Table 2.
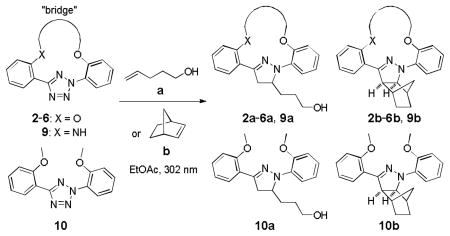
Photoinduced 1,3-Dipolar Cycloaddition Reactions of Macrocyclic and Acylic Tetrazoles with Alkenes[a].
 | |||||
|---|---|---|---|---|---|
| entry | alkene | tetrazole | pyrazoline | time[b] (min) | yield[c] (%) |
| 1 | 2 | 2a | 120 | 59 | |
| 2 | 3 | 3a | 75 | 71 | |
| 3 | 4 | 4a | 84 | 60 | |
| 4 | 5 | 5a | 180 | 70 | |
| 5 | 6 | 6a | 120 | 46[d] | |
| 6 | 9 | 9a | 300 | 43 | |
| 7 | 10 | 10a | 120 | 58 | |
| 8 |  |
2 | 2b | 108 | 95 |
| 9 | 3 | 3b | 54 | 91 | |
| 10 | 4 | 4b | 120 | 81 | |
| 11 | 5 | 5b | 240 | 60 | |
| 12 | 6 | 6b | 90 | 84 | |
| 13 | 9 | 9b | 120 | 84 | |
| 14 | 10 | 10b | 60 | 76 | |
Reactions were conducted by irradiating 0.1 mmol of tetrazole and 10 mmol of alkene dipolarophile in 250 mL EtOAc with a 302-nm UV lamp in quartz flask.
Time was determined by tracing the disappearance of the starting materials on TLC.
Isolated yields.
The pyrazoline adduct was unstable upon standing.
