Abstract
Estrogenic effects are mediated through two estrogen receptor (ER) subtypes, ERα and ERβ. Estrogens are the most commonly prescribed drugs to treat menopausal conditions, but by non-selectively triggering both ERα and ERβ pathways in different tissues they can cause serious adverse effects. The different sizes of the binding pockets and sequences of their activation function domains indicate that ERα and ERβ should have different specificities for ligands and biological responses that can be exploited for designing safer and more selective estrogens. ERα and ERβ regulate different genes by binding to different regulatory elements and recruiting different transcription and chromatin remodeling factors that are expressed in a cell-specific manner. ERα- and ERβ-selective agonists have been identified that demonstrate that the two ERs produce distinct biological effects. ERα and ERβ agonists are promising new approach for treating specific conditions associated with menopause.
Introduction
Estrogens have important actions in non-reproductive tissues, including the brain, urogenital tract and bone. Because of their actions in these tissues, estrogens have been used for over 50 years to prevent and treat a variety of conditions affecting postmenopausal women, including hot flashes, urogenital atrophy and osteoporosis. Estrogens would be the clear drug of choice for treating menopausal symptoms if they did not cause some serious adverse effects. The most troublesome side-effect of estrogens is the increased risk of breast and endometrial cancer [1,2]. Estrogens also increase blood clotting which can lead to venous thromboembolisms, and possibly strokes and heart disease, particularly in older women [1].
Estrogens in hormone therapy (HT) were formulated long before there was a significant understanding of the mechanism of action of estrogens. The identification of ERα and ERβ (Figure 1) and the crystal structures of their ligand binding domain (LBD), the discovery of variety of coregulatory proteins involved in the genomic pathway and the demonstration of the nongenomic actions of estrogens [3,4] provide an extraordinary opportunity to design a new generation of estrogens that are safer and more selective. Estrogen receptor subtype agonists (ERSAs) [5–10] have been identified (Figure 2) which might represent new classes of drugs to treat menopausal conditions. Here we will review ERα and ERβ regulation of genes and the actions of several ERSAs and their potential clinical applications.
Figure 1.
Comparison of the structures and homology between ERα and ERβ. Human ERα contains 595 amino acids whereas ERβ contains 530 amino acids. The DNA binding domains are nearly identical whereas the A/B domain and LBD, which contains AF-1 and AF-2, respectively, have the least homology.
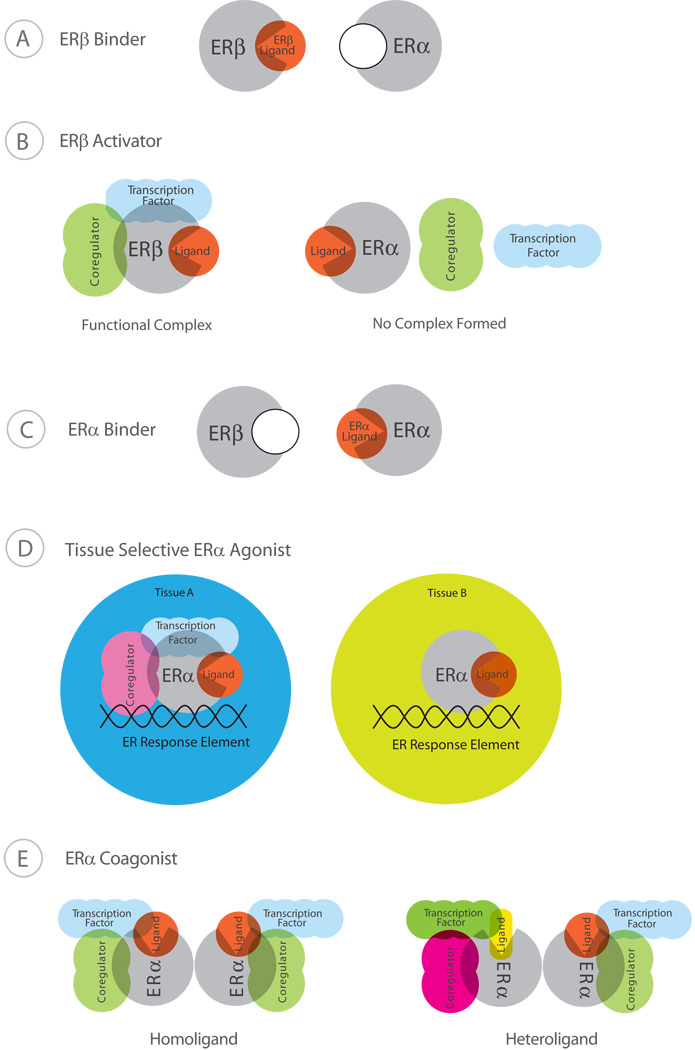
Figure 2.
Potential classes of estrogen receptor subtype agonists (ERSAs) for drug therapy. Potential ERβ-selective estrogens. (A) ERβ binders (ERB-041) are estrogens that are selective because they bind to ERβ with a much higher affinity than ERα. (B) ERβ activators (MF101, liquiritigenin) bind to ERα and ERβ with a similar affinity, but form a functional complex when bound to ERβ (left panel), but not ERα (right panel). An ERβ binder/activator (DPN) selectively binds to (A) and activates ERβ (B). Potential ERα-selective agonists. (C) ERα binders (PPT) bind to ERα with a much higher affinity than ERβ. (D) Tissue selective ERα agonists (Radix Glycyrrhiza and Radix Pueraria) form a functional transcription complex at response elements with ERα in some tissues (left panel), but not in other tissues (right panel). (E) A ligand such as E2 binds to both ERα subunits that leads to the recruitment of coregulators and transcription factors (left panel). In the presence of an ERα coagonist (chalcone) E2 binds to one subunit and the coagonist binds to the other subunit (right panel). The heteroliganded ERα could create a different conformation than the homoliganded ERα which leads to the recruitment of different coregulators and/or transcription factors.
Differences in ERα and ERβ are important for designing ERSAs
ERs are composed of three major modular domains; an A/B domain, a DNA binding domain (DBD), and a LBD. Several features differ between ERα and ERβ that might be important for designing ERSAs. First, the size of the ERα and ERβ binding pocket for ligands are different providing a structural basis for designing ligands that selectively bind to each ER. Second, the two activation function (AF-1 and AF-2) domains that are responsible for regulating gene transcription are located in the least homologous regions (Figure 1). The A/B domain contains AF-1 has only 17% homology, whereas the LBD which contains the AF-2 is 55% homologous. Differences in AF-1 and AF-2 could allow drugs to be designed that recruit different cofactors to ERα and ERβ, and thereby causing a different pattern of genes regulated.
ERα and ERβ have distinct cellular actions, which provide a rationale for developing ERSAs. This has been demonstrated with microarrays that showed ERα and ERβ regulate different genes [11–14]. Only 40% of genes regulated by estradiol (E2) in U2OS cells that express ERα are also regulated by ERβ [12]. Furthermore, ERα and ERβ regulate different classes of genes suggesting that the two ERs have distinct physiological roles. Another feature that distinguishes ERβ from ERα is that ERβ regulates three classes of genes, whereas ERα regulates a single class of genes [15]. U2OS cell lines stably transfected with a doxycycline-inducible ERα or ERβ [15] were used to measure the effects of unliganded ER in cells treated only with doxycycline or liganded-ER when cells were treated with both doxycycline and E2. Unliganded ERα produced a small upregulation of only 1 gene and downregulation of 3 genes, whereas the addition of E2 to doxycycline treated U2OS-ERα cells resulted in the activation of 518 genes and repression of 157 genes. These data indicate that ERα requires the ligand to regulate gene transcription in U2OS cells. In contrast, three classes of genes were regulated in U2OS-ERβ cells. 453 genes were regulated by unliganded ERβ (Class I genes). 258 genes were not regulated by unliganded ERβ, but regulated by E2-bound ERβ (Class II genes). 83 genes were regulated by unliganded ERβ and potentiated by the addition of E2 (Class III genes). The unliganded effect of ERβ is mediated by AF-2, because it is lost when the ERβ AF-2 is replaced by the ERα AF-2 [16]. These results demonstrate that intrinsic differences in AF-2 of ERα and ERβ can lead to a different set of genes regulated.
ERα and ERβ regulate different genes by binding to distinct regulatory elements
A major question is how do ERα and ERβ regulate different genes. The first step required for estrogens to regulate gene transcription involves the binding of ligand to the LBD. This causes a conformational change that allows the ligand-ER complex to bind to regulatory elements in target genes. ERα and ERβ might regulate different genes by binding to different regulatory elements on target genes. To explore this possibility, ChIP-sequencing was performed in U2OS cells that express a stably transfected ERα or ERβ to identify ER binding sites. 11,975 binding sites were found for ERβ in response to E2 [15] and 15,947 binding sites for ERα (unpublished data). There was approximately a 30% overlap between ERα and ERβ binding sites. Different ERα and ERβ binding sites were also observed in MCF-7 cells [17,18]. There were 4,405 ERα and 1,897 ERβ binding sites, of which 1,386 binding sites were common. These results demonstrate that many ERα and ERβ binding sites are unique in U2OS and MCF-7 cells.
Tiling arrays [19–21] and ChIP-seq [15,22] studies demonstrated that many ER binding are more diverse and complex than the classical estrogen responsive element (ERE), requiring multiple different transcription factors for activity, such as AP1, FoxA1 and Sp1 [15,19–23]. The complexity is exemplified by the regulatory element in the NKG2E gene which requires a collaboration between c-jun, heat-shock factor 2, and CCAAT/enhancer-binding protein beta and a unique variant ERE for full activation by E2 [24]. In MCF-7 cells T-cell factor and p53 motifs were present only in ERα binding sites [17], whereas forkhead transcription factors and Sp1 sites were enriched in ERα and ERβ sites, respectively [18]. These observations suggest that transcription factor binding elements are a major determinant of whether ERα or ERβ will bind to a particular gene.
ERα and ERβ regulate different genes by recruiting distinct coregulators and chromatin remodeling factors
Once the ER complex attaches to a regulatory element it functions as a docking site for the recruitment of coregulatory proteins, and transcription and chromatin remodeling factors to form a large protein complex that regulates transcription [25,26]. Even if ERα and ERβ bind to the same site they could regulate different genes because differences in their conformation might lead to the recruitment of different coregulatory proteins at the same genes. For example, liquiritigenin (LIQ) caused the recruitment of the coactivator, NCOA2 to the CECR6, NKG2E, and NKD genes in U2OS-ERβ cells, but not in U2OS-ERα cells [9]. Furthermore, GIOT-4 has been identified as an ERβ specific coactivator [27], whereas a member of the SWI/SNF chromatin remodeling complex, BAF57 selectively regulates ERα-mediated transcription [28].
Identification of three classes of ERβ-selective agonists
Multiple ERβ-selective agonists have been synthesized [10]. 2,3-bis(4-hydroxyphenyl)-propionitrile (DPN) has 70-fold higher relative binding affinity and 170-fold higher relative potency in transfection assays with ERβ compared to ERα [7]. Wyeth synthesized a number of ERβ-selective compounds [29]. ERB-041 has been the most studied. It has over a 200-fold greater selectivity for binding to ERβ compared to ERα [6]. In addition to synthetic compounds, a plant extract, MF101 contains ERβ-selective agonists [8], several of which have been identified, including liquiritigenin and nyasol [9,13]. Based on binding and functional studies, we proposed that these compounds can be grouped into three classes [13] (Figure 1). One class is represented by ERB-041 which is selective because it binds to ERβ at a much higher affinity than ERα (Figure 1A). We termed it an ERβ binder. MF101, LIQ and nysasol bind to both ERα and ERβ similarly, but they only activate ERβ [13]. When these compounds bind to ERα they produce an inactive conformation that prevents ERα from forming a functional complex and recruiting coactivators [8,9] (Figure 1B). These are termed ERβ activators. DPN is selective because it binds ERβ with higher affinity, but also more potently activates ERβ than ERα. We termed it an ERβ binder/activator. While most genes regulated by DPN, ERB-041, MF101, LIQ and nyasol are the same, these three classes of ERβ agonists regulate some different genes [13]. Importantly, many genes regulated by these ERβ agonists in U2OS-ERβ cells are distinct from those regulated by E2. This observation is consistent with the finding that ERβ binding sites are different when it is bound to ERB-041 compared to E2 in MCF-7 cells [17]. From these results, it can be expected that different classes of ERβ agonists will produce different biological and clinical effects from one another and non-selective estrogens used in HT.
ERβ-selective agonists for hot flashes
Estrogens are the most effective treatment for hot flashes. However, it is unclear if this effect is mediated through ERα, ERβ or both ERs. This has been difficult to address experimentally because of inadequate animal models to test drugs on spontaneous hot flashes. Most studies used rat models that measure tail skin temperature as a surrogate marker for hot flashes. In a morphine-addicted rat model two ERB-041 analogs were ineffective [30], whereas DPN was effective in another rat model [31]. A Phase II clinical trial with 217 postmenopausal women having moderate to severe hot flashes was conducted with the ERβ-selective plant extract, MF101. After 12 weeks, there was a statistically significant median 11.9% reduction in hot flashes and a 67% reduction in night sweats in women treated with MF101 compared to those treated with placebo [32]. Taken together, these results suggest that ERβ agonists might have beneficial effects on hot flash prevention.
ERβ-selective agonists for breast cancer prevention
Multiple studies showed that ERα mediates the proliferative effects of estrogens in breast cells. Anti-proliferative effects of ERβ have been demonstrated in breast cancer cells [33,34]. In MCF-7 breast cancer cells, ERβ causes a G2 cell cycle arrest [34] by inhibiting the activity of cyclin dependent kinase 1 (CDK1) which is essential for cells to progress from G2 phase to mitosis. The major activator of CDK1 is cyclin B1. ERβ inhibits the transcription of the cyclin B1 gene which leads to a reduction in cyclin B1 protein levels (submitted). CDK1 is inhibited by the tumor suppressor proteins, GADD45A and BTG2. ERβ binds to the promoter of these genes leading to increased transcription (submitted). Ultimately, the reduction in cyclin B1 and increased production of GADD45A and BTG2 leads to the inactivation of CDK1 and a G2 cell cycle arrest.
ERB-041 did not produce proliferative effects in the rat mammary gland [6,8,9]. MF101 did not stimulate growth promoting genes, such as c-myc and cyclin D1 in MCF-7 cells [8]. Furthermore, MF101 or LIQ did not increase MCF-7 cell tumor formation in mouse xenograft models [8,9]. These results demonstrate that ERβ agonists do not promote proliferation of normal mouse mammary epithelial and human breast cancer cells. ERβ inhibits ERα-mediated activation of reporter genes in transfection assays [35], suggesting that one mechanism whereby ERβ exerts an anti-proliferative action is by interfering with the action of ERα. This was examined in MCF-7 cells that express ERα, ERβ or both ERs [17]. These studies showed that ERα and ERβ competed for the same genomic binding sites and that the presence of both ERs produced new binding sites for ERα and ERβ homodimers, which likely leads to a different gene expression profile that is observed when the two ERs are coexpressed in cells [36]. These findings suggest that ERβ agonists might be useful for preventing breast cancer by antagonizing the proliferative action of ERα.
ERβ-selective agonists for inflammatory Diseases
One important action of estrogens that is relatively unappreciated is their anti-inflammatory effects. A number of diseases during menopause have an inflammatory component to their pathogenesis. These conditions include osteoporosis, cardiovascular disease, Alzheimer’s disease, obesity and atrophic vaginitis. Estrogens in HT are very effective at preventing osteoporosis and atrophic vaginitis, but controversy exists regarding their effects on cardiovascular disease, obesity and Alzheimer’s disease. The anti-inflammatory action of ERB-041 have been examined in multiple inflammatory rodent models, including endometriosis, rheumatoid arthritis, inflammatory bowel and sepsis [6,37,38]. These studies demonstrated that ERB-041 was very potent at blocking inflammation in these models and suggested that ERβ-selective agonists might be important drugs to treat a variety of disorders associated with inflammation. MF101 and synthetic ERβ agonists, including ERB-041 are potent repressors of pro-inflammatory genes [8,39], indicating that estrogens can produce anti-inflammatory actions through ERβ.
The effects of ERβ on inflammatory conditions associated with menopause, such as osteoporosis, obesity, cardiovascular disease and atrophic vaginitis is unclear. ERB-041 did not prevent ovariectomy-induced bone loss or weight gain in rats [6], suggesting that ERα mediates these effects. DPN decreases the size of infarcts in mouse hearts subjected to ischemia and reperfusion similar to E2 [40]. This cardioprotective effect of DPN was abolished in ERβ knockout mice [40]. These findings indicate that ERβ agonists might be useful for preventing cardiovascular disease. Another possible clinical indication for ERβ agonists, where an anti-inflammatory effect could be therapeutic is atrophic vaginitis. Our pre-clinical studies with mice indicate that ERβ agonists may play a role in the treatment of postmenopausal vaginal atrophy and dryness.
ERα is important for preventing osteoporosis, weight gain and insulin resistance
ERα is essential for preventing osteoporosis because a rare genetic mutation that inactivates ERα leads to severe osteoporosis in humans [41]. The observation that PPT, but not ERB-041 prevents bone loss in rats after ovariectomy provides additional evidence that ERα mediates the beneficial effects of estrogens in bone [6,42]. ERα also likely mediates the beneficial effects of estrogens in adipose tissue and on insulin resistance, because ERKO mice have increased weight gain, greater adipose tissue, insulin resistance and impaired glucose tolerance [43]. PPT prevents weight gain in rats and exerts anti-diabetic effects by improving insulin sensitivity and glucose intolerance [44].
ERα-selective agonists
The major concern for developing ERα agonists is that they will cause cell proliferation and increase the risk of cancer. In fact, PPT stimulates the proliferation of HC11 mouse mammary epithelial cells [45] and increases uterine weight in rats [42]. These findings indicate that ERα-selective binders (Figure 1C), like PPT might not be useful drugs for hormone therapy. Another strategy would be to design tissue selective ERα agonists that activate ERα in some tissues, such as the bone and adipose tissue, but not in the mammary gland and uterus (Figure 1D). An alternative strategy is to combine estrogens with other compounds that block the proliferative effects of estrogens in the mammary and uterus (Figure 1E). Progestins are effective at blocking the proliferative effects of estrogens in the uterus, but unfortunately they exacerbate the proliferative effects in the mammary gland.
Tissue Selective ERα agonists prevent weight gain without promoting cell proliferation
It is well established that estrogens exert tissue-specific effects, but the mechanism is unclear. Tiling arrays identified 1,090 ERα binding sites on chromosomes 1 and 6 in MCF-7 cells whereas 1,137 binding sites were found in U2OS cells [46]. Only 172 ERα binding sites were common to both cell types. The cell specific recruitment of ERα is mediated by the binding of the pioneer factor, FoxA1 that recognizes monomethylated and dimethylated histone H3. Once FoxA1 recognizes these methylated histones near an ER binding site it interacts with ER to open up chromatin structure and facilitate the recruitment of transcription factors leading to increased transcription [46]. Because FoxA1 is expressed in MCF-7 cells, but not U2OS cells, the genes regulated by ERα are different [46]. These findings suggest that it might be possible to design tissue selective ERα modulators that mimic the agonist activity of E2 in some tissues, but not in other tissues.
We identified two plant extracts (PEs), Radix Glycyrrhiza and Radix Pueraria that behave as tissue selective ERα agonists (Figure 1D). These PEs activate ERα in transfection assays using an ERE upstream of the luciferase reporter and bind to purified ERα (In preparation). To test the effects of the PEs on weight loss, ovariectomized mice were fed a high fat diet (HFD). After the mice gained weight, they were treated orally for 6 weeks with the PEs separately while being maintained on the HFD. The vehicle treated control mice continued to gain weight, whereas the E2-treated mice, which served as positive controls, lost 20.5% of their weight. The body weight and abdominal fat of both PE treated mice was significantly reduced to levels similar to mice treated with E2. In contrast, no significant proliferative effects were found in the mammary gland and uterus. While further characterization and studies are needed with the PEs these studies suggest that it might be possible to develop tissue selective ERα agonists that retain the beneficial effects mediated by ERα without promoting breast and endometrial cancer.
ERα coagonists change the gene expression profile and proliferative response of E2
Another potential way to make estrogens safer for drug therapy is to add a second drug to alter the biological properties of estrogens after they interact with ERα. We screened plant extracts and found that a chalcone derivative dramatically changed the gene expression profile by E2 in U2OS cells expressing ERα. We termed the chalcone an ERα coagonist (Figure 1E), because it was inactive by itself, but it caused E2 to regulate genes that it did not activate in its absence and it potentiates the regulation of E2 on some genes. The coagonist blocked E2-mediated proliferation of MCF-7 cells, suggesting that the coagonist changes the proliferative response of E2 by causing ERα to regulate a different set of genes. While the mechanism of the coagonist is unclear, our studies suggest the possibility that it binds to ERα as heteroligand with one subunit binding to E2 and the other subunit binding to the chalcone (Figure 1E, right panel). The combination of two different ligands bound to ERα simultaneously likely produces different conformation then when ERα is bound to only E2 (Figure 1E, left panel) or the chalcone. While the effects of the coagonist on E2-mediated bone loss, weight loss, and mammary gland and endometrial cell proliferation in animals need to be investigated, it may be possible that coagonist compounds can alter the clinical responses to estrogens and make them safer.
Concluding remarks
Gene expression data, tiling arrays and ChIP-seq data shows that ERα and ERβ regulate different genes by binding to distinct regulatory elements and interacting with different coactivators and transcription factors. Animal studies demonstrated that ERα- and ERβ-selective agonists produce different biological effects. Three classes of ERβ-selective agonists have been identified; ERβ binder, ERβ activator and ERβ binder/activator. ERβ-selective agonists might be clinically useful for preventing breast cancer and treating hot flashes and inflammatory conditions associated with menopause. Because the proliferative effects of estrogens are mediated through ERα, the impetus to design ERα-selective agonists for clinical use has not been strong as ERβ-selective agonists. However, ERα is clearly important for preventing osteoporosis, weight gain and insulin resistance. Tissue selective ERα agonists or ERα coagonists may provide a safer approach if proven to activate ERα in tissues that are beneficial, such as the bone and adipose tissue, but not the mammary gland and uterus. While many additional studies are needed evaluate the safety and efficacy of ERα- and ERβ-selective agonists they offer a new therapeutic approach for preventing and treating specific menopausal conditions.
Acknowledgements
We thank the National Center for Complementary and Alternative Medicine for funding our research.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: O.I.V. and C.B.H have nothing to declare. S.P., E.F.S., I.C., and M.T., are employees of Bionovo, Inc. D.C.L. and T.P.S. are on the Scientific Advisory Board of Bionovo, Inc. D.C.L. has received financial support for research from Bionovo, Inc.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
References
- 1. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Jama. 2002;288:321–333. doi: 10.1001/jama.288.3.321. This seminal study was the first randomized, placebo controlled study to evaluate the effects of hormone therapy on postmenopausal women. The study found that the risks of hormone therapy exceed the benefits.
- 2.Shang Y. Molecular mechanisms of oestrogen and SERMs in endometrial carcinogenesis. Nat Rev Cancer. 2006;6:360–368. doi: 10.1038/nrc1879. [DOI] [PubMed] [Google Scholar]
- 3.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 4.Levin ER. Plasma membrane estrogen receptors. Trends Endocrinol Metab. 2009;20:477–482. doi: 10.1016/j.tem.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- 6. Harris HA, Albert LM, Leathurby Y, Malamas MS, Mewshaw RE, Miller CP, Kharode YP, Marzolf J, Komm BS, Winneker RC, et al. Evaluation of an estrogen receptor-beta agonist in animal models of human disease. Endocrinology. 2003;144:4241–4249. doi: 10.1210/en.2003-0550. This important study showed that a highly selective ERβ agonist, ERB-041 had potent antiinflammatory effects in multiple inflammation models and suggested that ERβ agonists could be useful for treating inflammatory diseases.
- 7.Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- 8. Cvoro A, Paruthiyil S, Jones JO, Tzagarakis-Foster C, Clegg NJ, Tatomer D, Medina RT, Tagliaferri M, Schaufele F, Scanlan TS, et al. Selective activation of estrogen receptor-beta transcriptional pathways by an herbal extract. Endocrinology. 2007;148:538–547. doi: 10.1210/en.2006-0803. This study demonstrated that plants contain ERβ agonists, which do not stimulate the proliferation of human breast cancer cells or stimulate growth of the uterus in mice.
- 9.Mersereau JE, Levy N, Staub RE, Baggett S, Zogric T, Chow S, Ricke WA, Tagliaferri M, Cohen I, Bjeldanes LF, et al. Liquiritigenin is a plant-derived highly selective estrogen receptor beta agonist. Mol Cell Endocrinol. 2008;283:49–57. doi: 10.1016/j.mce.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Minutolo F, Macchia M, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor beta ligands: Recent advances and biomedical applications. Med Res Rev. 2009 doi: 10.1002/med.20186. This important review summarizes the synthesis and biological properties of the ERβ-selective compounds that have been developed.
- 11.Monroe DG, Getz BJ, Johnsen SA, Riggs BL, Khosla S, Spelsberg TC. Estrogen receptor isoform-specific regulation of endogenous gene expression in human osteoblastic cell lines expressing either ERalpha or ERbeta. J Cell Biochem. 2003;90:315–326. doi: 10.1002/jcb.10633. [DOI] [PubMed] [Google Scholar]
- 12.Kian Tee M, Rogatsky I, Tzagarakis-Foster C, Cvoro A, An J, Christy RJ, Yamamoto KR, Leitman DC. Estradiol and Selective Estrogen Receptor Modulators Differentially Regulate Target Genes with Estrogen Receptors {alpha} and {beta} Mol Biol Cell. 2004;15:1262–1272. doi: 10.1091/mbc.E03-06-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paruthiyil S, Cvoro A, Zhao X, Wu Z, Sui Y, Staub RE, Baggett S, Herber CB, Griffin C, Tagliaferri M, et al. Drug and cell type-specific regulation of genes with different classes of estrogen receptor beta-selective agonists. PLoS One. 2009;4:e6271. doi: 10.1371/journal.pone.0006271. This study demonstrates that different ERβ agonists regulate different genes from each other and from estradiol, suggesting that they will have different clinical effects.
- 14.Stossi F, Barnett DH, Frasor J, Komm B, Lyttle CR, Katzenellenbogen BS. Transcriptional profiling of estrogen-regulated gene expression via estrogen receptor (ER) alpha or ERbeta in human osteosarcoma cells: distinct and common target genes for these receptors. Endocrinology. 2004;145:3473–3486. doi: 10.1210/en.2003-1682. [DOI] [PubMed] [Google Scholar]
- 15. Vivar OI, Zhao X, Saunier EF, Griffin C, Mayba OS, Tagliaferri M, Cohen I, Speed TP, Leitman DC. Estrogen receptor [beta] binds to and regulates three distinct classes of target genes. J Biol Chem. 2010 doi: 10.1074/jbc.M110.114116. in press. This study demonstrates that there is a single class of genes regulated by ERα that requires the ligand, whereas ERβ regulates three classes of genes, including genes regulated by unliganded ERβ, genes regulated only by liganded ERβ and genes regulated by both unliganded and liganded ERβ.
- 16.Levy N, Paruthiyil S, Zhao X, Vivar OI, Saunier EF, Griffin C, Tagliaferri M, Cohen I, Speed TP, Leitman DC. Unliganded estrogen receptor-beta regulation of genes is inhibited by tamoxifen. Mol Cell Endocrinol. 315:201–207. doi: 10.1016/j.mce.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 17. Charn TH, Liu ET, Chang EC, Lee YK, Katzenellenbogen JA, Katzenellenbogen BS. Genome-wide dynamics of chromatin binding of estrogen receptors alpha and beta: mutual restriction and competitive site selection. Mol Endocrinol. 24:47–59. doi: 10.1210/me.2009-0252. This important study shows that there is mutual competition for binding sites when both ERα and ERβ are present and that presence of both receptors creates novel binding sites for ERα and ERβ homodimers.
- 18.Liu Y, Gao H, Marstrand TT, Strom A, Valen E, Sandelin A, Gustafsson JA, Dahlman-Wright K. The genome landscape of ERalpha- and ERbeta-binding DNA regions. Proc Natl Acad Sci U S A. 2008;105:2604–2609. doi: 10.1073/pnas.0712085105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122:33–43. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 20. Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, et al. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38:1289–1297. doi: 10.1038/ng1901. This was an important study that first identified genome-wide ERα binding sites and showed that ER binding sites are diverse and complex, requiring the interaction of multiple different transcription factors.
- 21.Lupien M, Eeckhoute J, Meyer CA, Wang Q, Zhang Y, Li W, Carroll JS, Liu XS, Brown M. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell. 2008;132:958–970. doi: 10.1016/j.cell.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welboren WJ, van Driel MA, Janssen-Megens EM, van Heeringen SJ, Sweep FC, Span PN, Stunnenberg HG. ChIP-Seq of ERalpha and RNA polymerase II defines genes differentially responding to ligands. Embo J. 2009;28:1418–1428. doi: 10.1038/emboj.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin CY, Vega VB, Thomsen JS, Zhang T, Kong SL, Xie M, Chiu KP, Lipovich L, Barnett DH, Stossi F, et al. Whole-genome cartography of estrogen receptor alpha binding sites. PLoS Genet. 2007;3:e87. doi: 10.1371/journal.pgen.0030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy N, Zhao X, Tang H, Jaffe RB, Speed TP, Leitman DC. Multiple Transcription Factor Elements Collaborate with Estrogen Receptor {alpha} to Activate an Inducible Estrogen Response Element in the NKG2E Gene. Endocrinology. 2007;148:3449–3458. doi: 10.1210/en.2006-1632. [DOI] [PubMed] [Google Scholar]
- 25.Metivier R, Penot G, Hubner MR, Reid G, Brand H, Kos M, Gannon F. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell. 2003;115:751–763. doi: 10.1016/s0092-8674(03)00934-6. [DOI] [PubMed] [Google Scholar]
- 26.Stanisic V, Lonard DM, O'Malley BW. Modulation of steroid hormone receptor activity. Prog Brain Res. 181:153–176. doi: 10.1016/S0079-6123(08)81009-6. [DOI] [PubMed] [Google Scholar]
- 27.Kouzu-Fujita M, Mezaki Y, Sawatsubashi S, Matsumoto T, Yamaoka I, Yano T, Taketani Y, Kitagawa H, Kato S. Coactivation of estrogen receptor beta by gonadotropin-induced cofactor GIOT-4. Mol Cell Biol. 2009;29:83–92. doi: 10.1128/MCB.00884-08. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Garcia-Pedrero JM, Kiskinis E, Parker MG, Belandia B. The SWI/SNF chromatin remodeling subunit BAF57 is a critical regulator of estrogen receptor function in breast cancer cells. J Biol Chem. 2006;281:22656–22664. doi: 10.1074/jbc.M602561200. [DOI] [PubMed] [Google Scholar]
- 29.Malamas MS, Manas ES, McDevitt RE, Gunawan I, Xu ZB, Collini MD, Miller CP, Dinh T, Henderson RA, Keith JC, Jr, et al. Design and synthesis of aryl diphenolic azoles as potent and selective estrogen receptor-beta ligands. J Med Chem. 2004;47:5021–5040. doi: 10.1021/jm049719y. [DOI] [PubMed] [Google Scholar]
- 30.Manas ES, Unwalla RJ, Xu ZB, Malamas MS, Miller CP, Harris HA, Hsiao C, Akopian T, Hum WT, Malakian K, et al. Structure-based design of estrogen receptor-beta selective ligands. J Am Chem Soc. 2004;126:15106–15119. doi: 10.1021/ja047633o. [DOI] [PubMed] [Google Scholar]
- 31.Bowe J, Li XF, Kinsey-Jones J, Heyerick A, Brain S, Milligan S, O'Byrne K. The hop phytoestrogen, 8-prenylnaringenin, reverses the ovariectomy-induced rise in skin temperature in an animal model of menopausal hot flushes. J Endocrinol. 2006;191:399–405. doi: 10.1677/joe.1.06919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grady D, Sawaya GF, Johnson KC, Koltun W, Hess R, Vittinghoff E, Kristof M, Tagliaferri M, Cohen I, Ensrud KE. MF101, a selective estrogen receptor beta modulator for the treatment of menopausal hot flushes: a phase II clinical trial. Menopause. 2009 doi: 10.1097/gme.0b013e31818e64dd. [DOI] [PubMed] [Google Scholar]
- 33.Strom A, Hartman J, Foster JS, Kietz S, Wimalasena J, Gustafsson JA. Estrogen receptor beta inhibits 17beta-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc Natl Acad Sci U S A. 2004;101:1566–1571. doi: 10.1073/pnas.0308319100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC. Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 2004;64:423–428. doi: 10.1158/0008-5472.can-03-2446. [DOI] [PubMed] [Google Scholar]
- 35.Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 36.Monroe DG, Secreto FJ, Subramaniam M, Getz BJ, Khosla S, Spelsberg TC. Estrogen receptor alpha and beta heterodimers exert unique effects on estrogen- and tamoxifen-dependent gene expression in human U2OS osteosarcoma cells. Mol Endocrinol. 2005;19:1555–1568. doi: 10.1210/me.2004-0381. [DOI] [PubMed] [Google Scholar]
- 37.Cristofaro PA, Opal SM, Palardy JE, Parejo NA, Jhung J, Keith JC, Jr, Harris HA. WAY-202196, a selective estrogen receptor-beta agonist, protects against death in experimental septic shock. Crit Care Med. 2006;34:2188–2193. doi: 10.1097/01.CCM.0000227173.13497.56. [DOI] [PubMed] [Google Scholar]
- 38.Harris HA, Bruner-Tran KL, Zhang X, Osteen KG, Richard Lyttle C. A selective estrogen receptor-{beta} agonist causes lesion regression in an experimentally induced model of endometriosis. Hum Reprod. 2004 doi: 10.1093/humrep/deh711. [DOI] [PubMed] [Google Scholar]
- 39.Cvoro A, Tatomer D, Tee MK, Zogovic T, Harris HA, Leitman DC. Selective estrogen receptor-agonists repress transcription of proinflammatory genes. J Immunol. 2008;180:630–636. doi: 10.4049/jimmunol.180.1.630. [DOI] [PubMed] [Google Scholar]
- 40.Lin J, Steenbergen C, Murphy E, Sun J. Estrogen receptor-beta activation results in S-nitrosylation of proteins involved in cardioprotection. Circulation. 2009;120:245–254. doi: 10.1161/CIRCULATIONAHA.109.868729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grumbach MM, Auchus RJ. Estrogen: consequences and implications of human mutations in synthesis and action. J Clin Endocrinol Metab. 1999;84:4677–4694. doi: 10.1210/jcem.84.12.6290. [DOI] [PubMed] [Google Scholar]
- 42.Harris HA, Katzenellenbogen JA, Katzenellenbogen BS. Characterization of the biological roles of the estrogen receptors, ERalpha and ERbeta, in estrogen target tissues in vivo through the use of an ERalpha-selective ligand. Endocrinology. 2002;143:4172–4177. doi: 10.1210/en.2002-220403. [DOI] [PubMed] [Google Scholar]
- 43.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lundholm L, Bryzgalova G, Gao H, Portwood N, Falt S, Berndt KD, Dicker A, Galuska D, Zierath JR, Gustafsson JA, et al. The estrogen receptor {alpha}-selective agonist propyl pyrazole triol improves glucose tolerance in ob/ob mice; potential molecular mechanisms. J Endocrinol. 2008;199:275–286. doi: 10.1530/JOE-08-0192e. [DOI] [PubMed] [Google Scholar]
- 45.Helguero LA, Faulds MH, Gustafsson JA, Haldosen LA. Estrogen receptors alfa (ERalpha) and beta (ERbeta) differentially regulate proliferation and apoptosis of the normal murine mammary epithelial cell line HC11. Oncogene. 2005;24:6605–6616. doi: 10.1038/sj.onc.1208807. [DOI] [PubMed] [Google Scholar]
- 46. Krum SA, Miranda-Carboni GA, Lupien M, Eeckhoute J, Carroll JS, Brown M. Unique ERalpha cistromes control cell type-specific gene regulation. Mol Endocrinol. 2008;22:2393–2406. doi: 10.1210/me.2008-0100. This important study demonstrated that the pioneer factor FoxA1 is important for cell-specific regulation of genes by estrogens.