Abstract
Background
Left ventricular (LV) remodeling following myocardial infarction (MI) is associated with increased levels of specific matrix metalloproteinases (MMPs) and relative reduction of endogenous tissue inhibitors of the MMPs (TIMPs). However, transcriptional mechanisms for the disparate post-MI MMP/TIMP expression remain unknown. Using murine constructs designed to report gene promoter activation, this study tested the hypothesis that distinctly different temporal profiles of MMP-2, MMP-9, and TIMP-1 transcription occurs post-MI.
Methods/Results
Transcriptional activity (β-galactosidase (β-gal) reporter constructs) of MMP-2 (n=49), MMP-9 (n=62), or TIMP-1 (n=40) was assayed at 1 hour (acute), and 1 – 28 days after MI (coronary ligation) in transgenic reporter mice. At 7 days post-MI, the area of promoter activation normalized to LV area was increased from acute values for MMP-2 (63.4±5.8 vs 1.1±1.0 %, p<0.05) and MMP-9 (53.1±6.1 vs 1.3±0.9 %, p<0.05). While TIMP-1 promoter activation at 7 days post-MI increased from acute values (3.6±1.3 vs 0.3±0.5%, p<0.05), this increase was smaller than that for MMP-2 or MMP-9 (both p<0.05). MMP-2 promoter activation peaked in the MI region at 7 days post-MI and MMP-9 promoter activation was highest in the border region at 7 and 14 days post-MI. TIMP-1 promoter activation peaked within the MI region at 7 days post-MI and within the remote region at 14 days post-MI.
Conclusions
These findings provided direct in vivo evidence that discordant changes in temporal and spatial patterns of MMP/TIMP transcription occurs with MI. Restoration of TIMP-1 promoter activation may represent a molecular therapeutic target to attenuate/prevent adverse post-MI LV remodeling.
Keywords: Matrix metalloproteinase, Tissue inhibitor of metalloproteinase, Myocardial infarction, Remodeling
INTRODUCTION
A number of changes in left ventricular (LV) geometry and within the extracellular matrix (ECM) occur following myocardial infarction (MI) (1–5). The matrix metalloproteinases (MMPs) and the endogenous tissue inhibitors of the metalloproteinases (TIMPs) are key components involved in remodeling of the ECM (2, 4, 6). Past studies have clearly documented that changes in the protein levels of MMPs and TIMPs occur post-MI (7–12). While the levels of certain MMPs are increased post-MI, levels of the TIMPs have been reported to be decreased at the border and MI regions (13). Therefore, the stoichiometric imbalance between MMP and TIMP levels may contribute to adverse LV remodeling post-MI.
Through pharmacological inhibition of MMPs and/or utilization of transgenic models with targeted deletion of certain MMPs and TIMPs, causal relationships for the role of these proteins in post-MI remodeling have been established (14–19). For example, post-MI LV dilatation and/or post-MI mortality due to LV rupture were attenuated in mice deficient in MMP-2 or MMP-9 (14, 15). Conversely, mice deficient in TIMP-1 demonstrated an accelerated LV dilation in the post-MI period (19). Taken together, these past studies provided evidence that changes in MMP-2, MMP-9, and TIMP-1 likely contribute to pathologic LV remodeling post-MI. Nevertheless, it remains unknown whether the imbalance in MMP/TIMP stoichiometry is due to dysregulation of gene promoter activation. Accordingly, through the use of transgenic promoter reporter constructs, the goal of the present study was to determine the temporal as well as spatial patterns for the activation of the promoter regions of MMP-2, MMP-9, and TIMP-1 genes post-MI.
MATERIALS AND METHODS
This study was designed to examine the spatial as well as temporal induction of gene promoters for matrix metalloproteinase (MMP)-2, MMP-9, and tissue inhibitor of the metalloproteinases (TIMP)-1 following myocardial infarction (MI). In this study, transgenic mice were used in which appropriate β-galactosidase (β-gal) reporter constructs for MMP-2, MMP-9, or TIMP-1 were inserted into the genome. All animals were treated and cared for in accordance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” (National Research Council, Washington, 1996), and the protocol was approved by the Institutional Animal Care and Use Committee.
Reporter mice
The MMP-2 gene promoter reporter mice were developed and described by Alfonso-Jaume et al. (20). Mice with the MMP-9 reporter-lacz construct were developed and described by Mohan et al. (21). Mice with the TIMP-1 reporter-lacz construct were developed and described by Flenniken et al. (22). All three mouse lines were generated and maintained on the CD-1 background. As internal controls, transgene expression was ascertained by β-gal elaboration (X-gal reacted) at the cut edges of excised tail tips.
MI induction and experimental design
To provide equivalent gender distribution, MI was surgically induced in male and female mice (age: 108±3 days, weight: 35.5±0.5 g) by ligation of the coronary artery as described previously (3, 8, 19). This technique has been previously documented to cause an MI of 35±4% of the LV (3, 8, 19). Five mice from each of the reporter groups were included to serve as non-MI reference controls.
Terminal studies on the mice were performed at 1 hour (acute), 1 day, 3 days, 7 days, 14 days, and 28 days post-MI. At the assigned post-MI timepoint, the mice were deeply anesthetized using inhalation isoflurane and weighed. The thoracic cavity was opened, 0.2 mL of a 0.1M CdCl2 solution injected into the LV, and the heart extirpated. The heart was blotted dry, weighed, and sectioned along the long axis such that the line of dissection bisected the MI. One half of the heart was used to determine the extent of β-gal staining (formalin fixation followed by reaction against the X-gal substrate) and the other was frozen in OTC embedding compound (Tissue-Tek). MI size was determined from hematoxylin and eosin stained sections of the LV long axis. Briefly, the endocardial length of the MI segment was measured through the determination of the extent of myocyte necrosis and normalized to endocardial length. In subsets of mice from each of the transgenic lines, echocardiographic determinations of LV volumes (Vevo 660, VisualSonics) were performed at baseline (pre-MI) and at 28 days post-MI. Briefly, parasternal long and short axis views of the LV together with a recording of the surface ECG was acquired to disk. LV volumes were determined by manual planimetry of the LV endocardial border in the long axis view at end-diastole (frame with R-wave) and end-systole (smallest LV area in cardiac cycle) and application of Simpson’s algorithm for volume determination (23, 24).
β-gal staining and quantitation
Gene promoter activation from each of the groups was assayed as a function of β-gal elaboration in one half of each heart by overnight incubation in a substrate buffer (X-gal, Sigma) as previously described (8, 21). Briefly, the LV was photographed (5 Mpixel camera with macro attachment) and the area and intensity of β-gal staining on the LV epicardium as well as the LV epicardial area were determined by digital planimetry (SigmaScan v4.0, SPSS). The area of β-gal staining was normalized to LV epicardial area and expressed as a percentage. Spatial elaboration of β-gal staining in each of the hearts was quantitated with respect to the center of the MI region as described previously (8). Briefly, the color images of the β-gal stained hearts were converted to gray scale such that regions of β-gal staining projected as lighter areas on a darker background. The MI region was digitally planimetered to determine the major axis of the MI region as well as the center of mass, which was then defined as the center of the MI region. A 5-pixel wide region-of-interest extending from the center of the MI region to the remote myocardium along the major axis was demarcated (8). Defining the center of the MI region as the origin, gray scale intensities (level 0 = black, level 255 = white) at 2.0 mm (border region) and at 3.0 mm (remote region) were determined.
Data Analysis
MI was induced in a combined total of 168 mice that elaborated reporter constructs for either MMP-2, MMP-9, or TIMP-1 gene promoters. A total of 17 (11 male and 6 female, p=0.134 with Pearson’s χ2 analysis and p=0.212 using Fisher’s exact test) mice died in the post-MI period, with 8 dying at 3 days post-MI, 3 at 5 days post-MI, 2 at 6 days post-MI, 2 at 7 days post-MI, and 1 each on days 9 and 13 post-MI. There was no difference between the numbers of male and female mice that died in the post-MI period (χ2 p=0.43). The final sample size of mice at each post-MI timepoint is provided in Table 1. Changes in MI size, heart mass indices, and the area of β-gal staining were compared between groups using analysis of variance (ANOVA), where the two main blocks were reporter group and time post-MI. For analysis of spatial distribution of β-gal staining, myocardial region was included as an additional factor for the ANOVA analysis. Gender-dependent differences with respect to the extent of β-gal staining was examined using multiway ANOVA where the interactions between gender and reporter strain as well as gender and post-MI timepoint were examined. Pairwise comparisons were performed by Bonferroni adjusted t-tests. Statistical tests were performed using the Stata software package (Stata Intercooled, v8.0, College Station, TX). Results are presented as mean ± standard error of the mean (SEM). Values of p<0.05 were considered to be statistically significant.
TABLE 1.
Sample sizes for mice in each of the metalloproteinase (MMP)-2, MMP-9, and tissue inhibitor of the metalloproteinases (TIMP)-1 reporter mice used at each post myocardial infarction (MI) timepoint
| Days Post-MI |
||||||
|---|---|---|---|---|---|---|
| 0 (Acute, 1 Hour) | 1 | 3 | 7 | 14 | 28 | |
| Sample size | ||||||
| MMP-2 Reporter | 5 (2/3) | 6 (3/3) | 7 (4/3) | 12 (5/7) | 13 (8/6) | 6 (2/4) |
| MMP-9 Reporter | 9 (5/4) | 6 (4/2) | 10 (4/6) | 14 (6/8) | 12 (7/5) | 11 (4/7) |
| TIMP-1 Reporter | 6 (3/3) | 6 (4/2) | 8 (3/5) | 8 (4/4) | 6 (4/2) | 6 (3/3) |
Values presented as: total sample size (male/female)
RESULTS
Heart mass for the referent control mice in the MMP-2, MMP-9, and TIMP-1 reporter groups were 158±10 mg, 172±12 mg, and 166±10 mg, respectively, with no difference between groups. The number of mice studied at each time point following myocardial infarction (MI) in each of the 3 reporter groups is summarized in Table 1. The number of male and female mice were similar for each post-MI timepoint (Table 1). In sections obtained at 1 hour post-MI (acute), myocyte necrosis was not readily observed, and MI size could not be computed. Compared to MI size at 1 day post-MI (20±4%), MI size was increased by 3 days post-MI (28±2%, p<0.05), increased further by 7 days post-MI (35±3%, p<0.05 vs. acute and 1 day post-MI values), and remained elevated through 28 days post-MI (37±2%, p<0.05 vs. acute and 1 day post-MI values). There were no between group differences in MI size at any post-MI time point. Left ventricular (LV) end-diastolic volume increased from the pre-MI value of 64±2 μL to 146±11 μL (p<0.05) at 28 days post-MI and LV ejection fraction decreased from the pre-MI value of 58±1 % to 16±3 % (p<0.05) at 28 days post-MI, with no differences between reporter groups.
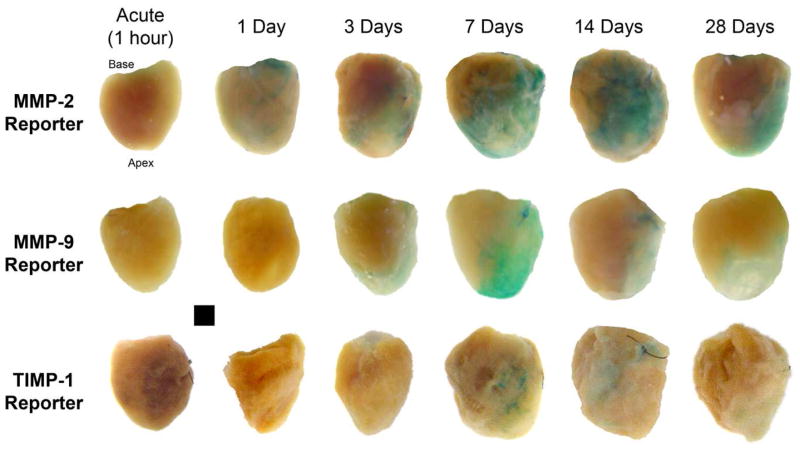
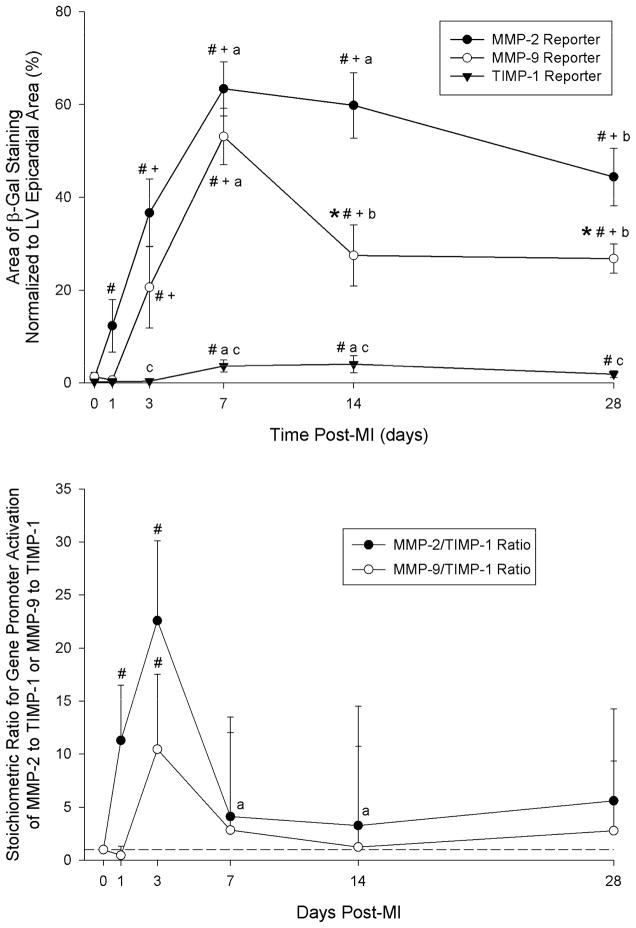
Representative photographs of LVs with areas of β-galactosidase (β-gal) staining, which reflect either MMP-2 or MMP-9 or TIMP-1 promoter activation are shown in Figure 1. In referent, non-MI hearts from any of the reporter groups, no β-gal staining was evident on the LV. In the MMP-2 reporter group, β-gal staining was increased by 1 day post-MI, increased further through 7 days, and fell at longer post-MI timepoints (Figure 2, top). MMP-9 promoter activation increased from acute and 1 day post-MI values by 3 days and peaked at 7 days post-MI. TIMP-1 promoter activation could not be detected either acutely or at 1 day post-MI, but was significantly higher than 3 day or acute values at 7 days post-MI. TIMP-1 reporter activation was not significantly different from 7 days post-MI values at either 14 or 28 days post-MI. The change in β-gal staining at each post-MI timepoint was similar between male and female mice for each of the reporter strains (Table 2). Ratios for promoter activation levels for MMP-2 to TIMP-1 and for MMP-9 to TIMP-1 were computed using the average value of TIMP-1 promoter activation detected at each post-MI timepoint (Figure 2, bottom). The maximum change in MMP-2/TIMP-1 or MMP-9/TIMP-1 ratios occurred at 3 days post-MI and normalized at later post-MI durations.
Figure 1.
TOP: Photographs of left ventricles (LV) from the MMP-2, MMP-9, and TIMP-1 gene promoter reporter mice showing regions of β-galactosidase staining following myocardial infarction (MI). Hearts were extirpated at the indicated post-MI time points.
Figure 2.
TOP: Summary data for area of positive β-galactosidase staining normalized to LV epicardial area. Sample sizes for each group at each post-MI time point are presented in Table 1. # p<0.05 vs. Acute (1 hour post-MI), + p<0.05 vs. 1 day post-MI, a p<0.05 vs. 3 days post-MI, b p<0.05 vs. 7 days post-MI, *p<0.05 vs. MMP-2 Reporter values only, c p<0.05 vs. MMP-2 and MMP-9 Reporter values. BOTTOM: Ratios of MMP-2 to TIMP-1 promoter activation and MMP-9 to TIMP-1 promoter activation at each post-MI timepoint. These ratios were computed as a function of the average β-galactosidase staining recorded in the TIMP-1 reporter group at each respective post-MI timepoint. The maximum change in MMP-2/TIMP-1 or MMP-9/TIMP-1 ratios occurred at 3 days post-MI and was normalized at later post-MI durations. # p<0.05 vs. Acute (1 hour post-MI), + p<0.05 vs. 1 day post-MI, a p<0.05 vs. 3 days post-MI.
TABLE 2.
Time-dependent changes in intensity of positive β-galactosidase staining in matrix metalloproteinase (MMP)-2, MMP-9, and tissue inhibitors of the metalloproteinases (TIMP)-1 reporter mice following myocardial infarction (MI): Effect of mouse gender
| Days Post-MI |
||||||
|---|---|---|---|---|---|---|
| 0 (Acute) | 1 | 3 | 7 | 14 | 28 | |
| MMP-2 Reporter | ||||||
| Male | 0±-- | 11±9# | 27±9#+ | 68±8#+a | 59±7#+a | 48±16#+a |
| Female | 2±2 | 14±7# | 21±13# | 58±9#+a | 55±15#+ | 42±11#+ |
| MMP-9 Reporter | ||||||
| Male | 1±1 | 1±1* | 14±8#+ | 63±12#+a | 28±8*#+b | 27±6*#+b |
| Female | 1±1 | 1±1* | 25±11#+ | 50±10#+a | 20±7*#+b | 18±6*#+b |
| TIMP-1 Reporter | ||||||
| Male | 0±-- | 1±1* | 2±1c | 9±4#ac | 9±3#+ac | 4±2#c |
| Female | 0±-- | 2±1* | 3±2c | 11±3#+ac | 6±4#c | 5±3c |
Values presented as Mean ± SEM. Sample sizes presented in Table 1.
LV: Left ventricular,
p<0.05 vs. Day 0 (Acute, 1 hour post-MI),
p<0.05 vs. 1 day post-MI,
p<0.05 vs. 3 days post-MI,
p<0.05 vs. 7 days post-MI,
p<0.05 vs. gender-matched MMP-2 Reporter values only,
p<0.05 vs. gender-matched MMP-2 and MMP-9 Reporter values
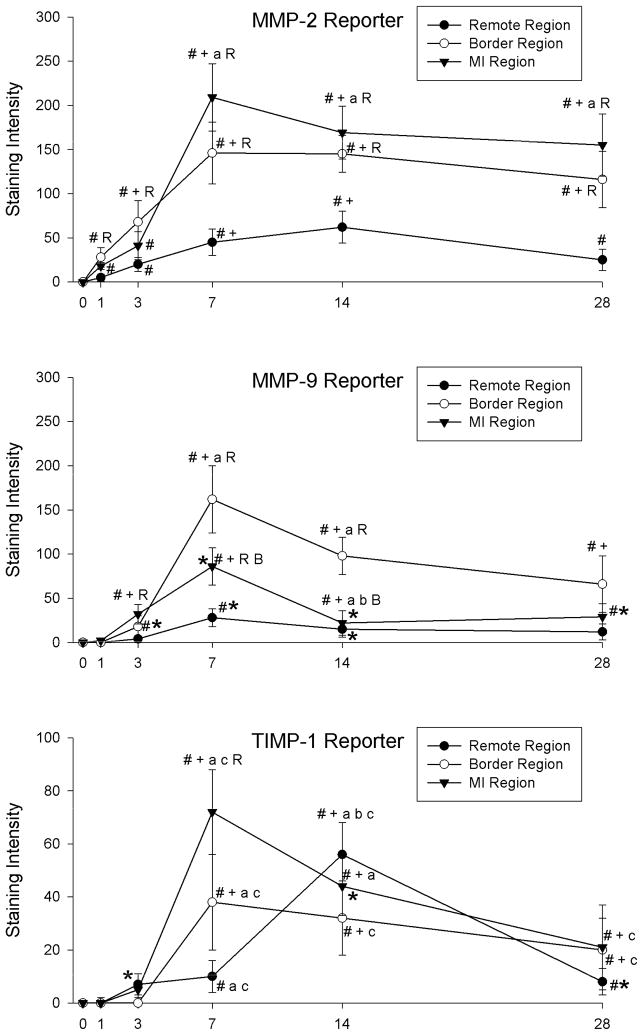
There were distinct patterns of spatial expression of MMP-2, MMP-9, and TIMP-1 promoter activation with respect to localization within the remote, border, and MI regions (Figure 3). MMP-2 promoter activation within the border region was higher than acute values by 1 day post-MI remained higher at longer post-MI durations. MMP-2 promoter activation within the MI region was higher than acute values by 3 days post-MI. MMP-9 promoter activation in the border and MI regions peaked at 7 days post-MI and was highest in the border region compared to the remote or MI regions. TIMP-1 promoter activation within the MI region peaked at 7 days post-MI, but was lower than region-matched promoter activation in the MMP-2 and MMP-9 reporter groups. TIMP-1 promoter activation in the remote region was highest at 14 days post-MI, but remained lower than corresponding levels in the MMP-2 and MMP-9 reporter groups.
Figure 3.
Spatial distribution of β-galactosidase staining intensity following MI in the remote, border, and MI regions of LVs from MMP-2 (TOP), MMP-9 (MIDDLE), and TIMP-1 (BOTTOM) reporter lines. MMP-2 promoter activation peaked in the MI region at 7 days post-MI and MMP-9 promoter activation was highest in the border region at 7 and 14 days post-MI. TIMP-1 promoter activation peaked within the MI region at 7 days post-MI and within the remote region at 14 days post-MI. Please note different y-axis scale for TIMP-1 reporter graph. # p<0.05 vs. Acute (1 hour post-MI), + p<0.05 vs. 1 day post-MI, a p<0.05 vs. 3 days post-MI, b p<0.05 vs. 7 days post-MI, R p<0.05 vs. strain-matched remote region, B p<0.05 vs. strain-matched border region, *p<0.05 vs. MMP-2 Reporter values only, c p<0.05 vs. MMP-2 and MMP-9 Reporter values.
DISCUSSION
The matrix metalloproteinases (MMPs), which are endopeptidases capable of degrading components of the extracellular matrix (ECM), have been demonstrated to be causative in the adverse LV remodeling post-MI (2, 14, 15, 17, 18, 25). For example, transgenic deletion of certain MMPs or pharmacological MMP inhibition can attenuate of LV dilation post-MI (2, 5, 14, 15, 17, 18, 25). Since binding of the MMPs to the tissue inhibitors of the metalloproteinases (TIMPs) can inhibit MMP activity, the TIMPs represent an important regulatory step in the control of MMP activity (2, 3, 19). Therefore, these past observations provide evidence that alterations in the stoichiometric balance between MMPs and TIMPs following MI may determine the extent of adverse LV remodeling post-MI. However, whether the post-MI imbalance between MMPs and TIMPs occurred due to differential transcriptional activation of specific MMP and TIMP gene promoters remained unknown. Using transgenic reporter constructs (8, 20–22), the main findings of the present study were that activation of MMP-2, MMP-9, and TIMP-1 promoters occurred with distinct temporal trajectories in the post-MI period and that the spatial distribution patterns for MMP-2, MMP-9, and TIMP-1 gene promoter activation post-MI were unique. Moreover, the stoichiometric balance for promoter activation of these two MMP species and TIMP-1 favored increased MMP elaboration post-MI. These findings provided direct in vivo evidence that discordant MMP/TIMP transcription occurs in the early post-MI period.
Changes that occur within the myocardial ECM following MI have been well documented (2, 4, 26, 27). Briefly, initial cellular necrosis is followed by the deposition of granulation tissue, clearing of the granulation tissue, and finally, increased fibrosis within the MI region (2, 11, 25, 27). These dynamic post-MI changes within the ECM are associated with time-dependent changes in the expression and abundance of the MMPs and the TIMPs (7–10, 12). Specifically, the levels of certain MMP species, such as MMP-2 and MMP-9, have been reported to be increased post-MI (4, 7, 9, 12). Concomitant to these post-MI changes in myocardial MMP levels, changes in the protein levels of the TIMPs has been reported (8, 9, 11, 19). For example, TIMP-1 protein levels are increased through 3 days post-MI and then fell to below non-MI values at longer post-MI durations (8). Importantly, adverse LV remodeling post-MI has been reported to be attenuated through pharmacological inhibition or transgenic deletion of certain MMPs and to be exacerbated by transgenic overexpression of MMP-1 or deletion of TIMP-1 (3, 19, 28). Taken together, these past findings provide evidence that alterations in MMP and TIMP elaboration can lead to inappropriate ECM remodeling post-MI. However, it must be recognized that the balance between MMPs and TIMPs may become discordant due to the many regulatory steps that determine net ECM proteolytic activity. These regulatory steps can include transcription of MMP/TIMP genes, post-transcriptional and translational regulation, intracellular trafficking of the proteins, secretion and activation of the proteins, and finally, protein-protein interactions between the MMPs and TIMPs (6, 29–31). While it is likely that many of these regulatory steps contribute to the stoichiometric imbalance between MMP and TIMP protein levels post-MI, the findings of the present study that activation of the gene promoters for MMP-2 and MMP-9 were higher than that for TIMP-1 suggest that an imbalance in the activation of these gene promoters play a significant role in regulating downstream protein levels.
Changes in TIMP-1 protein and mRNA levels have been reported to occur within the post-MI myocardium (9, 11). Moreover, this laboratory has reported exacerbated post-MI LV dilation in TIMP-1 deficient mice, suggesting a key role for this protein in post-MI remodeling (3, 19). In the present study, TIMP-1 promoter activation was detectable at 3 days post-MI, increased further through 14 days post-MI and remained higher than acute values through 28 days post-MI. These findings are consistent with those reported by Sun et al., where TIMP-1 mRNA levels in rats were increased from acute MI levels by 3 days post-MI and remained elevated at longer post-MI durations (11). The present study builds on the existing knowledge of post-MI MMP and TIMP-1 regulation in two important ways. First, this study demonstrated that at least one cause of changes in the stoichiometric balance between MMPs and TIMPs is a differential activation of MMP and TIMP-1 gene promoters. Second, the findings of this study demonstrate that there are distinct spatial differences with respect to the induction of the MMP-2, MMP-9, and TIMP-1 gene promoters post-MI.
The gelatinases, MMP-2 and MMP-9, have been uniformly demonstrated to contribute to post-MI remodeling and changes in mRNA and protein levels of MMP-2 and MMP-9 occur post-MI (4, 13–15, 25). Importantly, cause-effect relationships for MMP-2 and MMP-9 in post-MI LV remodeling have been reported (4, 14, 15, 25). For example, Ducharme et al. demonstrated that post-MI LV dilation was attenuated in MMP-9 deficient mice and Hayashidani et al. have reported a reduction in post-MI mortality in MMP-2 deficient mice (4, 14, 15). However, MMP-2 and MMP-9 levels and activity can change as a function of post-MI duration and unique temporal profiles of protein and mRNA expression post-MI have been identified (7, 9). Moreover, MMP activity is regulated through the binding of activated MMPs to the TIMPs (32). Therefore, changes in TIMP elaboration and binding of TIMPs to activated MMPs may influence the “activational state” for the MMPs. For example, if changes in the elaboration of MMPs and TIMPs were to occur proportionally in the same direction, then it is likely that the stoichiometric balance between MMPs and TIMPs would be maintained and that adverse ECM remodeling may not occur. Conversely, the balance between ECM deposition and degradation may be determined by relative shifts in changes in the balance between MMP and TIMP expression and elaboration. In the present study, the ratios of promoter activation levels between MMP-2 to TIMP-1 and MMP-9 to TIMP-1 were computed. The maximum change in both, the MMP2 to TIMP1 promoter activation ratio and the MMP-9 to TIMP-1 promoter activation ratio, occurred in the early post-MI period, achieving a peak at 3 days post-MI and normalizing at longer post-MI durations. The significance of these findings is two-fold. First, these findings suggest that the initial increase in MMP promoter activation relative to TIMP-1 promoter activation dictate early post-MI infarct expansion. Second, normalization of the promoter activation ratios for the MMPs and TIMP-1 at later post-MI periods may lead to a shift in balance from ECM degradation to ECM deposition within the MI region. However, these issues remain speculative and future studies would be required to determine whether post-MI changes in the balance between MMP and TIMP-1 promoter activation correspond directly to changes in the stoichiometric balance between MMP and TIMP proteins.
LV remodeling following MI is a heterogeneous process with respect to temporal as well as spatial changes in geometry of the MI, border zone, and remote myocardial regions (4, 27, 33). Specifically, the MI region can progressively become thinner and expand in the post-MI period, which is associated with increased fibrosis (4, 17, 34). The border zone, which provides a tissue interface between the non-contractile MI region and the viable remote region, is subjected to differential stress and strain patterns, which in turn is considered to be a mechanical impetus for infarct expansion (35). The myocardium of the viable remote region invariably becomes hypertrophied with an increase in the collagen content relative to hearts not subjected to MI (26, 27, 36). These regional changes in myocardial geometry in the LV post-MI are associated with differential changes in the cellular composition of the MI, border zone, and MI regions (27, 34, 36). For example, the MI region is richly populated with fibroblasts/myofibroblasts (36), while the predominant cell type in the remote myocardium remains the myocytes (36). In the present study, there were distinct differences in the spatial pattern for the activation of the gene promoters of MMP-2, MMP-9, and TIMP-1 in the post-MI period. MMP-2 promoter activation was highest within the MI region and peak MMP-9 promoter activation occurred within the border zone. TIMP-1 promoter activation in the MI region peaked at 7 days post-MI and was highest in the remote region at 14 days post-MI. These spatiotemporal changes in the activation of these MMP and TIMP gene promoters suggest that the heterogeneous remodeling of the LV post-MI is associated with specific time-dependent alterations in the balance between MMPs and TIMPs and occurs as a function of the release of these MMP and TIMP types from cells that are resident within each of the regions during the progress of LV remodeling post-MI.
There are several limitations of the present study that must be recognized. First, the transgenic reporter mouse lines used in this study were useful in detecting the spatiotemporal induction of MMP-2, MMP-9, or TIMP-1 transcription and not levels of these proteins or activity levels of MMP-2 or MMP-9. Second, it must be recognized that there are likely species-dependent differences in MMP/TIMP transcriptional regulation between humans and mice. Third, mice of both sexes were included in order to determine the post-MI activation of the MMP-2, MMP-9, or TIMP-1 gene promoters independent of potential sex-dependent differences. While post-MI changes in MMP-2, MMP-9, and TIMP-1 promoter activation were similar between male and female mice, it must be recognized that group sizes, when subdivided by gender, were relatively small. A future study with larger sample sizes would be required to adequately power a more careful examination of the role of gender in post-MI activation of the MMP-2, MMP-9, or TIMP-1 gene promoters. Finally, it must be recognized that the cellular sources for MMP-2, MMP-9, and TIMP-1 are not only likely to be different, but may also change during the course of post-MI LV remodeling. In the present study, the distinct region-dependent differences in the activation of MMP-2, MMP-9, and TIMP-1 gene promoters suggest that different cell types resident in these myocardial regions contribute differentially to the expression of MMP and TIMP proteins during the progression of LV remodeling post-MI. Therefore, a future study in which the cellular sources for post-MI activation of the MMP-2, MMP-9, and TIMP-1 promoters is warranted. These limitations notwithstanding, this study provided unique visual evidence that the post-MI induction of the MMP-2, MMP-9, and TIMP-1 gene promoters occurred in a region and time specific manner. Moreover, the ratio of MMP-2 or MMP-9 to TIMP-1 promoter activation favored a shift in balance towards MMP-mediated ECM degradation in the early post-MI period. Thus, restoration of TIMP-mediated inhibition of the MMPs, either through increased TIMP-1 gene promoter activation or attenuating MMP-2 or MMP-9 promoter activation, may represent a molecular approach to target adverse LV remodeling post-MI.
Acknowledgments
This study was supported by National Institutes of Health grants HL-66029 (RM), HL-45024, HL-97012, and PO1-HL48788 (FGS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cleutjens JP, Kandala JC, Guarda E, Guntaka RV, Weber KT. Regulation of collagen degradation in the rat myocardium after infarction. J Mol Cell Cardiol. 1995;27:1281–1292. doi: 10.1016/s0022-2828(05)82390-9. [DOI] [PubMed] [Google Scholar]
- 2.Cleutjens JP, Creemers EE. Integration of concepts: cardiac extracellular matrix remodeling after myocardial infarction. J Card Fail. 2002;8:S344–348. doi: 10.1054/jcaf.2002.129261. [DOI] [PubMed] [Google Scholar]
- 3.Creemers EE, Davis JN, Parkhurst AM, Leenders P, Dowdy KB, Hapke E, Hauet AM, Escobar PG, Cleutjens JP, Smits JF, Daemen MJ, Zile MR, Spinale FG. Deficiency of TIMP-1 exacerbates LV remodeling after myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2003;284:H364–371. doi: 10.1152/ajpheart.00511.2002. [DOI] [PubMed] [Google Scholar]
- 4.Creemers EE, Cleutjens JP, Smits JF, Daemen MJ. Matrix metalloproteinase inhibition after myocardial infarction: a new approach to prevent heart failure? Circ Res. 2001;89:201–210. doi: 10.1161/hh1501.094396. [DOI] [PubMed] [Google Scholar]
- 5.Spinale FG, Wilbur NM. Matrix metalloproteinase therapy in heart failure. Curr Treat Options Cardiovasc Med. 2009;11:339–346. doi: 10.1007/s11936-009-0034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;87:1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Tung CH, Allport JR, Chen S, Weissleder R, Huang PL. Near-infrared fluorescent imaging of matrix metalloproteinase activity after myocardial infarction. Circulation. 2005;111:1800–1805. doi: 10.1161/01.CIR.0000160936.91849.9F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukherjee R, Mingoia JT, Bruce JA, Austin JS, Stroud RE, Escobar GP, McClister DM, Jr, Allen CM, Alfonso-Jaume MA, Fini ME, Lovett DH, Spinale FG. Selective spatiotemporal induction of matrix metalloproteinase-2 and matrix metalloproteinase-9 transcription after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;291:H2216–2228. doi: 10.1152/ajpheart.01343.2005. [DOI] [PubMed] [Google Scholar]
- 9.Peterson JT, Li H, Dillon L, Bryant JW. Evolution of matrix metalloprotease and tissue inhibitor expression during heart failure progression in the infarcted rat. Cardiovasc Res. 2000;46:307–315. doi: 10.1016/s0008-6363(00)00029-8. [DOI] [PubMed] [Google Scholar]
- 10.Romanic AM, Burns-Kurtis CL, Gout B, Berrebi-Bertrand I, Ohlstein EH. Matrix metalloproteinase expression in cardiac myocytes following myocardial infarction in the rabbit. Life Sci. 2001;68:799–814. doi: 10.1016/s0024-3205(00)00982-6. [DOI] [PubMed] [Google Scholar]
- 11.Sun Y, Cleutjens JP, Diaz-Arias AA, Weber KT. Cardiac angiotensin converting enzyme and myocardial fibrosis in the rat. Cardiovasc Res. 1994;28:1423–1432. doi: 10.1093/cvr/28.9.1423. [DOI] [PubMed] [Google Scholar]
- 12.Tao ZY, Cavasin MA, Yang F, Liu YH, Yang XP. Temporal changes in matrix metalloproteinase expression and inflammatory response associated with cardiac rupture after myocardial infarction in mice. Life Sci. 2004;74:1561–1572. doi: 10.1016/j.lfs.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 13.Wilson EM, Moainie SL, Baskin JM, Lowry AS, Deschamps AM, Mukherjee R, Guy TS, St John-Sutton MG, Gorman JH, 3rd, Edmunds LH, Jr, Gorman RC, Spinale FG. Region- and type-specific induction of matrix metalloproteinases in post-myocardial infarction remodeling. Circulation. 2003;107:2857–2863. doi: 10.1161/01.CIR.0000068375.40887.FA. [DOI] [PubMed] [Google Scholar]
- 14.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashidani S, Tsutsui H, Ikeuchi M, Shiomi T, Matsusaka H, Kubota T, Imanaka-Yoshida K, Itoh T, Takeshita A. Targeted deletion of MMP-2 attenuates early LV rupture and late remodeling after experimental myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;285:H1229–1235. doi: 10.1152/ajpheart.00207.2003. [DOI] [PubMed] [Google Scholar]
- 16.Yarbrough WM, Mukherjee R, Brinsa TA, Dowdy KB, Scott AA, Escobar GP, Joffs C, Lucas DG, Crawford FA, Jr, Spinale FG. Matrix metalloproteinase inhibition modifies left ventricular remodeling after myocardial infarction in pigs. J Thorac Cardiovasc Surg. 2003;125:602–610. doi: 10.1067/mtc.2003.197. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee R, Brinsa TA, Dowdy KB, Scott AA, Baskin JM, Deschamps AM, Lowry AS, Escobar GP, Lucas DG, Yarbrough WM, Zile MR, Spinale FG. Myocardial infarct expansion and matrix metalloproteinase inhibition. Circulation. 2003;107:618–625. doi: 10.1161/01.cir.0000046449.36178.00. [DOI] [PubMed] [Google Scholar]
- 18.Rohde LE, Ducharme A, Arroyo LH, Aikawa M, Sukhova GH, Lopez-Anaya A, McClure KF, Mitchell PG, Libby P, Lee RT. Matrix metalloproteinase inhibition attenuates early left ventricular enlargement after experimental myocardial infarction in mice. Circulation. 1999;99:3063–3070. doi: 10.1161/01.cir.99.23.3063. [DOI] [PubMed] [Google Scholar]
- 19.Ikonomidis JS, Hendrick JW, Parkhurst AM, Herron AR, Escobar PG, Dowdy KB, Stroud RE, Hapke E, Zile MR, Spinale FG. Accelerated LV remodeling after myocardial infarction in TIMP-1-deficient mice: effects of exogenous MMP inhibition. Am J Physiol Heart Circ Physiol. 2005;288:H149–158. doi: 10.1152/ajpheart.00370.2004. [DOI] [PubMed] [Google Scholar]
- 20.Alfonso-Jaume MA, Bergman MR, Mahimkar R, Cheng S, Jin ZQ, Karliner JS, Lovett DH. Cardiac ischemia-reperfusion injury induces matrix metalloproteinase-2 expression through the AP-1 components FosB and JunB. Am J Physiol Heart Circ Physiol. 2006;291:H1838–1846. doi: 10.1152/ajpheart.00026.2006. [DOI] [PubMed] [Google Scholar]
- 21.Mohan R, Rinehart WB, Bargagna-Mohan P, Fini ME. Gelatinase B/lacZ transgenic mice, a model for mapping gelatinase B expression during developmental and injury-related tissue remodeling. J Biol Chem. 1998;273:25903–25914. doi: 10.1074/jbc.273.40.25903. [DOI] [PubMed] [Google Scholar]
- 22.Flenniken AM, Williams BR. Developmental expression of the endogenous TIMP gene and a TIMP-lacZ fusion gene in transgenic mice. Genes Dev. 1990;4:1094–1106. doi: 10.1101/gad.4.7.1094. [DOI] [PubMed] [Google Scholar]
- 23.Nichols K, Lefkowitz D, Faber T, Folks R, Cooke D, Garcia EV, Yao SS, DePuey EG, Rozanski A. Echocardiographic validation of gated SPECT ventricular function measurements. J Nucl Med. 2000;41:1308–1314. [PubMed] [Google Scholar]
- 24.Tortoledo FA, Quinones MA, Fernandez GC, Waggoner AD, Winters WL., Jr Quantification of left ventricular volumes by two-dimensional echocardiography: a simplified and accurate approach. Circulation. 1983;67:579–584. doi: 10.1161/01.cir.67.3.579. [DOI] [PubMed] [Google Scholar]
- 25.Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, Dyspersin GD, Cleutjens JP, Shipley M, Angellilo A, Levi M, Nube O, Baker A, Keshet E, Lupu F, Herbert JM, Smits JF, Shapiro SD, Baes M, Borgers M, Collen D, Daemen MJ, Carmeliet P. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med. 1999;5:1135–1142. doi: 10.1038/13459. [DOI] [PubMed] [Google Scholar]
- 26.Jugdutt BI, Joljart MJ, Khan MI. Rate of collagen deposition during healing and ventricular remodeling after myocardial infarction in rat and dog models. Circulation. 1996;94:94–101. doi: 10.1161/01.cir.94.1.94. [DOI] [PubMed] [Google Scholar]
- 27.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 28.D’Armiento J. Matrix metalloproteinase disruption of the extracellular matrix and cardiac dysfunction. Trends Cardiovasc Med. 2002;12:97–101. doi: 10.1016/s1050-1738(01)00160-8. [DOI] [PubMed] [Google Scholar]
- 29.Tyagi SC, Kumar SG, Haas SJ, Reddy HK, Voelker DJ, Hayden MR, Demmy TL, Schmaltz RA, Curtis JJ. Post-transcriptional regulation of extracellular matrix metalloproteinase in human heart end-stage failure secondary to ischemic cardiomyopathy. J Mol Cell Cardiol. 1996;28:1415–1428. doi: 10.1006/jmcc.1996.0132. [DOI] [PubMed] [Google Scholar]
- 30.Deschamps AM, Yarbrough WM, Squires CE, Allen RA, McClister DM, Dowdy KB, McLean JE, Mingoia JT, Sample JA, Mukherjee R, Spinale FG. Trafficking of the membrane type-1 matrix metalloproteinase in ischemia and reperfusion: relation to interstitial membrane type-1 matrix metalloproteinase activity. Circulation. 2005;111:1166–1174. doi: 10.1161/01.CIR.0000157149.71297.3A. [DOI] [PubMed] [Google Scholar]
- 31.Deschamps AM, Spinale FG. Pathways of matrix metalloproteinase induction in heart failure: bioactive molecules and transcriptional regulation. Cardiovasc Res. 2006;69:666–676. doi: 10.1016/j.cardiores.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 33.Lutgens E, Daemen MJ, de Muinck ED, Debets J, Leenders P, Smits JF. Chronic myocardial infarction in the mouse: cardiac structural and functional changes. Cardiovasc Res. 1999;41:586–593. doi: 10.1016/s0008-6363(98)00216-8. [DOI] [PubMed] [Google Scholar]
- 34.Camelliti P, Devlin GP, Matthews KG, Kohl P, Green CR. Spatially and temporally distinct expression of fibroblast connexins after sheep ventricular infarction. Cardiovasc Res. 2004;62:415–425. doi: 10.1016/j.cardiores.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 35.Blom AS, Pilla JJ, Arkles J, Dougherty L, Ryan LP, Gorman JH, 3rd, Acker MA, Gorman RC. Ventricular restraint prevents infarct expansion and improves borderzone function after myocardial infarction: a study using magnetic resonance imaging, three-dimensional surface modeling, and myocardial tagging. Ann Thorac Surg. 2007;84:2004–2010. doi: 10.1016/j.athoracsur.2007.06.062. [DOI] [PubMed] [Google Scholar]
- 36.Yang F, Liu YH, Yang XP, Xu J, Kapke A, Carretero OA. Myocardial infarction and cardiac remodelling in mice. Exp Physiol. 2002;87:547–555. doi: 10.1113/eph8702385. [DOI] [PubMed] [Google Scholar]