from anoxia-induced apoptosis (from 17.7% to 12.8% for early apoptotic activity and from 5.39% to 1.78% for late apoptotic activity). The bottom panel shows that Annexin V-FITC fluorescent intensity decreased significantly in the late apoptotic cell population in cells exposed to anoxia and treated with HB-EGF compared to non-HB-EGF-treated cells (p<0.001; Student's t-test). This experiment was repeated three times with similar results.
HB-EGF Protects Pericytes from Injury after Intestinal I/R
In adult mice, the intestinal villi contain an abundance of capillary plexuses, allowing for morphological study of pericytes. With found that double staining with antibodies to the pericyte-specific markers PDGFR-β and desmin resulted in visualization of pericytes in the lamina propria of the intestinal villi (Figure 5). I/R-induced tissue damage, manifested as edematous villi with damage to the villous architecture, resulted in decreased pericyte cell numbers. However, intraluminal administration of HB-EGF led to a significant increased in pericyte numbers, as well as decreased intestinal histologic injury.
Figure 5.
HB-EGF protects pericytes for I/R injury in vivo. Shown are representative examples of ileal tissue sections immunostained with antibodies to the pericyte-specific markers PDGFR-β and desmin from: A–D) mice subjected to sham surgery; E–H) mice subjected to I/R injury; I-L) mice subjected to I/R injury with administration of HB-EGF (1200 μg/kg). A, E, I) tissue stained for PDGFR-β; B, F, J) tissue stained for desmin; C, G, K) merged images; D, H, L) merged images with DAPI counterstaining of nuclei. Slides were visualized with fluorescent microscopy, 40× magnification. M) Quantification of immunohistochemical results. Results were quantified by counting pericytes that stained positively for both PDGFR-β and desmin in 200 well-aligned villi for each group of animals. * p<0.001; Chi-square test.
DISCUSSION
The vasculature consists of an inner endothelium surrounded and intimately associated with pericytes in the microvasculature or with layers of vascular SMC in the larger vessels. Pericytes give support to the mature microvasculature, and are essential for normal blood vessel development (31). Pericytes play a pivot role in vessel maturation, and there is increasing evidence to indicate that pericytes increase blood vessel stability (25). Morphologic evidence suggests that pericytes are contractile perivascular cells located in capillaries and post-capillary venules (32). Recent studies have revealed that pericytes, together with arterioles, precapillary sphincters, and capillary endothelium, play an important role in regulating blood flow in the intestinal microvascular bed and other regions of the body due to their contractile filaments (33). Pericytes have been shown to regulate capillary blood flow and new capillary growth (6). Many studies support the notion that EC and their surrounding mural cells have significant “cross-talk”, enabling them to influence each other's behavior. Recruitment of vascular SMC by EC is essential for the process of angiogenesis.
HB-EGF, a member of the EGF family, exerts its biological effects by binding to cell-surface EGFR (16, 17) and to the HB-EGF-specific receptor Nardilysin (Nrdc) (18, 19). Many cell types including epithelial cells produce HB-EGF which acts as an autocrine growth factor for these cells (34). HB-EGF is known as a potent mitogen for a number of cell types, including smooth muscle cells, epithelial cells, fibroblasts, keratinocytes, and renal tubule cells (35). Interestingly, even though EC produce HB-EGF (21), and HB-EGF promotes tube formation in EC in vitro and angiogenesis in vivo (1), HB-EGF does not have direct mitogenic effects on EC. HB-EGF is the product of a hypoxia- and stress-inducible gene that is involved in reduction of ischemia/reperfusion injury and stress-induced tissue damage (36). Numerous lines of evidence exist to demonstrate that HB-EGF protects cells from injury in vitro and organs from injury in vivo. Our previous studies revealed that HB-EGF preserves mesenteric microvascular blood flow in adult rats subjected to intestinal I/R injury (2), HS/R (3), and experimental NEC (4). We have also shown that HB-EGF acts as a vasodilator of isolated terminal mesenteric arterioles via upregulation of (NO) production in vascular EC and via increased expression of endothelin B (ETB) receptors in vascular SMC (5).
Endothelial cells synthesize both platelet-derived growth factor A and B chains, basic fibroblast growth factor (bFGF) and HB-EGF (21), which may act in a paracrine fashion to stimulate mural cell proliferation or migration (6). Interaction between EC and mural cells leads to the activation of TGF-β1, which stimulates mesenchymal cells to express pericyte/SMC markers and also inhibits EC proliferation. HB-EGF promotes SMC migration by stimulating tyrosine phosphorylation of ErbB-1 and ErbB-2 in vascular SMC (20). Furthermore, neutralization of HB-EGF or inhibition of ErbB-1 or ErbB-2 decreases the ability of EC to stimulate SMC migration (20). This suggests a pivot role for HB-EGF and EGFR in the recruitment of pericytes/SMC by EC during angiogenesis. While Hirschi and D'Amore mention as unpublished observations that HB-EGF acts as a mitogen for pericytes (Hirschi and D'Amore, 1996), no previous publications specifically address this observation. Although a previous publication by Swinscoe et al. does mention a retinal endothelial cell-derived growth factor that had the ability to bind to heparin and to stimulate pericyte growth (37), we do not believe that they were looking at HB-EGF since their growth factor did not stimulate the growth of smooth muscle cells or Balb/c 3T3 fibroblast cells, as does HB-EGF. In the current study, we report for the first time that HB-EGF promotes pericyte proliferation and protects pericytes from apoptosis, even under conditions of anoxic injury. Furthermore, we show for the first time that HB-EGF protects pericytes from I/R injury in vivo.
The C3H/10T1/2 cell line is a multipotent mouse embryonic cell line that can be differentiated into pericytes upon exposure to TGF-β1, upon which the cells exhibit a pericyte-like morphology and expression of pericyte specific markers (25, 26, 31), making these cells a useful tool for in vitro pericyte studies. When C3H/10T1/2 cells are differentiated by addition of TGF-β1, they display an angiogenic program of gene expression, with up-regulation of several genes previously implicated in angiogenesis, including HB-EGF, vascular endothelial growth factor (VEGF), interleukin-6 (IL-6), VEGF-C, ephrin receptor A2, integrin α5, tenascin C and connective tissue growth factor (CTGF) (31). In the current study, we examined the effects of HB-EGF on pericyte-like cells, using a well-established in vitro model of TGF-β-stimulated differentiation of C3H/10T1/2 mouse mesenchymal cells into pericytes. We showed that HB-EGF exhibited strong proliferative effects on differentiated C3H/10T1/2 cells but had no effect on undifferentiated cells, demonstrating that HB-EGF selectively promotes pericyte-like cell proliferation.. In addition, the proliferative effects of HB-EGF on pericyte-like cells was prominent under both basal and injury conditions. The proliferation effect of HB-EGF on differentiated C3H/10T1/2 cells was mediated via EGFR, since these effects were completely blocked when cells were pretreated with the EGFR inhibitor AG1478. We also found that addition of HB-EGF to pericyte-like cells exposed to anoxia led to decreased apoptosis of the cells. In addition to having potent mitogenic effects on differentiated C3H/10T1/2 cells, HB-EGF also promoted the proliferation in primary pericyte cultures. Importantly, HB-EGF protected pericytes from injury in vivo in a rat model of SMAO.
Previous studies from our laboratory demonstrated that HB-EGF protects enterocytes from proinflammatory cytokine-induced apoptosis in vitro (38). One of the mechanisms by which HB-EGF exerts its potent cytoprotective effects is by decreasing the expression of inducible nitric oxide synthase (iNOS) and NO production upon intestinal epithelial cell injury (39). Others have shown that HB-EGF inhibited apoptosis of cytotrophoblast cells (40) and that mature, secreted HB-EGF inhibited apoptosis of granulosa cells (41). The current study demonstrates that HB-EGF also has anti-apoptotic effects in pericytes.
In conclusion, we have shown that HB-EGF significantly promotes cell proliferation of differentiated C3H/10T1/2 (pericyte-like) cells via interaction with cell surface EGF tyrosine kinase receptors, and protects pericyte-like cells from anoxia-induced apoptosis. The effect of HB-EGF on pericyte-like cells was confirmed in primary pericyte cultures. In addition, we have shown that HB-EGF protects pericytes from intestinal I/R injury in vivo. Given that pericytes play pivotal roles in the vasculature including regulation of capillary blood flow and new capillary growth, the effects of HB-EGF on microvascular blood flow may be due, at least in part, to its mitogenic and anti-apoptotic effects on pericytes.
ACKNOWLEDGEMENTS
The authors thank Dave Dunaway (Flow Cytometry Core, The Research Institute at Nationwide Children's Hospital) for assistance with flow cytometry, and Wei Wang (Biostatistics Core, The Research Institute at Nationwide Children's Hospital) for assistance with data analysis.
This work was supported by NIH R01DK074611 and NIH R01GM61193 (GEB) and by the Children's Hospital Firefighter's Endowment (XY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mehta VB, Besner GE. Growth factors. Vol. 25. Chur, Switzerland: 2007. HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways; p. 253. [DOI] [PubMed] [Google Scholar]
- 2.El-Assal ON, Paddock H, Marquez A, et al. Heparin-binding epidermal growth factor-like growth factor gene disruption is associated with delayed intestinal restitution, impaired angiogenesis, and poor survival after intestinal ischemia in mice. Journal of pediatric surgery. 2008;43:1182. doi: 10.1016/j.jpedsurg.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Assal ON, Radulescu A, Besner GE. Heparin-binding EGF-like growth factor preserves mesenteric microcirculatory blood flow and protects against intestinal injury in rats subjected to hemorrhagic shock and resuscitation. Surgery. 2007;142:234. doi: 10.1016/j.surg.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Yu X, Radulescu A, Zorko N, et al. Heparin-binding EGF-like growth factor increases intestinal microvascular blood flow in necrotizing enterocolitis. Gastroenterology. 2009;137:221. doi: 10.1053/j.gastro.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Y, Brigstock D, Besner GE. Heparin-binding EGF-like growth factor is a potent dilator of terminal mesenteric arterioles. Microvasc Res. 2009;78:78. doi: 10.1016/j.mvr.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirschi KK, D'Amore PA. Pericytes in the microvasculature. Cardiovascular research. 1996;32:687. [PubMed] [Google Scholar]
- 7.Majno G, Palade GE, Schoefl GI. Studies on inflammation. II. The site of action of histamine and serotonin along the vascular tree: a topographic study. J Biophys Biochem Cytol. 1961;11:607. doi: 10.1083/jcb.11.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maxwell DS, Kruger L. Small Blood Vessels and the Origin of Phagocytes in the Rat Cerebral Cortex Following Heavy Particle Irradiation. Exp Neurol. 1965;12:33. doi: 10.1016/0014-4886(65)90097-x. [DOI] [PubMed] [Google Scholar]
- 9.Herman IM, D'Amore PA. Microvascular pericytes contain muscle and nonmuscle actins. J Cell Biol. 1985;101:43. doi: 10.1083/jcb.101.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeNofrio D, Hoock TC, Herman IM. Functional sorting of actin isoforms in microvascular pericytes. J Cell Biol. 1989;109:191. doi: 10.1083/jcb.109.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tilton RG, Kilo C, Williamson JR. Pericyte-endothelial relationships in cardiac and skeletal muscle capillaries. Microvasc Res. 1979;18:325. doi: 10.1016/0026-2862(79)90041-4. [DOI] [PubMed] [Google Scholar]
- 12.Kelley C, D'Amore P, Hechtman HB, et al. Vasoactive hormones and cAMP affect pericyte contraction and stress fibres in vitro. J Muscle Res Cell Motil. 1988;9:184. doi: 10.1007/BF01773740. [DOI] [PubMed] [Google Scholar]
- 13.Haefliger IO, Zschauer A, Anderson DR. Relaxation of retinal pericyte contractile tone through the nitric oxide-cyclic guanosine monophosphate pathway. Invest Ophthalmol Vis Sci. 1994;35:991. [PubMed] [Google Scholar]
- 14.Besner G, Higashiyama S, Klagsbrun M. Isolation and characterization of a macrophage-derived heparin-binding growth factor. Cell regulation. 1990;1:811. doi: 10.1091/mbc.1.11.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higashiyama S, Abraham JA, Miller J, et al. Science. Vol. 251. New York, N.Y: 1991. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF; p. 936. [DOI] [PubMed] [Google Scholar]
- 16.Beerli RR, Hynes NE. Epidermal growth factor-related peptides activate distinct subsets of ErbB receptors and differ in their biological activities. The Journal of biological chemistry. 1996;271:6071. doi: 10.1074/jbc.271.11.6071. [DOI] [PubMed] [Google Scholar]
- 17.Elenius K, Paul S, Allison G, et al. Activation of HER4 by heparin-binding EGF-like growth factor stimulates chemotaxis but not proliferation. The EMBO journal. 1997;16:1268. doi: 10.1093/emboj/16.6.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishi E, Prat A, Hospital V, et al. N-arginine dibasic convertase is a specific receptor for heparin-binding EGF-like growth factor that mediates cell migration. The EMBO journal. 2001;20:3342. doi: 10.1093/emboj/20.13.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hospital V, Prat A. Nardilysin, a basic residues specific metallopeptidase that mediates cell migration and proliferation. Protein and peptide letters. 2004;11:501. doi: 10.2174/0929866043406508. [DOI] [PubMed] [Google Scholar]
- 20.Iivanainen E, Nelimarkka L, Elenius V, et al. Angiopoietin-regulated recruitment of vascular smooth muscle cells by endothelial-derived heparin binding EGF-like growth factor. FASEB J. 2003;17:1609. doi: 10.1096/fj.02-0939com. [DOI] [PubMed] [Google Scholar]
- 21.Yoshizumi M, Kourembanas S, Temizer DH, et al. Tumor necrosis factor increases transcription of the heparin-binding epidermal growth factor-like growth factor gene in vascular endothelial cells. The Journal of biological chemistry. 1992;267:9467. [PubMed] [Google Scholar]
- 22.Fujiyama S, Matsubara H, Nozawa Y, et al. Angiotensin AT(1) and AT(2) receptors differentially regulate angiopoietin-2 and vascular endothelial growth factor expression and angiogenesis by modulating heparin binding-epidermal growth factor (EGF)-mediated EGF receptor transactivation. Circulation research. 2001;88:22. doi: 10.1161/01.res.88.1.22. [DOI] [PubMed] [Google Scholar]
- 23.Mehta VB, Zhou Y, Radulescu A, et al. Growth factors. Vol. 26. Chur, Switzerland: 2008. HB-EGF stimulates eNOS expression and nitric oxide production and promotes eNOS dependent angiogenesis; p. 301. [DOI] [PubMed] [Google Scholar]
- 24.Darland DC, D'Amore PA. TGF beta is required for the formation of capillary-like structures in three-dimensional cocultures of 10T1/2 and endothelial cells. Angiogenesis. 2001;4:11. doi: 10.1023/a:1016611824696. [DOI] [PubMed] [Google Scholar]
- 25.Darland DC, Massingham LJ, Smith SR, et al. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol. 2003;264:275. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Hirschi KK, Rohovsky SA, D'Amore PA. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141:805. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dohgu S, Takata F, Yamauchi A, et al. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-beta production. Brain Res. 2005;1038:208. doi: 10.1016/j.brainres.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 28.Hall AP. Review of the pericyte during angiogenesis and its role in cancer and diabetic retinopathy. Toxicol Pathol. 2006;34:763. doi: 10.1080/01926230600936290. [DOI] [PubMed] [Google Scholar]
- 29.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circulation research. 2005;97:512. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 30.Betsholtz C, Lindblom P, Bjarnegard M, et al. Role of platelet-derived growth factor in mesangium development and vasculopathies: lessons from platelet-derived growth factor and platelet-derived growth factor receptor mutations in mice. Curr Opin Nephrol Hypertens. 2004;13:45. doi: 10.1097/00041552-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Kale S, Hanai J, Chan B, et al. Microarray analysis of in vitro pericyte differentiation reveals an angiogenic program of gene expression. FASEB J. 2005;19:270. doi: 10.1096/fj.04-1604fje. [DOI] [PubMed] [Google Scholar]
- 32.Joyce NC, Haire MF, Palade GE. Contractile proteins in pericytes. I. Immunoperoxidase localization of tropomyosin. J Cell Biol. 1985;100:1379. doi: 10.1083/jcb.100.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wille KH, Schnorr B. The occurrence of hemodynamic effective elements in the intestinal blood vessel system. Anat Histol Embryol. 2003;32:94. doi: 10.1046/j.1439-0264.2003.00463.x. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto K, Higashiyama S, Asada H, et al. Heparin-binding epidermal growth factor-like growth factor is an autocrine growth factor for human keratinocytes. The Journal of biological chemistry. 1994;269:20060. [PubMed] [Google Scholar]
- 35.Davis-Fleischer KM, Besner GE. Structure and function of heparin-binding EGF-like growth factor (HB-EGF) Front Biosci. 1998;3:d288. doi: 10.2741/a241. [DOI] [PubMed] [Google Scholar]
- 36.Feng J, El-Assal ON, Besner GE. Heparin-binding EGF-like growth factor (HB-EGF) and necrotizing enterocolitis. Seminars in pediatric surgery. 2005;14:167. doi: 10.1053/j.sempedsurg.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Swinscoe JC, Carlson EC. Capillary endothelial cells secrete a heparin-binding mitogen for pericytes. J Cell Sci. 1992;103(Pt 2):453. doi: 10.1242/jcs.103.2.453. [DOI] [PubMed] [Google Scholar]
- 38.Michalsky MP, Kuhn A, Mehta V, et al. Heparin-binding EGF-like growth factor decreases apoptosis in intestinal epithelial cells in vitro. Journal of pediatric surgery. 2001;36:1130. doi: 10.1053/jpsu.2001.25730. [DOI] [PubMed] [Google Scholar]
- 39.Xia G, Lara-Marquez M, Luquette MH, et al. Heparin-binding EGF-like growth factor decreases inducible nitric oxide synthase and nitric oxide production after intestinal ischemia/reperfusion injury. Antioxidants & redox signaling. 2001;3:919. doi: 10.1089/15230860152665073. [DOI] [PubMed] [Google Scholar]
- 40.Leach RE, Kilburn BA, Petkova A, et al. Diminished survival of human cytotrophoblast cells exposed to hypoxia/reoxygenation injury and associated reduction of heparin-binding epidermal growth factor-like growth factor. Am J Obstet Gynecol. 2008;198:471 e1. doi: 10.1016/j.ajog.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan B, Sengoku K, Goishi K, et al. The soluble and membrane-anchored forms of heparin-binding epidermal growth factor-like growth factor appear to play opposing roles in the survival and apoptosis of human luteinized granulosa cells. Mol Hum Reprod. 2002;8:734. doi: 10.1093/molehr/8.8.734. [DOI] [PubMed] [Google Scholar]






