Summary
MicroRNAs (miRNA) are small, non-coding RNAs that regulate gene expression post-transcriptionally. We investigated the hypothesis that Bone Morphogenetic Protein (Bmp)-signaling regulates miRNAs in cardiac progenitor cells. Bmp2 and Bmp4 regulate OFT myocardial differentiation via regulation of the miRNA 17-92 cluster. In Bmp mutant embryos, myocardial differentiation was delayed and multiple miRNAs encoded by miRNA 17-92 were reduced. We uncovered functional miRNA17-92 seed sequences within the 3′ UTR of cardiac progenitor genes such as Isl1 and Tbx1. In both Bmp and miRNA 17-92 mutant embryos, Isl1and Tbx1 expression failed to be correctly downregulated. Transfection experiments indicated that miRNA 17 and miRNA 20a directly repressed Isl1and Tbx1. Genetic interaction studies uncovered a synergistic interaction between miRNA 17-92 cluster and Bmp4 providing direct in vivo evidence for the Bmp-miRNA 17-92 regulatory pathway. Our findings indicate that Bmp-signaling directly regulates a miRNA-mediated effector mechanism that downregulates cardiac progenitor genes and enhances myocardial differentiation.
Keywords: Bone morphogenetic protein (Bmp), cardiovascular, microRNA
Introduction
The cardiac outflow tract (OFT), a developmentally complex structure that is often defective in patients with congenital heart disease, arises from Isl1-expressing second heart field (SHF) cardiac progenitors. Lineage tracing and retrospective clonal analysis uncovered two cardiac progenitor pools. The first heart field (FHF) contributes primarily to left ventricle while SHF forms outflow tract (OFT), right ventricle (RV), and inflow tract (Buckingham et al., 2005). SHF progenitors diversify into myocardium, endocardium, and vascular smooth muscle supporting the notion of a common precursor that gives rise to three lineages in vivo (Laugwitz et al., 2008).
The Bone morphogenetic protein (Bmp) signaling pathway regulates cardiac progenitor development both in vivo and in embryonic stem cell culture systems (Liu et al., 2004; Prall et al., 2007; Ying et al., 2003). Bmps signal via a heterodimeric receptor complex containing type I and type II receptors. In the canonical pathway, activated serine-threonine kinase type I receptor phosphorylates one of the Bmp receptor regulated Smads (R-Smads): Smad1, Smad5 or Smad8 (Derynck and Zhang, 2003). After phosphorylation, the R-Smad is released from the receptor complex, associates with the common Smad4, and translocates to the nucleus to regulate gene expression.
Recent findings indicate that Bmp signals through microRNA (miRNA)-mediated pathways to regulate gene expression in tissue culture either via the canonical pathway or by promoting processing of primary (pri)-miRNA to precursor (pre)-miRNA. The Smad1-Drosha complex interaction is independent of both C-terminal phosphorylation and Smad4 (Davis et al., 2008; Li et al., 2008).
Our data indicate that Bmp-signaling promotes OFT myocardial differentiation from cardiac progenitors by directly regulating miRNA 17-92 expression through a conserved Smad binding element. Our findings, suggesting that miRNA 17-92 directly downregulates Isl1 and Tbx1, reveal a mechanism for Bmp-regulated myocardial differentiation and identify a critical miRNA pathway under the control of Bmp signaling in vivo.
Results
Bmp2 and Bmp4 deletion in second heart field progenitors
We generated compound Bmp2 and Bmp4 conditional mutants using conditional null alleles and the Mef2ccre driver (Fig. S1 A, B). Bmp2 and Bmp4, vertebrate orthologs of Drosophila Decapentaplegic (dpp), are highly related with 92% identity within the C-terminal mature ligand domain. Whole mounts with Bmp2 exon 3 and Bmp4 exon 4 probes, the deleted regions of Bmp2 and Bmp4, indicated efficient inactivation of both genes by embryonic day (E) 9.5 (Fig. S1 C-F). P-Smad1/5/8 immunostaining revealed a strong reduction in Bmp-signaling in dorsal pericardium, OFT myocardium, and endocardium in Bmp2;Bmp4 (Bmp2/4) CKO mutant embryos (Fig. S1 G, H). Quantitative RT pcr (QRTpcr) indicated efficient deletion in E9.5 Bmp2/4 CKO mutant embryos (Fig. S1 I).
To determine if Bmp2 and Bmp4 deletion was deleterious for heart development, we collected embryos at multiple developmental stages. Viable Bmp2/4 CKO mutant embryos were recovered until E12.5 but not at later stages indicating that Bmp2/4 deletion was embryonic lethal (Table S1). Bmp2/4 CKO mutant embryos had severe pericardial edema indicating that these embryos had heart failure (Fig. S1 J, K).
Bmp-signaling promotes myocardial differentiation
Examination of myocardial differentiation revealed that sarcomeric myosin was drastically reduced in Bmp2/4 CKO mutant OFT (Compare Fig. 1 A, B to F, G). Using a Bmp4 gain of function allele (Bmp4 OE) (see Materials and Methods), we observed more intense immunofluorescence and thickened myocardium in both OFT and RV of Bmp4 OE embryos (Compare Fig. 1 C, D, E to H, I, J and Fig. 1K). QRTpcr revealed that myocardial differentiation markers were downregulated in Bmp 2/4 CKO mutants (Fig. 1 L) and upregulated in Bmp4 OE embryos (Fig. 1 M). These data, indicating that Bmp2 and Bmp4 promote OFT myocardial differentiation, support previous observations (reviewed in (Dyer and Kirby, 2009)).
Figure 1. Myocardial differentiation in Bmp mutant embryos.
(A-J) Immunofluorescence with MF20 (green, denoted by arrow). Genotypes are shown. (K) Myocardial thickness at E10.5. (L,M) Bar graph for QRTPCR assay. (N-S) Immunofluorescence with Isl1 antibody (green, arrows) at E9.75. (T-W) Whole mount in situ hybridization at E9.5. ba, branchial arch; e, eye. Nuclei stained with TO-PRO-3 iodide (TP3) (red) in all Immunofluorescence. *, (P < 0.05; error bars represent SEM).
Failure to silence cardiac progenitor genes in Bmp mutant embryos
In control embryos, immunostaining with an Isl1 antibody indicated that SHF progenitors in dorsal pericardium, pharyngeal mesoderm and distal OFT myocardium express Isl1 (Fig. 1 N, O, P) (Cai et al., 2003). In proximal OFT myocardium, Isl1 expression was extinguished although rare proximal myocardial cells expressed Isl1. In contrast, Isl1 was expressed throughout the whole length of the Bmp2/4 CKO mutant OFT myocardium indicating a failure to downregulate Isl1 in OFT (Fig. 1 Q, R, S). Downregulation of other cardiac progenitor genes, such as Tbx1 and Fgf10, was also defective in Bmp2/4 CKO mutant embryos (Fig. 1 T, U, V, W and Fig. S1 L).
In contrast, the Wnt11-Tgf2 pathway, important for OFT morphogenesis, was significantly reduced in Bmp2/4 CKO mutants (Fig. S1 L) (Zhou et al., 2007). The progenitor cell marker, Hand1 was reduced to 30% of control but Hand2 and Nkx2.5 were unchanged in Bmp2/4 CKO mutants (Fig. S1 L). These data, indicating that Bmp-signaling induces some progenitor genes while inhibiting others, suggested distinct mechanisms for Bmp-regulated gene expression in cardiac progenitors.
Bmp regulates the miRNA 17-92 complex
Expanded progenitor gene expression, such as Isl1 and Tbx1, in Bmp2/4 CKO mutants suggested that Bmp-signaling might regulate progenitor genes through a miRNA-mediated mechanism. MiRNA profiling indicated that multiple mature miRNAs encoded by the miRNA 17-92 complex and its two related complexes were drastically reduced (Fig. 2 A). The miRNA 17-92 complex encodes six miRNAs that are processed from a common primary miRNA (Fig. 2 C). Two homologous clusters, miRNA 106b-25 and miRNA 106a-363, are found in the mouse and human genomes (Fig. 2 C). Previous experiments indicated that miRNA 17-92 complex promotes lung progenitor proliferation and cardiac septation (Lu et al., 2007; Ventura et al., 2008).
Figure 2. Bmp-signaling regulates miRNAs.
(A) miRNA array from Bmp2/4 CKO embryos. MiRNAs from the miR-17-92 complex and homologous complexes (arrows). Other cardiovascular miRNAs (asterisk). (B) QRTPCR validation of miRNA array data at E9.5. *, statistically significant difference (P < 0.05). (C) Genomic structure for miR-17-92 and two orthologous clusters (left) and sequences of mature miRNA sequences of miR-17 family with seed sequence yellow highlighted (right) (Modified from Mendell 2008). (D-I) In situ hybridization: (D) miR-17 is expressed in OFT (upper arrow) and SHF (lower arrow) at E9.5. (E) Blank control for E9.5 miR-17 in situ hybridization. (F, H) pri-miR-17-92 in situ whole mount (F) and section (H) at E10.5. Proximal OFT (arrow). (G, I) Sense control for pri-miR-17-92 in situ. ba, branchial arch; oft, outflow tract. (J-L) MiR-17-92 5′ upstream Luciferase reporter (miR-17-92-pGL3) activity co-transfected with constitutively active Alk3 (caAlk3) (J), pcDNA3.1-Smad6 (K), and pSR siSmad1 (L). *, (P <0.05; error bars represent SEM). (M) In vivo chromatin immunoprecipitation (ChIP) using E9.5 hearts with indicated antibodies to IP chromatin fragment containing the miR-17-92 5′ upstream Bmp/Smad element.
Because the miRNA 17-92 cluster encodes the predominant function of the three related clusters, we focused our analysis on this cluster (Ventura et al., 2008). QRT-pcr indicated that pri-miRNA 17-92 was reduced, as was pre- and mature miRNA 17 and miRNA 20a suggesting that Bmp-signaling regulates pri-miRNA 17-92 expression (Fig. 2 B). In situ analysis indicated that at E9.5 mature miRNA 17 was expressed in OFT myocardium and SHF (Fig. 2 D, E). At E10.5, pri-miRNA17-92 expression was detected in proximal OFT myocardium in cells that normally silence Isl1 (Fig. 2 F-I).
To determine if Bmp-signaling directly regulated pri-miRNA 17-92, we used a bioinformatics approach to uncover a region of miRNA17-92 that contained a number of phylogenetically conserved Smad recognition elements ((Karaulanov et al., 2004) and Fig. S2 I). This miRNA17-92 region was cloned into a Luciferase reporter vector and tested for Bmp inducibility. Co-transfection of a constitutively active Bmpr1a construct with the miRNA17-92 Luc reporter resulted in approximately 4-fold induction (Fig. 2 J). Co-transfection of the miRNA17-92 Luc reporter with inhibitory Smad6 and Smad1 shRNA abolished miRNA17-92 Luc reporter activity (Fig. 2 K, L). To determine whether Smad1 or Smad5 directly bound to miRNA17-92, we performed in vivo ChIP using embryonic heart extracts. We noted enrichment in the anti-Smad1/5 immunoprecipitated chromatin compared to control indicating that Smad1/5 directly bound miRNA17-92 chromatin (Fig. 2 M). These findings support the hypothesis that miRNA 17-92 is a direct target of Bmp-signaling.
MiRNA17-92 regulates cardiac progenitor gene expression
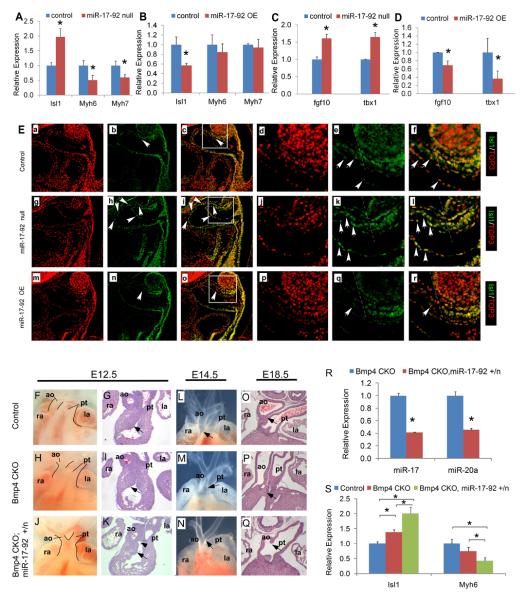
We examined embryos that were homozygous for a null miRNA17-92 allele, miRNA17-92null, to determine if cardiac progenitor genes were upregulated in the absence of miRNA17-92 (Ventura et al., 2008). Homozygous miRNA17-92null mutant embryos failed to express miRNA17 and miRNA20a (not shown). Isl1 expression was upregulated twofold while Myh6 and Myh7 were significantly downregulated in miRNA17-92null −/− mutant OFT (Fig. 3 A). Both Fgf10 and Tbx1 were upregulated in miRNA17-92null −/− mutant embryos (Fig. 3 C).
Figure 3. MiR-17-92 cardiac phenotype and Bmp4 genetic interaction.
(A-D) Real time qPCR analysis. *, statistically significant difference (P < 0.05). (E) Immunofluorescence at E9.5. Boxed areas in c, i, o are correspondingly shown at higher magnification in d-f, j-l and p-r. Tangent line with branchial arch landmark (dashed line), reveals Isl1-expressing progenitors toward proximal OFT (left of dashed line) were persistent in miR-17-92 null mutants (arrows in h, i, k and l) while decreased in controls (arrows in b, c, e and f) and almost extinguished in miR-17-92 OE mutants (arrows in n, o, q and r). Persistent Isl1-expressing progenitors in endocardium of miR-17-92 null mutants (arrowheads in h, i, k and l). ao, aorta; la, left atrium; ra, right atrium; pt, pulmonary trunk. (F-K) Whole mount (F, H, J) and sections (G, I, K) of embryos with the indicated genotypes and stages. Arrows in G, I, K denote the proximal cushion mesenchyme and arrowhead in K the OFT septum. (L-N) E14.5 embryos showing normal and defective OFT alignment (arrows). (O, P, Q) Transverse sections through OFT showing normal and defective separation between the aorta and pulmonary trunk (arrows). (R, S) Real time qPCR analysis. *, (P < 0.05; error bars represent SEM).
We crossed the miRNA17-92 OE allele, that contains the human miRNA17-92 cluster introduced into the rosa26 locus, to the Mef2cCre transgenic line to conditionally overexpress miRNA17-92 in SHF and OFT myocardium (Xiao et al., 2008). QRTpcr experiments indicated that miRNA17 and miRNA20a were upregulated by approximately 1.7 to 2.0 fold in the Mef2ccre; miRNA17-92OE embryos (not shown). QRTpcr indicated that in Mef2ccre; miRNA17-92OE embryos Isl1 expression was reduced to 60% of control (Fig. 3 B). Although Isl1 was reduced, Myh6 and Myh7 expression levels were unchanged in the Mef2ccre; miRNA17-92OE embryos indicating that modestly reduced Isl1 levels failed to change the E9.5 myocardial differentiation program (Fig. 3 B). Fgf10 and Tbx1 were reduced in the Mef2ccre; miRNA17-92OE to 75% and 40% of wild type levels (Fig. 3 D). These gene expression changes are insufficient to cause myocardial defects since cardiac OFT defects are first observed in Tbx1 mutant mouse embryos when Tbx1 levels are reduced to 20% of wild type (Zhang and Baldini, 2008). Fgf10 mutants don't have an OFT phenotype (Watanabe et al., 2010).
Immuofluorescence indicated that in controls, Isl1 positive cells were primarily located in distal OFT and were only rarely found in proximal OFT (Fig. 3 E d-f) while in miRNA17-92null −/− mutant OFT Isl1 expression was expanded proximally (Compare Fig. 3 E a-f to Fig 3 E g-l). In contrast, in miRNA17-92OE embryos Isl1 expression was confined to the distal OFT and was not expressed in proximal OFT (Fig. 3 E m-r).
Functional interaction between Bmp4 and miRNA 17-92 in vivo
We crossed the miRNA 17-92 null allele onto Mef2ccre ;Bmp4 conditional mutant background to test for genetic interaction in compound mutant embryos. Although miRNA 17-92 null homozygous mutants have ventricular septal defects, small right ventricle, and double outlet right ventricle (Fig. S2 A-H and (Ventura et al., 2008)), miRNA 17-92 null heterozygous embryos do not have a heart phenotype. Bmp4 CKO mutant embryos have a mild OFT phenotype due to redundancy with Bmp2 ((McCulley et al., 2008); this work). In addition to its overlapping function with Bmp4, Bmp2 is known to have a distinct role in cardiac progenitor specification and growth regulation (Prall et al., 2007).
At E12.5, Bmp4 CKO mutant embryos had defective OFT remodeling with a deficiency in proximal OFT mesenchyme (Fig. 3 Compare F, G to H, I). In Bmp4 CKO; miRNA 17-92 +/null mutants, the proximal OFT mesenchyme deficiency was more severe, resulting in failure of fusion between proximal mesenchyme and OFT septum (Fig. 3 J, K). At E14.5 and E18.5, the Bmp4 CKO mutant had abnormal alignment of the aorta and PT with a deficiency in separation of proximal OFT (Fig. 3 Compare L, O to M, P). In Bmp4 CKO; miRNA17-92 +/null embryos, the deficiency in separation of the proximal OFT was more severe than Bmp4 CKO mutants (Fig. 3 Compare M, P to N, Q). QRT-pcr confirmed that mature miRNA 17 and miRNA 20a were reduced in the Bmp4 CKO; miRNA17-92 +/null embryos when compared to Bmp4 CKO mutants (Fig. 3 R). Isl1 was upregulated and Myh6 was reduced in the Bmp4 CKO; miRNA17-92 +/null compared to Bmp4 CKO mutants (Fig. 3 S). These data add further support for our model that Bmp-signaling regulates the miRNA 17-92 complex during myocardial differentiation in vivo.
Direct regulation of cardiac progenitor genes by miRNA17-92
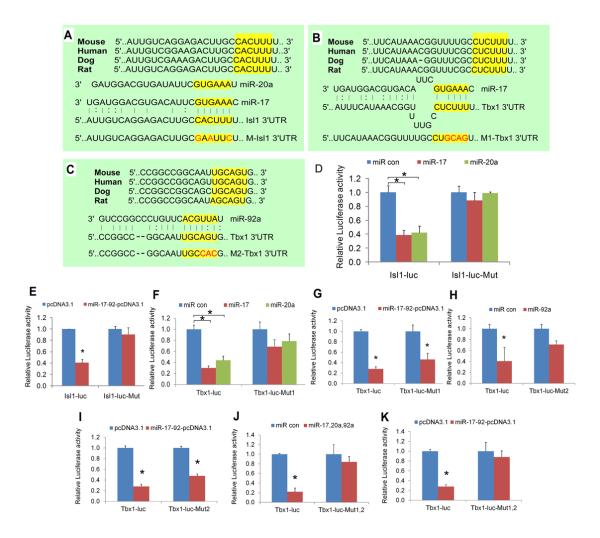
Database searches revealed conserved miRNA 17 and miRNA 20a seed sequence in the Isl1 3′UTR (Fig. 4 A). The Tbx1 3′UTR contained both a miRNA 17 family seed site and a miRNA 92 family seed site (Fig. 4 B, C). To test miRNA seed sequence function, we cloned the Isl1 and Tbx1 3′UTRs into luciferase reporter plasmids. Transfection with miRNA mimics, and a miRNA 17-92 expression plasmid resulted in drastic reduction in Luciferase activity for both Isl1 and Tbx1 reporter plasmids (Fig. 4 D, E, F, G, H). Mutation of miRNA 17 and miRNA 20a seed sequences within Isl1 3′UTR resulted in loss of repression by miRNA 17 and miRNA 20a and the miRNA 17-92 expression plasmid (Fig. 4 D, E). Likewise, mutation of individual seed sites in the Tbx1 reporter resulted in loss of repression by the corresponding miRNA mimic (Fig. 4 F, H). The Tbx1 reporter became refractory to the miRNA 17-92 expression plasmid or mixtures of miRNA mimics only when both seed sites were simultaneously mutated (Fig. 4 G, I, J, K). These data suggest that the miRNA 17-92 complex directly inhibits Isl1 and Tbx1.
Figure 4. Isl1 and Tbx1 are miR-17-92 targets.
(A-C) Phylogenetic sequence alignment and mutagenesis of miR-17-92 family seed sequence in Isl1 3′ UTR (A) and Tbx1 3′ UTR (B, C). (D-K) Luciferase reporter assays with reporters and miR's as labelled. *, (P < 0.05; error bars represent SEM).
Discussion
Bmp-signaling regulates the miRNA 17-92 complex to control a pathway promoting SHF myocardial differentiation. As a result of defective miRNA activity, physiologic repression of cardiac progenitor genes such as Isl1 is relieved in Bmp2/4 CKO mutant OFT. We propose that increased cardiac progenitor gene activity results in defective myocardial differentiation. Our findings uncover a mechanism underlying Bmp-regulated OFT myocardial differentiation and reveal that Bmp-regulated miRNA activity is critical for fine tuning the dose of cardiac progenitor genes.
The role of miRNAs in cardiac development
MiRNAs are critical regulators of gene expression in diverse organisms (Bartel, 2009). Embryos with a Dicer deletion in the developing heart had dilated cardiomyopathy uncovering a physiologic role for miRNAs in myocardial development (Chen et al., 2008). Other experiments looking at individual cardiac miRNAs focused on the role of miRNA 1 and miRNA 133. MiRNA 1 regulates cardiac morphogenesis, growth and conduction system development in part via regulation of Hand2 (Zhao et al., 2007; Zhao et al., 2005). Other studies indicated that miRNA 133 was necessary for cardiac differentiation and growth in mice and zebrafish (Chen et al., 2006; Liu et al., 2008; Mishima et al., 2009).
Cell non-autonomous mechanisms regulating miRNA expression have been much less characterized. Bmp-signaling promotes pri-miRNA processing to pre-miRNA in cultured smooth muscle cells through a mechanism involving Smad1 binding to the Drosha complex (Davis et al., 2008). Our results suggest that Bmp-signaling regulates miRNA 17-92 expression through Smad-mediated pri-miRNA transcriptional activation rather than processing.
Although we focused on miRNA 17-92 complex in our studies, our profiling data reveal that other important cardiovascular miRNAs, such as miRNA 143 and miRNA 145, are downregulated in Bmp 2/4 CKO mutants (Boettger et al., 2009; Cordes et al., 2009; Elia et al., 2009; Xin et al., 2009). Other miRNAs are upregulated in the Bmp 2/4 CKO mutants suggesting that Bmp-signaling likely regulates multiple events in cardiac development through miRNA effector pathways.
Tight regulation of cardiac progenitor gene dose is critical for normal cardiac development
Timely downregulation of cardiac progenitor genes, such as Isl1 and Tbx1, is required for myocardial differentiation. Transgenic Tbx1 overexpression in mouse embryos increased Fgf10 expression, inhibited cardiomyocyte differentiation, and resulted in embryonic lethality (Chen et al., 2009; Hu et al., 2004; Vitelli et al., 2009; Zhang and Baldini, 2008)). Tbx1 also directly regulates Fgf10 expression in a cell culture system (Xu et al., 2004). Tbx1 overexpression in the range of 4.5 to 5 fold resulted in phenotypes that were reminiscent of the loss of function phenotype (Liao et al., 2004). Interestingly, Tbx1 interferes with the Smad1-Smad4 interaction required for canonical Bmp-signaling suggesting a tightly controlled feedback mechanism to concurrently refine both Bmp-signaling responsiveness and Tbx1 levels (Fulcoli et al., 2009).
Although less is known about Isl1, overexpression of Isl1 has been shown to inhibit differentiation of craniofacial skeletal muscle (Harel et al., 2009). Our findings indicate that low level overexpression of multiple progenitor genes such as Isl1 and Tbx1 inhibits cardiomyocyte differentiation.
The miRNA 17-92 complex in cardiac development
Little is known about the physiologic role of miRNA17-92 cluster in cardiac development (Mendell, 2008; Petrocca et al., 2008). MiRNA 17-92 complex mutant embryos are perinatal lethal and have ventricular septal defects. Our findings indicate that miRNA 17-92 mutant embryos also have double outlet right ventricle (DORV), a common phenotype in embryos with defective SHF development. One of the two related complexes, miRNA 106b-25, was shown to have functional overlap with miRNA 17-92 during heart development (Ventura et al., 2008). Genetic data indicate that the miRNA 17-92 cluster encodes most of the function for the three clusters since one copy of the miRNA 17-92 cluster is enough to support normal mouse development (Ventura et al., 2008). Of the four miRNA families encoded by the three loci, the miRNA 17 family is most affected in the Bmp2/4 CKO mutants with downregulation of five out of the six miRNAs.
Our data demonstrate that Isl1 and Tbx1 are regulated by miRNAs. In other contexts, different miRNA 17-92 targets have been identified, such as Rbl2 in the developing lung and the proapoptotic gene Bim in B lymphocytes (Carraro et al., 2009; Lu et al., 2007). MiRNA 92a regulates angiogenesis in the adult by regulating the Integrin α5 target gene (Bonauer et al., 2009). MiRNA 17 also regulates fibronectin at later stages of organogenesis (Shan et al., 2009). This is consistent with conservative estimates that miRNAs have as many as 300 target genes each when analyzed using stringent criteria (Bartel, 2009).
Our data indicate that the miRNA 17 and miRNA20a recognize a 6 nucleotide (nt) seed sequence in the Isl1 3′ UTR. It is generally thought, based in large part on computational grounds, that 7 nt is optimal for target site recogition (Bartel, 2009). Nonetheless, there is strong experimental evidence supporting the importance of 6 nt seed site recognition in target gene regulation (Lim et al., 2005). Our findings add further support for the importance of 6 nt seed site recognition.
Relevance for cardiac renewal and regeneration
Recent C-14 dating experiments revealed that adult cardiomyocytes have a small capacity to self renew (Bergmann et al., 2009; Parmacek and Epstein, 2009). Also, Isl1-lineage cardiac progenitors have a limited proliferative potential suggesting an intrinsic mechanism to modulate progenitor growth (Domian et al., 2009). Importantly, loss of proliferative potential corresponded to activation of differentiation markers and silencing of progenitor genes such as Isl1. The capacity for cardiac progenitors to expand is an absolute requirement for therapeutic utility. Our findings indicate that Isl1 and Tbx1 expression levels may be manipulated by antagonizing miRNA 17 and miRNA 20a activity.
Isl1 is generally though to be an anti-differentiation factor in cardiac development. Work from Prall and colleagues uncovered defective Isl1 downregulation in Nkx2.5 mutant embryos during SHF specification (Prall et al., 2007). The developmental context for the Isl1 expansion reported by Prall et al. is distinct from our observation. Since we used the Isl1-dependent Mef2cCre driver to delete Bmp2 and Bmp4, initiation of Isl1 expression occurs prior to Bmp2/4 deletion. It will be interesting to determine if miRNA mediated regulation also underlies expanded Isl1 in the Nkx2.5 mutant embryos. Recent data indicate that Isl1 may also promote myocardial differentiation in embryoid bodies supporting the idea that Isl1 function is context dependent (Cai et al., 2003; Kwon et al., 2009; Laugwitz et al., 2008). Taken together, our data indicate that a major signaling pathway, Bmp-signaling, controls the timing of progenitor gene silencing in OFT myocardium by regulating the expression of miRNAs. If this progenitor gene downregulation is delayed then OFT myocardial differentiation is suppressed.
Materials and Methods
Mouse alleles and transgenic lines
The Bmp2 and Bmp4 conditional null, miRNA17-92 alleles, and Mef2c cre line were described (Liu et al., 2004; Ma and Martin, 2005; Verzi et al., 2005; Ventura et al., 2008). The Bmp4tetO gain of function allele will be described elsewhere (MBC and JFM unpublished data, supplement).
In situ hybridization for miRNA
Tissue preparation and automated miRNA in situ hybridization were as described (Yaylaoglu et al., 2005). miRCURY LNA™ probe mmu-miR-17 and mmu-miR-20a purchased from Exiqon and used per manufacturer's guidelines.
X-gal staining and Histology
For histology, embryos were fixed overnight in buffered formalin, dehydrated through graded ethanol and paraffin embedded. Sections were cut at 7-10 microns and stained with H&E. Staining for LacZ as previously described (Lu et al., 1999).
Immunohistochemistry and Immunofluorescence
Embryos were fixed, embedded and sectioned, followed by immunohistochemistry or immunofluorescence (detailed information in supplement).
Real time quantitative polymerase chain reaction
Total RNA from embryonic hearts was isolated using RNeasy® Micro Kit (QIAGEN) and real-time thermal cycling performed using Mx3000P thermal cycler (Stratagene). For miRNAs, TaqMan microRNA assay kits (Applied Biosystems) were used according to manufacturer's guidelines. For other real time q-PCR assays, Super ScriptTM II Reverse Transcriptase (invitrogen) was used for RT-PCR and SYBR® Green JumpStart TM Taq ReadyMix TM (SIGMA) was used for real-time thermal cycling. All error bars represent SEM. Primer sequences available upon request.
Microarray for miRNAs
Total RNA from mouse embryonic hearts was extracted and purified using mirVana™ miRNA Isolation Kit (Ambion). RNA samples were labeled and analyzed by LC Sciences using a μParaflo® Microfluidic Biochip technology as previously described (van Rooij et al., 2008).
Site-directed mutagenesis
Site-directed mutagenesis of the seed sites in Isl1 3′ UTR and Tbx1 3′ UTR were performed with QuikChange II Site-Directed Mutagenesis kit (Stratagene) (detail information in supplement).
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) was performed with a Smad158 antibody on E9.5 hearts using a ChIP assay kit (Upstate) (detail information in supplement).
Luciferase activity assay
Expression and reporter plasmids were described above. MiRNA mimics were purchased from Thermo Scientific. Constitutively active Alk3 (caAlk3) was gift from Dr. Massague's lab (Memorial Sloan Kettering). pcDNA3.1-Smad6 expression plasmid, pSUPER RNAi vector and pSR siSmad1 plasmid were gifts from Dr. Feng's lab (Baylor College of Medicine). P19 cells and 293T cells were transfected using Lipofectamine 2000 (Invitrogen). Luciferase activity assays were performed using the luciferase Assay System (Promega).
Doxycycline administration
Pregnant females were supplied for 12 or 24 hours with doxycycline (Sigma cat# D9891) in the drinking water (2 mgDox/ml) and in the food (Bio-Serv cat# S388; 200mgDox/kg).
Supplementary Material
Acknowledgements
We thank S. Evans, R. Kelly, and D. Srivastava for in situ probes. Supported by NIH grants 2R01DE12324-12 and R01HL093484-01 (JFM), T32 DE15355-04 (MBC) and CA096824 (FW); AHA0655077Y from The American Heart Association (FW). JW was supported by grant 09PRE2150024 from the South Central Affiliate of the American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, Braun T. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraro G, El-Hashash A, Guidolin D, Tiozzo C, Turcatel G, Young BM, De Langhe SP, Bellusci S, Shi W, Parnigotto PP, Warburton D. miR-17 family of microRNAs controls FGF10-mediated embryonic lung epithelial branching morphogenesis through MAPK14 and STAT3 regulation of E-Cadherin distribution. Dev Biol. 2009 doi: 10.1016/j.ydbio.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Murchison EP, Tang R, Callis TE, Tatsuguchi M, Deng Z, Rojas M, Hammond SM, Schneider MD, Selzman CH, et al. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc Natl Acad Sci U S A. 2008;105:2111–2116. doi: 10.1073/pnas.0710228105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Fulcoli FG, Tang S, Baldini A. Tbx1 regulates proliferation and differentiation of multipotent heart progenitors. Circ Res. 2009;105:842–851. doi: 10.1161/CIRCRESAHA.109.200295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009 doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Domian IJ, Chiravuri M, van der Meer P, Feinberg AW, Shi X, Shao Y, Wu SM, Parker KK, Chien KR. Generation of functional ventricular heart muscle from mouse ventricular progenitor cells. Science. 2009;326:426–429. doi: 10.1126/science.1177350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer LA, Kirby ML. The role of secondary heart field in cardiac development. Dev Biol. 2009;336:137–144. doi: 10.1016/j.ydbio.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia L, Quintavalle M, Zhang J, Contu R, Cossu L, Latronico MV, Peterson KL, Indolfi C, Catalucci D, Chen J, et al. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ. 2009 doi: 10.1038/cdd.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcoli FG, Huynh T, Scambler PJ, Baldini A. Tbx1 regulates the BMP-Smad1 pathway in a transcription independent manner. PLoS One. 2009;4:e6049. doi: 10.1371/journal.pone.0006049. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Harel I, Nathan E, Tirosh-Finkel L, Zigdon H, Guimaraes-Camboa N, Evans SM, Tzahor E. Distinct origins and genetic programs of head muscle satellite cells. Dev Cell. 2009;16:822–832. doi: 10.1016/j.devcel.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T, Yamagishi H, Maeda J, McAnally J, Yamagishi C, Srivastava D. Tbx1 regulates fibroblast growth factors in the anterior heart field through a reinforcing autoregulatory loop involving forkhead transcription factors. Development. 2004;131:5491–5502. doi: 10.1242/dev.01399. [DOI] [PubMed] [Google Scholar]
- Karaulanov E, Knochel W, Niehrs C. Transcriptional regulation of BMP4 synexpression in transgenic Xenopus. Embo J. 2004;23:844–856. doi: 10.1038/sj.emboj.7600101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C, Qian L, Cheng P, Nigam V, Arnold J, Srivastava D. A regulatory pathway involving Notch1/beta-catenin/Isl1 determines cardiac progenitor cell fate. Nat Cell Biol. 2009 doi: 10.1038/ncb1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugwitz KL, Moretti A, Caron L, Nakano A, Chien KR. Islet1 cardiovascular progenitors: a single source for heart lineages? Development. 2008;135:193–205. doi: 10.1242/dev.001883. [DOI] [PubMed] [Google Scholar]
- Li Z, Hassan MQ, Volinia S, van Wijnen AJ, Stein JL, Croce CM, Lian JB, Stein GS. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc Natl Acad Sci U S A. 2008;105:13906–13911. doi: 10.1073/pnas.0804438105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J, Kochilas L, Nowotschin S, Arnold JS, Aggarwal VS, Epstein JA, Brown MC, Adams J, Morrow BE. Full spectrum of malformations in velo-cardio-facial syndrome/DiGeorge syndrome mouse models by altering Tbx1 dosage. Hum Mol Genet. 2004;13:1577–1585. doi: 10.1093/hmg/ddh176. [DOI] [PubMed] [Google Scholar]
- Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- Liu N, Bezprozvannaya S, Williams AH, Qi X, Richardson JA, Bassel-Duby R, Olson EN. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev. 2008;22:3242–3254. doi: 10.1101/gad.1738708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Selever J, Wang D, Lu MF, Moses KA, Schwartz RJ, Martin JF. Bmp4 signaling is required for outflow-tract septation and branchial-arch artery remodeling. Proc Natl Acad Sci U S A. 2004;101:4489–4494. doi: 10.1073/pnas.0308466101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MF, Pressman C, Dyer R, Johnson RL, Martin JF. Function of Rieger syndrome gene in left-right asymmetry and craniofacial development. Nature. 1999;401:276–278. doi: 10.1038/45797. [DOI] [PubMed] [Google Scholar]
- Lu Y, Thomson JM, Wong HY, Hammond SM, Hogan BL. Transgenic over-expression of the microRNA miR-17-92 cluster promotes proliferation and inhibits differentiation of lung epithelial progenitor cells. Dev Biol. 2007;310:442–453. doi: 10.1016/j.ydbio.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Martin JF. Generation of a Bmp2 conditional null allele. Genesis. 2005;42:203–206. doi: 10.1002/gene.20132. [DOI] [PubMed] [Google Scholar]
- McCulley DJ, Kang JO, Martin JF, Black BL. BMP4 is required in the anterior heart field and its derivatives for endocardial cushion remodeling, outflow tract septation, and semilunar valve development. Dev Dyn. 2008;237:3200–3209. doi: 10.1002/dvdy.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell JT. miRiad roles for the miR-17-92 cluster in development and disease. Cell. 2008;133:217–222. doi: 10.1016/j.cell.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima Y, Abreu-Goodger C, Staton AA, Stahlhut C, Shou C, Cheng C, Gerstein M, Enright AJ, Giraldez AJ. Zebrafish miR-1 and miR-133 shape muscle gene expression and regulate sarcomeric actin organization. Genes Dev. 2009;23:619–632. doi: 10.1101/gad.1760209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmacek MS, Epstein JA. Cardiomyocyte renewal. N Engl J Med. 2009;361:86–88. doi: 10.1056/NEJMcibr0903347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrocca F, Vecchione A, Croce CM. Emerging role of miR-106b-25/miR-17-92 clusters in the control of transforming growth factor beta signaling. Cancer Res. 2008;68:8191–8194. doi: 10.1158/0008-5472.CAN-08-1768. [DOI] [PubMed] [Google Scholar]
- Prall OW, Menon MK, Solloway MJ, Watanabe Y, Zaffran S, Bajolle F, Biben C, McBride JJ, Robertson BR, Chaulet H, et al. An nkx2-5/bmp2/smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell. 2007;128:947–959. doi: 10.1016/j.cell.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan SW, Lee DY, Deng Z, Shatseva T, Jeyapalan Z, Du WW, Zhang Y, Xuan JW, Yee SP, Siragam V, Yang BB. MicroRNA MiR-17 retards tissue growth and represses fibronectin expression. Nat Cell Biol. 2009 doi: 10.1038/ncb1917. [DOI] [PubMed] [Google Scholar]
- van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132:875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzi MP, McCulley DJ, De Val S, Dodou E, Black BL. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol. 2005;287:134–145. doi: 10.1016/j.ydbio.2005.08.041. [DOI] [PubMed] [Google Scholar]
- Vitelli F, Huynh T, Baldini A. Gain of function of Tbx1 affects pharyngeal and heart development in the mouse. Genesis. 2009;47:188–195. doi: 10.1002/dvg.20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Miyagawa-Tomita S, Vincent SD, Kelly RG, Moon AM, Buckingham ME. Role of mesodermal FGF8 and FGF10 overlaps in the development of the arterial pole of the heart and pharyngeal arch arteries. Circ Res. 2010;106:495–503. doi: 10.1161/CIRCRESAHA.109.201665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, Henderson JM, Kutok JL, Rajewsky K. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008;9:405–414. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF, Richardson JA, Bassel-Duby R, Olson EN. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23:2166–2178. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Morishima M, Wylie JN, Schwartz RJ, Bruneau BG, Lindsay EA, Baldini A. Tbx1 has a dual role in the morphogenesis of the cardiac outflow tract. Development. 2004;131:3217–3227. doi: 10.1242/dev.01174. [DOI] [PubMed] [Google Scholar]
- Yaylaoglu MB, Titmus A, Visel A, Alvarez-Bolado G, Thaller C, Eichele G. Comprehensive expression atlas of fibroblast growth factors and their receptors generated by a novel robotic in situ hybridization platform. Dev Dyn. 2005;234:371–386. doi: 10.1002/dvdy.20441. [DOI] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Baldini A. In vivo response to high-resolution variation of Tbx1 mRNA dosage. Hum Mol Genet. 2008;17:150–157. doi: 10.1093/hmg/ddm291. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, Srivastava D. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell. 2007;129:303–317. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436:214–220. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- Zhou W, Lin L, Majumdar A, Li X, Zhang X, Liu W, Etheridge L, Shi Y, Martin J, Van de Ven W, et al. Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family-mediated transcriptional activation of TGFbeta2. Nat Genet. 2007;39:1225–1234. doi: 10.1038/ng2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.