Abstract
Patients receiving chemoradiation for cervical cancer are at risk for distress, chemoradiation-related side-effects, and immunosuppression. This prospective randomized clinical trial examined effects of a complementary therapy, Healing Touch (HT), versus relaxation training (RT) and usual care (UC) for 1) supporting cellular immunity, 2) improving mood and quality of life (QOL), and 3) reducing treatment-associated toxicities and treatment delay in cervical cancer patients receiving chemoradiation. Sixty women with stages IB1 to IVA cervical cancer were randomly assigned to receive UC or 4×/weekly individual sessions of either HT or RT immediately following radiation during their 6-week chemoradiation treatment. Patients completed psychosocial assessments and blood sampling before chemoradiation at baseline, weeks 4 and 6. Multilevel regression analyses using orthogonal contrasts tested for differences between treatment conditions over time. HT patients had a minimal decrease in natural killer cell cytotoxicity (NKCC) over the course of treatment whereas NKCC of RT and UC patients declined sharply during chemoradiation (group by time interaction: p=0.018). HT patients showed greater decreases in 2 different indicators of depressed mood (CESD depressed mood subscale and POMS depression scale) compared to RT and UC (group by time interactions: p < 0.05). No between group differences were observed in QOL, treatment delay, or clinically-rated toxicities. HT may benefit cervical cancer patients by moderating effects of chemoradiation on depressed mood and cellular immunity. Effects of HT on toxicities, treatment delay, QOL, and fatigue were not observed. Long-term clinical implications of findings are not known.
Keywords: cervical cancer, Healing Touch, complementary and alternative medicine, psychoneuroimmunology, relaxation, NK cell cytotoxicity
1. Introduction
Cervical cancer is the second most common female cancer worldwide (Kamangar et al., 2006). Due to screening and vaccination, mortality rates have declined in developed countries, but remain high in the rest of the world (Canavan et al., 2000; Kamangar et al., 2006). Although concomitant chemoradiation treatment is potentially curative (Eifel et al., 2004), acute and late side-effects are common and are associated with compromised quality of life (QOL) and psychological distress (Hodgkinson et al., 2007; Vistad et al., 2006).
Immune compromise, including depletion of natural killer (NK) cell activity, is a common side-effect of chemoradiation (Santin et al., 2000). NK cells play an important role in the cellular immune response to cervical cancer (Textor et al., 2008). Downregulated expression of the NK activating receptors NKp30, NKp46 and NKG2D has been reported in cervical cancer (Garcia-Iglesias et al., 2009) and impaired NK cell activity is associated with a more aggressive disease course in cervical cancer (Balaram et al., 1988; Garzetti et al., 1995; Pillai, et al, 1990). Therefore, identification of interventions to reduce toxicities, distress, and maintain maximal immunocompetence in cervical cancer patients during chemoradiation has great therapeutic relevance.
Complementary and Alternative Medicine (CAM) therapies are commonly used by cancer patients (Boon et al., 2000; Fouladbakhsh et al., 2005). Biofield therapies, which manipulate hypothesized “energy fields” around the body (NCCAM, 2005), are frequently used by cancer patients to reduce pain, fatigue, and other treatment side-effects (Jain and Mills, 2010; Molassiotis et al., 2005; Sparber et al., 2000). Healing Touch (HT) is a standardized biofield therapy that uses gentle touch and movements in the patient's “energy field” with the goal of restoring balance in the patient's energy system and strengthening the patient's “healing capacity” (Mentgen, 2002). HT has been shown to increase well-being in cervical and breast cancer patients during radiation (Cook et al., 2004) and to reduce distress and fatigue during chemotherapy (Astin et al., 2000; Post-White et al., 2003). However, little is known about effects of HT on the immune response during chemoradiation. According to a recent review of biofield therapies, their effects on quality of life, fatigue, and physiological outcomes have been understudied in cancer populations (Jain and Mills, 2010). The objective of this prospective randomized clinical trial was to examine effects of HT on NK cell activity, mood, and specific clinical and QOL outcomes among women with locally advanced cervical cancer receiving a standard 6-week course of chemoradiation. Primary outcome variables were NK cell activity and chemoradiation-related toxicities; secondary variables were mood, leukocyte and erythrocyte counts, treatment delay, and QOL. Relaxation (RT), which has known beneficial physiological effects (Dusek et al., 2008; Kiecolt-Glaser et al., 1985; Luebbert et al., 2001), served as an active control for social support and the expectation of receiving a plausible alternative treatment.
2. Methods
2.1. Patients
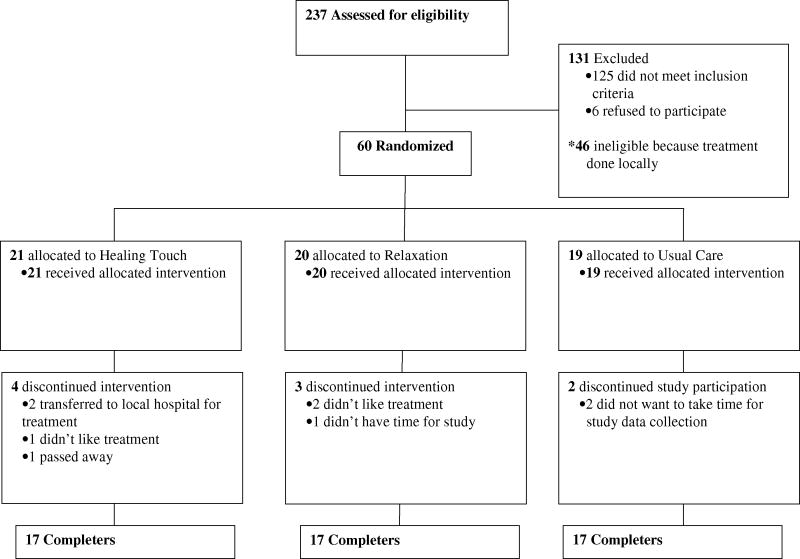
This study was approved by the University of Iowa IRB. Patients over 18 years of age with stages IB1 through IVA cervical squamous or adeno-carcinoma were eligible. Patients were recruited prior to chemoradiation through the Gynecologic Oncology service at the University of Iowa Hospitals and Clinics between May 2002 and March 2007. Exclusion criteria included conditions affecting the immune system (e.g. multiple sclerosis, lupus), systemic steroid medication within a month of study entry, radiation treatment elsewhere, refusal of radiotherapy, and lack of English fluency. Sixty patients were randomized and 17 in each group completed the study.a (Figure 1).
Figure 1.
Experimental design and flow diagram of study participation.
2.2. Procedure
Patients received a description of HT and RT when consented. They were randomized by the Cancer Center statistical core using permuted block randomization (kept in sealed envelopes) to one of 3 conditions.b Patients were informed of group assignment after completing initial questionnaires and blood sampling. All participants received standard medical treatment consisting of weekly platinum-based chemoradiation,c external beam radiation (total dose approx. 45-50.4 Gy), and brachytherapy. Low dose rate (LDR) brachytherapy was administered before September 2005; afterwards high dose rate (HDR) brachytherapy was administered, reflecting new treatment technology. HT or RT interventions were administered individually, 4 days/week throughout chemoradiation on non-chemotherapy days, immediately following radiation, in a quiet room in the Clinical Research Center before May 2005 and subsequently in the Radiation Oncology Center. Morning blood samples (35cc) were taken pre-chemotherapy in weeks 1, 4, and 6. Psychosocial surveys were completed before each blood draw. Laboratory personnel and physicians were blind to group assignment and all samples were coded.
2.3. Interventions
2.3.1. Healing Touch
HT sessions (approximately 20-30 minutes) were conducted by experienced practitioners and included five specific HT techniques designed to promote calm and facilitate balancing of energy flow in the body (Mentgen, 2002; Menthe and Bulbrook, 2002). The techniques included the following: 1) “grounding and centering”, in which the practitioners set their intent to support the patient's healing and healthy function of the immune system; 2) “pain drain”, a technique designed to reduce congestion of energy in a specific area to assist in removing toxins from the liver; 3) “chakra connection”, a technique designed to facilitate opening and balancing the energy centers and restoring the body's normal pattern of energy flow; 4) “magnetic unruffling”, a technique used to cleanse and clear congested energy, toxins, and emotional distress; and 5) “mind clearing”, a technique using eleven sequential hand positions on the head, neck, and face which is thought to promote relaxation, peacefulness, and focus. Techniques 2, 3 and 5 involve physical touch. Use of additional techniques was determined by patient presentation. For maximum efficiency, HT sessions were generally provided by a team of two practitioners (63.5% of sessions). Three nurses who were certified Healing Touch practitioners were the primary providers of the intervention.
2.3.2. Relaxation Training
RT sessions (approximately 20-25 minutes) were conducted by a trained research assistant or graduate student who was present during the entire session and guided the relaxation process using manualized relaxation scripts. The manualized relaxation intervention, adapted from previously used protocols (Antoni, 2003), included passive progressive relaxation, autogenic relaxation, relaxation with nature imagery, and relaxation with imagery of a patient-selected special place. Three primary therapists provided the intervention. For both RT and HT we aimed for consistency of therapists from session to session for each patient.
2.3.3. Usual Care (UC)
Patients had assessments and blood sampling at the same time-points as HT and RT.
2.4. Psychosocial Assessments
2.4.1. Depressed mood
The Center for Epidemiological Studies Depression Scale (CES-D) is a 20-item questionnaire assessing depressive symptomatology over the last week (Radloff, 1977). Scores of 16 or greater indicate “probable cases of depression” (Weissman et al., 1977). In addition to the total score, for better understanding of facets of depression that might be affected by the intervention, three of the four subscales (depressed mood, positive mood, and vegetative depression) (Sheehan et al., 1995) were examined. We also used two subscales from the Profile of Mood States-Short Form (POMS-SF) to differentiate effects on anxiety vs. depressed mood. The POMS-SF includes 37 adjectives describing mood over the past week (Curran et al., 1995).
2.4.2. Quality of Life
The Functional Assessment of Cancer Therapy (FACT) is a well-validated 27-item scale measuring QOL over the last week (Cella, 1994; Cella et al., 1993). The Fatigue Symptom Inventory (FSI) is a 13-item scale assessing intensity and frequency of fatigue in cancer patients during the last week (Hann et al., 1999). Mean fatigue and fatigue duration were examined.
2.4.3. Expectations
A 5-item scale, modified from the Credibility of Therapy scale (Borkovec and Nau, 1972), was administered at study entry to assess patient expectations before receiving group assignment. Items were rated on a Likert scale from “0” (not at all) to “7” (very much). Pre-treatment questions asked “How logical does this therapy seem to you? How confident are you that this treatment will be successful in helping you with side effects of your cancer treatment? How confident are you that this treatment will help you keep up your strength during your cancer treatment? How confident are you that this treatment will reduce your distress and/or increase your sense of well-being during your cancer treatment? How confident would you be in recommending this treatment to a friend who was going through cancer treatment? The post-treatment questions were similar but worded in the past tense, e.g., “How logical did this treatment seem to you? How successful was this treatment in helping you with side effects of your cancer treatment?” etc.
2.5. Psychophysiology: Manipulation Checks
Extent of relaxation during interventions was assessed by a mean of three blood pressure measurements taken at two minute intervals before and three measurements taken after the second RT or HT session in weeks 1, 3, and 5. A Dinamap Pro 100 (Critikon, Tampa, FL) vital signs monitor applied to the non-dominant arm was used as in previous work from this laboratory (Lutgendorf et al., 2000). At these time-points, UC patients watched a 20-25 minute neutral video in the same room used for HT and RT manipulation checks, with equivalent assessments before and after the video, sitting in a relatively supine position to decrease positional artifacts.
2.6. Clinical and Demographic Information
Clinical information was abstracted from medical records. Assessment of chemoradiation-related toxicities (NCI, 1999) and treatment delay (days) was performed by a radiation oncologist on the treatment team who was blind to group assignment (G.J.). Patients reported demographic characteristics and health behaviors including average sleep and consumption of cigarettes, alcohol, and coffee during the week before each blood draw.
2.7. Immune Measures
2.7.1. NK Cell Activity
Fresh peripheral blood mononuclear cells were tested for cytolytic activity against the NK-sensitive K562 cells and the NK-resistant LAK-sensitive ZKBL cells in a standard 4-hour 51Chromium-release assay as reported previously (Lutgendorf et al., 2005). Results (NKCC) are reported as percent specific lysis at the 100:1 effector:target (E:T) ratio (maximum specific lysis) using the formula: percent lysis=(Experimental CPM-Spontaneous CPM/Maximal CPM-Spontaneous CPM) ×100, where CPM equals gamma counts per minute. As a secondary measure, NK activity was expressed as area under the curve across all E:T ratios (NKAUC) calculated using the trapezoidal method (Lutgendorf et al., 2005; Sheeran, et al., 1988). The correlation between NKAUC and NKCC was greater than 0.97 at each time-point. Leukocyte (WBC) and erythrocyte (RBC) counts were assessed in the UIHC pathology laboratory.
2.8. Statistical Analyses
SPSS version 17.0 (Statistical Program for the Social Sciences, Chicago, IL) and SAS version 9.1 (Statistical Analysis System, Cary, NC) were used for data analysis. Distributions were examined for outliers and non-normality. Differences between groups were tested using one-way analyses of variance (ANOVA) for continuous variables and with Chi-square analyses for categorical variables. Baseline comparisons tested equivalence of groups on outcome variables at study entry. Relationships between health behaviors and outcomes were tested at all time-points and subsequent analyses adjusted for significant health behavior correlates. All models included age and disease stage a priori as covariates.
Multilevel regression analyses using SAS Proc Mixed were conducted to evaluate effects of treatment condition, time, and treatment by time interactions on dependent variables. This approach has the advantage of fitting models using all available data. Model 1 evaluated the impact of covariates on outcomes. Model 2 added treatment condition as a predictor, with two orthogonal contrast variables employed to test for differences among the three treatment conditions. Contrast 1 tested for differences between HT and the two control conditions (RT and UC). Contrast 2 tested for differences between the two control conditions (RT vs. UC). Model 3 added time (weeks since treatment initiation) as a predictor variable. Model 4 tested for interactions between treatment condition (represented by the two contrast variables) and time. Statistically significant interaction effects were followed by analyses of simple effects where changes over time for each condition were tested for statistical significance. For tests of statistical mediation (whether effects of treatment condition on NK cell activity were due to the indirect effect of depression), a bootstrap analysis was conducted (MacKinnon et al., 2007; Preacher and Hayes, 2008).
3. Results
3.1. Patient Characteristics
Demographic and clinical characteristics of participants are shown in Table 1. There were no significant differences between conditions with respect to age (p=0.42), education (p=0.32), income (p=0.11), disease stage (p=0.64), or body mass index (p=0.62). Baseline differences between the three groups were also non-significant with regard to health behaviors over the previous week (all p values > 0.41). Analyses examining relationships between health behavior covariates and outcome variables over time indicated significant relationships between cigarettes and higher WBC (p=0.01); sleep and higher NK cell percentage (p=0.003); coffee and higher RBC (p=0.003), higher QOL (p=0.01), and lower mean fatigue (p=0.02); no other health behaviors were significantly related to outcomes (all p values > 0.10). Relevant health behaviors were included as covariates in analyses where they were related to outcomes. There were no significant differences between conditions in numbers of chemotherapy cycles received before the final blood draw (p=.57). The dose of external beam radiation received (p=0.67), and the number of HDR (p=0.81) or LDR brachytherapy (p=0.24), or vaginal cylinder treatments (p=0.45) before the final blood draw was equivalent between conditions.
Table 1. Demographic characteristics and health behaviors of patients at study entry.
| Measure | Healing Touch | Relaxation | Usual Care |
|---|---|---|---|
| Age years (standard deviation) | 48.1 (16.0) | 43.1 (9.6) | 48.0(13.8) |
| (n=21,20,19) | Range 25-82 | Range 24-60 | Range 26-77 |
| Education (n=18,20,17) | |||
| Less than high school | 0.0% | 10.0% | 0.0% |
| some high school | 16.7% | 0.0% | 5.9% |
| high school graduate | 38.9% | 35.0% | 35.3% |
| trade school/some college | 27.8% | 35.0% | 29.4% |
| college graduate | 11.1% | 15.0% | 23.5% |
| post-graduate | 5.5% | 5.0% | 5.9% |
| Annual Income (n=20,20,15) | |||
| Less than $10,000 | 40.0% | 35.0% | 20.0% |
| $10,001-$20,000 | 10.0% | 20.0% | 20.0% |
| $20,001-$30,000 | 25.0% | 15.0% | 13.3% |
| $30,001-$40,000 | 15.0% | 0.0% | 13.3% |
| $40,001-$50,000 | 5.0% | 35.0% | 20.0% |
| More than $50,000 | 5.0% | 5.0% | 13.3% |
| Race (n=21,20,19) | |||
| American Indian/Alaskan Native | 0.0% | 0.0% | 5.3% |
| Asian/Pacific Islander | 0.0% | 5.0% | 0.0% |
| African American | 0.0% | 0.0% | 0.0% |
| Caucasian | 100.0% | 95.0% | 94.7% |
| Ethnicity (n=21,20,19) | |||
| Hispanic | 9.5% | 0.0% | 0.0% |
| Non Hispanic | 90.5% | 100.0% | 100.0% |
| FIGO Stage (n=21,20,19) | |||
| IB1 | 33.3% | 15.0% | 15.8% |
| IB2 | 23.8% | 15.0% | 36.8% |
| IIA | 0.0% | 15.0% | 5.3% |
| IIB | 23.8% | 35.0% | 5.3% |
| IIIA | 0.0% | 5.0% | 0.0% |
| IIIB | 14.3% | 15.0% | 31.5% |
| IVA | 4.8% | 0.0% | 5.3% |
|
Body Mass Index (kg/m2) (n=21,19,19) |
|||
| Underweight (< 18.5) | 0.0% | 10.5% | 5.3% |
| Normal Weight (18.5-24.9) | 23.8% | 36.8% | 31.5% |
| Overweight (25.0-29.9) | 33.3% | 21.2% | 15.8% |
| Obese (≥ 30) | 42.9% | 31.5% | 47.4% |
| Sleep in past week, hours/nite, mean (SD) (n=18,19,17) | 6.72 (1.27) |
6.76 (1.90) |
7.00 (2.24) |
| Cigarettes, packs/day, mean (SD) (n=19,20,17) | 0.25 (0.38) |
0.30 (0.59) |
0.38 (0.49) |
| Caffeine, cups/day, mean (SD) (n=18,20,17) | 1.33 (1.94) |
1.38 (1.88) |
1.88 (2.39) |
| Alcohol, drinks/day, mean (SD) (n=19,19,16) | 0.26 (0.65) |
0.26 (0.65) |
0.13 (0.50) |
| Cycles of chemotherapy received before final blood draw | 4.11 (1.18) |
4.21 (.79) |
4.43 (.63) |
3.2. Manipulation Checks
HT participants received on average 15.25 (±6.97) sessions vs. 11.75 (±5.20) sessions for RT (p=0.08). There were no significant differences in the pre- to post-intervention changes in SBP or DBP in weeks 1, 3, or 5 for either Contrast 1 (HT vs. RT/UC) or 2 (RT vs. UC), all p values > 0.20.
3.2.1. Expectations
Prior to randomization, patients in all conditions rated the anticipated interventions (RT and HT) from the descriptions they had received during recruitment. As seen in Table 2, patients rated interventions as relatively logical, and were highly confident that the interventions would help them reduce side effects and treatment-associated distress, would enable them to keep up their strength, with no significant differences in ratings between patients ultimately assigned to each condition (p values > 0.13). At week 6 patients receiving either RT or HT did not differ in their perceptions that the intervention they had personally received was logical (p=0.99), useful in reducing distress (p=0.85) or keeping up their strength (p=0.16), or was a treatment they would recommend to a friend going through cancer treatment (p=0.27). HT was rated more successful than RT in reducing treatment side-effects, but this difference was not significant (p=0.06).
Table 2. Age- and Stage-Adjusted Means of Psychological Measures.
| Measure | Week | HT Mean (S.D.) | Relaxation Mean (S.D.) | Usual Care Mean (S.D.) |
|---|---|---|---|---|
| CESD Total | 1 | 19.96 (16.53) | 21.54 (11.26) | 21.80 (8.89) |
| 4 | 15.84 (12.66) | 15.22 (8.55) | 16.04 (7.78) | |
| 6 | 12.65 (6.56) | 16.83 (10.18) | 21.37 (13.88) | |
| CESD positive subscale | 1 | 7.87 (4.22) | 7.60 (2.29) | 6.70 (2.52) |
| 4 | 7.84 (3.43) | 7.33 (3.47) | 8.23 (3.18) | |
| 6 | 8.70 (1.87) | 7.07 (3.40) | 7.17 (4.28) | |
| CESD depressed affect subscale | 1 | 5.30 (5.10) | 6.34 (4.14) | 6.33 (3.74) |
| 4 | 3.95 (4.28) | 3.12 (3.19) | 4.02 (2.45) | |
| 6 | 2.71 (2.10) | 3.62 (3.01) | 4.56 (4.30) | |
| CESD vegetative subscale | 1 | 8.58 (5.88) | 8.59 (4.29) | 8.28 (4.29) |
| 4 | 6.68 (4.26) | 6.91 (3.62) | 6.90 (2.75) | |
| 6 | 6.40 (2.94) | 7.43 (4.01) | 10.27 (4.44) | |
| POMS Depression | 1 | 11.43 (10.45) | 9.78 (7.82) | 11.22 (6.39) |
| 4 | 7.42 (8.64) | 4.15 (7.03) | 5.90 (5.12) | |
| 6 | 3.81 (2.19) | 5.98 (7.49) | 8.34 (9.36) | |
| POMS anxiety | 1 | 12.04 (6.23) | 11.26 (6.36) | 13.63 (5.26) |
| 4 | 7.64 (4.55) | 6.57 (6.09) | 7.41 (4.80) | |
| 6 | 8.47 (3.91) | 7.99 (5.64) | 7.20 (6.51) | |
| FACT (QOL) a | 1 | 92.54 (14.01) | 88.17 (17.03) | 84.58 (18.42) |
| 4 | 91.97 (16.35) | 94.88 (13.46) | 83.64 (17.20) | |
| 6 | 90.54 (15.80) | 87.46 (18.94) | 87.93 (18.38) | |
| FSI Average Fatiguea | 1 | 4.23 (2.08) | 4.61 (1.93) | 5.15 (2.50) |
| 4 | 3.92 (1.77) | 4.95 (1.48) | 4.43 (1.93) | |
| 6 | 4.77 (1.85) | 4.78 (2.01) | 5.48 (1.86) | |
| FSI Fatigue Duration | 1 | 4.03 (3.38) | 4.69 (2.37) | 5.22 (3.18) |
| 4 | 4.22 (2.08) | 4.63 (2.29) | 4.85 (2.48) | |
| 6 | 5.33 (1.69) | 4.59 (2.86) | 4.87 (2.58) | |
|
Expectations: Treatment seems Logical |
0 | 5.94 (1.52) | 5.42 (1.43) | 6.25 (1.00) |
| Tx will help with side effects | 0 | 6.00 (1.58) | 5.10 (1.52) | 5.93 (1.24) |
| Tx will help with strength | 0 | 5.35 (1.69) | 5.16 (1.21) | 5.25 (1.34) |
| Tx will reduce distress | 0 | 5.71 (1.26) | 5.53 (1.22) | 5.56 (1.53) |
| Recommend to friend | 0 | 5.59 (1.77) | 5.84 (1.30) | 5.94 (1.29) |
|
Evaluation Treatment was Logical |
6 | 5.39 (1.34) | 5.93 (1.10) | 5.55 (1.88) |
| Tx helped with side effects | 6 | 6.19 (1.33) | 5.33 (1.11) | 4.67 (1.94) |
| Tx helped with strength | 6 | 5.44 (1.50) | 4.67 (1.49) | 4.11 (2.09) |
| Tx reduced distress | 6 | 5.69 (1.35) | 5.60 (1.24) | 4.67 (1.80) |
| Would recommend tx to friend | 6 | 6.69 (.79) | 6.33 (.97) | 5.75 (1.49) |
Adjusted for coffee consumption
3.3. Physiological Outcomes
3.3.1. NK cells
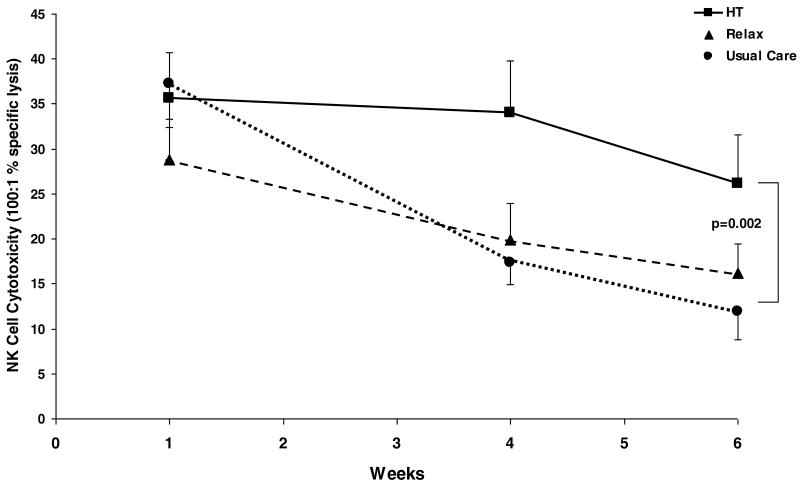
At study entry, there were no significant differences in NKCC (p=.48), NKAUC (p=.34), or NK cell percentages (p=.48) between conditions. Age was associated with higher (p=.002) and stage with lower (p=.002) NKCC. There was a significant Contrast 1 (HT vs. RT and UC) by time interaction for NKCC, b=1.01, t(87)=2.42, p=.018 (Figure 2). Simple effects demonstrated relative preservation in NKCC over time for HT (a 4.5% decrease from week 1 to 4 and a 26.6% decrease from week 1 to week 6), b= -.68, t(87)= -.64, p=0.52. In contrast, NKCC dropped significantly over time in both control conditions (RT: b= -3.16, t(87)= -3.21, p=.002; UC: b= -4.23, t(87)=-4.20, p<.001). This represents a 68% drop from week 1 to week 6 in UC and a 43.7% decrease over the same time period in RT. At week 6, the orthogonal contrast between the HT and the RT/UC patients showed significantly higher NKCC in the HT condition, t(84)=3.15, p=0.002. (Means for physiological outcome measures are reported in Table 3).
Figure 2.
NK cell cytotoxicity (NKCC: percent specific lysis at 100:1 E:T) in cervical cancer patients randomized to Healing Touch (■), relaxation (▲) or usual care (●) during chemoradiation. HT patients show relative preservation of NKCC over time (p=0.52); both control groups show significant drops in NKCC during treatment (RT: p=0.002, usual care: p=0.001). Orthogonal contrast for HT vs. RT/UC groups at Week 6 (p=.002).
Table 3.
Age- and Stage-Adjusted Means of Physiological Measures
| Measure | Week | HT Mean (S.D.) | Relaxation Mean (S.D.) | Usual Care Mean (S.D.) |
|---|---|---|---|---|
| NK Cytotoxicity % specific lysis 100:1 (NKCC) | 1 | 35.64 (22.99) | 28.82 (19.96) | 37.27 (21.33) |
| 4 | 34.02 (21.51) | 19.87 (15.11) | 17.31 (9.61) | |
| 6 | 26.14 (19.65) | 16.22 (13.14) | 11.90 (12.57) | |
| NK Cytotoxicity Area Under the Curve (NKAUC) | 1 | 3033.90 (1959.71) | 2076.98 (1447.25) | 2689.23 (1646.21) |
| 4 | 2454.21 (1709.90) | 1494.04 (1082.06) | 1361.31 (785.31) | |
| 6 | 2000.39 (1581.16) | 1036.40 (714.27) | 842.61 (1082.57) | |
| NK cell percentagea | 1 | 14.07 (6.12) | 11.43 (5.45) | 14.36 (9.62) |
| 4 | 10.04 (5.82) | 8.41 (7.67) | 8.20 (4.89) | |
| 6 | 9.90 (4.63) | 11.14 (7.26) | 7.68 (6.33) | |
| White blood cells (K/mL)b | 1 | 8.24 (3.14) | 7.94 (3.77) | 8.46 (3.69) |
| 4 | 5.14 (1.66) | 4.19 (1.37) | 4.89 (1.73) | |
| 6 | 3.51 (2.05) | 3.45 (1.88) | 2.91 (1.06) | |
| Red blood cells (M/mL)a | 1 | 4.09 (.63) | 4.29 (.45) | 4.25 (.35) |
| 4 | 4.22 (.34) | 4.39 (.33) | 4.13 (.39) | |
| 6 | 3.91 (.40) | 3.89 (.31) | 3.43 (.33) | |
| Clinically Rated Toxicities (CTC) severity | ||||
| 0 | 6 | 10 | 10 | 12 |
| 1 | 2 | 4 | 1 | |
| 2 | 4 | 1 | 4 | |
| 3 | 3 | 1 | 1 | |
| 4 | 0 | 2 | 1 | |
| Blood Pressure (Clinic) | ||||
| SBP | 0 | 133.29 (15.53) | 130.88 (15.69) | 132.62 (15.34) |
| DBP | 82.50 (11.13) | 76.40 (11.27) | 74.19 (11.04) | |
| Pre-Session SBP | 1 | 129.58 (14.06) | 122.40 (14.18) | 122.24 (13.89) |
| 3 | 128.99 (17.95) | 119.63(18.29) | 119.38 (17.92) | |
| 5 | 129.51 (13.06) | 117.71 (13.74) | 117.42 (13.25) | |
| Post-Session SBP | 1 | 129.71 (13.31) | 121.72 (13.41) | 117.59 (13.14) |
| 3 | 129.06 (19.72) | 116.49 (20.09) | 122.07 (19.69) | |
| 5 | 129.87 (14.37) | 117.22 (15.12) | 116.33 (14.58) | |
| Pre-Session DBP | 1 | 74.50 (10.01) | 67.63 (10.25) | 66.63 (10.04) |
| 3 | 72.46 (10.57) | 67.06 (10.77) | 65.97 (10.56) | |
| 5 | 74.35 (8.17) | 68.02 (8.60) | 69.93 (8.29) | |
| Post-Session DBP | 1 | 72.79 (8.69) | 68.70 (8.77) | 66.18 (8.59) |
| 3 | 72.33 (9.35) | 68.31 (9.39) | 67.83 (9.26) | |
| 5 | 72.99 (9.84) | 66.62 (10.37) | 70.52 (10.00) | |
Adjusted for hours sleep
Adjusted for number of cigarette packs per day
For NKAUC, significant age (p=.003), and stage (p=.001) effects were found as above. There was also a significant Contrast 1 (HT vs. RT and UC) by time interaction effect, b=61.61, t(87)=1.99, p=.049. Simple effects indicated no significant change in NKAUC over time for HT, b= -86.57, t(87)= -1.10, p=0.28, but significant declines in NKAUC were seen in patients receiving RT, b=-239.14, t(87)= -3.29, p=.002, and UC, b= -301.68, t(87)= -4.04, p<.001. At week 6, the orthogonal contrast between the HT and the RT/UC patients showed significantly higher NKAUC in the HT condition, t(84)=3.20, p=0.002. Contrast 2 (RT vs. UC) by time interactions for both NKCC and NKAUC were non-significant (p=0.44, p=0.55, respectively), indicating that RT and UC patients did not differ significantly in their change in NK cell activity or NKAUC over time. Despite differences in NK cell activity, all conditions showed a significant drop in NK percentage over time, b= -.68, t(68)= -3.02, p=.004, adjusting for age, stage, and sleep, but changes in NK cell percentage over time did not differ significantly between conditions.
At baseline there were no significant differences between conditions in WBC (p=0.15) or RBC (p=0.29); both WBC and RBC declined in all conditions during the 6 weeks of chemoradiation (p < 0.001). There were no significant condition or condition by time interaction effects for these parameters.
3.4. Clinically Rated Toxicities and Treatment Delay
There were no significant differences between conditions in number or severity of clinically-rated toxicities during treatment (p=0.93) or in days of treatment delay (p=0.94).
3.5. Psychological, QOL, and Clinical Outcomes
3.5.1. Depression
Descriptive statistics for psychological variables are reported in Table 2. At baseline, no significant differences between conditions in total depression scores (CES-D) were observed (p=0.48). Older patients reported less depression (p=.005), but there were no significant main effects of condition or time. There was a marginally significant interaction between Contrast 1 (HT vs. RT and UC) and time, b= -.38, t(88)= -1.71, p=0.09. Analyses of simple effects demonstrated a significant decline in depression among HT patients over time, b= -1.24, t(88) = -2.25, p=0.03, but not in the other two conditions. By week 6, mean CES-D scores of HT patients were below 16 (the clinical depression cutoff), whereas mean RT and UC scores were still in the depressed range (p=0.07; Figure 3). The Contrast 2 (RT vs. UC) by time interaction for CESD was nonsignificant (p=0.96).
Figure 3.
Center for Epidemiological Studies Depression (CES-D) total scores in cervical cancer patients randomized to Healing Touch (■), relaxation (▲) or usual care (●) during chemoradiation. HT patients show a significant drop in depression over time (p = 0.03) and mean values are in the non-depressed range (mean scores below 16) at week 6. Mean RT and UC scores remain in the depressed range at week 6.
Further analyses were conducted on the depressed mood, positive mood, and vegetative CES-D subscales. There were no significant differences between conditions for these subscales at baseline (all p values < 0.28). Analyses examining condition and time effects indicated that the effects described above for CES-D were specific to the depressed mood subscale. There was a significant interaction between Contrast 1 (HT vs. RT and UC) and time for the depressed mood subscale, b= -.18, t(88)= -2.07, p=0.042, with simple effects demonstrating a significant decrease in depressed mood only for HT, b= -.75, t(88)= -3.46, p < 0.001. At week 6, the orthogonal contrast between the HT and RT/UC patients showed lower depressed mood in the HT condition, b= -.84, t(85)= -1.94, p=0.056. The Contrast 2 (RT vs. UC) by time interaction for the CESD depressed mood scale was non-significant (p=0.84). None of the condition, time, or condition by time interaction effects for either contrast were significant for other CES-D subscales (p > 0.21).
Similar analyses were conducted on the POMS depression and anxiety subscales. There were no significant differences between conditions on these scales at baseline (all p > 0.37). Older patients reported less depression (p=.01) and anxiety (p=.005). For POMS depression, there was a significant interaction between Contrast 1 (HT vs. RT and UC) and time, b= -.29, t(92)= -2.03, p=0.046, with simple effects indicating a significant decrease in depression over time for HT, b= -1.37, t(92) = -3.92, p < 0.001, but not for the other 2 conditions. At week 6, the orthogonal contrast between the HT and RT/UC patients showed lower depressed mood in the HT condition, but this was non-significant, b= -2.23, t(85)= -1.84, p=0.069. The Contrast 2 (RT vs. UC) by time interaction for the POMS depression scale was non-significant (p=0.68). Anxiety significantly decreased in all conditions over time, b= -.1.01, t(94)= -5.87, p<.0001, but there were no significant condition or condition by time interaction effects for either contrast.
3.5.2. Quality of Life and Fatigue
There were no significant differences between the three conditions at baseline in QOL (p=0.65), mean fatigue (p=.31), or average fatigue duration (p=0.42). Although older patients reported better QOL (p=0.006), they also reported greater mean fatigue (p< 0.001) and fatigue duration (p=0.028). None of the tests involving condition or time or their interactions showed significant effects on QOL or fatigue.
3.6. Mediation Analyses
A test of mediation (MacKinnon et al., 2007) examined whether effects of HT on NK cell activity was due to effects of the intervention on depression. In this test, a value of zero indicates that there is no indirect effect of HT on NK cell activity via depression. For change in NKCC, the 95% confidence interval for the indirect effect ranged from -1.58 to 2.56. Since this interval included zero, it demonstrates that change in total CES-D scores did not mediate effects of HT on changes in NKCC. For NKAUC, the 95% confidence interval ranged from -244.22 to 67.25, similarly indicating lack of mediation by depression on NKAUC changes.
4. Discussion
The key findings of this study were that cervical cancer patients randomized to a Healing Touch intervention during chemoradiation demonstrated relatively preserved NK cell activity during treatment, whereas patients receiving RT and UC showed significant declines in NK cell activity, with the extent of decrements potentially clinically significant as well (Garzetti et al., 1995; Pillai et al., 1990). Changes in NK cell activity were not paralleled by alterations in NK number, suggesting that observed changes were not secondary to increased NK cell availability but more likely reflected increased activity in available cells. HT patients also showed a greater decline over time in 2 subscales of depressive mood as compared to controls. By week 6, mean CES-D total depression scores were within a non-depressed range for HT, whereas mean scores remained in the depressed range for both comparison groups. Effects of HT appear to be specific to depressed mood rather than reductions in other aspects of depression or anxiety. Effects of HT on toxicities, leukocyte and erythrocyte counts, treatment delay, QOL, and fatigue were not observed.
These data parallel effects of cognitive behavioral stress management interventions in producing positive psychological and physiological responses in cervical (Antoni et al., 2008; Nelson et al., 2008) and breast cancer patients (Andersen et al., 2008; Antoni et al., 2009; McGregor et al., 2004; Thornton et al., 2007; Witek-Janusek et al., 2008), although behavioral modulation of NKCC during chemoradiation has rarely been observed (Hernandez-Reif, et al., 2005). With some notable exceptions (e.g., Thornton, et al., 2009), psychosocial interventions have more commonly shown effects on functional measures of immunity or on cytokine activity (Antoni et al., 2009; Nelson et al., 2008) than on cell numbers; thus it is not surprising that the immune effects reported here were specific to a functional immune measure (NK cell activity) as opposed to NK cell number or total white and red blood cell counts.
Previous research has shown positive effects of HT on QOL of cancer patients (Cook et al., 2004; Danhauer et al., 2008; Post-White et al., 2003) as well as symptom relief for neurological ailments (Curtis et al., in press). The absence of HT effects on QOL in the current study was surprising, given that effects due to expectation would more likely influence self-reported QOL and fatigue ratings than physiological data.
There are a number of possible explanations for these findings. As HT involves both touch and non-touch techniques, positive immunologic effects may be secondary to beneficial effects of physical contact on the immune response and on neuroendocrine stress hormones. This is consistent with findings showing that regular massage following active treatment increased NK cell numbers (but not NK cell activity) and decreased depression in breast cancer patients who were at least 3 months post treatment (Hernandez-Reif et al., 2005). However, even intensive massage did not have effects on immunity or distress in cancer patients during chemoradiation (Billhult et al., 2008). In contrast, HT appears to demonstrate effects even during active cancer treatment.
Another possibility is that HT diminished levels of stress, thereby protecting against potential immunosuppression by neuroendocrine stress hormones. However, there were no differences among the 3 conditions in pre-post intervention blood pressure changes, suggesting that effects of HT may not have been attributable to stress-reduction alone. Relaxation-related enhancement of NK cell activity was observed among breast cancer patients who were at least 3 months post-treatment (Hernandez-Reif et al., 2005); however, relaxation effects on NK cell activity in the present study were inferior to effects of HT, again suggesting that stress reduction alone does not account for effects of HT.
Another explanation is that non-specific expectation effects may have contributed to these findings. Expectation did not differ between conditions at study entry and ratings of how “logical” interventions were did not differ between HT and RT at the end of the study. However, HT practitioners were trained professionals with HT certification and substantial experience. In contrast, relaxation was provided by research assistants or doctoral students trained to reliability administer the manualized intervention. Thus, differences in professional credibility of providers may have contributed to findings.
The fact that HT patients received on average slightly more sessions than RT patients may also have contributed to findings. However, results were re-analyzed controlling for number of sessions (data not shown), and findings remained essentially the same, indicating that differences in intervention “dose” were not responsible for findings. Nonspecific effects of social support may also have contributed to effects reported here. Although part of the rationale for using an attention-time matched relaxation condition was to control for support and perceived treatment efficacy, use of a team of HT practitioners for a majority of sessions may have generated non-specific effects of attention in HT not seen in RT. Thus, we cannot completely rule out effects of social support. However, a previous 6-week social support intervention showed no effects on NK cell activity (Kiecolt-Glaser et al., 1985), suggesting that the HT effects on NK cell activity seen here are unlikely due to social support alone.
An alternative interpretation of these findings is that there was some effect of manipulation of hypothesized biofields, resulting in preservation of the innate immune response in HT recipients. This interpretation would be consistent with findings demonstrating positive effects of a similar therapy, Therapeutic Touch, on bone cells in vitro (Jhaveri et al., 2008) and the effects of another biofield treatment, external QiGong, on signaling and apoptosis in cancer cells (Kiang et al., 2005; Yan et al., 2008; Yan et al., 2006). Such well-controlled interventions demonstrated effects on in vitro cultures in the absence of physical contact with the cells. Mechanisms underlying such effects are poorly understood although a recent report in a clinical setting has shown changes in heart rate variability in healthy adults following HT treatments (Tang et al., in press). Protocols for direct assessment of biofield energies are not yet well developed and thus this interpretation cannot be critically evaluated. It is also possible that effects of touch, expectation, and social support may have acted synergistically with any biofield effects.
The clinical significance of NK cell activity in cervical cancer is evidenced in the known role of NK cells in eliminating virus-infected and tumor cells (Arreygue-Garcia et al., 2008; Smyth et al., 2005), the general diminution of NK cell activity reported among cervical cancer patients (Cheriyan et al., 2009; Garzetti et al., 1995; Textor et al., 2008) and associations of decreased NK cell activity with disease progression in cervical cancer (Balaram et al., 1988; Garzetti et al., 1995; Pillai et al., 1990; Textor et al., 2008; Vaquer et al., 1990). For instance, Garzetti and colleagues (l995) found that cervical cancer patients who developed disease recurrence over a 40-month period following initial treatment demonstrated NKCC that was approximately 85% below means of patients who did not develop recurrent disease. Our findings of an average 68% drop in NKCC in the UC group and a 43.7% drop in the RT group are of a lesser magnitude than that seen in Garzetti et al's recurrent patients, but nevertheless may represent potential compromise if these decrements were maintained over time.
An algorithmically based immunological staging system, including NK cell activity as well as several other parameters of cellular immunity, was shown to have prognostic significance for recurrence of cervical cancer (Pillai et al., 1990). Recent findings have shown that NK cell activity was related to progression free survival in breast cancer patients treated with trastuzumab (Beano et al., 2008) and that NK cell IFNγ production after treatment with Imatinib independently predicted long term survival in gastrointestinal stromal tumors (Menard et al., 2009), suggesting a role for NK activity in survival for other cancers. As NK cells interact with multiple factors in the tumor microenvironment, in future research it would be useful to examine NK cell data in the context of other aspects of the immune response and tumor environment, including cell signaling pathways and receptor expression, in order to illuminate underlying mechanisms.
Partial recovery of NKCC has been observed following radiotherapy for cervical cancer in some reports (Garzetti et al., 1995; Pillai et al., 1990; Satam et al, 1986); thus, longitudinal data would be necessary to understand the full clinical significance of the present findings. Moreover, as the HT treatment was discontinued following the end of radiation therapy, long-term stability of the observed changes is not known. Thus, implications of the present findings for disease progression are unclear.
4.1. Methodological considerations
Although some complementary medicine research has utilized sham treatments to control for expectancy (Cook et al., 2004), such protocols are often unwieldy. Because of its beneficial physiological effects (Blanchard et al., 1982; Dusek et al., 2008; Kiecolt-Glaser et al., 1985), relaxation was thought to be a plausible control for the expectation of active treatment. Nevertheless, the lack of patient blinding to treatment condition could have produced bias. We also do not have data on adequacy of blinding of medical personnel.
Sample size was determined prior to the study based on effect sizes in the literature. A final sample size of 64 patients was recommended for adequate power; thus the current findings may under-represent relationships that were present.
4.2. Conclusions
The present findings suggest that intensively administered HT has positive effects in preserving NK cell activity and reducing depressed mood during chemoradiation for advanced cervical cancer patients. Expected effects of HT on toxicities, treatment delay, WBC and RBC, QOL, and fatigue were not seen. Long-term clinical implications of these findings are not known. Because direct assessment of hypothesized biofield changes was not possible, the interpretation that the observed effects were secondary to changes in patient biofields cannot be evaluated. These data require replication and support the need for future research examining dose, putative mechanisms, role of expectations, and methodological refinement of HT research.
Research Highlights.
This prospective randomized clinical trial examined effects of a complementary therapy, Healing Touch (HT), versus relaxation training (RT) and usual care (UC) in cervical cancer patients receiving chemoradiation.
HT demonstrated positive effects in preserving natural killer cell cytotoxicity (NKCC) over the course of treatment whereas NKCC of RT and UC patients declined sharply during chemoradiation.
HT patients showed greater reductions in 2 different indicators of depressed mood compared to RT and UC.
No effects of HT were observed for QOL, treatment delay, or clinically-rated toxicities.
HT may benefit cervical cancer patients by moderating effects of chemoradiation on depressed mood and cellular immunity. Long-term clinical implications of findings are not known.
Acknowledgments
This research was funded in part by NIH grant #R21AT0095801 to Susan Lutgendorf, NIH grant #P20AT75601 to Karen Prestwood, and grant #UL1RR024979 from the National Center for Research Resources, NIH. We gratefully acknowledge John Buatti, M.D., Heena Maiseri, M.S.N., Stephanie McGinn, B.S., Patrick Henderson, B.S., Brian Weinberg, B.A., Emily Schlitter, B.S., Mildred Freel, M.S., R.N., C.H.T.P., Marjorie Fearing, R.N., C.H.T.P., Carol Flack, L.M.T., C.H.T.P., Dixie Ecklund, R.N., Michael Bosch, R.N., B.A., and the Clinical Research Center staff for their valuable contributions to the study.
Footnotes
Two additional patients were randomized but became ineligible before starting study procedures when their medical care was transferred elsewhere. Their randomization was given to the next eligible patient. These 2 patients are included in the CONSORT chart among those not meeting inclusion criteria.
Initially the study was funded as a 2-group protocol (HT vs. UC). Five patients (4 UC, 1 HT) were randomized during this time. Subsequently, additional NIH funding allowed extension of this trial to a 3-group intervention. Previously recruited patients were kept in the study and a new randomization scheme was implemented as described above starting with participant #6.
Six patients (5 RT, 1 HT) received cisplatin/gemcitabine and radiation therapy as part of Gynecologic Oncology Group Phase I Trial #9912. Significant analyses were rerun without these patients; test-statistics associated with statistically significant interaction effects were only minimally affected by removing these patients from analyses, suggesting that effects reported in the manuscript are not driven by these group-related disparities in chemotherapy.
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen B, Yang H, Farrar W. Psychologic intervention improves survival for breast cancer patients. Cancer. 2008;113:3450–3458. doi: 10.1002/cncr.23969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH. Stress management intervention for women with breast cancer. Washington, D.C.: American Psychological Association; 2003. [Google Scholar]
- Antoni MH, Lechner S, Diaz A, Vargas S, Holley H, Phillips K, McGregor B, Carver CS, Blomberg B. Cognitive behavioral stress management effects on psychosocial and physiological adaptation in women undergoing treatment for breast cancer. Brain Behav Immun. 2009;23:580–591. doi: 10.1016/j.bbi.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Pereira DB, Marion I, Ennis N, Andrasik MP, Rose R, McCalla J, Simon T, Fletcher MA, Lucci J, Efantis-Potter J, O'Sullivan MJ. Stress management effects on perceived stress and cervical neoplasia in low-income HIV-infected women. J Psychosom Res. 2008;65:389–401. doi: 10.1016/j.jpsychores.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arreygue-Garcia NA, Daneri-Navarro A, del Toro-Arreola A, Cid-Arregui A, Gonzalez-Ramella O, Jave-Suarez LF, Aguilar-Lemarroy A, Troyo-Sanroman R, Bravo-Cuellar A, Delgado-Rizo V, Garcia-Iglesias T, Hernandez-Flores G, Del Toro-Arreola S. Augmented serum level of major histocompatibility complex class I-related chain A (MICA) protein and reduced NKG2D expression on NK and T cells in patients with cervical cancer and precursor lesions. BMC Cancer. 2008;8:16–26. doi: 10.1186/1471-2407-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astin J, Harkness E, Ernst E. The efficacy of “Distant Healing”: A systematic review of randomized trials. Ann Int Med. 2000;132:903–910. doi: 10.7326/0003-4819-132-11-200006060-00009. [DOI] [PubMed] [Google Scholar]
- Balaram P, Pillai R, Padmanabhan T, Abraham T, Hareendran N, Nair M. Immune function in malignant cervical neoplasia: A multiparameter analysis. Gyn Oncol. 1988;31:409–423. doi: 10.1016/s0090-8258(88)80025-8. [DOI] [PubMed] [Google Scholar]
- Beano A, Signorino E, Evangelista A, Brusa D, Mistrangelo M, Polimeni MA, Spadi R, Donadio M, Ciuffreda L, Matera L. Correlation between NK function and response to trastuzumab in metastatic breast cancer patients. J Transl Med. 2008;6:25–35. doi: 10.1186/1479-5876-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billhult A, Lindholm C, Gunnarsson R, Stener-Victorin E. The effect of massage on cellular immunity, endocrine and psychological factors in women with breast cancer: A randomized controlled clinical trial. Autonomic Neurosci. 2008;140:88–95. doi: 10.1016/j.autneu.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Andrasik F, Neff DF, Arena JG, Ahles TA, Jurish SE, Pallmeyer TP, Saunders NL, Teders SJ, Barron KD, Rodichok LD. Biofeedback and relaxation training with three kinds of headache: treatment effects and their prediction. J Consult Clin Psychol. 1982;50:562–575. doi: 10.1037//0022-006x.50.4.562. [DOI] [PubMed] [Google Scholar]
- Boon H, Stewart M, Kennard MA, Gray R, Sawka C, Brown JB, McWilliam C, Gavin A, Baron RA, Aaron D, Haines-Kamka T. Use of complementary/alternative medicine by breast cancer survivors in Ontario: prevalence and perceptions. J Clin Oncol. 2000;18:2515–2521. doi: 10.1200/JCO.2000.18.13.2515. [DOI] [PubMed] [Google Scholar]
- Borkovec T, Nau S. Credibility of analogue therapy rationales. J Behav Ther Exper Psychiatry. 1972;1:257–260. [Google Scholar]
- Canavan T, Doshi N. Cervical cancer. Am Family Physician. 2000;61:1369–1376. [PubMed] [Google Scholar]
- Cella D. Manual for the functional assessment of Cancer Therapy, (FACT) and Functional Assessment of HIV Infection, (FAHI) Measurement System. Chicago, IL: Rush-Presbyterian St. Lukes Medical Center; 1994. [Google Scholar]
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J, Eckberg E, Lloyd S, Purl S, Blendowski C, Goodman M, Barnicle M, Stewart I, McHale M, Bonomi P, Kaplan E, Taylor SR, Thomas CR, Harris J. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- Cheriyan VT, Krishna SM, Kumar A, Jayaprakash PG, Balaram P. Signaling defects and functional impairment in T-cells from cervical cancer patients. Cancer Biother Radiopharm. 2009;24:667–73. doi: 10.1089/cbr.2009.0660. [DOI] [PubMed] [Google Scholar]
- Cook CA, Guerrerio JF, Slater VE. Healing touch and quality of life in women receiving radiation treatment for cancer: a randomized controlled trial. Alt Ther Health Med. 2004;10:34–41. [PubMed] [Google Scholar]
- Curran SL, Andrykowski MA, Studts JL. Short form of the profile of mood states, (POMS-SF): Psychometric information. Psychol Assess. 1995;7:80–3. [Google Scholar]
- Curtis AR, Tegeler C, Burdette J, Yosipovitch G. Holistic approach to treatment of intractable central neuropathic itch. J Am Acad Dermatol. doi: 10.1016/j.jaad.2010.02.023. in press. [DOI] [PubMed] [Google Scholar]
- Danhauer SC, Tooze JA, Holder P, Miller C, Jesse MT. Healing touch as a supportive intervention for adult acute leukemia patients: a pilot investigation of effects on distress and symptoms. J Soc Integr Oncol. 2008;6:89–97. [PMC free article] [PubMed] [Google Scholar]
- Dusek JA, Otu HH, Wohlhueter AL, Bhasin M, Zerbini LF, Joseph MG, Benson H, Libermann TA. Genomic Counter-Stress Changes Induced by the Relaxation Response. PLoS One. 2008;3:e2576. doi: 10.1371/journal.pone.0002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, Rotman M, Gershenson D, Mutch DG. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol. 2004;22:872–880. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- Fouladbakhsh JM, Stommel M, Given BA, Given CW. Predictors of use of complementary and alternative therapies among patients with cancer. Oncol Nurs Forum. 2005;32:1115–1122. doi: 10.1188/05.ONF.1115-1122. [DOI] [PubMed] [Google Scholar]
- Garcia-Iglesias T, Del Toro-Arreola A, Albarran-Somoza B, Del Toro-Arreola S, Sanchez-Hernandez PE, Ramirez-Dueñas MG, Balderas-Peña LM, Bravo-Cuellar A, Ortiz-Lazareno PC, Daneri-Navarro A. Low NKp30, NKp46 and NKG2D expression and reduced cytotoxic activity on NK cells in cervical cancer and precursor lesions. BMC Cancer. 2009;9:186. doi: 10.1186/1471-2407-9-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzetti G, Ciavattini A, Goteri G, Muzzioli M, Mannello B, Romanini C. Natural killer cell activity in patients with invasive cervical carcinoma: Importance of a longitudinal evaluation in follow-up. Gynecol Obstet Invest. 1995;40:133–138. doi: 10.1159/000292322. [DOI] [PubMed] [Google Scholar]
- Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: Evaluation of the Center for Epidemiological studies Depression Scale. J Psychosom Res. 1999;46:437–443. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- Hernandez-Reif M, Field T, Ironson G, Beutler J, Vera Y. Natural killer cells and lymphocytes increase in women with breast cancer following massage therapy. Int J Neurosci. 2005;115:495–510. doi: 10.1080/00207450590523080. [DOI] [PubMed] [Google Scholar]
- Hodgkinson K, Butow P, Fuchs A, Hunt GE, Stenlake A, Hobbs KM, Brand A, Wain G. Long-term survival from gynecologic cancer: psychosocial outcomes, supportive care needs and positive outcomes. Gyn Oncol. 2007;104:381–9. doi: 10.1016/j.ygyno.2006.08.036. [DOI] [PubMed] [Google Scholar]
- Jain S, Mills PJ. Biofield therapies: helpful or full of hype? A best evidence synthesis. Int J Behav Med. 2010;17:1–16. doi: 10.1007/s12529-009-9062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri A, Wang Y, McCarthy MB, Gronowicz GA. Therapeutic Touch affects proliferation and bone formation of human osteoblasts in vitro. J Orthop Res. 2008;26:1541–1546. doi: 10.1002/jor.20688. [DOI] [PubMed] [Google Scholar]
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- Kiang JG, Ives JA, Jonas WB. External bioenergy-induced increases in intracellular free calcium concentrations are mediated by Na+/Ca2+ exchanger and L-type calcium channel. Mol Cell Biochem. 2005;271:51–29. doi: 10.1007/s11010-005-3615-x. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J, Glaser R, Wiliger D, Stout J, Messick G, Sheppard S, Ricker D, Romisher SC, Briner W, Bonnell G. Psychosocial enhancement of immunocompetence in a geriatric population. Health Psychol. 1985;4:25–41. doi: 10.1037//0278-6133.4.1.25. [DOI] [PubMed] [Google Scholar]
- Luebbert K, Dahme B, Hasenbring M. The effectiveness of relaxation training in reducing treatment-related symptoms and improving emotional adjustment in acute non-surgical cancer treatment: a meta-analytical review. Psychooncology. 2001;10:490–502. doi: 10.1002/pon.537. [DOI] [PubMed] [Google Scholar]
- Lutgendorf S, Logan H, Kirchner H, Rothrock N, Svengalis S, Iverson K, Lubaroff DM. Effects of relaxation and stress on the capsaicin-induced local inflammatory response. Psychosom Med. 2000;62:524–534. doi: 10.1097/00006842-200007000-00011. [DOI] [PubMed] [Google Scholar]
- Lutgendorf S, Sood AK, Anderson B, McGinn S, Maiseri H, Dao M, Sorosky J, DeGeest K, Ritchie J, Lubaroff DM. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J Clin Oncol. 2005;23:7105–7113. doi: 10.1200/JCO.2005.10.015. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Ann Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor B, Antoni M, Boyers A, Alferi S, Blomberg B, Carver C. Cognitive-behavioral stress management increases benefit finding and immune function among women with early-stage breast cancer. J Psychosom Res. 2004;56:1–8. doi: 10.1016/S0022-3999(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Ménard C, Blay JY, Borg C, Michiels S, Ghiringhelli F, Robert C, Nonn C, Chaput N, Taïeb J, Delahaye NF, Flament C, Emile JF, Le Cesne A, Zitvogel L. Natural killer cell IFN-gamma levels predict long-term survival with imatinib mesylate therapy in gastrointestinal stromal tumor-bearing patients. Cancer Res. 2009;69:3563–3569. doi: 10.1158/0008-5472.CAN-08-3807. [DOI] [PubMed] [Google Scholar]
- Mentgen J. Healing Touch. Nursing Clinics of North America. 2002;36:143–157. [PubMed] [Google Scholar]
- Menthe J, Bulbrook M. Healing Touch Level I notebook. revised. North Carolina Center for Healing Touch; Carrboro NC: 2002. [Google Scholar]
- Molassiotis A, Fernadez-Ortega P, Pud D, Ozden G, Scott JA, Panteli V, et al. Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol. 2005;16:655–663. doi: 10.1093/annonc/mdi110. [DOI] [PubMed] [Google Scholar]
- NCCAM. 2005 http://nccam.nih.gov/health/backgrounds/energymed.htm.
- NCI. Common Toxicity Criteria Manual Version 2.0. Washington, DC: National Cancer Institute; 1999. [Google Scholar]
- Nelson EL, Wenzel LB, Osann K, Dogan-Ates S, Chantana N, Reina-Patton A, Laust AK, Nishimoto KP, Chicz-DeMet A, duPont N, Monk BJ. Stress, immunity, and cervical cancer: Biobehavioral outcomes of a randomized clinical trial. Clin Cancer Res. 2008;14:2111–2118. doi: 10.1158/1078-0432.CCR-07-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai M, Balaram P, Chidambaram S, Padmanabhan T, Nair M. Development of an immunological staging system to prognosticate disease course in malignant cervical neoplasia. Gyn Oncol. 1990;37:200–205. doi: 10.1016/0090-8258(90)90333-g. [DOI] [PubMed] [Google Scholar]
- Post-White J, Kinney ME, Savik K, Berntsen J, Wilcox C, Lerner I. Therapeutic massage and healing touch improve symptoms in cancer. Integrative Cancer Ther. 2003;2:332–344. doi: 10.1177/1534735403259064. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling procedures for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: A self-report depression scale for research in the general population. App Psychol Meas. 1977;1:385–401. [Google Scholar]
- Santin AD, Hermonat PL, Ravaggi A, Bellone S, Roman J, Pecorelli S, Cannon M, Parham G. Effects of concurrent cisplatinum administration during radiotherapy vs. radiotherapy alone on the immune function of patients with cancer of the uterine cervix. Int J Radiat Oncol Biol Phys. 2000;48:997–1006. doi: 10.1016/s0360-3016(00)00769-0. [DOI] [PubMed] [Google Scholar]
- Satam M, Suraiya J, Nadkarni J. Natural killer and antibody-dependent cellular cytotoxicity in cervical carcinoma patients. Cancer Immunol Immunother. 1986;23:56–59. doi: 10.1007/BF00205556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan TJ, Fifield J, Reisine S, Tennen H. The measurement structure of the Center for Epidemiologic Studies depression scale. J Pers Assess. 1995;64:507–521. doi: 10.1207/s15327752jpa6403_9. [DOI] [PubMed] [Google Scholar]
- Sheeran TP, Jackson FR, Dawes PT, Collins M, Shadforth MF. Measurement of natural killer cell cytotoxicity by area under a cytotoxic curve. A method suitable for rheumatoid arthritis. Journal of Immunological Methods. 1988;115:95–98. doi: 10.1016/0022-1759(88)90314-6. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Cretney E, Kelly JM, Westwood JA, Street SE, Yagita H, Takeda K, van Dommelen SL, Degli-Esposti MA, Hayakawa Y. Activation of NK cell cytotoxicity. Mol Immunol. 2005;42:501–510. doi: 10.1016/j.molimm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Sparber A, Bauer L, Curt G, Eisenberg D, Levin T, Parks S, Steinberg SM, Wootton J. Use of complementary medicine by adult patients participating in cancer clinical trials. Oncol Nurs Forum. 2000;27:623–630. [PubMed] [Google Scholar]
- Tang R, Tegeler C, Larrimore D, Cowgill S, Kemper K. Improving the wellbeing of nursing leaders through healing touch training. J Altern Complement Med. doi: 10.1089/acm.2009.0558. in press. [DOI] [PubMed] [Google Scholar]
- Textor S, Dürst M, Jansen L, Accardi R, Tommasino M, Trunk MJ, Porgador A, Watzl C, Gissmann L, Cerwenka A. Activating NK cell receptor ligands are differentially expressed during progression to cervical cancer. Int J Cancer. 2008;123:2343–2353. doi: 10.1002/ijc.23733. [DOI] [PubMed] [Google Scholar]
- Thornton LM, Andersen BL, Crespin TR, Carson WE. Individual trajectories in stress covary with immunity during recovery from cancer diagnosis and treatments. Brain Behav Immun. 2007;21:185–194. doi: 10.1016/j.bbi.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquer S, Jordá J, López de la Osa E, Alvarez de los Heros J, López-García N, Alvarez de Mon M. Clinical implications of natural killer (NK) cytotoxicity in patients with squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 1990;36:90–92. doi: 10.1016/0090-8258(90)90114-z. [DOI] [PubMed] [Google Scholar]
- Vistad I, Fosså SD, Dahl AA. A critical review of patient-rated quality of life studies of long-term survivors of cervical cancer. Gynecol Oncol. 2006;102:563–72. doi: 10.1016/j.ygyno.2006.03.050. [DOI] [PubMed] [Google Scholar]
- Weissman M, Sholomskas D, Pottenger M, Prusoff B, Locke B. Assessing depressive symptoms in five psychiatric populations: A validation study. Am J Epidemiology. 1977;106:203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- Witek-Janusek L, Albuquerque K, Chroniak KR, Chroniak C, Durazo-Arvizu R, Mathews HL. Effect of mindfulness based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain Behav Immun. 2008;22:969–981. doi: 10.1016/j.bbi.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Shen H, Jiang H, Zhang C, Hu D, Wang J, Wu X. External Qi of Yan Xin Qigong induces G2/M arrest and apoptosis of androgen-independent prostate cancer cells by inhibiting Akt and NF-kB pathways. Mol Cell Biochem. 2008;310:227–234. doi: 10.1007/s11010-007-9684-2. [DOI] [PubMed] [Google Scholar]
- Yan X, Shen H, Jiang H, Zhang C, Hu D, Wang J, Wu X. External Qi of Yan Xin Qigong differentially regulates the Akt and extracellular signal-regulated kinase pathways and is cytotoxic to cancer cells but not to normal cells. Int J Biochem Cell Biol. 2006;38:2102–2113. doi: 10.1016/j.biocel.2006.06.002. [DOI] [PubMed] [Google Scholar]